Abstract
Background
Effective therapeutics for respiratory viruses are needed. Early data suggest that nitazoxanide (NTZ) may be beneficial for treating acute respiratory viral illness.
Methods
From March 2014 through March 2017, a double-blind, placebo-controlled trial was conducted in 260 participants ≥1 year old hospitalized with influenza-like illness at 6 hospitals in Mexico. Participants were randomized 1:1 to NTZ (age ≥12 years, 600 mg twice daily; age 4–11 years and 1–3 years, 200 or 100 mg twice daily, respectively) or placebo for 5 days in addition to standard of care. The primary endpoint was time from first dose to hospital discharge. Influenza reverse-transcription polymerase chain reaction and Respifinder 22 multiplex test were used for virus detection.
Results
Of 260 participants enrolled, 257 were randomized and took at least 1 dose of study treatment (intention-to-treat population): 130 in the NTZ group and 127 in the placebo group. The Kaplan-Meier estimate of the median duration of hospitalization was 6.5 (interquartile range [IQR], 4.0–9.0) days in the NTZ group vs 7.0 (IQR, 4.0–9.0) days in the placebo group (P = .56). Duration of hospitalization between the 2 treatments was similar in children (P = .29) and adults (P = .62), influenza A and B (P = .32), and other respiratory viruses. Seven (5.4%) and 6 (4.7%) participants in the NTZ and placebo groups, respectively, reported serious adverse events.
Conclusions
Treatment with NTZ did not reduce the duration of hospital stay in severe influenza-like illness. Further analyses based on age and evaluations by virus did not reveal any subgroups that appeared to benefit from NTZ.
Clinical Trials Registration
Keywords: nitazoxanide, influenza-like illness, respiratory virus, hospitalized
Treatment of children and adults hospitalized with severe acute respiratory illness with nitazoxanide in addition to standard of care was safe, but did not reduce hospital stay, complications, nor shedding of influenza and other respiratory viruses.
Severe acute respiratory illness (SARI) has been among the top 3 causes of death and disability among both children and adults worldwide, and it is estimated that SARI causes nearly 4 million deaths annually [1, 2]. Besides causing severe complications and significant use of hospital services, SARI is responsible for major losses in productivity in part due to absenteeism [3, 4]. Respiratory viruses have been previously shown to cause 51% of hospitalizations for influenza-like illness (ILI) in adults and 65% in children in Mexico [5]. Of the viruses causing SARI, only influenza has effective treatments [6, 7]. In 2012, the World Health Organization (WHO) released a call to action, specifically stating that “an urgent need exists to support research for new, cost effective therapeutics to target specific respiratory viruses but also, if possible, to develop antivirals with broad spectrum activity” (WHO Battle against Respiratory Viruses [BRaVe] Initiative) [8].
Nitazoxanide (NTZ) is licensed in the United States and Latin America for the treatment of intestinal parasitic infections [9–11]. In recent studies, NTZ has shown to inhibit replication of a broad range of viruses, including influenza, but not rhinovirus [12]. The metabolite tizoxanide was shown to act by selectively blocking the maturation of the influenza viral hemagglutinin (HA), impairing HA intracellular trafficking and insertion of this protein into the host plasma membrane [13].
A phase 2 study of NTZ was conducted in 100 children aged 1–11 years with ILI symptoms of <72 hours’ duration and who were given NTZ 100–200 mg twice daily or placebo for 5 days. The NTZ cohort showed symptom resolution in 4 days vs >7 days in the placebo group (P < .001) [14]. A second phase 2 study was conducted in adults and adolescents (age ≥12 years) with ILI, in which 86 participants were randomized to either NTZ 500 mg twice daily or placebo for 5 days. Time to resolution of symptoms was a median of 4 days in the treatment arm vs 7 days in the placebo group (P = .04) [15]. A phase 2b/3 randomized, double-blind, placebo-controlled clinical trial in participants with confirmed influenza conducted at primary care clinics in the United States found that oral administration of NTZ 600 mg twice daily for 5 days reduced the duration of clinical symptoms (95.5 vs 116.7; P = .008) and reduced infectious virus titers over time (P = .0006) [16]. The same study also suggested a potential benefit for participants with ILI with unknown viral etiology (17.3 hours’ reduction of flu-like symptoms; P = .02).
We conducted a phase 2 clinical study to evaluate the use of NTZ as potential treatment for SARI.
METHODS
Study Design
The study was a randomized, double-blind, placebo-controlled clinical trial to evaluate the effect of NTZ in addition to standard of care (SOC) compared to placebo + SOC (control) for the treatment of hospitalized ILI. The study was designed to enroll a total of 290 participants randomized in a 1:1 ratio. The primary endpoint was duration of hospitalization (days and hours).
Study Population
The WHO 2014 definition of SARI [17] (acute respiratory infection with history of fever or measured fever of ≥38°C and cough with onset within the last 10 days and requiring hospitalization) was used as the basis of developing inclusion criteria for this study. Participants ≥1 year of age requiring hospitalization because of an acute ILI (defined as respiratory sign [cough, hypoxia, tachypnea; or in children < years of age included nasal flaring or chest retractions] and fever or other constitutional symptom) with onset within 7 days were invited to participate in the study. Hospitalization for ILI was up to the individual attending physician. All study participants signed written informed consent.
Randomization
The computer-generated randomization schema was incorporated into the sequence of the study medication kits, both prepared by Pharmavize NV, Belgium. All participants, site staff, and the remaining study team were masked to treatment allocation until the study database was locked.
Participants were stratified by age (1–11 years and ≥12 years) and allocated to 2 oral treatment groups: NTZ + SOC or control, twice daily for 5 days. The NTZ dose used for those ≥12 years of age was two 300-mg tablets; for those 4–11 years of age, the dose used was 200 mg NTZ oral suspension; in those 1–3 years of age, the dose was 100 mg NTZ suspension. Study treatments were identical in appearance. Standard of care included fluid replacement therapy, supplemental oxygen, anti-influenza antivirals, and antibiotics, as determined by the treating physician.
