Abstract
Purpose:
This article investigates the relationship between visual acuity (VA), total area of geographic atrophy (GA), and percentage of foveal GA.
Methods:
A multicenter, retrospective, cross-sectional study was conducted of patients with GA due to age-related macular degeneration. Demographics, VA, fundus autofluorescence (FAF), and spectral-domain optical coherence tomography (SD-OCT) images were collected. Using FAF images aided by SD-OCT, fovea-sparing status, GA pattern, total GA size, and percentage of GA covering the foveal area—within a 1.5-mm-diameter circle centered on the fovea centralis—were assessed. Univariable and multiple linear regression analyses were performed.
Results:
Fifty-four eyes (mean age, 78.7 ±7.7 years [SD], 60.0% female) were studied. Mean VA was 0.8 ± 0.6 logarithm of the minimum angle of resolution (Snellen equivalent 20/126 ± 20/80), mean total GA 8.8 ± 6.7 mm2, and mean percentage of foveal GA was 71.5 ± 30.9%. Of all assessed eyes, 48.2% (n = 26) presented with multifocal GA, and 18.5% (n =10) had foveal sparing. Multiple regression analysis revealed that, controlling for age and GA pattern, the percentage of foveal GA presented a statistically significant association with VA (ß =0.41, P = .004). No significant associations were observed with mean total GA size, while controlling for the same variables (ß=0.010, P = .440).
Conclusions:
Percentage of foveal GA was significantly associated with VA impairment, although the same was not verified for total GA area. These findings suggest that percentage of foveal GA may represent a more useful tool for assessing the impact of GA on VA. Further validation is needed in larger cohorts.
Keywords: dry AMD (nonneovascular), fovea, fundus autofluorescence, geographic atrophy, imaging, macula, OCT
Introduction
Age-related macular degeneration (AMD) is the leading cause of visual disability in elderly patients in industrialized countries.1 Geographic atrophy (GA) represents the late stage of dry AMD, and it is characterized by the irreversible loss of macular retinal tissue, retinal pigment epithelium (RPE), and choriocapillaris.2 Although this process is a slowly progressing one, it causes decreases in central vision over time,3 which rapidly accelerate when GA covers the foveal center.
GA is responsible for severe vision loss in approximately 20% of all patients with AMD, and more than 8 million people are affected worldwide.2,4 For not-well-understood reasons, atrophic macular diseases such as GA due to dry AMD can spare the foveal center until late in the disease course, and this so-called foveal sparing has been reported in about 20% of representative GA populations enrolled in clinical trials.5
Color fundus photography, fundus autofluorescence (FAF), and optical coherence tomography (OCT) imaging can be used to identify and follow GA lesions. However, FAF is considered by most to be the imaging of choice that allows a sharp discrimination of a lesion’s boundaries. This is primarily because FAF provides a good visualization of the high contrast between atrophic (hypofluorescent) and normal areas.4,6 On OCT, GA is typically characterized by thinning of the hyperreflective external bands due to attenuation/loss of the photoreceptors, ellipsoid zone, and RPE/Bruch complex, as well as deeper hyperreflectivity in the sub-RPE layers due to increased laser light penetration through the atrophic RPE.2
The total area of GA lesions is often used as an indicator of severity in late-stage dry AMD. However, this measure does not readily predict residual visual acuity (VA) nor VA decline rates.7 Fovea-sparing status has been shown to correlate better with VA than total GA size; nevertheless, its binary nature prevents it from being used to quantify the relationship between the continuous shrinking of the spared foveal area and the worsening of VA over time.8
To explore more sensitive anatomical predictors of VA in GA, we defined and analyzed the percentage of foveal GA and its association with VA. This approach may lead to more accurate outcome measures for clinical trials as well as for patient counseling.
Methods
Study Design
This is a multicenter, retrospective cross-sectional study. The research protocol was conducted in accordance with Health Insurance Portability and Accountability Act (HIPAA) requirements and the tenets of the Declaration of Helsinki. The institutional review boards of Massachusetts Eye and Ear and of Coimbra University Hospital approved this study. Informed consent was obtained when required.
