Abstract
Background
To estimate prescribing trends of and correlates independently associated with coprescribing of benzodiazepines and opioids among adults aged 65 years or older in office-based outpatient visits.
Methods
I examined a nationally representative sample of office-based physician visits by older adults between 2006 and 2015 (n = 109,149 unweighted) using data from the National Ambulatory Medical Care Surveys (NAMCS). National rates and prescribing trends were estimated. Then, I used multivariable logistic regression analyses to identify demographic and clinical factors associated with coprescriptions of benzodiazepines and opioids.
Results
From 2006 to 2015, 15,954 (14.6%) out of 109,149 visits, representative of 39.3 million visits nationally, listed benzodiazepine, opioid, or both medications prescribed. The rate of prescription benzodiazepines only increased monotonically from 4.8% in 2006–2007 to 6.2% in 2014–2015 (p < .001), and the rate of prescription opioids only increased monotonically from 5.9% in 2006–2007 to 10.0% in 2014–2015 (p < .001). The coprescribing rate of benzodiazepines and opioids increased over time from 1.1% in 2006–2007 to 2.7% in 2014–2015 (p < .001). Correlates independently associated with a higher likelihood of both benzodiazepine and opioid prescriptions included: female sex, a visit for chronic care, receipt of six or more concomitantly prescribed medications, and clinical diagnoses of anxiety and pain (p < .01 for all).
Conclusion
The coprescribing rate of benzodiazepines and opioids increased monotonically over time in outpatient care settings. Because couse of benzodiazepines and opioids is associated with medication burdens and potential harms, future research is needed to address medication safety in these vulnerable populations.
Keywords: Benzodiazepine, Opioid, Older adults, Prescribing, Outpatient care
In the past two decades, the opioid crisis has been one of the most serious public health problems in the United States (1). In 2016, about 46 people died every day from overdoses involving prescription opioids, and more than 40% of all opioid overdose deaths in the United States involved a prescription opioid (2). In addition, benzodiazepines, as one of the most frequently used coprescribed central nervous system depressants, contribute to nearly one third of all opioid overdose deaths in the United States (3–7). Coprescribing of benzodiazepines and opioids is common in diverse clinical settings (4,8), and the concomitant use of both benzodiazepines and opioids may pose even greater risks of morbidity and mortality in older adults.
The Beers criteria recommend against the use of all benzodiazepines because they can increase the risk of cognitive impairment, delirium, falls, and fractures in older adults (9). Furthermore, opioid analgesics are not recommended as they can cause central nervous system adverse effects, such as confusion and hallucinations (9). For these reasons, the concomitant use of benzodiazepines and opioids is considered potentially inappropriate prescribing in older adults (10).
There are a few pharmaco-epidemiologic studies investigating the concomitant use of benzodiazepines and opioids (4,6,7,11,12). Their findings suggest that the concomitant use of benzodiazepines and opioids was associated with the risks of overdose (7,11) and mortality (12). However, these studies focused on the Veterans Health Administration setting (12), emergency department (ED) setting (4), or nonelderly patients who are privately insured (11).
To address current gaps in the literature, I sought to address the following questions: (i) What are national rates and longitudinal trends of benzodiazepine-opioid coprescribing among older adults in office-based outpatient visits? (ii) What are demographic and clinical factors associated with coprescribing of benzodiazepines and opioids? This study is, thus, the first descriptive pharmaco-epidemiologic study investigating rates, correlates, and national trends of coprescribing of benzodiazepines and opioids among older adults in office-based outpatient settings.
Methods
Data Source and Study Sample
I used data from the 2006–2015 National Ambulatory Medical Care Survey (NAMCS), an annual cross-sectional survey of office-based physician visits. The NAMCS nationally represents ambulatory medical care services, including prescription trends (13). I limited the sample to all visits by adults aged 65 years or older (n = 109,149 unweighted). Using publicly available deidentified data, this study was exempted from the Institutional Review Board (#2000021850) at Yale School of Medicine. Further details of the survey, including descriptions, questionnaires, sampling methodology and data sets, are publicly available on the NAMCS website (13).
