Abstract
Background
Higher perivascular adipose tissue (PVAT) contributes to adverse physiologic alterations in the vascular wall, and thus could potentially limit normal physical function later in life. We hypothesize that higher PVAT volume at midlife is prospectively associated with slower gait speed later in life, independent of overall adiposity and other risk factors.
Methods
Participants from the Study of Women’s Health Across the Nation (SWAN) cardiovascular fat ancillary study were included. PVAT volume around the descending aorta was quantified using existing computed tomography scans at midlife, while gait speed was measured after an average of 10.4 ± 0.7 years.
Results
Two hundred and seventy-six women (aged 51.3 ± 2.8 years at PVAT assessment) were included. Mean gait speed was 0.96 ± 0.21 m/s. Adjusting for study site, race, education level, menopausal status, and length of descending aorta at PVAT assessment, and age, body mass index, difficulty paying for basics, overall health and smoking status at gait speed assessment, a higher midlife PVAT volume was associated with a slower gait speed later in life (p = .03). With further adjustment for presence of any comorbid conditions by the time of gait speed assessment, the association persisted; every 1SD increase in log-PVAT was associated with 3.3% slower gait speed (95% confidence interval: 0.3–6.3%; p = .03).
Conclusion
Greater PVAT in midlife women may contribute to poorer physical function in older age supporting a potential role of midlife PVAT in multiple domains of healthy aging. Additional research is needed to fully elucidate the physiologic changes associated with PVAT that may underlie the observed associations.
Keywords: Perivascular adipose tissue, Physical functioning, Walking speed, Midlife
Perivascular adipose tissue (PVAT), the fat depot surrounding the vasculature (1), is a metabolically active organ that contributes to vascular homeostasis and inflammation (2). It plays an integral role in both vascular health and disease through the release of inflammatory cells, adipokines, cytokines, and hormone-like factors (3). These biological markers could act in both autocrine and paracrine fashion impairing vascular function (3,4) and leading to vascular remodeling (5) and atherosclerosis (6).
Several lines of evidence support a physiologic link between vascular function impairments and physical functioning (7–10). In one study, higher arterial stiffness was associated with a lower walking speed (7). A higher ankle-brachial index was associated with a higher score of self-reported measures of physical functioning (8), and with a lower level of objective measures of physical performance (9). In participants with and without peripheral arterial disease, a decrease in ankle-brachial index was associated with lower but not upper extremity functional limitations (10). By contributing to adverse dynamic physiologic alterations in the vascular wall, PVAT might limit normal physical functioning later in life.
Midlife is a key period for women. It encompasses the menopausal transition, a pivotal marker in women’s aging process, when several hormonal and biological changes take place (11). Evidence showed that women are vulnerable to body fat redistribution, vascular remodeling, and physical functioning changes at midlife (12–14), with the menopausal transition and its related hormonal changes were found to contribute to this vulnerability (15). Given the coincidence increase risk of fat redistribution, vascular remodeling and physical functioning changes in women at midlife, evaluating a potential link between midlife PVAT and physical functioning status later in life is critical for women. No previous study has assessed this association in midlife women. The Study of Women’s Health Across the Nation (SWAN), a longitudinal study of the menopausal transition, provides a unique opportunity to assess the prospective relationship between midlife PVAT volume and later life gait speed, a measure of physical and functional limitations (16) that can predict mortality and morbidity in elderly population (17). We hypothesized that higher PVAT volume at midlife will be associated with slower gait speed later in life independent of overall adiposity and other risk factors.
Methods
Study Participants
SWAN is an ongoing, longitudinal, multisite, multiethnic study of the physiological and psychological changes during menopausal transition. The study design has been previously reported (18). In brief, between 1996 and 1997, 3,302 women aged 42–52 years were recruited from seven sites across the United States (Boston, MA; Detroit, MI; Chicago, IL, Pittsburgh, PA; Oakland, CA; Los Angeles, CA; and Newark, NJ). Eligibility criteria were: (i) an intact uterus and at least one ovary; (ii) at least one menstrual period within the last 3 months; (ii) not pregnant or breastfeeding at recruitment; (iv) no hormone therapy use within the last 3 months; and (v) self-identifies race as Caucasian, African American, Hispanic, Chinese, or Japanese.
