Abstract
The functionalization of primary C-H bonds has been a longstanding challenge in catalysis. Our group has developed a series of silylations of primary C-H bonds that occur with site selectivity and diastereoselectivity resulting from an approach to run the reactions as intramolecular processes. These reactions have become practical by using an alcohol or amine as a docking site for a hydrosilyl group, thereby leading to intramolecular silylations of C-H bonds at positions dictated by the presence common functional groups in the reactants. Oxidation of the C-Si bond leads to the introduction of alcohol functionality at the position of the primary C-H bond of the reactant. The development, scope, and applications of these functionalization reactions is described in this minireview.
Graphical Abstract

Introduction.
The functionalization of alkyl C-H bonds can create new synthetic strategies for the synthesis of both simple and complex molecules, and it can lead to the convenient modification of C-H bonds in existing structures.1–3 Among potential reactions that lead to functionalization, the dehydrogenative coupling of an alkyl C-H bond with the X-H bond of a reagent to form a C-X bond would be attractive, but most of these couplings lie far uphill.4 Thus, there is a large inherent barrier that makes the reactions challenging to achieve, even if the hydrogen were expelled from the system or consumed by an additional reagent, as in transfer dehydrogenation of alkanes.5 However, the more electropositive the heteroatom in the X-H bond, the smaller the difference between the C-X and H-X bond, and for some main-group elements, the C-X bond is stronger than the H-X bond.6–7 The difference in bond strengths for silanes is small enough that the thermodynamics can be driven by conducting intramolecular dehydrogenative couplings that are entropically favored,8 by conducting the reactions with a hydrogen acceptor, or by running the reactions in an open system.9–10 For this reason, the extension of our catalytic borylation of alkyl C-H bonds11–12 to the catalytic silylation of C-H bonds seemed feasible. Several design features described in this minireview led to systems for practical silylation of alkyl C-H bonds.
The resulting alkylsilanes undergo oxidation to the alcohol.13 Thus, the combination of silylation and oxidation leads to the hydroxylation of C-H bonds, and a comparison of the silylation to the direct oxidation of C-H bonds is warranted. In general, undirected oxidations of alkyl C-H bonds, with or without a catalyst, tend to occur more rapidly at weaker and more electron-rich C-H bonds, causing tertiary and secondary C-H bonds to be more reactive than primary C-H bonds.14 In contrast, the functionalizations of C-H bonds with main group reagents catalyzed by transition metal complexes that form metal-carbon bonds tend to occur selectively at primary over secondary C-H bonds.15 As described below, the silylation of secondary C-H bonds occurs, but it occurs in reactants lacking a primary C-H bond in a suitable position relative to the Si-H bond to react. Although many functionalizations of C-H bonds are directed by a Lewis basic functional group on the substrate, few directed hydroxylations of C-H bonds have been reported,16–18 and the directed reactions usually require separate steps with purifications of the intermediates to install and remove the directing group. As described in this minireview, the silylation and oxidation of C-H bonds leads to hydroxyl groups at positions controlled by the presence of an alcohol and the identity of the reagents or catalyst and, in many cases, do not require isolation of intermediates.
Discovery of the silylation of unactivated C-H Bonds.
The discovery of practical, iridiumcatalyzed silylations of alkyl C-H bonds was considered possible in part by our previous discovery of an intramolecular silylation of an alkyl C-H bond catalyzed by a Cp*-platinum(IV) catalyst.19 Dr. Naofomi Tsukada was investigating the silylation of aryl C-H bonds catalyzed by (TpMe2)Pt(Me)2H at high temperature with neat arene and was able to extend this C-H bond silylation to the reaction of tributyl silane (Scheme 1). The reaction of tributyl silane formed a five-membered ring silolane by formation of an alkyl-silicon bond at the position of a methyl C-H bond.
Scheme 1.