Study Population and Study Protocol
We identified and reviewed the medical records and images of eyes with GA. We adopted the most recent Age-Related Eye Disease Study (AREDS) definitions,9 namely that GA is present if the lesion has a diameter of 433 μm or more (AREDS circle I-2) and has at least 2 of the following features: absence of RPE pigment, circular shape, or sharp margins.
Individuals from 2 centers were considered. At Massachusetts Eye and Ear, we identified patients seen between September 2011 and June 2017 as part of the AMD biomarkers study and from the attending clinic (DV).10 We also considered individuals from Portugal participating in the AMD biomarkers study developed by the faculty of medicine, University of Coimbra, in collaboration with the Association for Innovation and Biomedical Research on Light and Image and the Centro Hospitalar e Universitário de Coimbra, Portugal.
For all considered participants, exclusion criteria included GA with choroidal neovascular membranes; diagnosis of any other vitreoretinal disease, active uveitis, or ocular infection; significant media opacities that precluded observation of the ocular fundus; refractive error equal to or greater than 6 diopters of spherical equivalent; history of any ocular surgery or intraocular procedure such as laser or intravitreal injections within 90 days prior to enrollment; and diagnosis of diabetes mellitus. Additionally, only eyes with FAF as well as OCT images according to a predefined protocol, available on at least one visit, were considered for this study. For FAF, we considered eyes with high-resolution 30° FAF, centered on the fovea. For OCT, we used high-resolution 30° spectral domain-optical coherence tomography (SD-OCT).
For the final included eyes, we reviewed medical records and collected the following information: age, sex, smoking status, AREDS supplementation, and Snellen VA at the same date as the considered images.
Imaging Analysis
We reviewed FAF and SD-OCT images of the eyes considered for this study. The foveal area was defined by a 1.5-mm-diameter circle, centered on the fovea centralis with the Heidelberg built-in circle tool (Heidelberg Engineering, GmbH). For determining the fovea centralis, SD-OCT cross-sectional images combined with the corresponding infrared images were used in parallel with the FAF images to help determine the location of the umbo/fovea centralis and the GA areas, and the pointer tool was used to mark points on the infrared image for the fovea centralis.
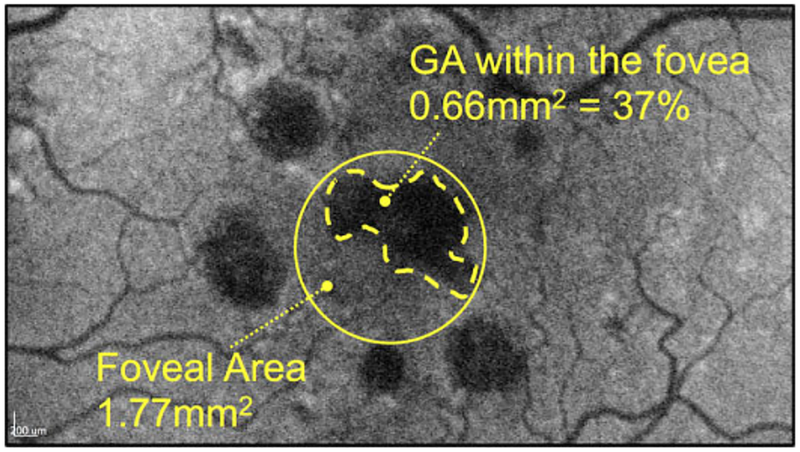
Using the Heidelberg built-in free-hand draw tool that automatically computes the enclosed area in square millimeters (mm2), 2 masked graders (S.B. and R.S.) independently measured the GA lesion within the foveal area of 1.77 mm2 (A=πr2 = π(1.5 mm/2)2 = 1.77 mm2), and calculated its value in percentage of the total foveal area (Figure 1). The total macular GA area was measured by the same graders. For marking of the GA areas, the pointer tool was used to mark points on the lesion borders for which SD-OCT cross-sectional scans showed loss of the RPE and ellipsoid zone. This was then transferred to the corresponding FAF image, which was subsequently connected via the free-hand draw tool in a multimodal approach.11 For analysis, average values of the 2 graders were used, except when values disagreed by more than 10%, in which case a third grader (I.L.) was used for adjudication. We graded for fovea-sparing status and GA pattern (focal or multifocal) in addition to collecting demographic information on age, sex, study eye, smoking status, and AREDS supplementation.