The SWAN Heart Study is an ancillary study at Pittsburgh and Chicago sites, where subclinical measures of atherosclerosis were measured at baseline and a follow-up visit (19). The SWAN Cardiovascular Fat ancillary study measured volumes of cardiovascular fat among SWAN Heart participants at the SWAN Heart baseline visit (coincident with SWAN visits 4–7). For this study, participants from the SWAN Cardiovascular Fat ancillary study who had available data on PVAT volume measured early at midlife (during SWAN visits 4–7) and gait speed measured later in life (at SWAN visit 13) were included. Of 521 women who had cardiovascular fat data available, 245 women were excluded for not having gait speed data. This resulted in a final study sample of 276 women. Participants who were excluded were younger at time of PVAT assessment and more likely to have a comorbidity by the time of gait speed assessment (Supplementary Table 1).
All participants provided written informed consent prior to enrollment and study protocols were approved by the institutional review boards at the Pittsburgh and Chicago sites.
Gait Speed Assessment
Gait speed was measured at SWAN visit 13, after a mean of 10.4 (± 0.7 SD) years since PVAT assessment. Four-meter walk time was performed on a level floor with tape markers indicating start and stop points, which were 4 m apart (20). Subjects were instructed to walk at their usual speed. Timing was stopped when the first foot completely crossed the stop mark. The average of two test results was reported. Gait speed was calculated by dividing the distance in meters (4 m) by average time in seconds (m/s). Gait speed has been established as a valid and reliable method for assessing physical functioning in older adults (21).
Cardiovascular Fat Assessment
PVAT was defined as the fat around the descending thoracic aorta. PVAT volume was quantified using preexisting electron beam computed tomography scans that were used to measure aortic artery calcification. PVAT volume was quantified as described before (19). In brief, the 6 mm trans-axial scans were read by two readers using the commercially available software Slice-O-Matic (Tomovision, Montreal, Canada). PVAT was distinguished from other tissue by a threshold of −190 to −30 Hounsfield Unit (HU) (22). The posterior border of PVAT was identified by its proximity to the anterior portion of the spinal foramen. The anterior border and the two lateral borders were identified by the location of left bronchus, esophagus, and crus of diaphragm. The proximal boundary of the descending aorta was defined by the carina, and the distal boundary was defined at the first lumbar vertebra. Analysis stops at the pedicles of L1. It is expected that the length of the evaluated part of the descending aorta within the anatomic landmarks described above (aorta length, cm) may vary across participants. Therefore, aorta length was estimated from table position number at first included CT slice and table position number at last included CT slice, and accounted for in multivariable analysis. Between and within reader Spearman correlation coefficients for PVAT were 0.99 indicating excellent reproducibility (23).
Study Covariates
Current age at SWAN visit 13 was calculated as the difference between the participant’s birthdate and SWAN visit 13 completion date. Self-reported race/ethnicity and education level were collected at SWAN baseline visit. Comorbid medical conditions including osteoarthritis, osteoporosis, any cancer, myocardial infarction, stroke, and angina were self-reported through questionnaires administered at each annual visit. Diabetes mellitus was defined as a fasting blood glucose ≥126 mg/dL, use of diabetes medication, or self-reported history of diabetes at any visit, while hypertension was defined as a systolic blood pressure ≥130 mmHg, a diastolic blood pressure ≥85 mm Hg or a report of antihypertensive medications at any visit. Presence of any comorbidity was defined as presence of any of these conditions at any time point until gait speed assessment visit. Ever use of hormone therapy was defined as self-reported use of hormone therapy at any time point until the gait speed assessment visit.