Study Procedures
After enrollment, baseline assessments were obtained including vital signs, safety laboratory values, and nasopharyngeal (NP) swabs for respiratory viral detection. Participants were then randomized by being assigned the next sequential treatment kit, and began treatment immediately. Participants recorded symptoms on diary cards, which were completed daily through day 14. Participants were followed at study days 3, 7, 14, and 28.
Nasopharyngeal Sample Collection
An NP swab (Copan, Brescia, Italy) for detection of respiratory pathogens was collected upon enrollment and at day 3.
Laboratory
All NP swabs were tested by real-time reverse-transcription quantitative polymerase chain reaction (PCR) to influenza (identification and subtyping) following the Centers for Disease Control and Prevention 2009 protocol [18]. For multipathogen detection, samples were tested with the RespiFinder 22 kit (PathoFinder B.V., Maastricht, the Netherlands). This multiplex PCR test can detect and differentiate 18 viruses (detailed in the Supplementary Appendix).
Ethical Considerations
The study protocol was approved by the local ethics and research committees of each participating institution. The study was conducted under an investigational new drug application with the US Food and Drug Administration and the Mexico Federal Commission for the Protection Against Sanitary Risk. This study is registered at ClinicalTrials.gov (identifier NCT02057757).
Statistical Analysis
A sample size of 258 participants was calculated to detect a 1.25-day improvement in the time to hospital discharge in the NTZ arm with 80% power. The study was originally written to enroll up to 290 participants, while allowing up to 10% loss to follow-up.
The intention-to-treat (ITT) population was defined as having been randomized and having received at least 1 dose of study drug. P values at baseline were described using the test of means for continuous variables, test of proportions for binary endpoints, or χ2 test for categorical endpoints. The primary endpoint was time from date of hospitalization to initial hospital discharge, censored at day 28.
RESULTS
From March 2014 throughout March 2017, 260 patients ≥1 year old with ILI admitted at one of the 6 tertiary care hospitals in Mexico participating in the Mexico Emerging Infectious Diseases Clinical Research Network (LaRed) (4 hospitals in Mexico City, 1 hospital in San Luis Potosí, and 1 hospital in Oaxaca) were enrolled in the study (Figure 1). Of them, 257 (99%) participants were randomized and received at least 1 dose of study drug, thus constituting the ITT population: 130 participants were randomized to the NTZ + SOC group and 127 participants to the placebo + SOC group. Of these, 129 of 130 (99.2%) in the NTZ + SOC group and 125 of 127 (98.4%) in the placebo + SOC arm had primary endpoint data. One hundred seventeen of 130 (90%) and 120 of 127 (94%) participants completed the study.
Figure 1.
Trial profile. Abbreviations: ITT, intention-to-treat; NTZ, nitazoxanide; PCB, placebo; SOC, standard of care.
Baseline and Clinical Characteristics of the ITT Population
Participants’ baseline and clinical characteristics were well balanced between study groups with the exception of sex; there were more female participants in the NTZ group than in the placebo group (52.3% vs 39.4%, respectively; P = .04; Table 1). Overall, 51% of participants were children <18 years old. Previous illnesses that were reported in ≥5% of participants included anemia, asthma, hypertension, pneumonia, and allergic rhinitis. Only 76 (29.6%) participants reported vaccination against influenza.
Table 1.
Baseline Demographics and Clinical Characteristics, by Study Treatment
| Characteristic | NTZ + SOC (n = 130) | Placebo + SOC (n = 127) | Total (N = 257) | P Value |
|---|---|---|---|---|
| Age, y | .75a | |||
| Mean (SD) | 22.6 (24.0) | 23.6 (24.4) | 23.1 (24.2) | |
| Median (IQR) | 9.5 (2.0–41.0) | 10 (2.0–42.0) | 10 (2.0–42.0) | |
| <2 | 23 (17.7) | 24 (18.9) | 47 (18.3) | .77b |
| 2 to <8 | 38 (29.2) | 38 (29.9) | 76 (29.6) | |
| 8 to <13 | 5 (3.8) | 2 (1.6) | 7 (2.7) | |
| 13 to <18 | 1 (0.8) | 0 | 1 (0.4) | |
| 18 to <65 | 54 (41.5) | 56 (44.1) | 110 (42.8) | |
| ≥65 | 9 (6.9) | 7 (5.5) | 16 (6.2) | |
| Sex | ||||
| Female | 68 (52.3) | 50 (39.4) | 118 (45.9) | .037b |
| Ethnicity | ||||
| Hispanic or Latino | 130 (100) | 127 (100) | 257 (100) | |
| BMI (in participants >18 y) | ||||
| Median (IQR) | 26.7 (22.3–30.9) | 26.5 (22.9–29.8) | 26.7 (22.9–30.1) | .85a |
| z score (in participants <18 y) | ||||
| Median (IQR) | 0.2 (–0.7 to 1.0) | –0.6 (–1.9 to 1.4) | –0.2 (–1.4 to 1.3) | .07a |
| Medical history occurring in >5% of participants | ||||
| Anemia | 8 (6.2) | 10 (7.9) | 18 (7.0) | .38b |
| Asthma | 39 (30.0) | 24 (18.9) | 63 (24.5) | |
| Hypertension | 11 (8.5) | 16 (12.6) | 27 (10.5) | |
| Allergic rhinitis | 7 (5.4) | 6 (4.7) | 13 (5.1) | |
| Influenza vaccination ≥ 14 d before hospitalization | ||||
| Yes | 33 (25.4) | 43 (33.9) | 76 (29.6) | .14b |
| No | 97 (74.6) | 84 (66.1) | 181 (70.4) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; IQR, interquartile range; NTZ, nitazoxanide; SD, standard deviation; SOC, standard of care.
aTest of means for continuous variables.
bTest of proportions for binary endpoints.
