Abstract
The lung is often overlooked as a metabolically active organ, yet biochemical studies have long demonstrated that glucose utilization surpasses that of many other organs, including the heart, kidney, and brain. For most cells in the lung, energy consumption is relegated to performing common cellular tasks, like mRNA transcription and protein translation. However, certain lung cell populations engage in more specialized types of energy-consuming behaviors, such as the beating of cilia or the production of surfactant. While many extrapulmonary diseases are now linked to abnormalities in cellular metabolism, the pulmonary community has only recently embraced the concept of metabolic dysfunction as a driver of respiratory pathology. Herein, we provide an overview of the major metabolic pathways in the lung and discuss how cells sense and adapt to low-energy states. Moreover, we review some of the emerging evidence that links alterations in cellular metabolism to the pathobiology of several common respiratory diseases.
Keywords: cellular metabolism, lung, glycolysis, energy, mitochondria, respiratory disease
INTRODUCTION
Though originally considered to be a passive conduit for gas exchange, it is now appreciated that the lung consumes, on a per gram basis, as much as or even more energy than almost any other organ in the body, including the brain, kidney, and liver. In general, energy consumption in the lung is relegated to performing usual cellular tasks, like rearrangement of cytoskeletal elements, gene transcription and protein translation, and replication and repair of DNA. However, the lung also contains several populations of highly specialized cells that engage in more unique forms of energy-consuming behaviors, such as airway clearance (phagocytosis and ciliary motility), bronchial gland secretion, constriction of airways and blood vessels, and production of pulmonary surfactant. As such, energy consumption in the lung is essential not just for regulating general cellular functions but also for maintaining the unique activities of the organ.
Until recently, the study of pulmonary metabolism was largely dependent on the use of ex vivo, whole-organ perfusion models or artificial in vitro model systems. However, recent technological advances enabled researchers to ask more sophisticated questions about how individual cells within the lung respond and adapt in health and disease. These include novel methods for separating and isolating highly purified populations of cells from digested lung preparations (1–3) so that qualitative and quantitative assessments of individual metabolites (nuclear magnetic resonance and mass spectrometry) and real-time monitoring of cellular metabolism (Agilent Seahorse XF Analyzers) are possible (4, 5). As a result, the last decade has seen an exponential rise in the number of studies related to cellular metabolism in respiratory medicine, reminiscent of the early days in our understanding of surfactant biology. What has emerged from these studies is not just a greater appreciation for the complexity of the lung but also a greater understanding of how alterations in cellular metabolism can contribute to complex pathological events, such as abnormal beating of cilia, acquisition of antiapoptotic behaviors in endothelial cells, phenotypic polarization of alveolar macrophages, and induction of cellular senescence in the alveolar epithelium.
Because the field of cellular metabolism is still emerging, and most lung biologists do not undergo formal training in the field, this review provides an overview of the major metabolic pathways and discusses the mechanisms through which cells normally gauge and adapt to nutrient stress. Moreover, we also review new evidence linking abnormalities in cellular metabolism to the pathobiology of four respiratory diseases: (a) the airway diseases chronic obstructive pulmonary disease (COPD) and asthma; (b) idiopathic pulmonary fibrosis (IPF); and (c) pulmonary hypertension (PH), while also proposing how targeting specific metabolic pathways could be a new avenue in the treatment of these, and other, respiratory conditions.
CELLULAR METABOLISM AND ITS MAJOR PATHWAYS
Cellular metabolism refers to the complex set of chemical reactions that permit cells, organs, and entire organisms to function and thrive. Although cellular metabolism is often discussed in the context of individual pathways, survival of an organism is ultimately dependent on the integration of all metabolic pathways. In fact, other than glycolysis, no major metabolic pathway functions entirely on its own; for example, the pentose phosphate pathway most commonly relies on glucose-6-phosphate from glycolysis in order to proceed, and lipid synthesis cannot move forward without input of both nicotinamide adenine dinucleotide phosphate (NADPH) and adenosine 5′-triphosphate (ATP) from at least two other metabolic pathways. While numerous other examples could be highlighted, the major point is that biochemical events in one metabolic pathway cannot be easily understood if discussed only in isolation. With this in mind, this section reviews each of the major metabolic pathways and also discusses how these pathways communicate with each other to ensure proper growth and functioning of a cell.
Glycolysis and the Warburg Effect
For most tissues, glucose serves as the primary source of energy, and this is also true for the lung (6). In fact, glucose oxidation has been estimated to be 40–50 μmol/(h·g) of dry lung weight, which is a value equal to or greater than most other metabolically active organs (7, 8). The metabolism of glucose begins with a 10-step reaction in the cytoplasm known as glycolysis, or more formally called the glycolytic pathway. In the end, this 10-step reaction converts one molecule of glucose into two 3-carbon pyruvate molecules (Figure 1). Glycolysis also yields two molecules of ATP and reduces two NAD+ molecules to nicotinamide adenine dinucleotide (NADH), which both serve as metabolic fuel for driving other biological reactions.
Figure 1.
Major metabolic pathways. Glycolysis is a 10-step reaction that converts glucose into pyruvate; pyruvate is then converted into lactate or enters the tricarboxylic acid (TCA) cycle as acetyl-CoA to yield NADH and FADH2. NADH and FADH2 are used by the electron transport chain (ETC) to generate ATP. Metabolic intermediates derived from glycolysis can also be diverted to the pentose phosphate pathway (PPP) to generate NADPH and ribose-5-phosphate. Fatty acid synthesis occurs in the endoplasmic reticulum and requires both NADPH and ATP from other metabolic pathways. During periods of energy deprivation, fatty acids are metabolized in mitochondria via β-oxidation, yielding large amounts of NADH and FADH2 for ATP synthesis in the ETC.
Once pyruvate is generated, it usually has one of two fates: Either it is delivered to mitochondria for breakdown via the tricarboxylic acid (TCA) cycle or it remains in the cytoplasm where it undergoes fermentation to lactate by the enzyme lactate dehydrogenase (LDH). By classic teaching, pyruvate is metabolized in mitochondria under aerobic conditions but is converted to lactate in the cytoplasm when oxygen tension is low. However, it is now clear that there are many scenarios in which this rule does not apply. Perhaps the best example of this is in cancer cells, in which the use of aerobic glycolysis is often dubbed the Warburg effect, after the German biochemist who first described the phenomenon (9, 10). Although initially believed to be due to a deficiency in mitochondrial respiration, it is now appreciated that mitochondrial function is often normal or even enhanced in many cancer cells (9, 11–13). Thus, the switch to aerobic glycolysis is now believed to be more of a survival mechanism than an adaptive response. For cancer cells, aerobic glycolysis and the production of lactate provide several important advantages over mitochondrial respiration. This includes the ability to replenish cellular NAD+ levels through the actions of LDH, thereby permitting glycolysis to continue, and the ability to generate ATP at a faster rate than oxidative phosphorylation (12, 14), providing cells with speed over quantity in energy production. Most importantly, glycolysis serves as a rich source of biosynthetic intermediates for building other parts of a cell (9), including the lipids, proteins, and nucleotides needed for growing and proliferating cancer cells.
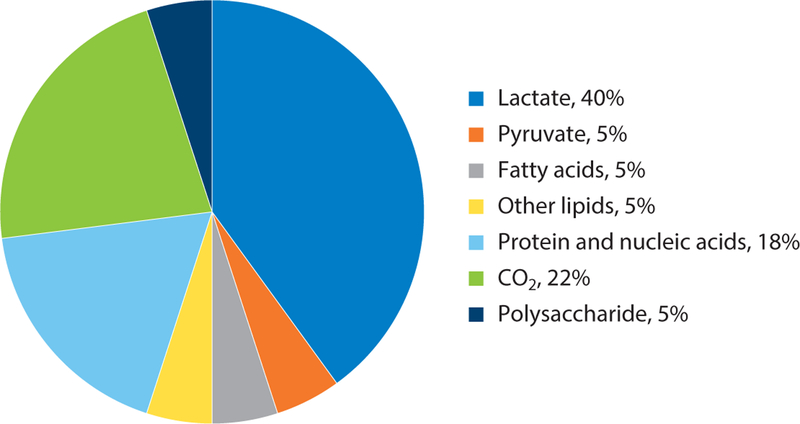
For reasons that remain unclear, lactate production in the normal lung is elevated when compared to many other tissues (15). In fact, carbon labeling studies have demonstrated that nearly 40% of all glucose consumed by the healthy rat lung is ultimately converted to lactate under normal oxygen conditions (Figure 2) (7, 16). This is particularly surprising when one considers that the lung’s need for biosynthetic materials is nowhere near that of most cancer cells, and cell turnover in the lung is actually low when compared to most other tissues (17–19). Interestingly, lactate production appears to be largely independent of oxygen concentration, as levels have been shown to increase only marginally when alveolar PO2 levels are markedly reduced (20, 21). It has been suggested that the lung might have evolved to utilize aerobic glycolysis as a means for minimizing local oxygen consumption, thereby enhancing overall oxygen delivery to other tissues. Additionally, it has been proposed that lactate production might serve as a source of energy for cells in the lung, in particular, for those lacking adequate access to nutrients in the pulmonary circulation. If true, then the healthy lung would be utilizing a strategy often ascribed to cancer cells, in which lactate secretion by the primary tumor cell is important for supporting the activities of other cells (e.g., stromal cells) in the tumor microenvironment (11). In these cells, the uptake of lactate through lactate transporters (also known as monocarboxylate transporters) drives the enzymatic activity of LDH in the opposite direction, yielding pyruvate in the process, which serves to not only feed the TCA cycle but also reduce dependence of these cells on blood glucose for pyruvate production.
Figure 2.
End products of glucose metabolism based on carbon atoms recovered during perfusion of isolated whole lung tissues. The data are represented as percent of total recovery. Data from Reference 10.
The Pentose Phosphate Pathway
The pentose phosphate pathway (PPP) is unique from all other metabolic pathways in that it neither consumes nor generates energy in the form of ATP. This pathway is also distinguished by its dependence on glucose-6-phosphate, which means that it often runs in parallel with glycolysis (Figure 1). Although the PPP performs several functions, it is probably best known for its role in reducing NADP to NADPH; notably, NADPH serves as a key biological reductant for a wide range of cellular reactions, including the regeneration of glutathione and the production of cholesterol and fatty acids. Reduction of NADP is mediated by the rate-limiting enzyme glucose-6-phosphate dehydrogenase (G6PD), whose levels fluctuate in response to various cellular perturbations (e.g., hypoxia) (22). While other enzymatic reactions, such as the conversion of malate to pyruvate by malic enzyme, and the conversion of isocitrate to α-ketoglutarate by isocitrate dehydrogenase, can also generate NADPH, the lung uses the PPP for the bulk of NADPH production (23). In fact, it is estimated that more than 10% of all glucose metabolized in the rat lung is ultimately delivered to the PPP for further breakdown (23–25). The PPP is also responsible for isomeric conversion of ribulose-5-phosphate to ribose-5-phosphate, which is the component sugar for the synthesis of all ribonucleotides. Thus, this makes the PPP an essential metabolic pathway for all transcriptionally active and proliferating cells.