Measures
Medications list
In NAMCS, up to eight medications prescribed in a randomly selected visit are recorded between 2006 and 2011. The number of prescription medications documented increased up to 10 in 2012 and 2013, and then up to 30 in 2014 and 2015. I examined all medications listed as prescribed in each visit. I included 13 benzodiazepine medications using their generic names (14): alprazolam; chloridiazepoxide (hydrochloride); clonazepam; clorazepate dipotassium; diazepam; estazolam; flurazepam hydrochloride; lorazepam; midazolam hydrochloride; oxazepam; quazepam; temazepam; and triazolam. For opioids, I only considered opiate agonists because opiate partial agonists (eg, buprenorphine) and opiate antagonists (eg, naloxone hydrochloride and naltrexone) are used to treat opiate use disorders (15,16). The following opiate agonists were included: alfentanil; butorphanol; codeine phosphate or codeine sulfate; dihydrocodeine; fentanyl, fentanyl citrate, or fentanyl hydrochloride; hydrocodone bitartrate; hydromorphone hydrochloride; levorphanol tartrate; meperidine hydrochloride; methadone hydrochloride; morphine sulfate; nalbuphine; opium; oxycodone, oxycodone hydrochloride, or oxycodone myrist; oxymorphone hydrochloride; pentazocine; propoxyphene; remifentanil hydrochloride; sufentanil citrate; tapentadol hydrochloride; tramadol hydrochloride; and their combined products (eg, droperidol-fentanyl). Based on these medications, I constructed an indicator variable for benzodiazepines only, opioids only, or both.
Covariates
Similar to previous pharmaco-epidemiologic studies using NAMCS, I selected covariates based on the potential for clinically relevant confounding (17–21). Demographic variables included: age; gender; race/ethnicity; primary source of payment (Private, Medicare, Medicaid, or other); and metropolitan statistical area (%) (22). Clinical characteristics included: physician specialty (primary care, psychiatry, or other); reason for visit (acute problem, routine chronic problem, preventive care, or surgical care); number of repeated visits within the past 12 months; and time spent with a doctor (in minutes). I also included number of chronic conditions (eg, arthritis, congestive heart failure, and diabetes), and number of concomitant medications prescribed as they are provided by the NAMCS.
In addition, I included visit diagnoses relevant to benzodiazepines or opioids. The NAMCS provides up to three clinical diagnoses at each visit using the International Classification of Diseases, 9th edition, Clinical Modification (ICD-9-CM) diagnostic codes. Using ICD-9-CM diagnostic codes, I constructed five diagnosis variables relevant to benzodiazepines or opioids: (i) cancer-related pain (140–239, 338.3X); (ii) pain other than cancer (338.XX, 350.1X-350.2X, 354.4X, 355.71, 379.91. 388.7X, 719.4X, 724.1X-724.2X, 729.1X, 780.96, 786.5X, 789.XX); (iii) anxiety disorder, including panic disorder (300.00–300.02, 300.09); (iv) insomnia (327.00–327.02, 327.09, 780.51); and (v) seizure (345.XX, 780.31–780.33, 780.39).
Data Analysis
I estimated if demographic and clinical characteristics differed by prescription status (no prescription, benzodiazepines only, opioids only, or both). For each characteristic, I used cross-tabulations and weight-corrected Pearson’s chi-squared statistics (ie, design-based F-tests) to investigate the differences in each prescription group. Using these raw p-values, I adjusted for and reported p-values using the false discovery rate (FDR) method to perform multiple comparisons across different prescription groups (23). Second, I assessed overall trends of benzodiazepine prescriptions, opioid prescriptions, and both prescriptions, and compared prescription rate changes between 2006 and 2007 and 2014 and 2015. Finally, I performed multivariable-adjusted logistic regression analyses to identify demographic and clinical correlates independently associated with a prescription of benzodiazepines, opioids, and both. I conducted all analysis using Stata MP/6-Core version 15.1 (College Station, TX) (24). I used svy commands to account for the survey sample design (eg, unequal probability of selection, clustering, and stratification). For multiple comparison tests, I used the Proc MULTTEST procedure in SAS 9.4 (Cary, NC) (25).