Platinum catalyzed intramolecular silylation of a methyl C-H bond.19
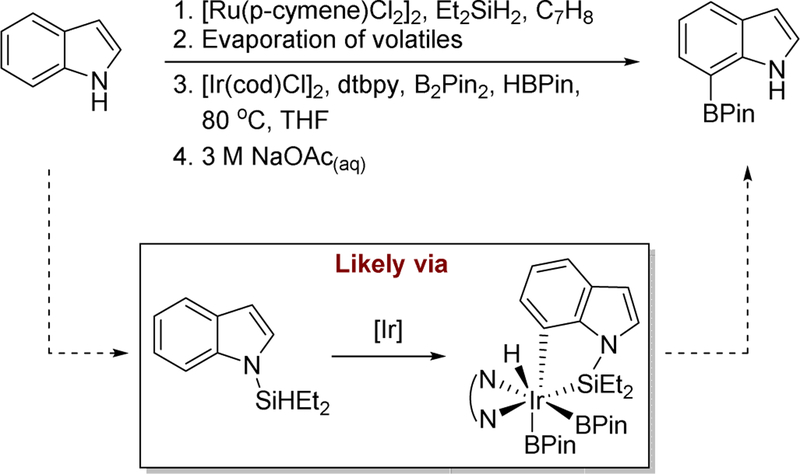
Our silylations of alkyl C-H bonds also was based in part on a silyl-directed borylation process discovered in our laboratory by Dr. Tim Boebel. Tim showed that the installation of a hydrosilyl group on a phenol enabled the coordination of the substrate to the iridium catalyst and site-selective borylation of the ortho C-H bonds (Scheme 2).20 As shown in Fig. 1, we assumed that the silane reacts with the iridium-boryl complex to install a silyl group derived from the reactant. The ortho C-H bond of the reactant would then add to iridium, and the product would form by reductive elimination to form a B-C bond. This silyl-directed borylation also enabled the site-selective borylation of indoles at the 7-position (Scheme 3).21
Scheme 2.
Iridium-catalyzed borylation of ortho C-H bonds of phenols directed by a hydrosilyl ether.20
Fig. 1.
Proposed mechanism for hydrosilyl-directed borylations of aromatic C-H bonds.20
Scheme 3.
Borylation of indoles at the 7-position of the heterocycle likely proceeds through a pathway directed by the hydrosilyl group.21
Dr. Eric Simmons in our group proposed that this type of functionalization of a C-H bond involving hydrosilyl ethers could be adapted to form an Si-C bond, rather than directing the formation of a B-C bond. After initial demonstration of this potential by forming aryl-Si bonds,22 he showed that this reaction could be extended to the construction of alkyl-Si bonds at a methyl group proximal to the hydroxyl group from which the silyl ether was formed (Scheme 4, top).23
Scheme 4.
Silylation of methyl C-H bonds of hydrosilyl ethers affords 1,3-diols after Tamao-Fleming oxidation of the intermediate 5-membered oxasilolanes (top). Scope of the reaction (bottom).23
The design of this silylation of C-H bonds is shown in Scheme 4. Although the borylation of alkyl C-H bonds has been established, and one might imagine that intramolecular borylation of C-H bonds would be faster than intermolecular borylation, it is difficult to envision how the borylation of C-H bonds could be conducted intramolecularly in a practical fashion. Hydroboranes undergo exchange of substituents at boron under mild conditions. Hydrosilanes are more stable to such exchange reactions. Thus, attachment of a silyl group to an alcohol by dehydrogenative silylation with a dihydrosilane formed a hydrosilane product that was stable to exchanges at elevated temperatures and underwent intramolecular silylation of an alkyl C-H bond. As designed, such reactions occurred to form five-membered oxasilolanes in high yield.
A summary of the demonstrated scope of the reactions is shown in Scheme 4 (bottom). These data show that the reaction sequence forms the 1,3 diols (isolated after acetylation for convenience) starting with tertiary or secondary alcohols. The reactions occur at primary C-H bonds in the presence of aryl groups that would form rings larger or smaller than a five-membered ring if the aryl C-H bond reacted. These data also show that the reaction occurs on a methyl group cis or trans to the alcohol on a cyclohexyl ring in nearly equivalent yields, but that the reaction occurs diastereoselectively at one of two methyl groups, even on an acyclic substrate.
Studies on reaction scope also showed that the silylation occurs at methyl groups proximal to the alcohols of terpenes. In the first set of reactions developed, the silylation of C-H bonds of methyl groups gamma to the oxygen of a hydrosilyl ether derived from the alcohol forms a five-membered ring, and oxidation of this oxasilolane leads to a 1,3-diol product. Reaction of methyl oleanate shows the efficiency of hydroxylation by this method (Scheme 5). In a three-step, one-pot protocol, this triterpenoid undergoes silylation at the alcohol to form the hydrosilyl ether and silylation at the methyl C-H bond to form the oxasilolane. This oxasilolane undergoes oxidation to form the natural product methyl hederagenate. The conversion of methyl oleanate to methyl hederagenate has been conducted previously by a sequence involving palladium-catalyzed directed acetoxylation,24 but this alternative sequence required eight steps.
Scheme 5.
Regioselective one-pot hydroxylation of a methyl C-H bond of methyl oleanate to afford methyl hederagenate in high yield.23
Silylations of Secondary C-H Bonds.