Figure 1.
Illustration of calculation of percentage of foveal geographic atrophy (GA). A 1.5-mm-diameter circle centered on the fovea centralis was determined with the use of optical coherence tomography cross-sectional and infrared images (see Methods). The outline of the GA within the foveal area was performed by 2 independent graders using the Heidelberg built-in free-hand draw tool (Heidelberg Engineering, GmbH), which automatically computes the enclosed area in square millimeters, and the percentage was calculated after division by the area defined by the standard foveal area of 1.5 mm diameter.
Statistical and Data Analysis
Traditional descriptive methods such as mean and SD for continuous variables, and percentages for dichotomous/categorical variables, were used to describe the clinical and demographic characteristics of the study population.
Regarding the inclusion of 2 eyes of the same patient for some cases, our statistical assessments were performed using multilevel mixed-effect models. By definition, these models are appropriate for research designs in which data for participants are organized at more than one level (ie, nested data). In this study, the units of analysis were considered the eyes (at a lower level), which are nested within patients who represent the contextual/aggregate units (at a higher level).12
Univariate analyses were initially performed for all the potential confounders such as age and GA pattern, and all variables with a P value less than or equal to .250 were included in the initial multiple model. A backward elimination procedure was then performed to achieve the multivariable models presented for the variables that were statistically significant in the univariate analysis.
For univariate and multivariable analyses, we report P values and beta coefficients. The beta coefficients represent the change in the outcome variable for one unit of change in the predictor variable (while holding other predictors in the model constant, in the case of multivariable analyses).13 This means, for example, given a continuous variable such as age, beta coefficients represent the change in VA per year increase in age. For binomial variables, such as smoking, AREDS supplementation, or foveal sparing, their absence was considered the reference term, so beta coefficients refer to the change in their presence. The reference term for the study eye was the right eye; for GA pattern, unifocal GA; and for sex, female sex.
All statistics were performed using Stata version 14.1 (StataCorp LP) and P values less than .05 were considered statistically significant.
Results
Study Population
We included 54 eyes from 35 patients (mean age, 78.7 ± 7.7 years, 60.0% female [n = 21]) with GA due to nonneovascular AMD. Mean VA was 0.81 (Snellen equivalent, 20/129) ± 0.63 (range, 0–2.60) logarithm of the minimum angle of resolution, mean total GA 8.79 ± 6.66 mm2 (range, 0.84–25.36 mm2), mean percentage of foveal GA was 71.53 ± 30.94% (range, 0%−100%). A total of 48.15% (n = 26) of assessed eyes presented with multifocal GA, and 18.52% (n = 10) had foveal sparing (see demographics in Table 1).
Table 1.
Demographic and Clinical Characteristics of the Included Study Eyes.
| Age at date of imaging, y (n = 54) | |
| Mean ± SD (range) | 78.7 ± 7.7 y (62–96 y) |
| Sex (n = 35) | |
| Female | 21 (60.0%) |
| Male | 14 (40.0%) |
| Smoking (n = 30) | |
| No | 27 (90.0%) |
| Yes | 3 (10.0%) |
| AREDS (n = 38) | |
| No | 14 (36.8%) |
| Yes | 24 (63.2%) |
| Study eye (n = 54) | |
| OD | 29 (53.7%) |
| OS | 25 (46.3%) |
| GA pattern (n = 54) | |
| Unifocal | 28 (51.9%) |
| Multifocal | 26 (48.2%) |
| Foveal sparing (n = 54) | |
| No | 44 (81.5%) |
| Yes | 10 (18.5%) |
| VA in logMAR (n = 54) | |
| Mean ± SD (range) | 0.8 ± 0.6 (0–2.6) |
Abbreviations: AREDS, Age-Related Eye Disease Study; GA, geographic atrophy; logMAR, logarithm of the minimum angle of resolution; OD, right eye; OS, left eye; VA, visual acuity.