Disease-related characteristics of participants up to hospital admission were similar for each of the 2 treatment groups (Table 2). Most participants reported cough (56.8%), fatigue (44.4%), and difficulty breathing (40.9%). Reporting of gastrointestinal symptoms (nausea, vomiting, and diarrhea) was infrequent. Twenty-three percent of participants hospitalized because of an acute ILI reported only mild (or absent) symptoms, and 13% of adult subjects reported functioning as well as they did before the respiratory illness that caused them to be hospitalized. At baseline, supplemental oxygen was required by 92% participants, and 57.2% were clinically diagnosed with pneumonia. Only 3 participants in the NTZ group and 2 participants in the placebo group were admitted to the intensive care unit (ICU) (P = .67). Oxygen saturation (88.3% [SD, 5.8] vs 88.4% [SD, 5.5]; P = .86), Sequential Organ Failure Assessment score (0.8 [SD, 1.2] vs 0.8 [SD, 1.1]; P = .81), Tal bronchitis score in children <24 months old (2.4 [SD, 1.3] vs 2.3 [SD, 1.3]; P = .85), and Charlson comorbidity index score (0.8 [SD, 1.2] vs 1.3 [SD, 2.1]) did not differ among the study groups.
Table 2.
Clinical and Disease-related Characteristics at Baseline
| Parameter | NTZ + SOC (n = 130) | Placebo + SOC (n = 127) | Total (N = 257) | P Valuea |
|---|---|---|---|---|
| Presence of symptoms | ||||
| Cough | 71 (54.6) | 75 (59.1) | 146 (56.8) | .43 |
| Fatigue | 60 (46.2) | 54 (42.5) | 114 (44.4) | .60 |
| Difficulty breathing | 54 (41.5) | 51 (40.2) | 105 (40.9) | .86 |
| Nasal discharge | 40 (30.8) | 25 (19.7) | 65 (25.3) | .045 |
| Sore throat | 29 (22.3) | 23 (18.1) | 52 (20.2) | .40 |
| Muscle pain | 28 (21.5) | 18 (14.2) | 46 (17.9) | .12 |
| Headache | 21 (16.2) | 13 (10.2) | 34 (13.2) | .16 |
| Nausea | 4 (3.1) | 8 (6.3) | 12 (4.7) | .22 |
| Vomiting | 5 (3.9) | 7 (5.6) | 12 (4.7) | .52 |
| Diarrhea | 4 (3.1) | 5 (3.9) | 9 (3.5) | .70 |
| Children | (n = 67) | (n = 64) | (n = 131) | |
| Cough | 41 (61.2) | 44 (68.8) | 85 (64.9) | .37 |
| Sore throat | 16 (23.9) | 14 (21.9) | 30 (22.9) | .75 |
| Fatigue | 30 (44.8) | 25 (39.1) | 55 (42.0) | .51 |
| Nasal discharge | 27 (40.3) | 15 (23.4) | 42 (32.1) | .04 |
| Difficulty breathing | 25 (37.3) | 27 (42.2) | 52 (39.7) | .57 |
| Headache | 10 (14.9) | 2 (3.1) | 12 (9.2) | .02 |
| Muscle pain | 11 (16.4) | 5 (7.8) | 16 (12.2) | .12 |
| Nausea | 3 (4.5) | 5 (7.8) | 8 (6.1) | .43 |
| Vomiting | 3 (4.5) | 4 (6.3) | 7 (5.3) | .65 |
| Diarrhea | 2 (3.0) | 2 (3.1) | 4 (3.1) | .96 |
| Adults | (n = 63) | (n = 63) | (n = 126) | |
| Cough | 30 (47.6) | 31 (49.2) | 61 (48.4) | .79 |
| Sore throat | 13 (20.6) | 9 (14.3) | 22 (17.5) | .37 |
| Fatigue | 30 (47.6) | 29 (46.0) | 59 (46.8) | .92 |
| Nasal discharge | 13 (20.6) | 10 (15.9) | 23 (18.3) | .51 |
| Difficulty breathing | 29 (46.0) | 24 (38.1) | 53 (42.1) | .40 |
| Headache | 11 (17.5) | 11 (17.5) | 22 (17.5) | .97 |
| Muscle pain | 17 (27.0) | 13 (20.6) | 30 (23.8) | .43 |
| Nausea | 1 (1.6) | 3 (4.8) | 4 (3.2) | .30 |
| Vomiting | 2 (3.2) | 3 (4.8) | 5 (4.0) | .63 |
| Diarrhea | 2 (3.2) | 3 (4.8) | 5 (4.0) | .63 |
| Scores | ||||
| Oxygen saturationb, median (IQR) | 89.0 (85.0–92.0) | 89.0 (88.6–92.0) | 89.0 (85.0–92.0) | .86c |
| SOFA score, median (IQR) | 0.0 (0.0–7.0) | 0.0 (0.0–6.0) | 0.0 (0.0–7.0) | .82c |
| Tal bronchitis score (<24 mo old), median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | .86c |
| Charlson comorbidity index, median (IQR) | 0.0 (0.0–1.5) | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) | .12c |
| Chronic oxygen use | 7 (5.4) | 6 (4.7) | 13 (5.1) | .79 |
| Supplemental oxygen required | 119 (91.5) | 118 (92.9) | 237 (92.2) | .68 |
| ICU admission required | 3 (2.3) | 2 (1.6) | 5 (2.0) | .67 |
| Presence of complications | ||||
| Pneumonia | 74 (56.9) | 73 (57.5) | 147 (57.2) | .93 |
| Respiratory failure requiring mechanical ventilation | 0 (0.0) | 0 (0.0) | 0 (0.0) | … |
| ARDS | 6 (4.6) | 6 (4.7) | 12 (4.7) | .97 |
| Sepsis | 0 (0.0) | 0 (0.0) | 0 (0.0) | … |
| Bronchitis | 2 (1.5) | 7 (5.5) | 9 (3.5) | .08 |
| Global assessment | ||||
| Participants ≥18 y old | ||||
| Felt as good as before illness? (yes) | 12 (19.1) | 11 (17.5) | 23 (18.3) | .85 |
| Functioning as well as before illness? (yes) | 10 (15.9) | 6 (9.5) | 16 (12.7) | .30 |
| Participants <18 y old | ||||
| Is child as active as before illness? (yes) | 6 (9.0) | 12 (18.8) | 18 (13.7) | .10 |
| Is the child eating as much as before illness? (yes) | 10 (14.9) | 12 (18.8) | 22 (16.8) | .56 |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: ARDS, acute respiratory distress syndrome; ICU, intensive care unit; IQR, interquartile range; NTZ, nitazoxanide; SARI, severe acute respiratory illness; SOC, standard of care; SOFA, Sequential Organ Failure Assessment.
aTest of proportions for binary endpoints unless otherwise noted.
bNormal oxygen saturation in Mexico City is 95%–99% per Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán hospital reference ranges but has been reported as a median of 91% in the literature [21].
cTest of means for continuous variables.