At each study visit, menopausal status was assessed based on frequency and regularity of menstrual bleeding and use of hormone therapy. Menopausal status at PVAT assessment was used as a covariate for the current analysis and women at related visits were either premenopausal (normal menses within the last 3 months), early perimenopausal (women with at least one menses within the last 3 months with perceived changes in cycle intervals), late perimenopausal (women with no menses for 3 consecutive months but with menstrual bleeding within the last 12 months), or natural postmenopausal (no menstrual cycles within the last 12 months).
At time of gait speed assessment, self-reported information on difficulty paying for the basics as food, housing and health care (somewhat/very hard versus not hard), perceived overall health (excellent/very good, good, or fair/poor), and smoking status were collected from questionnaires. Body mass index (BMI; kg/m2) was calculated as measured weight/height2.
Statistical Analysis
Characteristics of the study population were summarized as mean ± SD for normally distributed continuous variables or median (Q1, Q3) for skewed continuous variables. Categorical variables were presented as frequency (%). Distribution of PVAT was skewed and log-transformation was applied to reduce skewness.
Univariate linear regression analysis of gait speed and PVAT with study variables was conducted to identify potential covariates (variables found to be significantly (p < .05) associated with gait speed and/or PVAT) for multivariable analysis. Study site, education, and menopausal status at PVAT assessment were considered irrespective of their related p-values; study site is a SWAN design variable that is critical to account for potential differences across sites, while menopausal status has been strongly linked to body fat redistribution and physical functioning changes in women at midlife (11). Model was first adjusted for aorta length. This was followed by additional adjustment for site, race, education, menopausal status at PVAT assessment, and the following covariates at time of gait speed assessment: age, BMI, smoking status, difficulty paying for basics, and overall health status. Final model was additionally adjusted for presence of any comorbidity by time of gait speed assessment. To assess whether the menopausal status at time of PVAT assessment modify the association between PVAT and later gait speed, an interaction term between menopausal status and PVAT was included in the multivariable adjusted models.
To visually present the significant association between PVAT and gait speed, model-based means of gait speed by PVAT tertiles were estimated adjusting for aorta length, site, race, education, menopausal status at PVAT assessment, and the following covariates at visit of gait speed assessment: age, BMI, smoking status, difficulty paying for basics, overall health status, and presence of any comorbidity by the time of gait assessment. Post hoc Bonferroni adjustment was applied to correct for multiple comparisons. Statistical analyses were performed using SAS v9.3 (SAS Institute, Cary, NC).
Results
Characteristics of the Study Population
Women were 51.3 ± 2.8 years old at time of PVAT assessment and 61.3 ± 2.6 years old at time of gait speed assessment. Characteristics of study population are summarized in Table 1. Average gait speed was 0.96 ± 0.21 m/s; this is comparable to the results from other studies reporting gait speed in women at similar age (24).
Table 1.
Characteristics of Study Participants at PVAT and Gait Speed Assessments
| Study Variables | |
|---|---|
| Age at PVAT assessment (years), mean (±SD) | 51.3 (±2.8) |
| Age at gait speed assessment (years), mean (±SD) | 61.3 (±2.6) |
| White, n (%) | 173 (62.9%) |
| Education, n (%) | |
| High school | 44 (15.9%) |
| College | 131 (47.5%) |
| Graduate | 101 (36.6%) |
| How hard to pay for basics at gait speed assessment, n (%) | |
| Somewhat/very hard | 46 (17.4%) |
| Not hard | 218 (82.6%) |
| Menopausal Status at PVAT assessment, n (%) | |
| Premenopausal | 21 (7.6%) |
| Early perimenopausal | 140 (50.7%) |
| Late perimenopausal | 30 (10.9%) |
| Natural postmenopausal | 85 (30.8%) |
| BMI (kg/m2) at gait speed assessment, mean (±SD) | 30.6 (±7.0) |
| Smokers at gait speed assessment, n (%) | 17 (6.4%) |
| Overall health at gait speed assessment, n (%) | |
| Excellent/Very Good | 141 (52.6%) |
| Good | 94 (35.1%) |
| Fair/Poor | 33 (12.3%) |
| Presence of any medical comorbidities by gait speed assessment, Yes, n (%) | 252 (93.3%) |
| Use of hormone therapy by gait speed assessment, n (%) | 109 (39.5%) |
| PVAT Volume (cm3), median (Q1, Q3) | 29.44 (24.27, 39.06) |
| Length of the descending aorta (cm), mean (±SD) | 16.3 (±1.64) |
| Gait Speed (m/s), mean (±SD) | 0.96 (±0.21) |
Note: BMI = Body mass index; PVAT = Perivascular adipose tissue. N = 276: Numbers may not add up to 276 for all variables due to missing data for the following variables: smokers and overall health at gait speed assessment and presence of any medical comorbidities by gait speed assessment.