The Tricarboxylic Acid Cycle
The TCA cycle, also known as the citric acid or Krebs cycle, is a metabolic pathway that occurs in the mitochondrial matrix and is best known for its ability to maximize ATP production. While glycolysis nets only two ATPs, the two major by-products of the TCA cycle, NADH and flavin adenine dinucleotide (FADH2), allow for the production of additional 34 ATPs through oxidative phosphorylation in the electron transport chain (ETC). Most commonly, the entry point into the TCA cycle is through glucose-derived pyruvate or fatty acids, after conversion to acetyl coenzyme A (acetyl-CoA). However, various other factors such as glutamine, following conversion to α-ketoglutarate, and the deaminated amino acids such as alanine, cysteine, glycine, serine, and threonine, following conversion to pyruvate, can serve as substrates for the TCA cycle. In addition, the TCA cycle, like glycolysis, can be used to export biosynthetic intermediates to other parts of the cell through a process called cataplerosis. However, for cataplerosis to be sustained over long periods, ongoing input of multiple nutrients into the TCA cycle (also called anaplerosis) is often required.
Fatty Acid β-Oxidation
β-Oxidation is a catabolic process that predominantly occurs in the mitochondrial matrix and yields acetyl-CoA for the TCA cycle and NADH and FADH2 for ATP production by the ETC (26). Although some tissues preferentially utilize fatty acids for much of their energy production (e.g., heart), the lung is believed to primarily utilize this pathway during times of nutrient deprivation (15, 21). In this context, Shaw & Rhoades showed that β-oxidation of fatty acids increases by nearly 40% in the rat lung under starvation conditions (27). While β-oxidation is an excellent source of ATP (100 plus ATP molecules are generated from one 16 carbon fatty acyl chain), there are several notable disadvantages to relying on this pathway for the bulk of a cell’s energy production. For one, β-oxidation is generally more complicated than glycolysis, in that fatty acid molecules must first be conjugated to carnitine in the cytoplasm, and then delivered to mitochondria via a rate-limiting enzyme called carnitine palmitoyl transferase I (CPTI) before breakdown can even begin. Moreover, β-oxidation is also less efficient than glycolysis, at least in terms of oxygen consumption. For example, 23 molecules of O2 are consumed in the process of breaking down one palmitate molecule (yielding 129 ATP molecules), whereas only six O2 molecules are needed in the complete breakdown of one molecule of glucose (yielding 38 ATP molecules). Finally, the breakdown of fatty acids can cause significant oxidative stress to cells, in particular, for those not adequately equipped to handle an acute rise in reactive oxygen species (ROS) production. This is because fatty acid breakdown delivers a large bolus of electrons to the ETC from NADH and FADH2, thereby yielding superoxide as a by-product (28). Notably, very long chain fatty acids greater than 22 carbons and branched fatty acids are initially oxidized in peroxisomes rather than mitochondria (29). This ends in the production of octanoyl-CoA, which is then delivered to mitochondria for further breakdown. A distinct difference between β-oxidation in mitochondria and that in peroxisomes is that the latter does not involve ATP synthesis. Instead, NADH-carried electrons are transferred to O2 to produce H2O2, ultimately yielding H2O and O2 by the enzyme catalase (29). To date, the role of this catabolic process in the lung has not been adequately studied.
LIPID SYNTHESIS IN LUNG
Although most lung biologists consider lipid synthesis to be a process restricted to surfactantproducing cells, it is actually the case that most cells can generate the lipids they need for maintaining their own internal and external membranes. Because lipid production is an energy-consuming process, the activities of fatty acid synthesis pathways are normally closely coupled to the energy status of a cell. For example, when ATP levels are elevated, lipid synthesis is allowed to proceed, leading to an upregulation in the machinery for de novo lipid synthesis; this includes the enzymes fatty acid synthase and acetyl-CoA carboxylase (ACC) as well as the transcription factors sterol regulatory element-binding protein and the liver X receptor (LXR). Because fatty acids are mostly composed of long chains of carbon molecules, lipid synthesis also demands biosynthetic intermediates from other parts of the cell, such as pyruvate from glycolysis, or citrate from the TCA cycle, which are then converted to acetyl-CoA via pyruvate dehydrogenase complex (PDC) and ATP-citrate lyase, respectively (Figure 3). Once acetyl-CoA is made available, fatty acid synthesis begins by carboxylation of acetyl-CoA to malonyl-CoA by ACC, an irreversible reaction that consumes one molecule of ATP. The subsequent pathway of fatty acid synthesis from acetyl-ACP and malonyl-ACP involves the repetition of a 4-step reaction known as the condensation, reduction, dehydration, and reduction steps. In the end, 7 cycles of this 4-step reaction are performed in the synthesis of one 16-carbon fatty acid palmitate, requiring 14 molecules of NADPH and 7 molecules of ATP. Notably, fatty acyl chains longer than 16 carbons require the activity of elongases, a reaction that also consumes NADPH, whereas the conversion of saturated fatty acids to unsaturated fatty acids by desaturase enzymes involves just a hydrogenation reaction. Ultimately, most fatty acyl chains are incorporated into more complex molecules such as triglycerides, phospholipids, and cardiolipins that serve as building blocks for plasma membranes, lipid droplets, and other membrane-containing organelles. Although the formation of these more complex molecules does not usually consume significant amounts of energy, some elements, such as the conversion of choline to phosphocholine, do consume ATP, making even minor processes in the synthesis of lipids a challenge for cells under low-energy states.
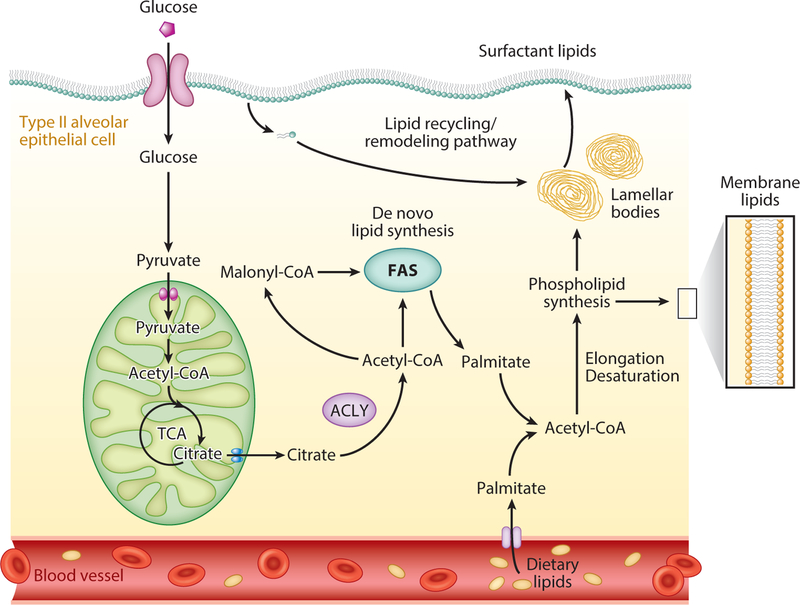
Figure 3.
Mechanisms for generating membrane and surfactant lipids in type II alveolar epithelial cells. These cells can engage in the de novo synthesis of lipids by utilizing pyruvate from glucose, or citrate from the TCA cycle. Mitochondrial citrate is mobilized to the cytoplasm and converted to acetyl-CoA by the enzyme ATP-citrate lyase. Acetyl-CoA undergoes carboxylation to malonyl-CoA by the enzyme acetyl-CoA carboxylase and subsequent steps require repetition of a 4-step reaction known as condensation, reduction, dehydration, and reduction. Fatty acyl chains can then undergo elongation and/or desaturation before being added to glycerol molecules to synthesize membrane and surfactant phospholipids. Fatty acyl chains for phospholipids can also be acquired from dietary sources or from the extracellular surfactant lipid pool. These diverse mechanisms are believed to explain how alveolar epithelial type II cells can maintain precise control over their surfactant lipid pool under a wide range of stress conditions. Abbreviations: ACLY, ATP-citrate lyase; FAS, fatty acid synthesis; TCA, tricarboxylic acid.
As type II alveolar epithelial cells are responsible for producing most pulmonary surfactant (some is produced by Clara cells in the mouse lung), these cells must continue to produce lipids even under metabolically unfavorable conditions (e.g., starvation). This is further compounded by the fact that turnover of surfactant phospholipids in the lung is exceedingly high. For example, Tierney and colleagues (8, 30) showed that the half-life of disaturated phosphatidylcholine is only 14 h in the healthy rat lung, and others have reported similar or even more rapid turnover rates in the newborn human lung and in injured rodent lung tissues (31). Because fatty acid synthesis is not feasible under a number of conditions (e.g., high intracellular AMP/ATP ratios), type II cells have evolved to utilize other less energy-costly methods for replenishing their surfactant lipid pool. This includes recycling old surfactant lipids back into the airway epithelium through a novel intracellular remodeling and resecretion process and incorporation of fatty acids from the circulation onto intracellular glycerol backbones (Figure 3) (32, 33). These alternative methods likely explain how type II cells are able to maintain precise control over their surfactant lipid pool while still engaging in other energy costly behaviors, such as the production of surfactant proteins, the detoxification of drugs and other chemicals, and the regeneration of epithelial cells lost to normal turnover or injury.
ENERGY-SENSING MECHANISMS
Metabolic flexibility describes the ability of an organism to respond or adapt to changes in energy demand. It was first used as a term to describe the capacity of worms to generate chemical energy under a variety of conditions but it is now appreciated that all organisms possess machinery for switching fuel sources (34, 35). Although many factors contribute to a cell’s ability to rapidly adjust to changes in nutrient availability, two of the major sensors that monitor energy status in mammalian cells are the enzymes 5′ adenosine monophosphate–activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR).
5′ Adenosine Monophosphate–Activated Protein Kinase
AMPK is a serine-threonine kinase comprised of one catalytic α subunit and two regulatory subunits, β and γ (36). This enzyme is most commonly activated by cellular stressors that deplete ATP stores, such as low glucose, hypoxia, ischemia, and inflammation. In these scenarios, utilization of ATP leads to binding of AMP (and to a lesser extent ADP) to its γ subunit, resulting in allosteric changes that activate the protein complex. These allosteric changes also make the catalytic α subunit more amenable to phosphorylation by upstream kinases, such as liver kinase B1 and calcium/calmodulin-dependent protein kinase 2 (37, 38). Importantly, these and other kinases are also capable of activating AMPK independently of changes in AMP to ATP ratios, thereby providing cells with an added layer of protection for controlling energy homeostasis.