Results
Selected Characteristics of the Sample
Between 2006 and 2015, 15,954 (14.6%) out of 109,149 visits by older adults, representative of 39.3 million visits nationally, had benzodiazepine, opioid, or both medications prescribed in outpatient settings (Table 1). Patient visits in which these medications prescribed were more likely to be made by younger older adults (65–74), women, non-Hispanic whites, Medicare beneficiaries, and those living in metropolitan areas. Furthermore, the majority of patient visits, in which these medications prescribed, listed a routine chronic problem as the main reason for visit, had two or more chronic conditions. More than 50% of visits in which benzodiazepines, opioids, or both medications prescribed had six or more medications prescribed at that visit, meeting the definition of a polypharmacy. This proportion was significantly higher than for visits where neither benzodiazepines nor opioids were prescribed (p < .001).
Table 1.
Selected Characteristics (weighted column %) of Older Adults by Benzodiazepine and Opioid Prescription Status in Office-Based Outpatient Settings, 2006–2015 NAMCS
| Total | No Prescription | p Value | Benzodiazepines (BZDs) Only | p Value | Opioids Only | p Value | Both BZDs and Opioids | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Sample size | |||||||||
| Unweighted sample | 109,149 | 93,195 | 5,740 | 8,295 | 1,919 | ||||
| Weighted visits | 262,142,969 | 222,799,164 | 14,271,289 | 20,658,478 | 4,414,038 | ||||
| Age | |||||||||
| 65–74 | 50.9 | 51.0 | .604 | 46.9 | .007 | 52.6 | .358 | 54.4 | .322 |
| 75–84 | 35.8 | 35.8 | 38.3 | 34.4 | 31.5 | ||||
| 85+ | 13.3 | 13.2 | 14.8 | 13.0 | 14.1 | ||||
| Gender | |||||||||
| Female | 57.0 | 55.8 | <.001 | 67.5 | <.001 | 61.4 | <.001 | 65.6 | <.001 |
| Male | 43.0 | 44.2 | 32.5 | 38.6 | 34.4 | ||||
| Race/ethnicity | |||||||||
| Non-Hispanic White | 79.0 | 78.9 | .300 | 81.8 | .006 | 77.3 | .118 | 83.7 | .011 |
| Non-Hispanic Black | 7.9 | 7.9 | 5.8 | 9.6 | 6.7 | ||||
| Hispanic | 8.4 | 8.4 | 9.7 | 7.8 | 7.6 | ||||
| Othera | 4.7 | 4.8 | 2.7 | 5.4 | 2.0 | ||||
| Primary source of payment | |||||||||
| Private | 15.3 | 15.6 | <.001 | 11.7 | <.001 | 13.7 | .112 | 14.9 | .709 |
| Medicare | 77.0 | 76.7 | 79.8 | 77.9 | 77.3 | ||||
| Medicaid | 2.5 | 2.5 | 2.5 | 2.5 | 2.0 | ||||
| Otherb | 2.1 | 2.0 | 2.6 | 2.0 | 1.9 | ||||
| Undocumented | 3.2 | 3.2 | 3.4 | 3.9 | 3.9 | ||||
| Metropolitan Statistical Area (%) | 88.5 | 88.6 | .420 | 87.2 | .420 | 87.9 | .423 | 87.0 | .423 |
| Physician specialty | |||||||||
| Primary care | 39.9 | 37.9 | <.001 | 51.4 | <.001 | 51.5 | <.001 | 54.0 | <.001 |
| Psychiatry | 1.3 | 1.0 | 7.7 | 0.2 | 0.7 | ||||
| Otherc | 58.7 | 61.1 | 40.9 | 48.3 | 45.3 | ||||
| Reason for visit | |||||||||