Univariate Analysis for Gait Speed and log-PVAT
Results of the univariate analysis are presented in Supplementary Table 2. White women, women who reported more difficulty paying for basics or who reported excellent/very good perceived health at time of gait speed assessment had significantly faster gait speed compared to reference groups. Higher BMI and presence of any comorbidity by time of gait speed assessment were significantly associated with slower gait speed and higher PVAT volume. Older age was significantly associated with higher PVAT volume, and a longer descending aorta was associated with faster gait speed and higher PVAT volume.
Associations Between PVAT and Gait Speed
Multivariable analysis is presented in Table 2. Adjusting for aorta length (Model 1), every 1 SD increase in log-PVAT was significantly associated with a 7.1% decrease in gait speed (95% confidence interval [CI]: −5.2%, −9.4%; p < .0001) relative to mean gait speed. Additional adjustment for site, race, education level, menopausal status at PVAT assessment, and age, BMI, difficulty paying for basics, overall health, and smoking status at gait speed assessment (Model 2) slightly reduced the effect size which remained statistically significant (−3.2%; 95% CI: −0.3%, −6.3%; p = .03). Adding presence of any comorbidity by time of gait speed assessment to the model did not alter the relation between midlife PVAT and gait speed later in life (Model 3).
Table 2.
Adjusted Change in Gait Speed per 1 SD Increase in log-PVAT
| Per 1 SD Increase in log-PVATa | Gait Speed (m/s) | ||
|---|---|---|---|
| Model | Change (95% CI) m/s | % Change Relative to Mean Gait Speed of 0.96 m/sa | p Value |
| Model 1 | −0.068 (−0.09, −0.05) | −7.1% (−9.4%, −5.2%) | <.0001 |
| Model 2 | −0.031 (−0.06, −0.003) | −3.2% (−6.3%, −0.3%) | .03 |
| Model 3 | −0.032 (−0.06, −0.003) | −3.3% (−6%, −0.3%) | .03 |
Note: PVAT = Perivascular adipose tissue.
Model 1: Adjustment for length of the descending aorta.
Model 2: Adjustment for Model 1+ study site, race, education, menopausal status at PVAT assessment, and the following covariates from the visit of gait speed assessment: age, BMI, smoking status., difficulty paying for basics, and overall health status.
Model 3: Adjustment for Model 2 + any comorbidity by gait speed assessment.
aPercent change in gait speed relative to mean gait speed was calculated as estimate of change in gait speed per 1 SD increase in log-PVAT divided by mean gait speed (0.96 m/s) multiplied by 100%.
Interaction between PVAT and menopausal status was not significant. Additional adjustment for ever use of hormone therapy and time difference between assessments did not alter the results (data not shown).