Although many functions have been ascribed to AMPK, its primary role in cellular metabolism is to restore energy balance by inhibiting ATP consumption and stimulating the production of new ATP molecules (36). As such, a major inhibitory target of AMPK is the fatty acid synthesis pathway. This is achieved through several distinct mechanisms, but most directly through phosphorylating ACC, effectively blocking the production of all new fatty acid molecules. In addition, AMPK also targets other ATP-consuming processes, such as protein translation (through phosphorylation of elongation factor 2), and inhibits most major anabolic pathways, including the mTOR pathway discussed below. In conjunction with these inhibitory events, AMPK also stimulates activities that result in ATP production; this includes breakdown of fatty acids, uptake of glucose from the environment, and the induction of macroautophagy (see below) (39).
In the lung, AMPK is expressed at high levels relative to many other tissues (40). It is tempting to speculate that this is important for both the health and functioning of the lung. For instance, one could envision a scenario in which high levels of AMPK enable type II cells to rapidly sense decreases in ATP levels and switch to more energy-efficient methods for producing surfactant lipids. Moreover, one could also envision that elevated AMPK levels might be important for controlling inflammatory responses in the lung. In other words, AMPK may serve as a “metabolic brake” on the energy-consuming processes that drive pulmonary inflammation, such as diapedesis, chemotaxis, phagocytosis, and the production of inflammatory mediators. Consistent with this line of reasoning, we and others have recently demonstrated that AMPK activators are extremely effective at attenuating inflammatory and injury responses to different types of pulmonary insults in the mouse lung (41–43).
Mammalian Target of Rapamycin
Whereas the activation of AMPK aims to conserve energy in cells, the mTOR pathway provides cells with the go-ahead to engage in energy-consuming behaviors. At the center of the mTOR pathway is the mTOR protein, a serine-threonine kinase whose expression, like that of AMPK, is conserved throughout evolution (44–46). The mTOR protein nucleates two distinct multiprotein complexes known as mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2); however, much of what we know about this pathway involves mTORC1 (47).
A unique feature of mTORC1 is that it is able to integrate signals from different pathways in order to make decisions on whether or not to engage in progrowth pathways. For example, mTORC1 receives signals from both growth factors and amino acids (leucine, arginine, glutamine, or serine) in its environment, thereby ensuring that anabolic pathways are constrained under less than optimal growth conditions. Moreover, mTORC1 activity can also be regulated by the activation state of AMPK, which when phosphorylated serves as a potent inhibitor of mTOR signaling (47).
Another important mechanism by which mTORC1 activity is regulated is through the tuberous sclerosis complex (TSC), a heterodimer complex comprising TSC1 (also known as hamartin) and TSC2 (also known as tuberin) proteins. Together, TSC1 and TSC2 function to negatively regulate mTORC1 signaling by converting a small Ras-related GTPase into its inactive GDP-bound state. Relevant to the lung, excessive mTOR activity is a prominent feature of lymphangioleiomyomatosis, a rare lung disease associated with the presence of numerous benign tumors and cystic air spaces and is closely linked to genetic inactivation of either the TSC1 or TSC2 gene. In fact, inhibition of mTORC1 activity with rapamycin is now considered a major treatment for this disease (48). Furthermore, as discussed in later sections, uncontrolled activation of the mTOR pathway is now linked to the development of various other pathological phenotypes in the lung, including the induction of epithelial senescence in COPD and the acquisition of antiapoptotic phenotypes in IPF fibroblasts (49–51).
MACROAUTOPHAGY AS A SOURCE OF ENERGY
Under most conditions, cells utilize nutrients obtained from the external environment; however, during starvation conditions, cells can also cannibalize their own parts to generate energy. This cannibalization process is called macroautophagy, or autophagy for short, and is a self-regulated process by which cells envelop their own cytosolic structures (e.g., proteins, organelles, cilia) in double-membrane vesicles, termed autophagosomes (52). These autophagosomes are then delivered to lysosomes for degradation, causing dissolution of cellular structures into reusable units such as sugars, amino acids, and lipids. For more details, we refer readers to one of several recent comprehensive reviews (52–54). Although autophagy is often driven by a need for energy, cells also utilize this process for the removal of dysfunctional or damaged organelles. Autophagy is generally considered a protective mechanism to either overcome low nutritional states or clear cells of damaged constituents, but it is now also appreciated that too much or too little autophagy can contribute to the development of many different diseases, including Parkinson’s disease, cancer, and Huntington’s disease as well as the respiratory diseases discussed below (54–57).
CELLULAR METABOLISM IN THE PATHOBIOLOGY OF LUNG DISEASE
The “metabolic theory” of disease posits that changes in the bioenergetics of cells lead to downstream consequences that not only alter the behavior of individual cells but also contribute to whole-organ dysfunction and development of disease. This theory has been most extensively tested in the cancer biology field but has more recently been applied to several nonmalignant diseases, including those in the lung (9, 58, 59). In this section, we discuss the emerging evidence that links abnormalities in cellular metabolism to four chronic lung diseases: (a) the airway diseases COPD and asthma, (b) IPF, and (c) PH. Moreover, we discuss how alterations in cellular metabolism can drive the development of complex pathological events and review emerging evidence that suggests targeting specific metabolic pathways could be employed for treatment of respiratory diseases.
Airway Diseases: Chronic Obstructive Pulmonary Disease and Asthma
COPD is a common respiratory condition that causes substantial morbidity and mortality by gradually reducing the ability of individuals to expel air from their lungs (60). COPD is the third leading cause of death in the United States and is estimated to be the third leading cause of death worldwide by the year 2030. By far, the biggest risk factor for developing COPD is cigarette smoking, but advanced age, genetic factors, and environmental exposures are also believed to play a significant role. Many theories have been proposed to explain the pathogenesis of COPD, including protease/antiprotease imbalances, persistence of lung inflammation, and excessive oxidative stress (60, 61). However, it has also long been observed that COPD-like phenotypes develop in individuals in the setting of severe malnutrition, such as anorexia nervosa and incarceration in concentration camps, suggesting that alterations in energy homeostasis can influence the onset and progression of this disease (62–64).
A primary target of cigarette smoke (CS) is the epithelium. In mouse models, CS has profound effects on both the structure and function of mitochondria in the lung epithelium. This includes a reduction in the number of cristae per mitochondria, an increase in organelle size, and a decrease in both mitochondrial membrane potential (MMP) and oxygen consumption rate (5). Importantly, similar structural changes in mitochondria have been observed in the lung epithelium of patients with COPD (65), suggesting that mouse models may mimic, in part, the metabolic changes seen in human disease. Consistent with the integrated nature of metabolic pathways, mitochondria dysfunction in mouse lung epithelium is associated with an upregulation in the activity of other energy-producing pathways, such as glycolysis, β-oxidation, and autophagy. The implications of these observations are discussed below.
Ciliary dysfunction is one of the hallmark features of the COPD lung and is believed to contribute significantly to the progression of disease by decreasing mucociliary clearance and increasing susceptibility to pulmonary infections (66). Although cilia have long been known to depend on ATP for proper functioning, it is only recently that decreased energy production has been directly linked to ciliary dysfunction in the COPD lung. Cloonan et al. (5) demonstrated that CS exposure causes mitochondrial dysfunction by inducing the activity of iron-responsive element binding protein 2 (IRP2) in the lung epithelium, which in turn causes mitochondrial iron overload and impaired ETC function. Moreover, mice treated with a mitochondrial iron chelator were found to be protected from the damaging effects of CS on mitochondrial function, as manifested by improved ciliary function and enhanced mucociliary clearance. Importantly, this same group previously identified IRP2 as a major susceptibility gene in genome-wide association studies and demonstrated that IRP2 protein levels were increased in the lungs of COPD patients, suggesting that mitochondrial iron chelation therapy may also be relevant for the treatment of human disease (67).
As mentioned, lung epithelial cells undergo several metabolic adjustments in association with CS exposure, including an upregulation in autophagy (55, 68). Lam et al. (69) recently showed that this upregulation contributes to ciliary dysfunction by nonselectively targeting cilia structures for destruction, through a process these investigators coined ciliophagy. However, increased ciliophagy in this study was not necessarily driven by mitochondrial dysfunction or a decrease in energy production, but rather by a need to remove toxic protein aggregates caused by CS from the cytoplasm. Nevertheless, inhibiting autophagy through several unique approaches was found to reverse the effects of CS exposure on ciliary function and mucociliary clearance in the lung (60). This included deleting beclin 1, which is involved in the nucleation of autophagosomes, or inhibiting hdac6, which facilitates retrograde transport of ubiquitinated proteins into aggresomes.
In addition to injuring the proximal lung epithelium, CS exposure also damages distal pulmonary epithelial cells, contributing to loss of gas-exchange surface area and the development of emphysema. In emphysema, apoptotic cell death is believed to play an important role in loss of distal alveolar structures that defines the disease. Although various factors can trigger the induction of apoptotic cell signaling pathways, mitochondrial ROS production induced by CS has been shown to be an important contributory factor (70, 71). Moreover, Jiang et al. (72) recently provided a direct link among alterations in cellular metabolism, ROS production, and apoptotic cell death. In this study, CS exposure was shown to induce β-oxidation in both lung endothelial and epithelial cell populations, in part, by upregulating the expression of Cpt1. Moreover, this increase in β-oxidation was associated with elevated ROS production, perhaps by increasing mitochondrial ETC activity (72). Likewise, pharmacological inhibition of Cpt1 was shown to reduce ROS production and ameliorate CS-induced apoptosis of lung epithelial cells. Importantly, these findings in mice may have clinical relevance, as FAM13A, which modulates the fatty acid oxidation pathway, was recently identified as a susceptibility gene in genome-wide association studies. Moreover, these investigators showed that Fam13A-deficient mice were protected against CS-induced cell death, suggesting that the link between FAM13A and COPD in humans is more than just an association (72, 73).
Another factor contributing to loss of the lung epithelium in COPD is the induction of cellular senescence, a physiologic state whereby cells lose their capacity to proliferate (74). Senescent cells exhibit a complex phenotype characterized by permanent cell-cycle arrest mediated by the p53 and p16/Rb pathways and also by the secretion of various inflammatory proteins (75). Thus, senescent cells are believed to contribute to disease by both compromising epithelial regeneration and driving chronic lung inflammation (76). Although various factors are thought to contribute to the induction of cellular senescence in COPD, including CS-induced DNA damage (77), emerging evidence suggests that chronic anabolic stimulation due to mTOR activation may also be a pathogenic factor. In a recent study, Houssaini et al. (51) showed that mTOR activity was increased in lung tissues of both COPD patients and mice after CS exposure. Further, inhibiting mTOR activity with low-dose rapamycin prevented the induction of senescence and reduced levels of proinflammatory senescence-associated proteins in the lung (51). Interestingly, overexpression of mTOR by itself was shown to induce lung cellular senescence and cause an emphysema-like phenotype in the mouse lung, suggesting that anabolic stimulation alone may be a driver of COPD pathology.