In all eyes, SD-OCT images allowed a clear identification of the umbo/fovea centralis as well as the GA lesion borders. Figure 1 presents an example of measurement of percentage of foveal GA.
The results of the univariate analysis considering all variables of interest and their association with VA are shown in Table 2.
Table 2.
Univariable Linear Regression Analysis Considering VA as the Outcome.
| β | P | 95% CI | |
|---|---|---|---|
| Age, y | 0.017 | .113 | −0.00409, 0.038529 |
| Sexa | −0.285 | .097 | −0.621 5504, 0.051 2273 |
| Study eyeb | −0.226 | .178 | −0.554347 1, −0.102 713 |
| Smokingc | −0.319 | .398 | −1.058263, 0.420522 6 |
| AREDSc | −0.412 | .051 | −0.826 5572, −0.001 8414 |
| GA patternd | −0.507 | .001* | −0.811 8193, −0.202 5564 |
| Foveal sparingc | −0.539 | .009* | –0.944041 5, −0.134 384 |
| Total GA (mm2) | 0.024 | .054 | −0.0004198, 0.048712 |
| Foveal GA (mm2) | 0.536 | <.001* | −0.262 5702, 0.8104187 |
| % of foveal GA | 0.010 | <.001* | 0.0049928, 0.0149448 |
Abbreviations: AREDS, Age-Related Eye Disease Study; GA, geographic atrophy; VA, visual acuity.
P <.05.
Female sex considered the reference term.
Right eye considered the reference term.
Reference term considered the absence of these variables.
Unifocal GA considered the reference term.
Percentage of Foveal GA
The mean percentage of foveal GA was statistically significantly associated with VA in univariate analysis (ß = 0.01, P < .001) (Table 2). This association remained significant on multivariable analysis, controlling for age and GA pattern (ß = 0.41, P = .004).
Total Macular GA
Univariate or multivariable analysis for total macular GA revealed there were no statistically significant associations with VA (ß= 0.02, P = .054, for univariate), (ß = 0.010, P = .440 for multivariable).
GA Pattern and Foveal Sparing
GA pattern presented a statistically significant association with VA (ß = −0.507187 9, P = .001) and so did foveal sparing (ß=−0.539212 7, P = .009).
Conclusions
We present a retrospective, cross-sectional study of 54 eyes diagnosed with GA due to nonneovascular AMD in which we used FAF and SD-OCT to examine the association of percentage of foveal GA and total macular GA lesion size with VA. Our results revealed that, after accounting for potential confounders such as age and GA pattern, the percentage of foveal GA was significantly associated with VA, although the same was not observed for total GA lesion size.
In GA clinical studies, the most common outcome measures for GA are changes in total GA, changes in square-root GA, or other phenotypic refinements.14,15 As our results show, total GA poorly correlates with VA, and potentially with patients’ overall quality of life. This finding is in agreement with previously published literature, which showed no relationship of total GA size with VA and has been investigated by multiple groups.8,16
Efforts have been made to study the association between VA and the distance between the edges of GA and the fovea,16,17 or to examine residual visual function in the presence of fovea-sparing lesions.16–19 Fovea-sparing status has been shown to have a stronger correlation with VA than total GA size; however, it does not quantify the extent to which the foveal area is affected nor the worsening of VA over time since it measures only presence or absence of GA in the anatomic foveola centralis.8,16,17 A recent investigation of the associations of VA with total GA size as well as fovea-sparing status in 65 eyes found no relationship between VA and total GA size as well as foveal island size.20 The same group also evaluated the width of the bridge—defined as the minimal linear dimension of intact RPE located within the residual foveal island— and found only a suggestion of a positive relationship in the range of 300 to 550 μm of bridge width and no relationship at all outside this range, leading to the conclusion that this measurement might not be an ideal outcome parameter for GA clinical trials.
Our study results suggest that using the percentage of foveal GA is potentially a more sensitive outcome parameter for association with VA. Our study is limited, however, by its modest size and retrospective design. As such, our results should be validated in larger, more representative populations before changes in percentage of foveal GA can be used more widely in clinical trials or clinical practice. Further studies should examine more precise evaluation of affected areas as well as evaluate the progression rate of percentage of foveal GA over time and examine predictive ability of such a tool on future VA changes.