Association Between PVAT Tertiles and Gait Speed
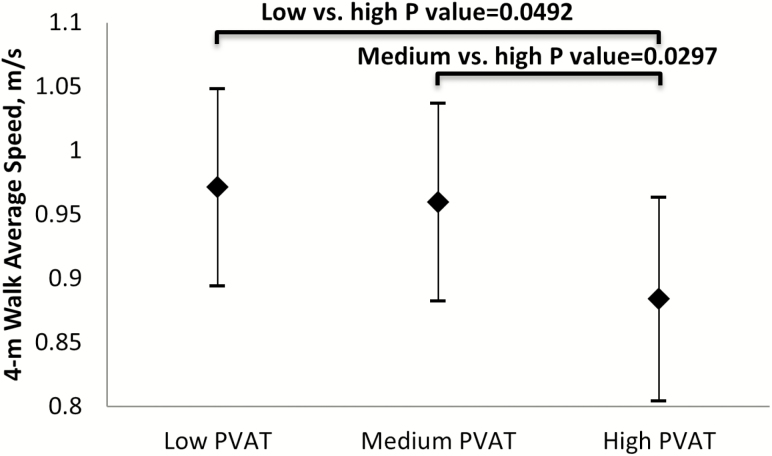
In the fully-adjusted model (Figure 1), PVAT volume tertiles at midlife were negatively associated with gait speed level later in life. Women in the highest PVAT volume tertile showed significantly slower gait speed later in life compared with those in the medium tertile (p = .049, Bonferroni adjusted) or the lowest tertile (p = .029, Bonferroni adjusted).
Figure 1.
Adjusted average gait speed mean by PVAT tertiles. aAdjusted for multiple testing; reference group is “high.” Model adjusted for length of the descending aorta, site, race, education, menopausal status at PVAT assessment, and the following covariates from the visit of gait speed assessment: age, BMI, smoking status, difficulty paying for basics, overall health status, and any comorbidity by gait speed assessment visit. Low PVAT: 13.163 cm3 ≤ PVAT volume< 25.949 cm3; Medium PVAT: 25.949 cm3 ≤ PVAT volume <35.625 cm3; High PVAT ≥ 35.625 cm3. BMI = Body mass index; PVAT = Perivascular adipose tissue.
Discussion
Our findings suggest that greater volume of periaortic fat in women at midlife is associated with slower gait speed later in life, independent of overall adiposity, comorbid conditions, and other possible confounders.
Although no previous study has assessed the association between gait speed and PVAT, few studies have evaluated the relationship between gait speed and other visceral fat depots, with inconsistent findings. Geisler and colleagues (25) found no cross-sectional association between abdominal visceral fat and gait speed in a healthy elderly population (mean age 71.7 ± 4.3 years; 50% women). In one longitudinal study, Beavers and colleagues (26) reported an inverse relation between baseline abdominal visceral adiposity and gait speed over 4 years in women (mean age 74.5 ± 2.8 years). Another longitudinal analysis (27) reported that in women (mean age 74.2 ± 2.9 years), abdominal visceral adipose tissue was not associated with later self-reported mobility limitations or slower gait speed after adjustment for BMI and other confounders. In the Framingham Study (28), it was similarly reported that the inverse relation between abdominal visceral fat and mobility disability and walking speed in women (mean age 66.3 ± 8.7 years) was attenuated after adjusting for BMI. This study assesses a younger population and evaluates a visceral fat depot that is virtually integrated into the vasculature. Location of visceral fat as well as the type of assessed physical functioning could be critical factors when evaluating this association.
PVAT is a metabolically active tissue that secretes adipokines and cytokines (29) which affects the contractility of vascular walls. PVAT prompts the increased secretion of the proinflammatory IL-6, IL-8, and MCP-1, while limiting the secretion of adiponectin (30). Adiponectin deficiency may be associated with accelerated atherosclerosis attributed to the insufficiency of vasodilatory effects (31). Moreover, higher IL-6 levels may be associated with slower walking speed in older women and with decline in walking speed over time (32).
The menopausal transition is a critical period during women’s lives when they are subject to changes in body fat composition (12), vascular remodeling (13) and a decline in their physical functioning (33). During the menopausal transition, concomitant increase in fat mass and a decline in physical functioning occurs (12,14). We have previously shown that during menopause, women experience vascular alterations and arterial remodeling as evidenced by unfavorable changes in carotid intima media thickness, arterial stiffness and adventitial diameter around menopause (13,34,35), and with significant physical function limitations in postmenopausal women (36,37). We have reported that in midlife women, higher inflammatory markers are associated with greater self-reported physical functioning limitations (38). Given the changes in fat depot volumes and distributions occurring at midlife (12), increases in volumes of periaortic fat could contribute to the increase in inflammation and thus, slower gaits speed that was found in this study.