Like COPD, asthma is a disease characterized by chronic airflow obstruction but differs in the fact that airflow obstruction is usually reversible (78, 79). Although asthma is now considered a heterogeneous disorder, a defining feature of disease is often the production of high levels of TH2 inflammatory cytokines, such as IL-4, IL-5 and IL-13 (79). These cytokines act on multiple cell types in the lung and, collectively, serve to induce most, if not all, hallmark features of disease, including eosinophilia, elevations in IgE levels, macrophage polarization, mast cell degranulation, airway hyperreactivity, and mucus hypersecretion. Indeed, long-standing asthma therapeutics work, in large part, by broadly inhibiting the synthesis of TH2 cytokines, and newer, more targeted asthma therapeutics act to specifically interfere with the activity of individual TH2 cytokines (80, 81).
A major factor driving the TH2 immune response is the CD4 T-lymphocyte. Like other T-cell effector subsets (TH1 and TH17 cells) TH2 cells are highly dependent on glucose for meeting their metabolic needs. However, TH2 cells are unique in that they utilize glucose to fuel the alternative TORC2 anabolic pathway, suggesting a unique molecular target in the treatment of asthma. Consistent with this line of reasoning, rapamycin, a nonselective inhibitor of the mTOR pathway, has recently been shown to markedly attenuate allergic airway responses in mice (82, 83). This includes reducing mucus production, decreasing IgE levels, and dramatically suppressing IL-5 and IL-13 levels.
Although asthma is best known as a disease of overactive effector T cells, it is also appreciated that the activity of regulatory T cells (Tregs) is diminished in animal models of this disease (84). Tregs are a subset of T-lymphocytes that play a very important role in mucosal immunity through their ability to dampen inflammatory responses in many different cell types, including effector T cells, macrophages, and other innate immune cells. Recently, several groups have demonstrated a reduction in Treg cells in the lungs of asthmatics. Moreover, Ostroukhova and colleagues (85) showed not only that Treg cells are reduced in the lungs of mice after low-dose allergen exposure but also that adoptive transfer of these cells into naïve mice can dramatically attenuate allergen sensitization in the lung. Although effector T cells depend on glucose to meet their metabolic demands, Treg cells appear to rely more on fatty acid β-oxidation. Moreover, this is associated with low levels of glucose receptors, a decrease in glycolytic flux (86), and high levels of activated AMPK (86, 87). Interestingly, the dependence of Treg cells on activation of AMPK might explain why mice deficient in the alpha subunit of this enzyme are more susceptible to developing asthma, and AMPK activators appear to be highly effective in suppressing eosinophilic inflammation and reducing airway remodeling in mice (88).
Along with the production of mucus, airway epithelial cells also synthesize high levels of TH2 cytokines and release high concentrations of nitric oxide (NO) into the airway during asthma. Indeed, exhaled NO concentrations are often used as a marker of disease activity in humans (89). NO is produced through the metabolism of arginine by the enzyme NO synthase. Because deficiency of NO synthase in mice does not increase asthma susceptibility, it is generally believed that NO production is just a by-product of disease rather than a causative agent. However, Xu et al. (90) recently showed that arginine metabolism may have a direct role in asthma pathobiology. Along with its conversion to NO, arginine is also catabolized to ornithine by the enzyme arginase. While arginase 1 is present only in the liver, arginase 2 is expressed in virtually all tissues in the body, including the lung. Xu et al. showed that arginase 2 levels are increased in the airway epithelium of mice and humans with asthma. Moreover, they showed that increased ornithine production by arginase 2 fed the TCA cycle, leading to an increase in α-ketoglutarate production. Increases in α-ketoglutarate were found to drive a host of downstream events, including the catalytic hydroxylation of hypoxia-induced factor (HIF) proteins, which reduced TH2 cytokine production by suppressing the activity of signal transducer and activator of transcription 6. Conversely, downregulation of arginase 2 was found to enhance airway inflammation and mucus production in mice, further bolstering the concept that arginine metabolism contributes directly to asthma pathogenesis. Interestingly, arginase 2 gene variants are one of the most well-recognized single nucleotide polymorphisms in genome-wide association studies of asthma patients (91, 92), suggesting that the findings in mice may be relevant to humans.
Another hallmark of asthma is that airway smooth muscle cells adopt a hyperproliferative and hypercontractile phenotype. These pathological responses play an integral role in the disease by decreasing the luminal diameter of airways and increasing airflow resistance. Similar to other contracting muscle populations, smooth muscle cells in asthma have increased metabolic demands and elevated levels of mitochondrial oxygen consumption. Moreover, increased mitochondrial mass has also been described in smooth muscle cells from asthmatics, which appears to be driven by an upregulation in various mitochondrial biogenesis factors, including peroxisome proliferator–activated receptor γ coactivator, nuclear respiratory factor 1, and mitochondrial transcription factor A (93). Interestingly, increased mitochondrial biogenesis has been associated with downregulation in the expression of CCAAT/enhancer binding proteins, which are known to regulate the expression of glucocorticoid receptors in smooth muscle cells (94). Taken together, these findings suggest that changes in mitochondrial dynamics may directly influence steroid responsiveness in asthma.
Finally, airway smooth muscle cells also exhibit an increase in autophagy in asthma. It has been suggested that autophagy may be needed so contracting smooth muscle cells can better meet their increased metabolic demands. Consistent with this line of reasoning, Cheng and colleagues (95) recently showed that reducing autophagy by delivering a beclin 1–targeted miRNA into the mouse lung could effectively reduce asthma severity and attenuate airway hyperreactivity in response to antigen challenge. Similarly, Pan and colleagues (96) demonstrated that blocking autophagic flux through activation of bitter taste receptors potently inhibited mitogenic-induced airway smooth muscle growth in vitro. Altogether, these findings suggest that targeting autophagy could be a novel way to reduce airway pathology in humans.
Idiopathic Pulmonary Fibrosis
IPF is a highly aggressive lung disease characterized by progressive dyspnea, hypoxemia, and respiratory failure due to pathological scarring of the lung (97). IPF develops almost exclusively in older individuals and has a prognosis worse than many cancers. According to the current paradigm, IPF is caused by repetitive insults (e.g., cigarette smoke, environmental exposures) to an aged, distal pulmonary epithelium in genetically susceptible adults. In time, the cumulative effects of these pulmonary insults are believed to reduce the regenerative capacity of the distal pulmonary epithelium, most likely by inducing death or senescence of type II alveolar progenitor cells (98, 99). As a result, a persistent wound healing response is triggered, manifesting as progressive organ fibrosis with severe respiratory functional limitations.
Because IPF is now believed to be due to age-related decline in distal pulmonary epithelial progenitor function, there has been a wealth of new investigations exploring how cellular metabolism is altered in type II cells in this disease. What is emerging from these studies is a greater appreciation for the wide range of metabolic changes that occur in the epithelium of IPF patients (100). At the heart of these changes appears to be mitochondrial dysfunction, as evidenced by the fact that large, dysmorphic and poorly functioning mitochondria have been found to accumulate in type II cells in this disease (101). Moreover, Yu et al. (102) demonstrated that restoring mitochondrial function by administering thyroid hormone, a known inducer of organelle biogenesis, effectively ameliorated pulmonary fibrosis in mice, suggesting that mitochondrial-targeted therapies may have a future in the treatment of fibrotic lung disease. In addition to these findings, it has also been demonstrated that mitochondrial dysfunction is associated with other metabolic perturbations in the alveolar epithelium, including an increase in both AMPK activation and lactate production (103, 104). Perhaps these metabolic alterations may explain why other pathological features develop in type II cells in IPF, such as the accumulation of misfolded or unfolded proteins in the endoplasmic reticulum (ER) (105, 106). It is known that protein translation and the unfolded protein response are both energy-consuming processes involving the activity of ATP-dependent chaperone proteins and the induction of lipid synthesis, respectively. Consistent with this, we recently showed that activation of AMPK is associated with a marked downregulation in the machinery for synthesizing lipids in the alveolar epithelium of mice after silica or bleomycin exposure. Similar directional changes in these proteins were also observed in whole lung tissues and isolated type II cells from the lungs of patients with IPF (4, 103, 107). More importantly, we found that augmenting lipid synthesis with an LXRα agonist not only reduced ER stress but also ameliorated fibrotic remodeling to silica dust in mice, supporting the notion that strategies aimed at restoring metabolic health to the lung epithelium might be effective in treating pulmonary fibrosis.
Although IPF is believed to originate in the lung epithelium, fibroblast dysfunction also plays an important role in the disease, as these cells are responsible for producing most, if not all, of the extracellular matrix material that makes up pulmonary scar tissues. However, in contrast to the case in the lung epithelium, fibroblasts display a decrease in AMPK activation in fibrotic lung tissues. (108). Moreover, this decrease in AMPK activation has been shown to contribute to decreasing mitochondrial function by reducing the production of critical mitochondrial complex proteins. Because mitochondrial dysfunction is now recognized to play a critical role in the acquisition of the profibrotic phenotype of myofibroblasts, it is not surprising that AMPK activators, such as metformin, have been shown to reduce the severity of pulmonary fibrosis in mice (108).
Consistent with having diminished mitochondrial function, fibroblasts from the lungs of mice with experimental pulmonary fibrosis and from patients with IPF are metabolically reprogrammed to maintain a high level of glycolytic activity. This metabolic reprogramming associates with elevated levels of key glycolytic enzymes, including hexokinase 2 (HK2), phosphofructokinase-1, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) and LDH, and glucose transporter 1 (104, 109, 110). Moreover, this increase in glycolytic activity has been mechanistically linked to the transdifferentiation of lung fibroblasts into fibrogenic myofibroblasts through several mechanisms (Figure 4). First, excess secretion of lactate due to enhanced glycolysis has been shown to create an acidic extracellular environment that causes the activation of latent transforming growth factor (TGF)-β1 (104). Second, increased delivery of pyruvate to the TCA cycle due to excessive glycolysis has been shown to yield metabolic intermediates, such as succinate, that contribute to promoting fibrotic responses. As an allosteric inhibitor of hypoxia-induced factor-1 alpha (HIF-1α) degradation, succinate not only aggravates glycolytic reprogramming by inducing HIF-1α–mediated expression of glycolytic genes but also promotes myofibroblastic differentiation by transcriptionally upregulating alpha-smooth muscle actin. The pivotal role of glycolysis in the pathogenesis of pulmonary fibrosis is further reinforced by several genetic and pharmacological studies showing that targeting HIF-1α, PFKFB3, or pyruvate dehydrogenase kinase 1 can ameliorate pulmonary fibrosis in rodents (109, 111). These recent discoveries have firmly advanced glycolytic manipulation to the forefront of novel metabolism–based approaches to combat pulmonary fibrosis, and optimism for this approach is only further bolstered by the safety of such approaches in clinical trials of cancer (112).