Pathogen Detection
At study day 0, 209 of the 257 (81.3%) participants enrolled were diagnosed with at least 1 respiratory pathogen: 101 (77.7%) and 108 (85.0%) in the NTZ and placebo groups, respectively (P = .23). Table 3 shows the frequency distribution of pathogens detected at day 0, by study group.
Table 3.
Confirmed Pathogens at Day 0 and Overall Frequency of Isolation at Day 3, by Treatment Group
| Confirmed Pathogen at Day 0 | NTZ + SOC (n = 130) | Placebo + SOC (n = 127) | Total (N = 257) | P Valuea |
|---|---|---|---|---|
| Influenza A | 25 (19.2) | 25 (19.7) | 50 (19.5) | .93 |
| H1N1 | 11 (8.5) | 14 (11.0) | 25 (9.7) | .40 |
| Non-H1N1 | 14 (10.8) | 11 (8.7) | 25 (9.7) | .40 |
| Influenza B | 5 (3.9) | 3 (2.4) | 8 (3.1) | .49 |
| RSV | 23 (17.7)b | 26 (20.5) | 49 (19.1) | .57 |
| RSV-A | 11 (8.5) | 19 (15.0) | 30 (11.7) | .07 |
| RSV-B | 13 (10) | 7 (5.5) | 20 (7.8) | .04 |
| Human metapneumovirus | 10 (7.7) | 16 (12.6) | 26 (10.1) | .19 |
| Rhinovirus/enterovirus | 31 (23.9) | 28 (22.1) | 59 (23.0) | .73 |
| Adenovirus | 2 (1.5) | 1 (0.8) | 3 (1.2) | .58 |
| Parainfluenza virus | 12 (9.2) | 12 (9.5) | 24 (9.3) | .95 |
| Type 1 | 1 (0.8) | 2 (1.6) | 3 (1.2) | .55 |
| Type 2 | 0 (0) | 1 (0.8) | 1 (0.4) | .31 |
| Type 3 | 7 (5.4) | 10 (7.9) | 17 (6.6) | .42 |
| Type 4 | 4 (3.1) | 0 (0) | 4 (1.6) | .05 |
| Bocavirus | 4 (3.1) | 1 (0.8) | 5 (2.0) | .18 |
| Coronavirus | 5 (3.9) | 12 (9.5) | 17 (6.6) | .07 |
| NL63 | 1 (0.8) | 5 (3.9) | 6 (2.3) | .09 |
| 229E | 1 (0.8) | 3 (2.4) | 4 (1.6) | .30 |
| OC43 | 3 (2.3) | 4 (3.2) | 7 (2.7) | .68 |
| Mycoplasma pneumoniae | 2 (1.5) | 2 (1.6) | 4 (1.6) | .98 |
| Chlamydophila pneumoniae | 0 (0) | 1 (0.8) | 1 (0.4) | .31 |
| Bordetella pertussis | 6 (4.6) | 0 (0) | 6 (2.3) | .01 |
| Pathogen category | ||||
| No pathogens identified | 29 (22.3) | 19 (15.0) | 48 (18.7) | … |
| 1 pathogen identified | 79 (60.8) | 89 (70.1) | 168 (65.4) | … |
| Coinfections identified | 22 (16.9) | 19 (15.0) | 41 (16.0) | .24 |
| Day 3 | ||||
| No detectable virus | 17 (21.5) | 19 (21.3) | 36 (21.4) | .98 |
| Same detectable virus | 56 (70.9) | 61 (68.5) | 117 (69.6) | … |
| Different detectable virus | 6 (7.6) | 9 (10.1) | 15 (8.9) | … |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: NTZ, nitazoxanide; RSV, respiratory syncytial virus; SOC, standard of care.
aTest of proportions for binary endpoints.
bOne participant was coinfected with RSV-A and RSV-B, and is noted under each individual virus, but only counted once for the main RSV category.
Adherence to Study Treatment and Concomitant Medication
A total of 221 of the 257 (86%) participants included in the ITT population took all doses of the study medication: 109 (83.8%) in the NTZ group and 112 (88.2%) in the placebo group (P = .07). Main reasons for not completing recommended treatment and use of concomitant anti-influenza antiviral use during the first 5 days are listed in Table 4; there were no significant differences between study groups (P = .68). One hundred (76.9%) participants in the NTZ group vs 88 (69.3%) in the placebo group were given other antibiotics. In 1 case in the placebo group, the investigator asked the participant to stop taking the study drug because of abnormal laboratory results at study entry.
Table 4.