Our study has limitations. Although the study design was cross-sectional, midlife PVAT was analyzed prospectively in relation with gait speed later in life. The lack of repeated measures of PVAT and gait speed limited our ability to assess for temporality and causality. The sample size was rather small; however, our findings are novel and call for further investigation. In our analysis, we tested for multiple covariates that could possibly impact the relation between PVAT and gait speed but we did not adjust for additional laboratory assessments such as adipokines and inflammatory markers which could be related to vascular dysfunction, and possibly mediate the relation between PVAT and gait speed (36). These measures were not available at the time of conducting this study. Future work will assess potential mediation effects of these biomarkers on the reported association in this paper. Participants were women of either a White or Black race; thus, these results are of limited generalizability to men and other racial/ethnic groups. Future work should assess whether similar association can be reported in midlife men and other racial/ethnic groups. Women who were excluded were more likely to have any comorbidity by the time of gait assessment which may have resulted in an underestimation of the assessed association. The strengths of the study are the novelty of the assessment, the use of high-quality method to quantify PVAT and the well-characterized SWAN cohort of midlife women.
This study is the first to demonstrate a prospective relationship between PVAT at midlife and gait speed level later in life in women independent of possible confounders including overall adiposity. A fast decline in gait speed has been recently defined as 2.4% decrease per year, and those with fast decline in gait speed had a 90% greater risk of mortality than those with slow decline (39). Our results could guide further understanding of the pathophysiology of impaired vascular function and the underlying mechanism for peripheral physical functioning limitation. Animal studies have shown that weight loss following caloric restriction is associated with reduced inflammation and increased nitric oxide synthase activity in PVAT (40); thus, lifestyle interventions which could alter the activity of PVAT may corroborate improvements in physical functioning.
In conclusion, greater PVAT in midlife women may contribute to poorer physical function in older age supporting a potential role of midlife PVAT in multiple domains of healthy aging. Additional research is needed to fully elucidate the physiologic changes associated with PVAT that may underlie the observed associations. Future longitudinal studies are necessary to assess the relations among PVAT, vascular health indices, and physical functioning, as well as how the changes in those measures may impact functionality.
Funding
This work was supported by an award from the American Heart Association Great River Affiliation Clinical Research Program: 12CRP11900031 (SWAN Cardiovascular Fat Ancillary Study). The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH); DHHS, through the National Institute on Aging (NIA); the National Institute of Nursing Research (NINR); and the NIH Office of Research on Women’s Health (ORWH) (grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). SWAN Heat was supported by the National Heart, Lung and Blood Institute (grants HL065581, HL065591). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
Author contributions: S.R.El.K. conceived and designed the manuscript. S.R.El.K. and A.N. drafted the manuscript. X.C. performed statistical analyses of the manuscript. S.R.El.K., K.S., I.J., and K.M. designed the SWAN Cardiovascular Fat Ancillary study. All coauthors read the manuscript and provided valuable comments and suggestions. All coauthors revised the manuscript for important intellectual content and provided final approval.
We thank the study staff at each site and all the women who participated in SWAN.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI. NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016 – present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers. Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 – present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA – Sonja McKinlay, PI 1995 – 2001. Steering Committee: Susan Johnson, Current Chair. Chris Gallagher, Former Chair.