The discovery of a process for the silylation of primary C-H bonds led to the question of whether this process could be extended to the silylation of secondary C-H bonds located gamma to the hydroxyl group via five-membered oxasilolanes. Indeed, Dr. Bijie Li showed that this reaction can be observed in high yield, although the current, published scope of this reaction is limited (Scheme 6).25 This method is currently limited to reactions of tertiary alcohols with steric properties that favor an intramolecular reaction. As shown in Scheme 6, reaction of the hydrosilyl ether derived from an acyclic secondary alcohol occurred at the secondary C-H bond in low yield, but the reaction of a more structurally constrained secondary alcohol occurred in good yield and with high diastereoselectivity. Reactions of an unhindered tertiary alcohol occurred at the secondary C-H bond in modest yield. As long as the two substituents on the alcohol are larger than a methyl group, then the reaction occurred in high yield, even for acyclic alcohols. We presume these larger substituents on the acyclic substrates favor cyclization for the same reason that geminal substituents favor cyclization in other process by the well-known “Thorpe-Ingold effect.26“ As shown in Scheme 7, reaction of a hydrosilyl ether containing three methyl C-H bonds gamma to the alcohol and four methylene C-H bonds gamma to the alcohol reacted preferentially at the methyl group by a factor of over 50, when correcting for the number of C-H bonds. Yet, the relative rates are close enough that further research should make this process broader in scope and capable of converting a range of chiral alcohols to 1,3 diols (or other diols) diastereoselectively.
Scheme 6.
Hydrosilyl ether-directed γ-C-H bond silylation efficiently yields 1,3-diols after oxidation.25
Scheme 7.
Competition experiments prove the preferential activation of methyl C-H bonds over methylene C-H bonds.25
Silylations to form 1,4-diols and 1,2-diols.
The discovery of a process to form 1,3 diols also led to the question of whether the reaction could occur to form diols separated by a different number of carbons. For example, we considered whether the process could form 1,4 diols or 1,2 diols. The formation of 1,4 diols by functionalization of a primary C-H bond in preference to the formation of 1,3 diols by functionalization of a secondary C-H bond was achieved by changing the catalyst from the iridium-phenanthroline system to a rhodium complex ligated by the bisphosphine Xantphos.27 Dr. Li investigated rhodium catalysts for this process because we had shown previously that rhodium complexes of bisphosphines catalyze the enantioselective silylation of aryl C-H bonds.28 Examples of the rhodium-catalyzed silylation to form 1,4 diols is shown in Scheme 8. The reaction required a tertiary alcohol and branching alpha to the reacting methyl group, presumably to ensure that one methyl group is proximal to the hydrosilyl ether group in the reaction intermediate. Despite this current constraint, the example of the functionalization of the C-H bond delta to the alcohol in cholesterol shows that the process can be applied to the functionalization of complex architectures.
Scheme 8.
δ-C-H bond silylation of hydrosilyl ethers catalyzed by the combination of rhodium and xantphos generates 1,4-diols after oxidation and can be applied to the hydroxylation of complex architectures.27
A detailed study of the origin of selectivity of this reaction was conducted by Caleb Karmel, a graduate student in our laboratory. His computational studies by DFT provided strong evidence that cleavage of the C-H bonds by the rhodium-Xantphos catalyst can be a reversible step prior to the formation of the C-Si bond (Fig. 2). Thus, the selectivity for the reaction to form a five or six-membered ring from reaction at secondary and primary positions, respectively, results from the relative rates for formation of the C-Si bond. Reductive elimination from the primary alkyl intermediate formed from addition of the C-H bond delta to the initial alcohol is followed by rapid reductive elimination to form the oxasilinane product. Addition of the secondary C-H bond gamma to the initial alcohol occurs with a lower barrier than addition of the more remote primary C-H bond, but the barrier to reductive elimination from the secondary alkyl intermediate is higher than that for either C-H bond cleavage or reductive elimination from the primary alkyl complex. Thus, this high barrier to reductive elimination from the secondary alkyl intermediate from the Xantphos-ligated rhodium catalyst prevents formation of the five-membered oxasilolane ring as the major product.
Fig. 2.