Figure 4.
Metabolic reprogramming of activated lung fibroblasts. Activated lung fibroblasts utilize aerobic glycolysis (Warburg effect) over mitochondrial glucose oxidation. Although this metabolic reprogramming event generates less ATP than mitochondrial respiration, it also supplies various biosynthetic intermediates for activated myofibroblasts. This includes the production of glucose-6-P, which is diverted to the pentose phosphate pathway for the production of NADPH and ribose-5-P, and the production of glycerate-3-P, which is diverted to the de novo serine and glycine synthesis pathway. Aerobic glycolysis also yields large amounts of lactate acid, which serves to activate latent TGF-β1 by reducing extracellular pH. By-products of aerobic glycolysis can also feed the TCA cycle, leading to an increase in succinate production. Succinate stabilizes HIF-1α, which then amplifies glycolysis by inducing the expression of various glycolytic genes and promotes myofibroblast differentiation via the induction of α-SMA expression. Activated myofibroblasts also exhibit enhanced glutaminase activity, leading to augmented glutaminolysis, which in turn promotes the conversion of glutamine to glutamate. Glutamine metabolism via the TCA cycle can yield high levels of α-KG, which activates mTOR, leading to increased transcription, translation, and hydroxylation of collagen. Abbreviations: α-KG, alpha-ketoglutarate; α-SMA, alpha-smooth muscle actin; GLUT1, glucose transporter 1; HIF-1α, hypoxia-induced factor-1 alpha; LDH, lactate dehydrogenase; mTOR, mammalian target of rapamycin; P, phosphate; PHGDH, phosphoglycerate dehydrogenase; PPP, pentose phosphate pathway; TCA, tricarboxylic acid; TGF-β1, transforming growth factor-beta 1.
Although increased glycolytic flux leads mostly to lactate or pyruvate production, upstream metabolic intermediates can also be diverted for the de novo syntheses of serine and glycine (Figure 4). The key enzymes that mediate this process include phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase 1, phosphoserine phosphatase, and serine hydroxymethyltransferase 2 (SHMT2), which are now recognized to be transcriptional targets of TGF-β1 in lung fibroblasts (113, 114). Mechanistically, it has been shown that carbon molecules derived from glucose are incorporated directly into newly synthesized collagen in response to TGF-β1 stimulation (114). As glycine is the most abundant amino acid residue in collagen (it accounts for approximately 33% of the content), fibroblasts must rely on the de novo serine/glycine synthesis pathways for collagen production. Relevant to humans, the expression of PHGDH and SHMT2 has also been shown to be upregulated in IPF lungs (114), indicative of the involvement of the de novo serine/glycine synthesis in the pathogenesis of this disease. This body of evidence has thus identified a number of potential therapeutic targets for treating pulmonary fibrosis. Indeed, follow-up studies have demonstrated that inhibition of PHGDH (by NCT-503) was highly efficacious in attenuating bleomycin-induced lung fibrosis in mice (113). Although these data support the notion of targeting PHGDH in pulmonary fibrosis, caution is still warranted given the unclear impact of inhibiting this metabolic enzyme in other cell populations in the lung.
As already discussed, metabolic intermediates are often diverted from the TCA cycle to be used for the biosynthesis of various compounds. However, for this process to continue, other metabolic intermediates must also re-enter the TCA cycle to fuel anaplerosis. There are two major anaplerotic reactions in cells; they include the formation of oxaloacetate from pyruvate and the formation of α-ketoglutarate from glutamate. One of the main sources of intracellular glutamate is glutamine, which is synthesized by a process called glutaminolysis involving the enzyme glutaminase (Gls). In recent studies, Gls1 expression was found to be induced by the profibrotic mediator TGF-β1 in lung fibroblasts (49, 115). Moreover, this upregulation was accompanied by an increase in glutaminolytic events, including augmented glutamine consumption and elevated intracellular glutamate levels (49). It has also been shown that α-ketoglutarate derived from glutaminolysis promotes protein translation of collagen by activating mTOR and stabilizes newly synthesized collagen molecules via increasing hydroxylation (49). Given additional findings showing that Gls1 is upregulated in fibroblasts within dense fibrotic scar tissue of mice after bleomycin and in fibroblastic foci of lung tissue from patients with IPF (49), it appears that glutamine metabolism is a key player in the pathogenesis of fibrotic lung disease. Therefore, targeting glutaminolysis may be an appealing strategy for attenuating pathological scar formation in IPF. Of note, similar strategies are already being investigated in treatment of other types of diseases, including cancer.
Not only is glycolysis increased in activated lung fibroblasts, it is also upregulated in macrophages from fibrotic lung tissues. Lung macrophages are now increasingly recognized to play an important role in pathogenesis of pulmonary fibrosis, as they have been shown to produce many of the profibrotic substances that drive fibroblast activation. In a recent study (116), we found that alveolar macrophages from mice with experimental pulmonary fibrosis exhibited a significant increase in glycolytic activity. Moreover, ex vivo experiments showed that inhibition of glycolysis with either HK2 or PFKFB3 inhibitors was highly effective in reversing the profibrotic M2-like phenotype of alveolar macrophages, whereas drugs inhibiting glutaminolysis or fatty acid oxidation had a negligible effect on the behavior of these cells (116). Interestingly, these findings appear to contradict observations in some other tissues in which M1 polarization is enhanced by augmenting glycolytic activity, suggesting that metabolic pathways controlling the behavior of macrophages may vary within different contexts or tissues (117).
Pulmonary Hypertension
PH is a heterogeneous group of disorders defined by a mean pulmonary artery pressure of greater than 25 mmHg at rest. Although patients with PH are often classified into various diagnostic groups based on their clinical and hemodynamic findings, most patients with PH manifest similar pathological features, including the muscularization of pulmonary blood vessels and the presence of perivascular immune cell infiltration (118). For decades, PH was considered to be a disease caused by increased blood vessel tone due to abnormalities in endothelial and smooth muscle signaling events, but now it is considered to be a hyperproliferative disorder, which induces constrictive events and remodeling of small-resistance blood vessels throughout the pulmonary circulation. Consistent with this, characteristic features of pulmonary vascular cells in PH include the adoption of a hyperproliferative and apoptosis-resistant phenotype. Indeed, several groups have demonstrated that pulmonary microvascular cells from PH patients grow at a much greater rate than control populations (119–123). Moreover, like fibroblasts in IPF, this hyperproliferative phenotype has been associated with metabolic reprogramming, which includes an increase in glycolytic activity and a reduction in mitochondrial respiration (119–124).
Although the mechanisms contributing to metabolic reprogramming in PH remain poorly understood, several recent investigations have linked these changes to a disruption in various key proteins, including the transcription factor STAT3 (signal transducer and activator of transcription 3). While STAT3 is best known for its role in regulating the expression of proinflammatory genes, recent work indicates that it also interferes with mitochondrial-dependent energy metabolism (125) by interacting with the mitochondrial complex I protein GRIM-19 (gene associated with retinoid interferon–induced mortality) of the ETC. Because STAT3 inhibition has been shown to restore mitochondrial function and reduce the hyperproliferative phenotype of pulmonary vascular cells from pulmonary arterial hypertension (PAH) patients in culture and to also attenuate experimental PH in monocrotaline-exposed rats, STAT3 inhibitors are now considered by some as a potential therapy for treatment of PH (126, 127).
Metabolic reprogramming in lung microvascular cells has also been linked to a downregulation in expression of several important genes, including bone morphogenetic protein receptor 2 (BMPR2) and the mitochondrial deacetylase sirtuin 3 (SIRT3). BMPR2 is a serine/threonine receptor kinase that binds members of the TGF-β superfamily of ligand and is the gene most closely linked to hereditary forms of PAH. Mice deficient in BMPR2 are more susceptible to developing hypoxia-induced PH, making this a relevant model for studying PH pathobiology (128). Interestingly, loss of BMPR2 has been shown to cause mitochondrial dysfunction by inducing p53-mediated upregulation of mitochondrial biogenesis genes (PGC-1α and TFAM), thereby promoting the activation of proinflammatory pathways through mitochondrial DNA inflammasome activation (128). In contrast to BMPR2 deficiency, loss of SIRT3 in pulmonary artery smooth muscle cells has been shown to induce mitochondrial dysfunction through reducing mitochondrial gene expression. In rodents, selective SIRT3 deficiency in pulmonary vascular cells is linked to the spontaneous development of PH, and restoring SIRT3 activity in either cultured human PAH cells or in experimental rodent models of PAH both restores mitochondrial homeostasis and attenuates the hyperproliferative behavior of pulmonary vascular cells (129).
Another important pathological feature of pulmonary vascular cells in PH is the development of apoptotic resistance. Recently, this pathological feature has been linked to an increase in glycolytic activity in endothelial cells through reducing MMP and preventing the release of proapoptotic factors (129–132). Michelakis and coworkers demonstrated that this increase glycolytic flux in PH resulted from a decrease in PDC activity, which is the enzyme responsible for converting pyruvate to acetyl-CoA before delivery to the TCA cycle (130, 132). Moreover, this group found that treatment of lung endothelial cells from PH patients with a PDC activator called dichloroacetate effectively reduced dependence of lung endothelial cells on glycolysis while also increasing their sensitivity to apoptosis. Consistent with this, dichloroacetate treatment has been shown to ameliorate chronic hypoxia and monocrotaline-induced PH in rodents, suggesting that similar approaches might be effective in the treatment of humans with pulmonary vascular disease. This notion is reinforced by a recent clinical trial showing that dichloroacetate treatment reduced mean pulmonary artery pressure and pulmonary vascular resistance and improved functional capacity in a portion of PAH patients (131). However, despite these promising results, it is worth mentioning that a large number of PH patients in this trial did not respond to dichloroacetate treatment, suggesting that this therapeutic approach might be effective for only a small fraction of patients with the disease.
In addition to a downregulation in PDC activity, another important mechanism linked to metabolic dysfunction in PH is upregulation of HIF-1α. For reasons that remain unclear, endothelial and smooth cells from patients with PAH exhibit a marked upregulation in the expression of HIF-1α, whether placed in normoxia or hypoxia conditions. Like IPF fibroblasts, the upregulation in HIF-1α is associated with increased dependence on glycolysis and the adoption of a hyperproliferative phenotype (133). Interestingly, Chettimada et al. (22) found that the activity of G6PD is important for regulating HIF-1α expression in pulmonary vascular cells and that inhibiting this enzyme with dehydroepiandrosterone was highly effective at ameliorating the hyperproliferative phenotype of hypoxic pulmonary vascular cells.