References
- 1. Lastra G, Manrique C. Perivascular adipose tissue, inflammation and insulin resistance: link to vascular dysfunction and cardiovascular disease. Horm Mol Biol Clin Investig. 2015;22:19–26. doi: 10.1515/hmbci-2015-0010 [DOI] [PubMed] [Google Scholar]
- 2. Lian X, Gollasch M. A clinical perspective: contribution of dysfunctional perivascular adipose tissue (PVAT) to cardiovascular risk. Curr Hypertens Rep. 2016;18:82. doi: 10.1007/s11906-016-0692-z [DOI] [PubMed] [Google Scholar]
- 3. Brown NK, Zhou Z, Zhang J, et al. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34:1621–1630. doi: 10.1161/ATVBAHA.114.303029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Szasz T, Bomfim GF, Webb RC. The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag. 2013;9:105–116. doi: 10.2147/VHRM.S33760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okamoto E, Couse T, De Leon H, et al. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–2235. [DOI] [PubMed] [Google Scholar]
- 6. Verhagen SN, Vink A, van der Graaf Y, Visseren FL. Coronary perivascular adipose tissue characteristics are related to atherosclerotic plaque size and composition. A post-mortem study. Atherosclerosis. 2012;225:99–104. doi: 10.1016/j.atherosclerosis.2012.08.031 [DOI] [PubMed] [Google Scholar]
- 7. Brunner EJ, Shipley MJ, Witte DR, et al. Arterial stiffness, physical function, and functional limitation: the Whitehall II Study. Hypertension. 2011;57:1003–1009. doi: 10.1161/HYPERTENSIONAHA.110.168864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. García-Ortiz L, Recio-Rodríguez JI, Mora-Simón S, et al. ; MARK Group Vascular structure and function and their relationship with health-related quality of life in the MARK study. BMC Cardiovasc Disord. 2016;16:95. doi: 10.1186/s12872-016-0272-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. [DOI] [PubMed] [Google Scholar]
- 10. McDermott MM, Tian L, Ferrucci L, et al. Associations between lower extremity ischemia, upper and lower extremity strength, and functional impairment with peripheral arterial disease. J Am Geriatr Soc. 2008;56:724–729. doi: 10.1111/j.1532-5415.2008.01633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thurston RC, Karvonen-Gutierrez CA, Derby CA, El Khoudary SR, Kravitz HM, Manson JE. Menopause versus chronologic aging: their roles in women’s health. Menopause. 2018;25:849–854. doi: 10.1097/GME.0000000000001143 [DOI] [PubMed] [Google Scholar]
- 12. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). 2008;32:949–958. doi: 10.1038/ijo.2008.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause. 2013;20:8–14. doi: 10.1097/gme.0b013e3182611787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sowers M, Tomey K, Jannausch M, Eyvazzadeh A, Nan B, Randolph J Jr. Physical functioning and menopause states. Obstet Gynecol. 2007;110:1290–1296. doi: 10.1097/01.AOG.0000290693.78106.9a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El Khoudary SR, Brooks MM, Thurston RC, Matthews KA. Lipoprotein subclasses and endogenous sex hormones in women at midlife. J Lipid Res. 2014;55:1498–1504. doi: 10.1194/jlr.P049064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chou CH, Hwang CL, Wu YT. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: a meta-analysis. Arch Phys Med Rehabil. 2012;93:237–244. doi: 10.1016/j.apmr.2011.08.042 [DOI] [PubMed] [Google Scholar]
- 17. Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23:314–322. doi: 10.1123/japa.2013-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sowers M, Crawford S, Sternfeld B, et al. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: Biology and Pathobiology. San Diego: Academic press; 2000:175–188. [Google Scholar]
- 19. El Khoudary SR, Shields KJ, Janssen I, et al. Cardiovascular fat, menopause, and sex hormones in women: the SWAN cardiovascular fat ancillary study. J Clin Endocrinol Metab. 2015;100:3304–3312. doi: 10.1210/JC.2015-2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pettee Gabriel K, Sternfeld B, Colvin A, et al. Physical activity trajectories during midlife and subsequent risk of physical functioning decline in late mid-life: the Study of Women’s Health Across the Nation (SWAN). Prev Med. 2017;105:287–294. doi: 10.1016/j.ypmed.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. [DOI] [PubMed] [Google Scholar]