Days of Hospitalization, by Viral Pathogen
| Pathogen | NTZ + SOC Subjects Discharged | Placebo + SOC Subjects Discharged | P Value | ||
|---|---|---|---|---|---|
| No. of Subjects | Days of Hospitalization, Median (IQR) | No. of Subjects | Days of Hospitalization, Median (IQR) | ||
| Influenza A and B | 27 | 7.5 (7.0–10.0) | 28 | 7.5 (5.5–9.5) | .32 |
| Influenza A | 24 | 8.0 (7.0–10.0) | 25 | 8.0 (6.0–9.0) | .47 |
| Influenza B | 5 | 9.0 (7.0–10.0) | 3 | 5.0 (3.0–12.0) | .51 |
| RSV | 23 | 6.0 (3.0–7.0) | 25 | 5.0 (4.0–8.0) | .68 |
| Human metapneumovirus | 10 | 6.5 (3.0–8.0) | 16 | 7.0 (4.0–9.5) | .46 |
| Rhinovirus | 29 | 6.0 (3.0–7.0) | 28 | 5.0 (4.0–7.5) | .78 |
| Adenovirus | 2 | 7.5 (7.0–8.0) | 1 | 9.0 (9.0–9.0) | .71 |
| Parainfluenza virus | 12 | 5.0 (4.5–7.0) | 11 | 7.5 (5.0–9.0) | .17 |
| Bocavirus | 4 | 7.0 (6.5–7.0) | 1 | 3.0 (3.0–3.0) | .41 |
| Coronavirus | 5 | 7.0 (7.0–9.0) | 12 | 6.0 (4.5–9.0) | .61 |
Abbreviations: IQR, interquartile range; NTZ, nitazoxanide; RSV, respiratory syncytial virus; SOC, standard of care.
Outcomes
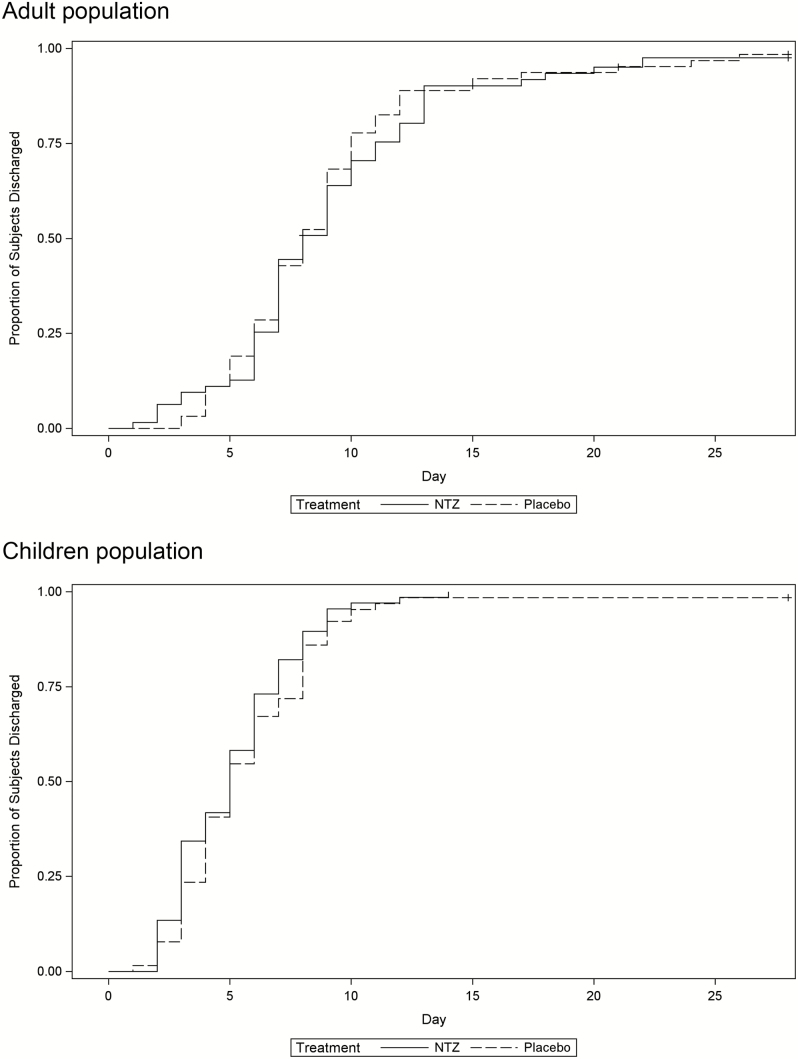
Median time to initial hospital discharge was 6.5 (interquartile range [IQR], 4.0–9.0) days in the NTZ group and 7.0 (IQR, 4.0–9.0) days in the placebo group (Fay-Shaw P = .56) [19]. In analysis by age group, for those ≥18 years old, median time to hospital discharge was 8.0 (IQR, 6.0–11.0) days in the NTZ group and 8.0 (IQR, 6.0–10.0) days in the placebo group (Fay-Shaw P = .62), whereas in children, the median time to hospital discharge was 5.0 (IQR, 3.0–7.0) days and 5.0 (IQR, 4.0–8.0) days in the NTZ and placebo groups, respectively (Fay-Shaw P = .30) (Figure 2). When analyzed by time to treatment (date/time of symptom to the first dose of study drug), the median time to hospital discharge was 6.0 (IQR, 4.0–8.0) days in the NTZ group and 6.1 (IQR, 4.0–8.5) days in the placebo group in those treated within 48 hours; 6.0 (IQR, 3.0–9.0) days vs 7.0 (IQR, 4.0–9.0) days in those treated within >48–96 hours; and 7.0 (IQR, 4.0–9.0) days vs 7.0 (IQR, 5.0–9.0) days in those treated within >96 hours.
Figure 2.
Time to hospital discharge (intention-to-treat population) in adults and children with severe influenza-like illness. Adult population: nitazoxanide (NTZ) + standard of care (SOC) (n = 63): Kaplan-Meier estimate of the median (8.0 [interquartile range {IQR}, 6.0–11.0]) vs placebo + SOC (n = 63): Kaplan-Meier estimate of the median (8.0 [IQR, 6.0–10.0]); Fay-Shaw formulation of the rank-sum statistic, P = .62. Child population: NTZ + SOC (n = 67): Kaplan-Meier estimate of the median (5.0 [IQR, 3.0–7.0]) vs placebo + SOC (n = 64): Kaplan-Meier estimate of the median (5.0 [IQR, 4.0–8.0]); Fay-Shaw formulation of the rank-sum statistic, P = .30.