Relative computed transition-state energies for the C-H bond-cleavage and C-Si bond-forming elementary reaction steps of rhodium-catalyzed silylation of primary and secondary C-H bonds.27
The formation of 1,2 diols by silylation of a C-H bond located beta to the hydroxyl group by an analogous strategy to the formation of 1,3 and 1,4 diols would require the reductive elimination of a strained four-membered oxasiletane (Scheme 9). Thus, an alternative approach would be needed to achieve the silylation of a C-H bond beta to an alcohol. Such a strategy was devised by Dr. Ala Bunescu in our laboratory.29 Ala converted the alcohol to an ester and then converted the ester to a hydrosilyl acetal by hydrosilylation of the ester. When this ester contained a long perfluoroalkyl group on the carbonyl carbon, the hydrosilyl acetal was sufficiently stable to achieve the silylation of a methyl C-H bond by the hydrosilyl group. The resulting six-membered dioxasilinane underwent Tamao-Fleming oxidation to form a 1,2 diol with concomitant hydrolysis of the acetal.
Scheme 9.
The strategy to access β-C-H bond silylation comprises the installation of a hydrosilyl acetal directing group and C-H bond silylation. The resulting dioxasilinanes have been oxidized to the corresponding 1,2-diols 29
Gevorgyan reported closely related reactions to form 1,4 diols from terminal alkenes (Scheme 10).30 This sequence begins with hydrosilylation of the alkene with dichlorodihydrosilane catalyzed by Wilkinson’s catalyst. Next, one tert-butyl and one picolinyl group are added to the resulting silane by addition of the corresponding organolithium reagents. Silylation of the C-H bond catalyzed by [IrCl(cod)]2, which is activated by coordination of the picolinyl group on the silane to iridium, forms a five-membered ring. Because of the presence of the picolinyl group on the silane, this tetraorganosilane can undergo oxidation to form a 1,4 diol. Of course, the use of dichlorosilane and the addition of organolithium reagents limits the functional-group compatibility of the process, but his sequence was suitable for the formation of a 1,4 diol from terpene hydrocarbons or a terpene containing a protected alcohol, as shown by the illustrative example in Scheme 10.
Scheme 10.
Hydrosilylation of alkenes affords precursors for Ir-catalyzed δ-C-H bond silylation to yield acetyl-protected 1,4-diols after oxidation.30
Following a strategy analogous to the silylation of C-H bonds directed by hydrosilyl ethers, our group investigated the silylation of C-H bonds in hydrosilyl amines. However, the first study led only to reactions at aryl and activated benzylic C-H bonds.31 The scope was limited because the Si-N bond is weaker and more kinetically labile than Si-O bonds, causing the hydrosilylamine to be unstable to the reaction conditions required for the silylation of alkyl C-H bonds. More recently, a graduate student in our laboratory, Taegyo Lee, rationalized that separating the nitrogen atom from the silicon atom by a methylene group (accessed by addition of the corresponding secondary amine to (chloromethyl)dimethylisopropoxysilane and reduction of the resulting silyl ether to the hydrosilane) should make the hydrosilyl unit more stable under catalytic conditions. The resulting substrates can then be transformed into silapyrrolidine heterocycles by an intramolecular silylation reaction of a methyl C-H bond β to the amine nitrogen. He and Dr. Su showed that this reaction was catalyzed by the phenanthroline ligands we recently published for the silylation of aryl C-H bonds and is applicable to a range of sterically and electronically diverse substrates (Scheme 11).32 In addition, this catalyst silylated the methyl C-H bond over the aryl C-H bond with a selectivity of 10:1 when the aryl C-H bond was positioned gamma to the amine nitrogen atom and the methyl group beta to the nitrogen. This methodology was also suitable for the regioselective silylation of the methyl ether derivative of the active pharmaceutical ingredient in Propranolol, a beta blocker for a range of indications, illustrating the ability of this catalyst to functionalize medicinally relevant compounds. The silapyrrolidine products can serve as precursors to 1,2-amino alcohols. Oxidation under Tamao-Fleming conditions generates the amino alcohol during a process in which the bridging methylene unit is presumably extruded as formaldehyde after cleavage of a hemiaminal.
Scheme 11.
Hydrosilyl-directed silylation of β-C-H bonds of tertiary amines followed by oxidation and protection of the resulting silapyrrolidines affords N-boc-1,2-amino alcohols.32
Enantioselective Silylation of Alkyl C-H Bonds.