| Acute problem | 25.1 | 25.1 | <.001 | 25.0 | <.001 | 26.1 | <.001 | 19.8 | <.001 |
| Routine chronic problem | 52.3 | 51.6 | 57.7 | 55.1 | 58.5 | ||||
| Preventive care | 10.4 | 10.8 | 8.5 | 7.6 | 7.1 | ||||
| Pre- or postsurgery | 7.1 | 7.3 | 3.8 | 7.2 | 5.3 | ||||
| Undocumented | 5.2 | 5.2 | 5.0 | 4.0 | 9.4 | ||||
| Repeat of visits in the past 12 mo | |||||||||
| 0 visit | 5.4 | 5.7 | <.001 | 4.2 | <.001 | 3.0 | <.001 | 4.8 | <.001 |
| 1–2 visits | 30.8 | 31.8 | 26.4 | 24.9 | 23.2 | ||||
| 3–5 visits | 29.8 | 29.6 | 32.4 | 30.5 | 27.0 | ||||
| 6+ visits | 22.6 | 21.2 | 28.9 | 31.2 | 33.3 | ||||
| Undocumented | 11.4 | 11.7 | 8.2 | 10.5 | 11.8 | ||||
| Time spent with doctor | |||||||||
| < 15 min | 18.1 | 18.7 | <.001 | 14.6 | <.001 | 14.8 | <.001 | 15.6 | .055 |
| 15–20 min | 48.7 | 48.6 | 48.7 | 50.7 | 46.4 | ||||
| 21–30 min | 21.4 | 21.1 | 22.6 | 23.8 | 25.5 | ||||
| > 30 min | 11.7 | 11.6 | 14.1 | 10.7 | 12.5 | ||||
| ≥2 chronic conditions (%)d | 59.3 | 57.5 | <.001 | 68.4 | <.001 | 68.6 | <.001 | 74.0 | <.001 |
| ≥6 medications (%) | 31.4 | 26.5 | <.001 | 63.6 | <.001 | 59.7 | <.001 | 73.3 | <.001 |
| Visit diagnosis | |||||||||
| Cancer-related pain (%) | 8.1 | 8.2 | .453 | 6.3 | .010 | 8.3 | .986 | 8.1 | .986 |
| Pain other than cancer (%) | 4.0 | 3.3 | <.001 | 3.8 | .134 | 10.6 | <.001 | 11.4 | <.001 |
| Anxiety disorder (%) | 1.3 | 0.6 | <.001 | 11.4 | <.001 | 0.5 | .488 | 5.9 | <.001 |
| Insomnia (%) | 0.6 | 0.5 | <.001 | 2.3 | <.001 | 0.5 | .996 | 1.1 | .034 |
| Seizure (%) | 0.3 | 0.3 | .930 | 0.5 | .183 | 0.1 | .026 | 0.2 | .868 |
Note: aincludes Asians, American Indian/Alaska Natives (AIANs), Native Hawaiian or Other Pacific Islanders (NHOPI), or 2+ reported racial/ethnic groups; bincludes worker’s compensation, self-pay, no charge, or others; cincludes obstetrics/gynecology, cardiovascular diseases, dermatology, urology, neurology, ophthalmology, otolaryngology, and others; and dwas based on 14 chronic conditions (yes/no) collected by the NAMCS (eg, arthritis, congestive heart failure, and diabetes).
National Trends of Benzodiazepine and Opioid Prescriptions
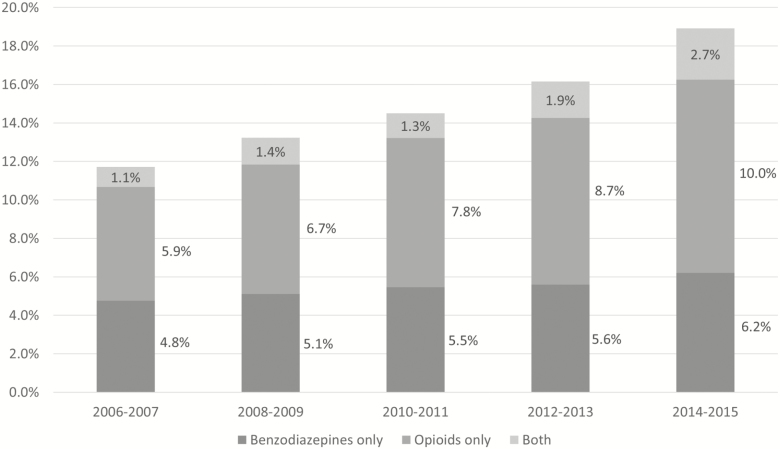
Figure 1 shows national trends of benzodiazepine and opioid prescriptions from 2006 to 2015. The overall rates increased from 11.7% of visits in 2006–2007 to 18.9% of visits in 2014–2015 (p < .001). In prescription benzodiazepines only, the prescribing rate increased monotonically from 4.8% of visits in 2006–2007 to 6.2% of visits in 2014–2015 (p < .001) among older adults. Similarly, the rate of prescription opioids only increased monotonically from 5.9% of visits in 2006–2007 to 10.0% of visits in 2014–2015 (p < .001). The coprescribing of benzodiazepines and opioids also increased monotonically from 1.1% of visits in 2006–2007 to 2.7% of visits in 2014–2015 (p < .001).