- 22. Murphy RA, Register TC, Shively CA, et al. Adipose tissue density, a novel biomarker predicting mortality risk in older adults. J Gerontol A Biol Sci Med Sci. 2014;69:109–117. doi: 10.1093/gerona/glt070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanley C, Matthews KA, Brooks MM, et al. Cardiovascular fat in women at midlife: effects of race, overall adiposity, and central adiposity. The SWAN Cardiovascular Fat Study. Menopause. 2018;25:38–45. doi: 10.1097/GME.0000000000000945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stringhini S, Carmeli C, Jokela M, et al. ; LIFEPATH Consortium Socioeconomic status, non-communicable disease risk factors, and walking speed in older adults: multi-cohort population based study. BMJ. 2018;360:k1046. doi: 10.1136/bmj.k1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geisler C, Schweitzer L, Muller MJ. Functional correlates of detailed body composition in healthy elderly subjects. J Appl Physiol (1985). 2018;124:182–189. doi: 10.1152/japplphysiol.00162.2017 [DOI] [PubMed] [Google Scholar]
- 26. Beavers KM, Beavers DP, Houston DK, et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr. 2013;97:552–560. doi: 10.3945/ajcn.112.047860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy RA, Reinders I, Register TC, et al. Associations of BMI and adipose tissue area and density with incident mobility limitation and poor performance in older adults. Am J Clin Nutr. 2014;99:1059–1065. doi: 10.3945/ajcn.113.080796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Therkelsen KE, Pedley A, Hoffmann U, Fox CS, Murabito JM. Intramuscular fat and physical performance at the Framingham Heart Study. Age (Dordr). 2016;38:31. doi: 10.1007/s11357-016-9893-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rittig K, Dolderer JH, Balletshofer B, et al. The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia. 2012;55:1514–1525. doi: 10.1007/s00125-012-2481-9 [DOI] [PubMed] [Google Scholar]
- 30. Chatterjee TK, Stoll LL, Denning GM, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6:27–35. doi: 10.1038/ncpcardio1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. [DOI] [PubMed] [Google Scholar]
- 33. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014(260):1–161. [PubMed] [Google Scholar]
- 34. Khan ZA, Janssen I, Mazzarelli JK, et al. Serial studies in subclinical atherosclerosis during menopausal transition (from the study of women’s health across the nation). Am J Cardiol. 2018;122:1161–1168. doi: 10.1016/j.amjcard.2018.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Samargandy S, Matthews K, Janssen I, et al. Abstract P362: central arterial stiffness increases within one year-interval of the final menstrual period in? midlife women: study of women’s health across the nation (SWAN) Heart. Circulation. 2018;137:AP362. [Google Scholar]
- 36. El Khoudary SR, Chen HY, Barinas-Mitchell E, et al. Simple physical performance measures and vascular health in late midlife women: the Study of Women’s Health across the nation. Int J Cardiol. 2015;182:115–120. doi: 10.1016/j.ijcard.2014.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tseng LA, El Khoudary SR, Young EA, et al. The association of menopause status with physical function: the Study of Women’s Health Across the Nation. Menopause. 2012;19:1186–1192. doi: 10.1097/gme.0b013e3182565740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McClure CK, El Khoudary SR, Karvonen-Gutierrez CA, et al. Prospective associations between inflammatory and hemostatic markers and physical functioning limitations in mid-life women: longitudinal results of the Study of Women’s Health Across the Nation (SWAN). Exp Gerontol. 2014;49:19–25. doi: 10.1016/j.exger.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. White DK, Neogi T, Nevitt MC, et al. Trajectories of gait speed predict mortality in well-functioning older adults: the Health, Aging and Body Composition study. J Gerontol A Biol Sci Med Sci. 2013;68:456–464. doi: 10.1093/gerona/gls197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bussey CE, Withers SB, Aldous RG, Edwards G, Heagerty AM. Obesity-related perivascular adipose tissue damage is reversed by sustained weight loss in the rat. Arterioscler Thromb Vasc Biol. 2016;36:1377–1385. doi: 10.1161/ATVBAHA.116.307210 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.