The iridium-catalyzed silylation of alkyl C-H bonds occurs with iridium catalysts ligated by planar nitrogen-donor ligands. However, these reactions can be rendered enantioselective by designing nitrogen-donor ligands that are chiral. Dr. Bo Su in our laboratory had previously used such ligands for the enantioselective borylation33 and silylation34 of aryl C-H bonds. Thus, he prepared a small library of such ligands (Scheme 12) and evaluated them for iridium-catalyzed enantioselective silylation of an isopropyl group containing enantiotopic methyl groups. The substrate aryl silanes were prepared by simple addition of dimethylchlorosilane to either aryl-lithium or aryl-Grignard reagents. He found that the ligand L1 depicted in Scheme 12 generated a catalyst for the reaction in the same scheme to form the cyclized product with ee values ranging from 86–96% in most cases.35 Previously, Takai and Murai,36 reported versions of such reactions with rhodium catalysts bearing more conventional bisphosphine ligands, but the enantioselectivities were less than 40%. This reaction with the iridium catalyst was applied to the late-stage functionalization of dehydroabietic methanoate in excellent yields and diastereoselectivity.
Scheme 12.
Ir-catalyzed hydrosilyl-directed silylation of enantiotopic methyl C-H bonds yields enantiopure products and can be applied to complex molecule scaffolds.35
The silylation of methyl C-H bonds in the hydrosilylmethylamines described in the previous section also can be rendered enantioselective (Scheme 13).32 In this case, an unusual pyridyl imidazoline ligand was prepared and used for this process. This N,N ligand generated a catalyst that was more active for this process than those from related pyridyl oxazoline ligands. The pyridyl imidazoline featuring a hindered pyridyl group generated a catalyst that was highly active and reacted with substantial enantioselectivity to form the silapyrrolidine products.
Scheme 13.
Use of a chiral ancillary ligand enables the enantioselective β-C-H bond silylation reaction to occur in high yields and moderate enantioselectivities.32
Applications of the silylation of alkyl C-H bonds.
The silylation of alcohols has been used by several research groups for the derivatization of natural products. In one report, the reaction has been reported to afford less available sugars from more abundant ones.37 The strategy was revealed initially, as shown in Scheme 14. Rare L-mannoside and L-galactoside derivatives were prepared in one pot by a four-step sequence starting with L-rhamnose and L-fucose. The unprotected alcohol of the rhamnoside was converted to the hydrosilyl ether, and the iridium-catalyzed process was conducted to form the oxasilolane unit. Oxidation and acetylation provided the final product. By a similar sequence, the more complex L-galactoside was prepared from a model fucoside. Again, the four steps were conducted in one pot, and a high 67% yield of the final product was obtained.
Scheme 14.
4-step, 1-pot reaction sequence affording fully protected sugars in good yields.37–38
In the second publication,38 the preparation of eight diastereomeric L-sugars from silylation and oxidation was reported. The structures of the sugars produced in this fashion are also shown in Scheme 14. In all eight cases, the silylation of an alcohol, C-H bond functionalization of the methyl C-H bond, oxidation and acetylation were conducted in one reaction vessel in sequence. These materials previously required more than 10 steps to prepare.39
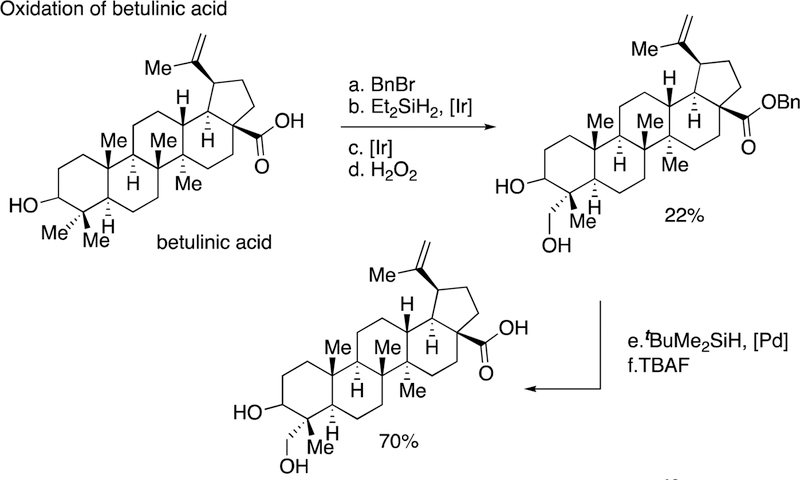
In a second set of applications, terpene natural products have been modified by the sequence we first published with methyl oleanate to modify the solubility and biological properties of terpene structures. In one case, benzyl-protected betulinic acid was converted to the C23-hydroxyl derivative by the combination of silylation of the secondary C3-hydroxyl group and subsequent silylation of the C23 methyl group (Scheme 15).40 Oxidation and debenzylation provided the hydroxylated natural product C23-hydroxybetulinic acid.
Scheme 15.