Preferential utilization of glycolysis in pulmonary vascular cells in PH has also been linked to a downregulation in expression of mitochondrial uncoupling proteins (UCPs). UCPs have important effects on mitochondria function, including regulation of calcium handling as well as the control of MMP. As previously mentioned, several groups have reported that MMP is increased in endothelial cells and smooth muscle cells from humans and rodents with PH. Similarly, MMP is also increased in pulmonary vascular cells in the setting of UCP2 deficiency, and mice lacking UCP2 expression manifest a more severe PH phenotype in response to intermittent hypoxia (134, 135). Taken together, these data suggest that targeting UCP could be effective in the treatment of pulmonary vascular diseases.
There is also emerging evidence to suggest that modulating cross talk between glucose metabolism and other metabolic events, such as fatty acid β-oxidation, can ameliorate pathological changes in pulmonary vascular cells in PH. This concept is based on the Randle cycle, also known as the glucose fatty acid cycle, which refers to the inverse relationship between fatty acid oxidation and glucose oxidation in most cells. Michelakis and coworkers (136) hypothesized that inhibiting fatty acid oxidation by knocking down malonyl-CoA decarboxylase (MCD) would increase glucose oxidation while simultaneously reducing dependence on glycolysis. Consistent with this line of reasoning, mice deficient in MCD exhibited an increase in mitochondrial respiration (rather than aerobic glycolysis) and were protected from developing hypoxia-induced PH. Similarly, pharmacological inhibition of the last enzymatic step of β-oxidation reduced the severity of PH in monocrotaline-treated rats, suggesting that pathological behaviors in pulmonary vascular cells can be modulated by simply limiting access to specific energy substrates.
It has been shown that the balance between production and clearance of mitochondria is altered in PH due to a simultaneous decrease in biogenesis (reduced PGC-1α levels) and an increase in organelle clearance through mitophagy. Notably, this increase in mitophagy has been linked to an upregulation in PINK1, which differs from the downregulation in PINK1 described by Bueno et al. in IPF lung tissues (101). Nevertheless, increased PINK1 expression is associated with the accumulation of this protein along the outer membrane of mitochondria, thereby leading to the recruitment of Parkin, an E3 ligase that targets mitochondria for destruction. Lee and colleagues (137) recently reported that loss of endothelial UCP2 contributes to increased PINK1-mediated mitophagy, suggesting an alternative mechanism by which UCP2 deficiency mediates mitochondrial dysfunction in PH.
Finally, like the role of fibroblasts in IPF, emerging evidence suggests that abnormalities in glutamine metabolism may play an important role in promoting vascular proliferation in PH. For example, Chan and coworkers (138, 139) showed that the master mechanosensing transcriptional coactivators YAP and TAZ that are often activated in pulmonary vascular cells in PH induce the transcriptional upregulation of Gls1, thereby stimulating glutaminolysis. It is presumed that this may be important for replenishing intracellular levels of aspartate, an amino acid critical for sustaining anabolic activities in proliferating and migrating cells (139). Consistent with this mechanism, pharmacologic inhibition of Gls1 has recently been shown to exhibit potent disease-modifying effects in a rodent model of PH (139).
FUTURE CHALLENGES AND DIRECTIONS
Significant progress has been made over the last several decades in our understanding of energy metabolism in the lung. Recent technological advances have enabled researchers to go beyond studying just whole organ metabolism and begin dissecting the metabolic events driving common and unique behaviors in individual cell populations in the lung. Though much has been learned, our understanding of pulmonary metabolism still lags behind that of many other fields. As such, even basic biological questions pertaining to pulmonary metabolism remain unanswered, such as: Why does the lung produce so much lactate, and how are type II cells and other metabolically active cells (ciliated cells) able to acquire the energy needed to perform critical duties under stress and disease conditions? To answer these and other critical questions, the pulmonary community will need to invest more heavily in the field, including taking advantage of recent technologies permitting quantitative assessment of lipids and other metabolites in individual cell populations. This information will, most likely, yield new biological insights and also help to identify previously unrecognized biological markers that can aid in the diagnosis, screening, and/or monitoring of respiratory diseases. Finally, from a treatment perspective, the pulmonary community needs to soon move beyond testing metabolic drugs in preclinical models and begin assessing the efficacy of these types of treatments in human lung diseases. Given recent work demonstrating the ability of such drugs to ameliorate conditions outside the lung, such as pancreatic cancer, diabetes (140), obesity, and autoimmune diseases (141), we are optimistic that similar results will be seen in the lung, not just for the four conditions discussed in this review, but also for a host of other pulmonary disorders, including lung cancer, pneumonia, sarcoidosis, and the acute respiratory distress syndrome.
SUMMARY POINTS.
The lung is a metabolically active organ.
Under well-oxygenated conditions, the lung produces large amounts of lactate relative to other organs.
Mitochondrial dysfunction is associated with the development of several common respiratory diseases.
Activated lung fibroblasts prefer aerobic glycolysis over mitochondria respiration but maintain an active TCA cycle.
High levels of AMPK in the lung may be important for controlling pulmonary inflammation.
Alterations in cellular metabolism drive complex pathological events in the lung, including the shortening of cilia, the profibrotic activation of lung fibroblasts and macrophages, and the induction of both cell death and cellular senescence in the lung epithelium.
Drugs targeting specific metabolic pathways have demonstrated remarkable efficacy while also exhibiting minimal toxicity in experimental models of lung disease.
FUTURE ISSUES.
How do different cell populations in the lung communicate their metabolic needs, and does breaking this communication impact organ function or contribute to the development of disease?
Why does the lung produce so much lactate, and is this important for proper growth and functioning of the lung?
Can targeting AMPK (or other key regulators of cellular metabolism) put a metabolic break on inflammatory responses in the lung and can AMPK activators be used to treat pulmonary fibrosis?
How are type II cells and other metabolically active cells able to adjust to nutrient deprivation while still being able to perform their other essential duties (e.g., production of pulmonary surfactant)?
Does the Warburg effect impart a more aggressive phenotype in lung mesenchymal cells, and can this be targeted for the treatment of disease?
What is the impact of aging, cigarette smoke, and other environmental insults on cellular metabolism in different cell populations in the lung, and how does this ultimately influence the onset or progression of disease?
Can glutaminolysis be targeted to reduce pathological scar tissue formation in the lung?
Will detailed profiling of pulmonary metabolites prove useful as either a diagnostic or prognostic tool for patients with lung disease?
Cellular metabolism:
the full set of chemical reactions that occur in living organisms that regulate energy homeostasis
Epithelium:
a type of tissue that forms the outer layer of the body’s surface and the lining of organs that communicates with fibroblasts, immune cells, and endothelium
COPD:
chronic obstructive pulmonary disease is characterized by fixed airflow obstruction and often associated with cigarette smoking
IPF:
idiopathic pulmonary fibrosis is chronic lung disorder that typically develops in older individuals and causes progressive scarring of the pulmonary parenchyma
PH:
pulmonary hypertension is a group of diseases defined by elevated pulmonary artery pressures and leads to progressive remodeling of blood vessels
Glycolysis:
a 10-step reaction involving the breakdown of glucose by enzymes, leading to the release of energy and the generation of pyruvate
ATP:
adenosine 5′-triphosphate is an organic compound that serves as a primary source of energy for cells
Mitochondria:
organelles found in almost all cells in which cellular respiration and energy production occur. They serve as a molecular sensor to other parts of the cell
TCA:
tricarboxylic acid is a cycle of enzymatic reactions that occur in the mitochondrial matrix which yield metabolic intermediates, NADH and FADH2
Warburg effect:
an eponym used to describe the conversion of pyruvate to lactic acid under aerobic conditions
PPP:
pentose phosphate pathway is a metabolic pathway parallel to glycolysis that generates NADPH and 5-carbon sugar molecules which serve as a precursor to the synthesis of nucleotides
ETC:
electron transport chain is a series of protein complexes that transfer electrons across the mitochondrial inner membrane to generate ATP
Catabolic:
a process by which complex molecules are broken down into smaller components
β-oxidation:
a catabolic process through which fatty acid molecules are broken down in mitochondria to generate acetyl coenzyme A yielding NADH and FADH2
Fatty acid synthesis:
a series of enzymatic reactions that form fatty acids from acetyl-CoA and reduced nicotinamide adenine dinucleotide phosphate (NADPH)
AMPK:
5′ adenosine monophosphate–activated protein kinase is a serine/threonine protein kinase that acts to regulate energy homeostasis in cells
mTOR:
mammalian target of rapamycin integrates both intracellular and extracellular signals and serves as a central regulator of cell metabolism, growth, proliferation and survival
Anabolic:
a process by which complex molecules are generated from simpler compounds
Autophagy:
a process by which cells consume their own components to remove damaged parts and acquire additional substrates for energy production
Lung fibroblast:
a cell type found in the mesenchyme of the lung that produces collagen and other extracellular matrix components
ACKNOWLEDGMENTS
The authors acknowledge funding from the US National Institutes of Health (grant R01 HL131784) to R.S. and (grant R35HL135830) to G.L.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, et al. 2001. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 128:5181–88 [DOI] [PubMed] [Google Scholar]
- 2.Summer R, Fitzsimmons K, Dwyer D, Murphy J, Fine A. 2007. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am. J. Respir. Cell Mol. Biol 37:152–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. 2003. Side population cells and Bcrp1 expression in lung. Am. J. Physiol. Lung Cell. Mol. Physiol 285:L97–104 [DOI] [PubMed] [Google Scholar]
- 4.Romero F, Hong X, Shah D, Kallen CB, Rosas I, et al. 2018. Lipid synthesis is required to resolve ER stress and limit fibrotic responses in the lung. Am. J. Respir. Cell Mol. Biol 59 10.1165/rcmb.2017-0340OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cloonan SM, Glass K, Laucho-Contreras ME, Bhashyam AR, Cervo M, et al. 2016. Mitochondrial iron chelation ameliorates cigarette smoke-induced bronchitis and emphysema in mice. Nat. Med 22:163–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhoades RA. 1974. Net uptake of glucose, glycerol, and fatty acids by the isolated perfused rat lung. Am. J. Physiol 226:144–49 [DOI] [PubMed] [Google Scholar]
- 7.O’Neil JJ, Tierney DF. 1974. Rat lung metabolism: glucose utilization by isolated perfused lungs and tissue slices. Am. J. Physiol 226:867–73 [DOI] [PubMed] [Google Scholar]