No significant time differences to hospital discharge were observed for most virology groups (Table 4): influenza A or B positive (n = 55; 8.6 vs 8.0 days; P = .32), rhinovirus/enterovirus positive (n = 57; 5.7 vs 5.6 days; P = .78), respiratory syncytial virus (RSV) positive (n = 48; 5.4 vs 5.8 days; P = .68), human metapneumovirus positive (n = 26; 6.6 vs 7.1 days; P = .46), parainfluenza virus positive (n = 23; 5.6 vs 7.0 days; P = .17), and coronavirus positive (n = 17; 8.8 vs 6.6 days; P = .62).
The duration of symptoms (time until all symptoms were graded 0–1) was a median of 155 (IQR, 78–246) hours in the NTZ group compared with 147 (IQR, 76–224) hours in the placebo group (P = .036). The delay in clinical resolution was most pronounced in the adult population (169 [IQR, 78–385] hours vs 132 [IQR, 0–247] hours) compared with children (153 [IQR, 73–221] hours vs 154 [IQR, 85–224] hours). Duration of supplemental oxygen (110 [IQR, 49–163] hours [n = 119] vs 118 [IQR, 67–174] hours [n = 118]; P = .51) and duration of ICU (45 [IQR, 40–156] hours [n = 3] vs 155 [IQR, 144–166] hours [n = 2]; P = .28) were not different between groups. It took a median of 7 (IQR, 2–28) days for adult subjects to function as well as before they had the respiratory illness (Global Assessment) in the NTZ group, compared with 4 (IQR, 1–14) days in the placebo + SOC arm (P = .04).
Six participants were rehospitalized before study day 28, 2 (1.54%) in the NTZ + SOC group and 4 (3.14%) in the placebo + SOC group (P = .44). Reasons in the NTZ group were an episode of aspergillosis and a case of viral pneumonia, whereas in the placebo group, 3 participants had a relapse of the respiratory illness and 1 participant was readmitted for stem cell transplantation.
The majority of complications occurred prior to study enrollment (Supplementary Table 1). Approximately 13% of participants developed complications after study enrollment, of which pneumonia was the most common complication noted, and 91% occurring by day 3. The NTZ group had similar rates of developing complications on study as those receiving SOC.
Viral Shedding
Of the 241 virus results detected in 168 participants on day 0, 36 (21.4%) patients had no pathogen identified at day 3, and 117 (69.6%) had the same virus still detected. There was no difference in treatment groups (P = .98; Table 3).
Safety
There were 2 deaths in the NTZ group: a 47-year-old participant with septic shock secondary to hospital-acquired pneumonia at day 6 of randomization, and a 51-year-old participant with respiratory failure at day 20 of randomization. In both cases, the investigator judged the event as not related to the study drug. Eighty-three of 130 (63.8%) participants had 205 adverse events in the NTZ group, and 80 of 127 (63.0%) participants had 185 adverse events in the placebo group (P = .89). The frequency of adverse events was similar between the 2 treatment groups, the most common being gastrointestinal disorders (31.9% [82 cases overall]), infections and infestations (16.3% [42 cases]), and respiratory and thoracic disorders (16.3% [42 cases]). There were 13 (5.06%) adverse events classified as serious: 7 (5.4%) and 6 (4.7) in the NTZ and placebo groups, respectively (P = .81; Table 5). Baseline and follow-up laboratory values did not change significantly between treatment groups (data not shown).
Table 5.
Recommended Study Treatment, and Serious Adverse Events by Medical Dictionary for Regulatory Activities System Organ Class and Preferred Term (Safety Population)
| Characteristic | NTZ + SOC (n = 130) | Placebo + SOC (n = 127) | Total (N = 257) | P Valuea |
|---|---|---|---|---|
| No. of study treatment doses received | .07 | |||
| 1–3 | 6 (4.6) | 1 (0.8) | 7 (2.7) | … |
| 4–6 | 1 (0.8) | 5 (3.9) | 6 (2.3) | … |
| 7–9 | 12 (9.2) | 9 (7.1) | 21 (8.2) | … |
| 10 | 109 (83.9) | 112 (88.2) | 221 (86.0) | |
| Unknownb | 2 (1.5) | 0 | 2 (0.8) | … |
| Concomitant antiviral use during first 5 d | ||||
| Oseltamivir | 46 (35.4) | 45 (35.4) | 91 (35.4) | … |
| Zanamivir | 0 | 0 | 0 | … |
| Participants with at least 1 AE during study duration | ||||
| Total no. of subjects reporting AEs | 83 (63.8) | 80 (63.0) | 163 (63.4) | .88 |
| SAEs: MedDRA system organ class preferred term | ||||
| Total no. of SAEs reported | 7 | 6 | 13 | .81 |
| Blood and lymphatic system disorders | ||||
| Neutropenia | 0 | 1 | 1 | … |
| Infections and infestations | ||||
| Aspergillosis | 1 | 0 | 1 | … |
| Pneumonia | 1 | 2 | 3 | … |
| Viral pneumonia | 1 | 0 | 1 | … |
| Septic shock | 1 | 2 | 3 | … |
| Neoplasm (including cyst and polyps) | ||||
| Immune reconstitution | 0 | 1 | 1 | … |
| Inflammatory syndrome associated to Kaposi sarcoma | … | |||
| Psychiatric disorders | ||||
| Delirium | 1 | 0 | 1 | … |
| Respiratory, thoracic, and mediastinal disorders | ||||
| Respiratory failure | 2 | 0 | 2 | … |
| Total deaths | ||||
| Adults (≥18 y of age) | 2 | 0 | 2 | … |
| Children (<18 y of age) | 0 | 0 | 0 | … |
Abbreviations: AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; NTZ, nitazoxanide; SAE, serious adverse event; SOC, standard of care.
aTest of proportions for binary endpoints.
bParticipants withdrew from study before study drug completion.