In conclusion, here we propose for the first time the use of percentage of foveal GA as a possible predictor of VA in GA. Our data suggest such a measure may have a stronger association with VA impairment than total GA size. Therefore, with future research, it might represent a better tool to measure VA decline over time compared with fovea-sparing status. Nonetheless, VA has limitations as a tool to assess visual function in patients with GA, and other outcomes may be better suited.21
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.B. has received personal fees from OliX Pharmaceuticals, outside the submitted work. R.S. has served as a consultant for Allergan, Alimera, Bayer, Novartis, and Thea Pharmaceuticals. D.H. has served as a consultant for Allergan, Genentech, and Omeicos Therapeutics. J.W.M. has received personal fees from Amgen Inc, KalVista Pharmaceuticals Ltd, Alcon Research Institute, Bausch + Lomb, Sunovion, Genentech/Roche Valeant Pharmaceuticals/Massachusetts Eye and Ear, and ONL Therapeutics, LLC; and grants from Lowy Medical Research Institute, Ltd, outside the submitted work. In addition, J.W.M. has patents US 5 798 349; US 6 225 303; US 6 610 679; CA 2 185 644; CA 2 536 069 with royalties paid by Valeant Pharmaceuticals to Massachusetts Eye and Ear, and a patent US 7 811 832 with royalties paid by ONL Therapeutics to Massachusetts Eye and Ear.L.S. has received grants from the National Institutes of Health/National Eye Institute, during the conduct of the study, and personal fees from Alkeus Pharmaceuticals, outside the submitted work. D.E. has served as a consultant for Alcon, Alimera, Allergan, Dutch Ophthalmic, Genentech, Regenxbio, has received research funding from Neurotech, and is a stockholder of Aldeyra Therapeutics and Pykus Therapeutics. D.G.V. has served as a consultant for OliX Pharmaceuticals Inc and Valitor Inc, holds equity with Theia Therapeutics, LLC, and has received funding from the NIH R01EY025362. The other authors have nothing to declare.
Footnotes
This paper was presented in part at the 2017 Annual Meeting of the Association for Research in Vision and Ophthalmology, May 9, 2017, in Baltimore, Maryland.
The research protocol was conducted in accordance with Health Insurance Portability and Accountability Act requirements and the tenets of the Declaration of Helsinki. The institutional review boards of Massachusetts Eye and Ear and Coimbra University Hospital approved this study.
Statement of Informed Consent
Informed consent was obtained when required.
References
- 1.Miller JW, Bagheri S, Vavvas DG. Advances in age-related macular degeneration understanding and therapy. US Ophthalmic Rev. 2017;10(2):119–130. doi: 10.17925/USOR.2017.10.02.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacconi R, Corbelli E, Querques L, Bandello F, Querques G. A review of current and future management of geographic atrophy. Ophthalmol Ther. 2017;6(1):69–77. doi: 10.1007/s40123-017-0086-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz-Valckenberg S, Sahel JA, Danis R, et al. Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression Study). Ophthalmology. 2016;123(2):361–368. doi: 10.1016/j.ophtha.2015.09.036 [DOI] [PubMed] [Google Scholar]
- 4.Sunness JS, Margalit E, Srikumaran D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114(2):271–277. doi: 10.1016/j.ophtha.2006.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunness JS. Reading and macular disease. [published online September 1, 2009]. Retinal Physician. https://www.retinalphysician.com/issues/2009/september-2009/reading-and-macular-disease. Accessed August 6, 2019. [Google Scholar]