C-H bond silylation of betulinic acid occurs in modest yield.40
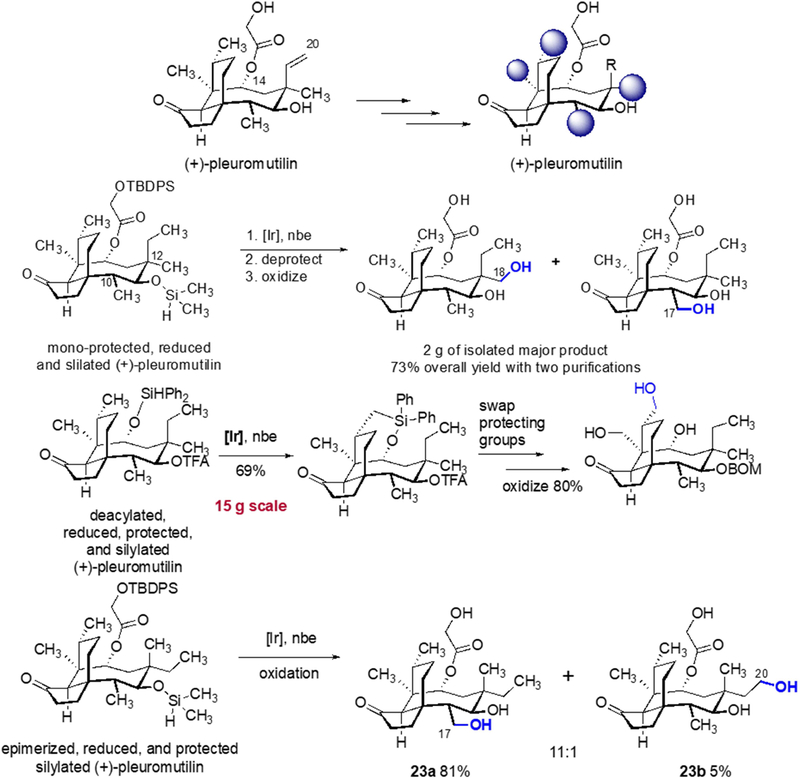
By a similar strategy, the natural product pleuromutilin has been modified to generate a series of hydroxyl derivatives (Scheme 16).41 In this case, strategic protection of one hydroxyl group and silylation of another hydroxyl group leads to the hydrosilyl ether that undergoes iridium-catalyzed silylation of a methyl C-H bond. These reactions were conducted on multi-gram scale in several cases. By the first sequence shown, the primary alcohol of pleuromutilin was protected, and the secondary alcohol was silylated with dimethylchlorosilane. By the second sequence, the C12 epimer was converted to the analogous dimethyl hydrosilyl ether, and silylation of a C-H bond occurred selectively at the C17 methyl group. In a third sequence, the primary alcohol of pleuromutilin was converted to a diphenyl hydrosilyl ether, and the secondary alcohol was protected as the trifluoro acetate. In this case, the methyl group on the cyclohexyl ring was modified to the alcohol on 15 g scale. This alcohol could then be used in a second silylation and oxidation of the angular methyl group.
Scheme 16.
Summary of the strategic C-H bond silylations of (+)-pleuromutilin.41
Silylations catalyzed by complexes of other metals.
Although this mini-review focuses on iridium-catalyzed silylation of alkyl C-H bonds, we note that some directed silylations of C-H bonds have been reported to occur with palladium and ruthenium catalysts.42–47 Palladium acetate has been shown to catalyze the silylation of primary C-H bonds located beta or gamma to the carbonyl group of amidoquinolines (Daugulis auxiliary)48 (Scheme 17). In particularly striking examples, amino acids and polypeptides have been reported to undergo palladium-catalyzed silylation at a terminal methyl groups directed by the amido-quinoline group of polypeptides (Scheme 18).49 These palladium-catalyzed reactions have been conducted with hexamethyl disilane as the silylating reagent. Thus, the products are tetraorganosilanes that are difficult to further functionalized by reaction of the C-Si bond. However, they are potentially valuable silyl derivatives of amino acids and peptides. Ruthenium-catalyzed silyation with triethyl silane occurs at primary C-H bond of alkyl groups in the 2-position of a pyridine (Scheme 19).45 Several additional reactions catalyzed by ruthenium complexes directed by pyridyl groups occur at activated benzylic positions50 or C-H bonds alpha to a boron atom.51 Thus, the potential scope and utility of the silylation of C-H bonds is broad.
Scheme 17.
Pd-catalyzed directed-silylation of primary C-H bonds located beta or gamma to the directing group.42–44,47
Scheme 18.