- 8.Tierney DF. 1974. Intermediary metabolism of the lung. Fed. Proc 33:2232–37 [PubMed] [Google Scholar]
- 9.Vander Heiden MG, Cantley LC, Thompson CB. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warburg O, Wind F, Negelein E. 1927. The metabolism of tumors in the body. J. Gen. Physiol 8:519–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faubert B, Li KY, Cai L, Hensley CT, Kim J, et al. 2017. Lactate metabolism in human lung tumors. Cell 171:358–71.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zu XL, Guppy M. 2004. Cancer metabolism: facts, fantasy, and fiction. Biochem. Biophys. Res. Commun 313:459–65 [DOI] [PubMed] [Google Scholar]
- 13.Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E. 2007. Energy metabolism in tumor cells. FEBS J 274:1393–418 [DOI] [PubMed] [Google Scholar]
- 14.Hume DA, Weidemann MJ. 1979. Role and regulation of glucose metabolism in proliferating cells. J. Natl. Cancer Inst 62:3–8 [PubMed] [Google Scholar]
- 15.Fisher AB. 1984. Intermediary metabolism of the lung. Environ. Health Perspect 55:149–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tierney DF. 1971. Lactate metabolism in rat lung tissue. Arch. Intern. Med 127:858–60 [PubMed] [Google Scholar]
- 17.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, et al. 2013. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig 123:3025–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowden DH. 1983. Cell turnover in the lung. Am. Rev. Respir. Dis 128:S46–48 [DOI] [PubMed] [Google Scholar]
- 19.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, et al. 2015. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517:621–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassett DJ, Fisher AB. 1976. Metabolic response to carbon monoxide by isolated rat lungs. Am. J. Physiol 230:658–63 [DOI] [PubMed] [Google Scholar]
- 21.Fisher AB, Steinberg H, Bassett D. 1974. Energy utilization by the lung. Am. J. Med 57:437–46 [DOI] [PubMed] [Google Scholar]
- 22.Chettimada S, Gupte R, Rawat D, Gebb SA, McMurtry IF, Gupte SA. 2015. Hypoxia-induced glucose-6-phosphate dehydrogenase overexpression and -activation in pulmonary artery smooth muscle cells: implication in pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol 308:L287–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher AB, Itakura N, Dodia C, Thurman RG. 1981. Pulmonary mixed-function oxidation: stimulation by glucose and the effects of metabolic inhibitors. Biochem. Pharmacol 30:379–83 [DOI] [PubMed] [Google Scholar]
- 24.Bassett DJ, Fisher AB. 1976. Pentose cycle activity of the isolated perfused rat lung. Am. J. Physiol 231:1527–32 [DOI] [PubMed] [Google Scholar]
- 25.Tierney D, Ayers L, Herzog S, Yang J. 1973. Pentose pathway and production of reduced nicotinamide adenine dinucleotide phosphate. A mechanism that may protect lungs from oxidants. Am. Rev. Respir. Dis 108:1348–51 [DOI] [PubMed] [Google Scholar]
- 26.Carracedo A, Cantley LC, Pandolfi PP. 2013. Cancer metabolism: fatty acid oxidation in the limelight. Nat. Rev. Cancer 13:227–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw ME, Rhoades RA. 1977. Substrate metabolism in the perfused lung: response to changes in circulating glucose and palmitate levels. Lipids 12:930–35 [DOI] [PubMed] [Google Scholar]
- 28.Eaton S, Bartlett K, Pourfarzam M. 1996. Mammalian mitochondrial beta-oxidation. Biochem. J 320 (Part 2):345–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wanders RJ, Waterham HR. 2006. Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem 75:295–332 [DOI] [PubMed] [Google Scholar]
- 30.Abe M, Tierney DF. 1977. Lung lipid metabolism after 7 days of hydrocortisone administration to adult rats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol 42:202–5 [DOI] [PubMed] [Google Scholar]
- 31.Bohlin K, Merchak A, Spence K, Patterson BW, Hamvas A. 2003. Endogenous surfactant metabolism in newborn infants with and without respiratory failure. Pediatr. Res 54:185–91 [DOI] [PubMed] [Google Scholar]
- 32.Harayama T, Eto M, Shindou H, Kita Y, Otsubo E, et al. 2014. Lysophospholipid acyltransferases mediate phosphatidylcholine diversification to achieve the physical properties required in vivo. Cell Metab 20:295–305 [DOI] [PubMed] [Google Scholar]
- 33.Haagsman HP, van Golde LM. 1991. Synthesis and assembly of lung surfactant. Annu. Rev. Physiol 53:441–64 [DOI] [PubMed] [Google Scholar]
- 34.Goodpaster BH, Sparks LM. 2017. Metabolic flexibility in health and disease. Cell Metab 25:1027–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Köhler P 1985. The strategies of energy conservation in helminths. Mol. Biochem. Parasitol 17:1–18 [DOI] [PubMed] [Google Scholar]
- 36.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, et al. 2011. Structure of mammalian AMPK and its regulation by ADP. Nature 472:230–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, et al. 2005. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2:9–19 [DOI] [PubMed] [Google Scholar]
- 38.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, et al. 2003. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol 13:2004–8 [DOI] [PubMed] [Google Scholar]
- 39.Garcia D, Shaw RJ. 2017. AMPK: Mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 66:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, et al. 1996. Mammalian AMP-activated protein kinase subfamily. J. Biol. Chem 271:611–14 [DOI] [PubMed] [Google Scholar]
- 41.Konter JM, Parker JL, Baez E, Li SZ, Ranscht B, et al. 2012. Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J. Immunol 188:854–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah D, Romero F, Duong M, Wang N, Paudyal B, et al. 2015. Obesity-induced adipokine imbalance impairs mouse pulmonary vascular endothelial function and primes the lung for injury. Sci. Rep 5:11362. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, et al. 2008. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol 295:L497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, et al. 1994. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 369:756–58 [DOI] [PubMed] [Google Scholar]
- 45.Heitman J, Movva NR, Hall MN. 1991. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253:905–9 [DOI] [PubMed] [Google Scholar]
- 46.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. 1994. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78:35–43 [DOI] [PubMed] [Google Scholar]
- 47.Saxton RA, Sabatini DM. 2017. mTOR signaling in growth, metabolism, and disease. Cell 169:361–71 [DOI] [PubMed] [Google Scholar]
- 48.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, et al. 2011. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N. Engl. J. Med 364:1595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ge J, Cui H, Xie N, Banerjee S, Guo S, et al. 2018. Glutaminolysis promotes collagen translation and stability via α-ketoglutarate-mediated mTOR activation and proline hydroxylation. Am. J. Respir. Cell Mol. Biol 58:378–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houssaini A, Adnot S. 2017. mTOR: A key to both pulmonary vessel remodeling and right ventricular function in pulmonary arterial hypertension? Am. J. Respir. Cell Mol. Biol 57:509–11 [DOI] [PubMed] [Google Scholar]
- 51.Houssaini A, Breau M, Kebe K, Abid S, Marcos E, et al. 2018. mTOR pathway activation drives lung cell senescence and emphysema. JCI Insight 3:e93203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galluzzi L, Pietrocola F, Levine B, Kroemer G. 2014. Metabolic control of autophagy. Cell 159:1263–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cloonan SM, Lam HC, Ryter SW, Choi AM. 2014. “Ciliophagy”: the consumption of cilia components by autophagy. Autophagy 10:532–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levine B, Kroemer G. 2008. Autophagy in the pathogenesis of disease. Cell 132:27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, et al. 2010. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. PNAS 107:18880–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crighton D, Wilkinson S, O’Prey J, Syed N, Smith P, et al. 2006. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 126:121–34 [DOI] [PubMed] [Google Scholar]
- 57.Martinez-Vicente M, Cuervo AM. 2007. Autophagy and neurodegeneration: when the cleaning crew goes on strike. Lancet Neurol 6:352–61 [DOI] [PubMed] [Google Scholar]
- 58.Rabinovitch M 2012. Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Investig 122:4306–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zank DC, Bueno M, Mora AL, Rojas M. 2018. Idiopathic pulmonary fibrosis: aging, mitochondrial dysfunction, and cellular bioenergetics. Front. Med 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes PJ, Burney PG, Silverman EK, Celli BR, Vestbo J, et al. 2015. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 1:15076. [DOI] [PubMed] [Google Scholar]
- 61.Michaeloudes C, Kuo CH, Haji G, Finch DK, Halayko AJ, et al. 2017. Metabolic re-patterning in COPD airway smooth muscle cells. Eur. Respir. J 50:1700202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coxson HO, Chan IH, Mayo JR, Hlynsky J, Nakano Y, Birmingham CL. 2004. Early emphysema in patients with anorexia nervosa. Am. J. Respir. Crit. Care Med 170:748–52 [DOI] [PubMed] [Google Scholar]
- 63.Sahebjami H, Wirman JA. 1981. Emphysema-like changes in the lungs of starved rats. Am. Rev. Respir. Dis 124:619–24 [DOI] [PubMed] [Google Scholar]
- 64.Stein J, Fenigstein H. 1979. Pathological anatomy of hunger disease. Curr. Concepts Nutr 7:207–34 [PubMed] [Google Scholar]
- 65.Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, et al. 2014. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Investig 124:3987–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yaghi A, Dolovich MB. 2016. Airway epithelial cell cilia and obstructive lung disease. Cells 5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DeMeo DL, Mariani T, Bhattacharya S, Srisuma S, Lange C, et al. 2009. Integration of genomic and genetic approaches implicates IREB2 as a COPD susceptibility gene. Am. J. Hum. Genet 85:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ito S, Araya J, Kurita Y, Kobayashi K, Takasaka N, et al. 2015. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy 11:547–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, et al. 2013. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J. Clin. Investig 123:5212–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirkham PA, Barnes PJ. 2013. Oxidative stress in COPD. Chest 144:266–73 [DOI] [PubMed] [Google Scholar]
- 71.Zinellu E, Zinellu A, Fois AG, Carru C, Pirina P. 2016. Circulating biomarkers of oxidative stress in chronic obstructive pulmonary disease: a systematic review. Respir. Res 17:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang Z, Knudsen NH, Wang G, Qiu W, Naing ZZC, et al. 2017. Genetic control of fatty acid β-oxidation in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol 56:738–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yun JH, Morrow J, Owen CA, Qiu W, Glass K, et al. 2017. Transcriptomic analysis of lung tissue from cigarette smoke-induced emphysema murine models and human chronic obstructive pulmonary disease show shared and distinct pathways. Am. J. Respir. Cell Mol. Biol 57:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, et al. 2015. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J 29:2912–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, et al. 2016. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab 23:303–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nyunoya T, Mebratu Y, Contreras A, Delgado M, Chand HS, Tesfaigzi Y. 2014. Molecular processes that drive cigarette smoke-induced epithelial cell fate of the lung. Am. J. Respir. Cell Mol. Biol 50:471–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barnes PJ. 2017. Senescence in COPD and its comorbidities. Annu. Rev. Physiol 79:517–39 [DOI] [PubMed] [Google Scholar]
- 78.Fanta CH. 2009. Asthma. N. Engl. J. Med 360:1002–14 [DOI] [PubMed] [Google Scholar]