- 6.Domalpally A, Danis R, Agrón E, et al. Evaluation of geographic atrophy from color photographs and fundus autofluorescence images: Age-Related Eye Disease Study 2 Report Number 11. Ophthalmology. 2016;123(11):2401–2407. doi: 10.1016/j.ophtha.2016.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sunness JS, Gonzalez-Baron J, Applegate CA, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999; 106(9):1768–1779. doi: 10.1016/S0161-6420(99)90340-8 [DOI] [PubMed] [Google Scholar]
- 8.Sunness JS, Rubin GS, Zuckerbrod A, Applegate CA. Foveal-sparing scotomas in advanced dry age-related macular degeneration. J Vis Impair Blind. 2008;102(10):600–610. doi: 10.1177/0145482X0810201004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danis RP, Domalpally A, Chew EY, et al. Methods and reproducibility of grading optimized digital color fundus photographs in the Age-Related Eye Disease Study 2 (AREDS2 Report Number 2). Invest Ophthalmol Vis Sci. 2013;54(7):4548–4554. doi: 10.1167/iovs.13-11804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laíns I, Kelly RS, Miller JB, et al. Human plasma metabolomics study across all stages of age-related macular degeneration identifies potential lipid biomarkers. Ophthalmology. 2018;125(2): 245–254. doi: 10.1016/j.ophtha.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Channa R, Sophie R, Bagheri S, et al. Regression of choroidal neovascularization results in macular atrophy in anti-vascular endothelial growth factor-treated eyes. Am J Ophthalmol. 2015; 159(1):9–19.e1–e2. doi: 10.1016/j.ajo.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton P, Gurrin L, Sly P. Extending the simple linear regression model to account for correlated responses: an introduction to generalized estimating equations and multi-level mixed modelling. Stat Med. 1998;17(11):1261–1291. doi: [DOI] [PubMed] [Google Scholar]
- 13.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2011. [Google Scholar]
- 14.Holz FG, Bindewald-Wittich A, Fleckenstein M, et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143(3):463–472. doi: 10.1016/j.ajo.2006.11.041 [DOI] [PubMed] [Google Scholar]
- 15.Feuer WJ, Yehoshua Z, Gregori G, et al. Square root transformation of geographic atrophy area measurements to eliminate dependence of growth rates on baseline lesion measurements: a reanalysis of Age-Related Eye Disease Study Report No. 26. JAMA Ophthalmol. 2013;131(1):110–111. doi: 10.1001/jamaophthalmol.2013.572 [DOI] [PubMed] [Google Scholar]
- 16.Lindner M, Böker A, Mauschitz MM, et al. Directional kinetics of geographic atrophy progression in age-related macular degeneration with foveal sparing. Ophthalmology. 2015;122(7): 1356–1365. doi: 10.1016/j.ophtha.2015.03.027 [DOI] [PubMed] [Google Scholar]
- 17.Schmitz-Valckenberg S, Nadal J, Fimmers R, et al. Modeling visual acuity in geographic atrophy secondary to age-related macular degeneration. Ophthalmologica. 2016;235(4):215–224. doi: 10.1159/000445217 [DOI] [PubMed] [Google Scholar]
- 18.Hart WM Jr, Burde RM. Three-dimensional topography of the central visual field. Sparing of foveal sensitivity in macular disease. Ophthalmology. 1983;90(8):1028–1038. doi: 10.1016/S0161-6420(83)80031-1 [DOI] [PubMed] [Google Scholar]
- 19.Sayegh RG, Sacu S, Dunavölgyi R, et al. Geographic atrophy and foveal-sparing changes related to visual acuity in patients with dry age-related macular degeneration over time. Am J Ophthalmol. 2017;179:118–128. doi: 10.1016/j.ajo.2017.03.031 [DOI] [PubMed] [Google Scholar]
- 20.Lindner M, Nadal J, Mauschitz MM, et al. Combined fundus autofluorescence and near infrared reflectance as prognostic bio-markers for visual acuity in foveal-sparing geographic atrophy. Invest Ophthalmol Vis Sci. 2017;58(6):BIO61–BIO67. doi: 10.1167/iovs.16-21210 [DOI] [PubMed] [Google Scholar]
- 21.Sadda SR, Chakravarthy U, Birch DG, Staurenghi G, Henry EC, Brittain C. Clinical endpoints for the study of geographic atrophy secondary to age-related macular degeneration. Retina. 2016; 36(10):1806–1822. doi: 10.1097/IAE.0000000000001283 [DOI] [PMC free article] [PubMed] [Google Scholar]