Figure 1.
National trends of prescription benzodiazepines and opioids among older adults in office-based outpatient settings, 2006–2015 National Ambulatory Medical Care Surveys (NAMCS).
Multivariable Logistic Regression Analysis
Table 2 presents the results of multivariable-adjusted logistic regression analyses, which estimated the odds of receiving a benzodiazepine, an opioid, or both medications, respectively. For a benzodiazepine prescription only, being female and having Medicare as a primary source of payment was associated with a greater likelihood of receiving benzodiazepines when compared to older male adults and those covered by a private insurance plan, respectively (p < .05). When compared to non-Hispanic whites, both non-Hispanic blacks and other minority groups were associated with a lower likelihood of receiving benzodiazepines (p < .01). Turning to clinical factors, visits to psychiatrists were more likely to receive benzodiazepines (adjusted odds ratio [AOR] = 8.43; 95% confidence interval [CI] = 6.32, 11.25), whereas visits to other specialists were less likely to receive benzodiazepines (AOR = 0.71; 95% CI = 0.63, 0.80). Older adults taking six or more medications prescribed were more likely to receive benzodiazepines (AOR = 4.83; 95% CI = 4.25, 5.48). Visits in which anxiety disorders or insomnia were diagnosed had at least two times more likely to receive benzodiazepines (p < .001).
Table 2.
Adjusted Odds Ratios (AOR) of Receiving Benzodiazepine and Opioid Prescriptions Among Older Adults in Office-Based Outpatient Settings, 2006–2015 NAMCS
| Benzodiazepines (BZDs) Only | Opioids Only | Both BZDs and Opioids | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (Reference group in a parenthesis) | AOR | 95% CI | p Value | AOR | 95% CI | p Value | AOR | 95% CI | p Value |
| Age (65–74) | |||||||||
| 75–84 | 1.15 | 1.03, 1.29 | .017 | 0.84 | 0.75, 0.93 | .001 | 0.77 | 0.65, 0.92 | .003 |
| 85+ | 1.04 | 0.90, 1.21 | .601 | 0.79 | 0.70, 0.89 | <.001 | 0.70 | 0.53, 0.94 | .016 |
| Gender (Male) | |||||||||
| Female | 1.53 | 1.40, 1.67 | <.001 | 1.22 | 1.12, 1.33 | <.001 | 1.45 | 1.19, 1.77 | <.001 |
| Race/ethnicity (Non-Hispanic White) | |||||||||
| Non-Hispanic Black | 0.67 | 0.55, 0.82 | <.001 | 1.17 | 1.01, 1.37 | .040 | 0.80 | 0.54, 1.18 | .262 |
| Hispanic | 1.14 | 0.91, 1.42 | .269 | 0.97 | 0.81, 1.15 | .701 | 0.79 | 0.56, 1.10 | .166 |
| Othera | 0.58 | 0.40, 0.84 | .004 | 0.93 | 0.70, 1.24 | .623 | 0.37 | 0.18, 0.79 | .010 |
| Primary source of payment (Private) | |||||||||
| Medicare | 1.25 | 1.08, 1.44 | .003 | 1.08 | 0.96, 1.22 | .205 | 1.05 | 0.79, 1.39 | .759 |
| Medicaid | 1.35 | 0.97, 1.89 | .074 | 1.15 | 0.89, 1.49 | .289 | 1.17 | 0.64, 2.15 | .611 |
| Otherb | 0.99 | 0.70, 1.40 | .963 | 1.54 | 1.19, 2.00 | .001 | 1.57 | 0.85, 2.84 | .148 |
| Metropolitan Statistical Area (No) | 0.97 | 0.82, 1.14 | .677 | 1.03 | 0.84, 1.27 | .783 | 0.91 | 0.68, 1.22 | .531 |
| Physician specialty (Primary care) | |||||||||
| Psychiatry | 8.43 | 6.32, 11.25 | <.001 | 0.14 | 0.08, 0.23 | <.001 | 0.56 | 0.22, 1.42 | .222 |