Application of Pd-catalyzed C-H bond silylation to polypeptide substrates in modest to good yields.49
Scheme 19.

Ru-catalyzed silylation of the primary alkyl C(sp3)-H bond of pyridines.45
Conclusions and Future Directions.
The number of reported transition-metal-catalyzed C-H bond functionalization reactions has increased dramatically in recent years and now includes methods to conduct these reactions with complex structures. Despite this progress, catalysts that regioselectively react at unactivated primary C-H bonds are rare, and reactions that enable the installation of a hydroxyl group at a primary C-H bond are even less common. This minireview has shown that the combination of silylation and oxidation can generate products from hydroxylation of a primary C-H bond. This advance has been surprising because the borylation of C-H bonds has not been conducted intramolecularly in a practical fashion and has generally required a large excess of substrate for reactions at primary C-H bonds. Moreover, silylations of C-H bonds generally occur with slower rates, require more forcing reaction conditions, and are less favorable thermodynamically than the analogous borylation reactions. However, because of the greater stability of hydrosilyl ethers than hydroboranes to disproportionation, hydrosilanes and hydrosilyl ethers derived from alkenes and alcohols can serve as intermediates for intramolecular functionalization of C-H bonds and subsequent oxidation to the corresponding dihydroxy products. These reactions are robust and generally applicable, as demonstrated by the use of these silylation methods by other groups for the regioselective silylation of C-H bonds in complex biologically active organic molecules. We anticipate that future research in this field will further increase the scope of reactions listed in this minireview. Success in these endeavors, along with the continued discovery of associated derivatizations of the carbon-silicon bond, will further expand the potential of the silylation of C-H bonds to create valuable products.
Acknowledgements.
We acknowledge the NIH-NIGMS for support of this work (GM-1R35GM130387). EAR thanks the California Alliance for a postdoctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Newton CG, Wang S-G, Oliveira CC, Cramer N, Chem. Rev 2017; 117:8908. [DOI] [PubMed] [Google Scholar]
- [2].He J, Wasa M, Chan KSL, Shao Q, Yu J-Q, Chem. Rev 2017; 117:8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Park Y, Kim Y, Chang S, Chem. Rev 2017; 117:9247. [DOI] [PubMed] [Google Scholar]
- [4].Hartwig JF, Larsen MA, ACS Cent. Sci 2016; 2:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kumar A, Bhatti TM, Goldman AS, Chem. Rev 2017; 117:12357. [DOI] [PubMed] [Google Scholar]
- [6].Benson SW, Francis JT, Tsotsis TT, J. Phys. Chem 1988; 92:4515. [Google Scholar]
- [7].Rablen PR, Hartwig JF, J. Am. Chem. Soc 1996; 118:4648. [Google Scholar]
- [8].Walsh R, Acc. Chem. Res 1981; 14:246. [Google Scholar]
- [9].Cheng C, Hartwig JF, Chem. Rev 2015; 115:8946. [DOI] [PubMed] [Google Scholar]
- [10].Cheng C, Hartwig JF, J. Am. Chem. Soc 2015; 137:592. [DOI] [PubMed] [Google Scholar]
- [11].Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF, Chem. Rev 2010; 110:890. [DOI] [PubMed] [Google Scholar]
- [12].Hartwig JF, Acc. Chem. Res 2012; 45:864. [DOI] [PubMed] [Google Scholar]
- [13].Jones GR, Landais Y, Tetrahedron 1996; 52:7599. [Google Scholar]
- [14].Newhouse T, Baran PS, Angew. Chem. Int. Ed 2011; 50:3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hartwig JF, Chem. Soc. Rev 2011; 40:1992. [DOI] [PubMed] [Google Scholar]
- [16].Mo F, Trzepkowski LJ, Dong G, Angew. Chem. Int. Ed 2012; 51:13075. [DOI] [PubMed] [Google Scholar]