DISCUSSION
Nitazoxanide, in addition to antiparasitic activity, has broad-spectrum in vitro antiviral activity against influenza, RSV, norovirus, rotavirus, and hepatitis B and C viruses [11]. The broad-spectrum antiviral activity of NTZ can be attributed to the fact that it targets host cell mechanisms rather than the virus. In the case of influenza, NTZ inhibits the function of the endoplasmic reticulum protein ERp57, selectively blocking the maturation of the viral hemagglutinin at a stage preceding resistance to endoglycosidase H digestion, thus impairing hemagglutinin intracellular trafficking and insertion into the host plasma membrane, a step essential for posttranslational trafficking from the endoplasmic reticulum to the Golgi [12, 13]. Studies have shown that tizoxanide inhibits the maturation of rotavirus viral protein 7, a glycoprotein that forms the outer part of the virion and 1 of the 6 structural glycoproteins involved in rotavirus replication; alters viroplasm formation; and interferes with viral morphogenesis. NTZ also potentiates the production of type 1 interferons after viral infection [20], which could contribute to the observed preclinical antiviral properties.
Despite the scientific rationale and promising early studies, in this trial NTZ + SOC did not reduce the duration of hospital stay, supplemental oxygen use, or shedding of respiratory viruses on day 3 in patients with SARI. This finding was surprising given the previous 2 phase 2 ILI studies and 1 phase 2/3 study in patients with confirmed influenza, which all suggested clinical benefit [14–16]. When evaluating the outcome in just those participants with influenza, or those with other viruses, we did not appreciate a significant trend toward benefit in any group. Our study enrolled a sicker population, those admitted to the hospital due to the ILI, and evaluated a clinically relevant endpoint, duration of hospitalization. At least 50% of patients presented with pneumonia, and this complication may cause more time in respiratory symptoms resolution and hospital discharge. It is possible that the need for ongoing hospitalization is driven by factors other than respiratory symptoms, and thus the clinical benefit seen in earlier studies would not change duration of hospitalization. However, duration of symptoms was also no different between the 2 treatments in our study. Some viruses such as rhinovirus are not predicted to respond to NTZ [12]. In our study, rhinovirus constituted 23.0% of the population. However, we do not believe this materially changed the outcomes of the study, as subgroup analysis of participants with other viruses also failed to show benefit in reducing duration of hospitalization.
It is possible that inadequate exposure of the active metabolite tizoxanide might have contributed to the negative findings. Of those in the NTZ arm, 83% received all 10 doses and 93% received ≥8 doses, suggesting that administration was not affected. However, the absorption of NTZ is significantly improved when administered with food (Alinia prescribing information [https://www.alinia.com/wp-content/uploads/2017/08/prescribing-information.pdf]), and we do not know if food was administered with the medication during this study. Additionally, while there are no specific pharmacokinetic data in the hospitalized population, absorption of oral drugs may be impaired in seriously ill/hospitalized patients.
Limitations to our study include the heterogeneous patient population, a variety of factors affecting decisions related to the primary endpoint (hospital discharge), and limited sample size. This is a very challenging population for therapeutic studies (as has been demonstrated by other influenza hospitalization studies), and additional improvements to study design and endpoints may be required to detect potential treatment benefits in this setting. Two ongoing studies in an influenza population have been completed but not yet published (NCT01610245 and NCT02612922), and 2 additional studies (NCT03336619 and NCT03605862) are ongoing (1 in patients with uncomplicated influenza and another in patients with colds due to enterovirus/rhinovirus infection). These will be critical in understanding the clinical value of NTZ in an influenza-positive and ILI population.
CONCLUSIONS
In this trial, treatment with NTZ was safe in children and adults, but failed to show benefit in reducing duration of hospitalization or other endpoints in severe ILI. The totality of the evidence from all performed trials with NTZ should be evaluated prior to additional studies being commenced with this molecule in respiratory viral diseases.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Mexico Emerging Infectious Diseases Clinical Research Network (LaRed) members. Nitazoxanide–Severe Acute Respiratory Illness study chairs: M. Lourdes Guerrero, Ana E. Gamiño-Arroyo. Investigators and study staff by participating institution: Instituto Nacional de Enfermedades Respiratorias (67 participants): Alejandra Ramírez-Venegas, Nora Bautista, Angélica Nolasco-Reza; Instituto Nacional de Pediatría (64 participants): Beatriz Llamosas-Gallardo, Ana A. Ortiz-Hernández, Diana Andrade-Platas, Juliana Estevez-Jimenez; Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (49 participants): Arturo Galindo-Fraga, Bricia Roa-Martínez, Itzel Cruz-Gaona, Diana Aguilar-Cruz; Hospital Infantil de México Federico Gómez (33 participants): Sarbelio Moreno-Espinosa, Mónica González-Matus, Luis Mendoza-Garcés; Hospital Central Dr. Ignacio Morones Prieto, San Luis Potosí México (24 participants): Javier Araujo-Meléndez, Norma Perea-Guzmán, Ana Sandoval-Gutiérrez, Daniel Hernández-Ramírez, Pedro Gerardo Hernández-Sánchez, Juana del Carmen Baez-Cruz; Hospital General Dr Aurelio Valdivieso (23 participants): Yuri A. Roldán-Aragón, Alejandra N. Davila-Cruz; Central Laboratory at the Department of Infectious Diseases at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán: Violeta Ibarra-González, Julia Martínez-López, Luis A. García-Andrade; Coordinación de los Institutos Nacionales de Salud y Hospitales de Alta Especialidad, Secretaría de Salud, México: Guillermo M. Ruiz-Palacios; US National Institute of Allergy and Infectious Diseases (NIAID): John H. Beigel, Mary Smolskis, Sally Hunsberger, H. Sean McCarthy; Leidos Biomedical Research, Inc, in support of NIAID: Louis Grue, Gregory Burge, Roxanne Cox, Preston Holley, Jr; Social and Scientific Systems, Inc: Anthony Cristillo, Nasreen Nahed, Wendolyne López; LaRed Network Coordinating Center: Jessica Mascareñas-Ruiz, Eli Xchel Becerril-Ruiz, Peter Quidgley, Hugo Arroyo-Figueroa.