- 79.McCracken JL, Veeranki SP, Ameredes BT, Calhoun WJ. 2017. Diagnosis and management of asthma in adults: a review. JAMA 318:279–90 [DOI] [PubMed] [Google Scholar]
- 80.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, et al. 2014. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med 371:1189–97 [DOI] [PubMed] [Google Scholar]
- 81.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, et al. 2011. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med 365:1088–98 [DOI] [PubMed] [Google Scholar]
- 82.Mushaben EM, Kramer EL, Brandt EB, Khurana Hershey GK, Le Cras TD. 2011. Rapamycin attenuates airway hyperreactivity, goblet cells, and IgE in experimental allergic asthma. J. Immunol 187:5756–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Y, Jing Y, Qiao J, Luan B, Wang X, et al. 2017. Activation of the mTOR signaling pathway is required for asthma onset. Sci. Rep 7:4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lloyd CM, Hawrylowicz CM. 2009. Regulatory T cells in asthma. Immunity 31:438–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, et al. 2004. Tolerance induced by inhaled antigen involves CD4+ T cells expressing membrane-bound TGF-β and FOXP3. J. Clin. Investig 114:28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, et al. 2011. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol 186:3299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi LZ, Wang R, Huang G, Vogel P, Neale G, et al. 2011. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med 208:1367–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park CS, Bang BR, Kwon HS, Moon KA, Kim TB, et al. 2012. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem. Pharmacol 84:1660–70 [DOI] [PubMed] [Google Scholar]
- 89.Pendharkar S, Mehta S. 2008. The clinical significance of exhaled nitric oxide in asthma. Can. Respir. J 15:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu W, Ghosh S, Comhair SA, Asosingh K, Janocha AJ, et al. 2016. Increased mitochondrial arginine metabolism supports bioenergetics in asthma. J. Clin. Investig 126:2465–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H, Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Estela Del Rio-Navarro B, et al. 2006. Genetic polymorphisms in arginase I and II and childhood asthma and atopy. J Allergy Clin. Immunol 117:119–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vonk JM, Postma DS, Maarsingh H, Bruinenberg M, Koppelman GH, Meurs H. 2010. Arginase 1 and arginase 2 variations associate with asthma, asthma severity and β2 agonist and steroid response. Pharmacogenet. Genom 20:179–86 [DOI] [PubMed] [Google Scholar]
- 93.Trian T, Benard G, Begueret H, Rossignol R, Girodet PO, et al. 2007. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J. Exp. Med 204:3173–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roth M, Johnson PR, Borger P, Bihl MP, Rudiger JJ, et al. 2004. Dysfunctional interaction of C/EBPα and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. N. Engl. J. Med 351:560–74 [DOI] [PubMed] [Google Scholar]
- 95.Cheng Z, Wang X, Dai L, Jia L, Jing X, et al. 2017. Suppression of microRNA-384 enhances autophagy of airway smooth muscle cells in asthmatic mouse. Oncotarget 8:67933–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pan S, Sharma P, Shah SD, Deshpande DA. 2017. Bitter taste receptor agonists alter mitochondrial function and induce autophagy in airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol 313:L154–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wolters PJ, Collard HR, Jones KD. 2014. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. Mech. Dis 9:157–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gross TJ, Hunninghake GW. 2001. Idiopathic pulmonary fibrosis. N. Engl. J. Med 345:517–25 [DOI] [PubMed] [Google Scholar]
- 99.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, et al. 2010. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med 181:254–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang YP, Lee SB, Lee JM, Kim HM, Hong JY, et al. 2016. Metabolic profiling regarding pathogenesis of idiopathic pulmonary fibrosis. J. Proteome Res 15:1717–24 [DOI] [PubMed] [Google Scholar]
- 101.Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, et al. 2015. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J. Clin. Investig 125:521–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, et al. 2018. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat. Med 24:39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Romero F, Shah D, Duong M, Penn RB, Fessler MB, et al. 2015. A pneumocyte-macrophage paracrine lipid axis drives the lung toward fibrosis. Am. J. Respir. Cell Mol. Biol 53:74–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kottmann RM, Kulkarni AA, Smolnycki KA, Lyda E, Dahanayake T, et al. 2012. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. Am. J. Respir. Crit. Care Med 186:740–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burman A, Tanjore H, Blackwell TS. 2018. Endoplasmic reticulum stress in pulmonary fibrosis. Matrix Biol 68–69:355–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lenna S, Trojanowska M. 2012. The role of endoplasmic reticulum stress and the unfolded protein response in fibrosis. Curr. Opin. Rheumatol 24:663–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, et al. 2016. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1:e90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, et al. 2018. Metformin reverses established lung fibrosis in a bleomycin model. Nat. Med 24:1121–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xie N, Tan Z, Banerjee S, Cui H, Ge J, et al. 2015. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am. J. Respir. Crit. Care Med 192:1462–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cho SJ, Moon JS, Lee CM, Choi AM, Stout-Delgado HW. 2017. Glucose transporter 1-dependent glycolysis is increased during aging-related lung fibrosis, and phloretin inhibits lung fibrosis. Am. J. Respir. Cell Mol. Biol 56:521–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goodwin J, Choi H, Hsieh MH, Neugent ML, Ahn JM, et al. 2018. Targeting hypoxia-inducible factor-1α/pyruvate dehydrogenase kinase 1 axis by dichloroacetate suppresses bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol 58:216–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dunbar EM, Coats BS, Shroads AL, Langaee T, Lew A, et al. 2014. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Investig. New Drugs 32:452–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hamanaka RB, Nigdelioglu R, Meliton AY, Tian Y, Witt LJ, et al. 2018. Inhibition of phosphoglycerate dehydrogenase attenuates bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol 58:585–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nigdelioglu R, Hamanaka RB, Meliton AY, O’Leary E, Witt LJ, et al. 2016. Transforming growth factor (TGF)-β promotes de novo serine synthesis for collagen production. J. Biol. Chem 291:27239–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bernard K, Logsdon NJ, Benavides GA, Sanders Y, Zhang J, et al. 2018. Glutaminolysis is required for transforming growth factor-β1-induced myofibroblast differentiation and activation. J. Biol. Chem 293:1218–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xie N, Cui H, Ge J, Banerjee S, Guo S, et al. 2017. Metabolic characterization and RNA profiling reveal glycolytic dependence of profibrotic phenotype of alveolar macrophages in lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol 313:L834–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Galván-Peña S, O’Neill LAJ. 2014. Metabolic reprograming in macrophage polarization. Front. Immunol 5:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Farber HW, Loscalzo J. 2004. Pulmonary arterial hypertension. N. Engl. J. Med 351:1655–65 [DOI] [PubMed] [Google Scholar]
- 119.D’Alessandro A, El Kasmi KC, Plecitá-Hlavatá L, Ježek P, Li M, et al. 2018. Hallmarks of pulmonary hypertension: mesenchymal and inflammatory cell metabolic reprogramming. Antioxid. Redox Signal 28:230–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tuder RM, Davis LA, Graham BB. 2012. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am. J. Respir. Crit. Care Med 185:260–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fessel JP, Hamid R, Wittmann BM, Robinson LJ, Blackwell T, et al. 2012. Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulm Circ 2:201–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sutendra G, Michelakis ED. 2014. The metabolic basis of pulmonary arterial hypertension. Cell Metab 19:558–73 [DOI] [PubMed] [Google Scholar]
- 123.Stenmark KR, Tuder RM, El Kasmi KC. 2015. Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension. J. Appl. Physiol 119:1164–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Plecitá-Hlavatá L, Tauber J, Li M, Zhang H, Flockton AR, et al. 2016. Constitutive reprogramming of fibroblast mitochondrial metabolism in pulmonary hypertension. Am. J. Respir. Cell Mol. Biol 55:47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, et al. 2009. Function of mitochondrial Stat3 in cellular respiration. Science 323:793–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, et al. 2007. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol 293:L548–54 [DOI] [PubMed] [Google Scholar]
- 127.Paulin R, Meloche J, Jacob MH, Bisserier M, Courboulin A, Bonnet S. 2011. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol 301:H1798–809 [DOI] [PubMed] [Google Scholar]
- 128.Diebold I, Hennigs JK, Miyagawa K, Li CG, Nickel NP, et al. 2015. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab 21:596–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Paulin R, Dromparis P, Sutendra G, Gurtu V, Zervopoulos S, et al. 2014. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab 20:827–39 [DOI] [PubMed] [Google Scholar]
- 130.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, et al. 2004. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ. Res 95:830–40 [DOI] [PubMed] [Google Scholar]
- 131.Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, et al. 2017. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci. Transl. Med 9:eaao4583 [DOI] [PubMed] [Google Scholar]
- 132.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, et al. 2002. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation 105:244–50 [DOI] [PubMed] [Google Scholar]
- 133.Marsboom G, Wietholt C, Haney CR, Toth PT, Ryan JJ, et al. 2012. Lung 18F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med 185:670–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Haslip M, Dostanic I, Huang Y, Zhang Y, Russell KS, et al. 2015. Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler. Thromb. Vasc. Biol 35:1166–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pak O, Sommer N, Hoeres T, Bakr A, Waisbrod S, et al. 2013. Mitochondrial hyperpolarization in pulmonary vascular remodeling. Mitochondrial uncoupling protein deficiency as disease model. Am. J. Respir. Cell Mol. Biol 49:358–67 [DOI] [PubMed] [Google Scholar]
- 136.Dyck JR, Hopkins TA, Bonnet S, Michelakis ED, Young ME, et al. 2006. Absence of malonyl coenzyme A decarboxylase in mice increases cardiac glucose oxidation and protects the heart from ischemic injury. Circulation 114:1721–28 [DOI] [PubMed] [Google Scholar]
- 137.Zhang Y, Sauler M, Shinn AS, Gong H, Haslip M, et al. 2014. Endothelial PINK1 mediates the protective effects of NLRP3 deficiency during lethal oxidant injury. J. Immunol 192:5296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, et al. 2015. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Rep 13:1016–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, et al. 2016. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Investig 126:3313–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mathis D, Shoelson SE. 2011. Immunometabolism: an emerging frontier. Nat. Rev. Immunol 11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, et al. 2015. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl. Med 7:274ra18 [DOI] [PMC free article] [PubMed] [Google Scholar]