- [17].Trammell R, See YY, Herrmann AT, Xie N, Díaz DE, Siegler MA, Baran PS, Garcia-Bosch I, J. Org. Chem 2017; 82:7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang Y-H, Yu J-Q, J. Am. Chem. Soc 2009; 131:14654. [DOI] [PubMed] [Google Scholar]
- [19].Tsukada N, Hartwig JF, J. Am. Chem. Soc 2005; 127:5022. [DOI] [PubMed] [Google Scholar]
- [20].Boebel TA, Hartwig JF, J. Am. Chem. Soc 2008; 130:7534. [DOI] [PubMed] [Google Scholar]
- [21].Robbins DW, Boebel TA, Hartwig JF, J. Am. Chem. Soc 2010; 132:4068. [DOI] [PubMed] [Google Scholar]
- [22].Simmons EM, Hartwig JF, J. Am. Chem. Soc 2010; 132:17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Simmons EM, Hartwig JF, Nature 2012; 483:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].García-Granados A, López PE, Melguizo E, Parra A, Simeó Y, J. Org. Chem 2007; 72:3500. [DOI] [PubMed] [Google Scholar]
- [25].Li B, Driess M, Hartwig JF, J. Am. Chem. Soc 2014; 136:6586. [DOI] [PubMed] [Google Scholar]
- [26].Jung ME, Piizzi G, Chem. Rev 2005; 105:1735. [DOI] [PubMed] [Google Scholar]
- [27].Karmel C, Li B, Hartwig JF, J. Am. Chem. Soc 2018; 140:1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee T, Wilson TW, Berg R, Ryberg P, Hartwig JF, J. Am. Chem. Soc 2015; 137:6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bunescu A, Butcher TW, Hartwig JF, J. Am. Chem. Soc 2018; 140:1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ghavtadze N, Melkonyan FS, Gulevich AV, Huang C, Gevorgyan V, Nat. Chem 2014; 6:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li Q, Driess M, Hartwig JF, Angew. Chem. Int. Ed 2014; 53:8471. [DOI] [PubMed] [Google Scholar]
- [32].Su B, Lee T, Hartwig JF, J. Am. Chem. Soc 2018; 140:18032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Su B, Zhou T-G, Xu P-L, Shi Z-J, Hartwig JF, Angew. Chem. Int. Ed 2017; 56:7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Su B, Zhou T-G, Li X-W, Shao X-R, Xu P-L, Wu W-L, Hartwig JF, Shi Z-J, Angew. Chem. Int. Ed 2017; 56:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Su B, Hartwig JF, J. Am. Chem. Soc 2017; 139:12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Murai M, Takeshima H, Morita H, Kuninobu Y, Takai K, J. Org. Chem 2015; 80:5407. [DOI] [PubMed] [Google Scholar]
- [37].Frihed TG, Heuckendorff M, Pedersen CM, Bols M, Angew. Chem. Int. Ed 2012; 51:12285. [DOI] [PubMed] [Google Scholar]
- [38].Frihed TG, Pedersen CM, Bols M, Angew Chem Int Ed Engl 2014; 53:13889. [DOI] [PubMed] [Google Scholar]
- [39].Crich D, Li L, J. Org. Chem 2009; 74:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Michaudel Q, Journot G, Regueiro-Ren A, Goswami A, Guo Z, Tully TP, Zou L, Ramabhadran RO, Houk KN, Baran PS, Angew. Chem. Int. Ed 2014; 53:12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ma X, Kucera R, Goethe OF, Murphy SK, Herzon SB, J. Org. Chem 2018; 83:6843. [DOI] [PubMed] [Google Scholar]
- [42].Chen C, Guan M, Zhang J, Wen Z, Zhao Y, Org. Lett 2015; 17:3646. [DOI] [PubMed] [Google Scholar]
- [43].Deb A, Singh S, Seth K, Pimparkar S, Bhaskararao B, Guin S, Sunoj RB, Maiti D, ACS Cat 2017; 7:8171. [Google Scholar]
- [44].Kanyiva KS, Kuninobu Y, Kanai M, Org. Lett 2014; 16:1968. [DOI] [PubMed] [Google Scholar]
- [45].Li W, Huang X, You J, Org. Lett 2016; 18:666. [DOI] [PubMed] [Google Scholar]
- [46].Liu Y-J, Liu Y-H, Zhang Z-Z, Yan S-Y, Chen K, Shi B-F, Angew. Chem. Int. Ed 2016; 55:13859. [DOI] [PubMed] [Google Scholar]
- [47].Pan J-L, Li Q-Z, Zhang T-Y, Hou S-H, Kang J-C, Zhang S-Y, Chem. Commun 2016; 52:13151. [DOI] [PubMed] [Google Scholar]
- [48].Daugulis O, Roane J, Tran LD, Acc. Chem. Res 2015; 48:1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhan B-B, Fan J, Jin L, Shi B-F, ACS Cat 2019; 9:3298. [Google Scholar]
- [50].Kakiuchi F, Tsuchiya K, Matsumoto M, Mizushima E, Chatani N, J. Am. Chem. Soc 2004; 126:12792. [DOI] [PubMed] [Google Scholar]
- [51].Ihara H, Ueda A, Suginome M, Chem. Lett 2011; 40:916. [Google Scholar]