Acknowledgments.The authors are indebted to the patients who participated in the study.
Disclaimer.The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported by the NIAID of the National Institutes of Health (NIH); Consejo Nacional de Ciencia y Tecnología (FONSEC SSA/IMSS/ISSSTE 71260 and 127088); and the National Cancer Institute, NIH (contract numbers HHSN261200800001E> and HHSN261201500003I).
Potential conflicts of interest.All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Mexico Emerging Infectious Diseases Clinical Research Network (LaRed):
M Lourdes Guerrero, Ana E Gamiño-Arroyo, Alejandra Ramírez-Venegas, Nora Bautista, Angélica Nolasco-Reza, Beatriz Llamosas-Gallardo, Ana A Ortiz-Hernández, Diana Andrade-Platas, Juliana Estevez-Jimenez, Arturo Galindo-Fraga, Bricia Roa-Martínez, Itzel Cruz-Gaona, Diana Aguilar-Cruz, Sarbelio Moreno-Espinosa, Mónica González-Matus, Luis Mendoza-Garcés, Javier Araujo-Meléndez, Norma Perea-Guzmán, Ana Sandoval-Gutiérrez, Daniel Hernández-Ramírez, Pedro Gerardo Hernández-Sánchez, Yuri A Roldán-Aragón, Alejandra N Davila-Cruz, Violeta Ibarra-González, Julia Martínez-López, Luis A García-Andrade, Guillermo M Ruiz-Palacios, John H Beigel, Mary Smolskis, Sally Hunsberger, H Sean McCarthy, Louis Grue, Gregory Burge, Roxanne Cox, Preston Holley, Jr, Anthony Cristillo, Nasreen Nahed, Wendolyne López, Eli Xchel Becerril-Ruiz, Peter Quidgley, and Hugo Arroyo-Figueroa
References
- 1. Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997; 349:1498–504. [DOI] [PubMed] [Google Scholar]
- 2. Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McLean HQ, Peterson SH, King JP, Meece JK, Belongia EA. School absenteeism among school-age children with medically attended acute viral respiratory illness during three influenza seasons, 2012–2013 through 2014–2015. Influenza Other Respir Viruses 2017; 11:220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petrie JG, Cheng C, Malosh RE, et al. Illness severity and work productivity loss amoung working adults with medically attended acute respiratory illnesses: US Influenza Vaccine Effectiveness Network 2012–2013. Clin Infect Dis 2016; 62:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galindo-Fraga A, Ortiz-Hernández AA, Ramírez-Venegas A, et al. ; La Red ILI 002 Study Group. Clinical characteristics and outcomes of influenza and other influenza-like illnesses in Mexico City. Int J Infect Dis 2013; 17:e510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Englund JA. Antiviral therapy of influenza. Semin Pediatr Infect Dis 2002; 13:120–8. [DOI] [PubMed] [Google Scholar]
- 7. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 2015; 385:1729–37. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization. Battle against Respiratory Viruses (BRaVe) Initiative—concept paper. 2012. Available at: http://www.who.int/influenza/patient_care/clinical/BRAVE_Concept_Paper.pdf. Accessed 15 September 2018. [Google Scholar]
- 9. Ortiz JJ, Ayoub A, Gargala G, Chegne NL, Favennec L. Randomized clinical study of nitazoxanide compared to metronidazole in the treatment of symptomatic giardiasis in children from northern Peru. Aliment Pharmacol Ther 2001; 15:1409–15. [DOI] [PubMed] [Google Scholar]
- 10. Amadi B, Mwiya M, Musuku J, et al. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 2002; 360:1375–80. [DOI] [PubMed] [Google Scholar]
- 11. Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of nitazoxanide. J Infect Dis 2001; 184:103–6. [DOI] [PubMed] [Google Scholar]
- 12. Rossignol JF. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res 2014; 110:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rossignol JF, La Frazia S, Chiappa L, Ciucci A, Santoro MG. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem 2009; 284:29798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lopez-Chegne N, Julcamoro LM, Carrion M, Bardin M. Randomized, double blind, pilot study of nitazoxanide (NTZ) versus placebo (PCB) for the treatment of symptoms associated with viral respiratory infection (VRI) in children. Oral abstract 1341. In: Program and abstracts of the 49th Annual Meeting of the Infectious Diseases Society of America, Boston, MA, 20–23 October 2011.
- 15. Lopez-Chegne N, Julcamoro LM, Rossignol JF, Carrion M, Bardin M. Randomized, double blind, pilot study of nitazoxanide (NTZ) versus placebo (PCB) for the treatment of symptoms associated with viral respiratory infection (VRI) in adults and adolescents. Oral abstract 142. In: Program and abstracts of the 49th Annual Meeting of the Infectious Diseases Society of America, Boston, MA, 20–23 October 2011.
- 16. Haffizulla J, Hartman A, Hoppers M, et al. ; US Nitazoxanide Influenza Clinical Study Group. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis 2014; 14:609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. WHO surveillance case definitions for ILI and SARI 2014. Available at: https://www.who.int/influenza/surveillance_monitoring/ili_sari_surveillance_case_definition/en/. Accessed 8 December 2018.
- 18. World Health Organization. CDC protocol of real-time RT-PCR for influenza A(H1N1).2009. Available at: http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf. Accessed 15 September 2018.
- 19. Shaw PA, Fay MP. A rank test for bivariate time-to-event outcomes when one event is a surrogate. Stat Med 2016; 35:3413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clerici M, Trabattoni D, Pacei M, Biasin M, Rossignol JF. The anti-infective nitazoxanide shows strong immuno-modulating effects. J Immunol 2011; 186:21. [Google Scholar]
- 21. Rico Méndez FG, Urias A, Barquera S, et al. Valores espirométricos y gasométricos en una población geriátrica sana, a diferentes alturas sobre el nivel del mar, en la República Mexicana [in Spanish]. Rev Inst Nal Enf Resp Mex 2001; 14:90–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.