| Otherc | 0.71 | 0.63, 0.80 | <.001 | 0.74 | 0.65, 0.83 | <.001 | 0.74 | 0.60, 0.91 | .005 |
| Reason for visit (Acute problem) | |||||||||
| Routine chronic problem | 0.96 | 0.85, 1.09 | .559 | 0.96 | 0.87, 1.06 | .401 | 1.41 | 1.15, 1.71 | .001 |
| Preventive care | 0.83 | 0.70, 0.97 | .023 | 0.73 | 0.62, 0.85 | <.001 | 0.94 | 0.68, 1.31 | .715 |
| Pre- or postsurgery | 0.73 | 0.57, 0.94 | .015 | 1.29 | 1.05, 1.58 | .014 | 1.42 | 1.02, 1.98 | .038 |
| Repeat of visits in the past 12 mo (Never) | |||||||||
| 1–2 visits | 0.94 | 0.75, 1.19 | .624 | 1.31 | 1.06, 1.61 | .011 | 0.59 | 0.41, 0.84 | .003 |
| 3–5 visits | 1.02 | 0.81, 1.29 | .837 | 1.52 | 1.23, 1.87 | <.001 | 0.69 | 0.48, 1.00 | .053 |
| 6+ visits | 0.99 | 0.79, 1.25 | .949 | 2.03 | 1.63, 2.52 | <.001 | 1.03 | 0.71, 1.50 | .871 |
| Time spent with a doctor (<15 min) | |||||||||
| 15–20 min | 1.06 | 0.93, 1.22 | .378 | 1.17 | 1.03, 1.32 | .013 | 0.93 | 0.73, 1.19 | .569 |
| 21–30 min | 1.05 | 0.89, 1.24 | .580 | 1.18 | 1.01, 1.38 | .035 | 1.03 | 0.75, 1.42 | .842 |
| > 30 min | 1.10 | 0.90, 1.34 | .345 | 1.01 | 0.86, 1.19 | .876 | 1.07 | 0.76, 1.51 | .696 |
| ≥2 chronic conditionsd | 1.00 | 0.89, 1.11 | .934 | 0.94 | 0.85, 1.04 | .204 | 0.94 | 0.79, 1.12 | .483 |
| ≥6 medications | 4.83 | 4.25, 5.48 | <.001 | 3.53 | 3.20, 3.91 | <.001 | 7.25 | 5.62, 9.36 | <.001 |
| Visit diagnosis | |||||||||
| Cancer-related pain | 1.08 | 0.90, 1.30 | .392 | 1.26 | 1.10, 1.43 | .001 | 1.45 | 1.10, 1.92 | .009 |
| Pain other than cancer | 0.91 | 0.72, 1.16 | .460 | 3.33 | 2.86, 3.89 | <.001 | 3.81 | 2.84, 5.11 | <.001 |
| Anxiety disorder | 12.90 | 9.72, 17.13 | <.001 | 0.39 | 0.22, 0.68 | .001 | 6.36 | 3.82, 10.58 | <.001 |
| Insomnia | 2.96 | 1.77, 4.94 | <.001 | 0.72 | 0.43, 1.21 | .220 | 1.47 | 0.68, 3.19 | .332 |
| Seizure | 1.53 | 0.80, 2.91 | .199 | 0.44 | 0.25, 0.79 | .005 | 0.2 | 0.06, 0.68 | .011 |
Note: aincludes Asians, American Indian/Alaska Natives (AIANs), Native Hawaiian or Other Pacific Islanders (NHOPI), or 2+ reported racial/ethnic groups; bincludes worker’s compensation, self-pay, no charge, or others; cincludes obstetrics/gynecology, cardiovascular diseases, dermatology, urology, neurology, ophthalmology, otolaryngology, and others; and dwas based on 14 chronic conditions (yes/no) collected by the NAMCS (eg, arthritis, congestive heart failure, and diabetes).
For opioid prescriptions only, being older than those aged between 65 and 74 was less likely to receive opioids (p < .001). Being female and having the other type of insurance as a primary source of payment were more likely to receive opioids (p < .01). Turning to clinical factors, visits to psychiatrists and other specialists were less likely to involve with opioid prescriptions when compared to primary care visits (p < .001). Compared to those with acute problems, surgery-related visits were more likely to receive opioid prescriptions (AOR = 1.29; 95% CI = 1.05, 1.58). Those who had at least six visits in the past year were more likely to receive opioids than those who had not visited at all (AOR = 2.03; 95% CI = 1.63, 2.52). Older adults taking six or more medications prescribed were more likely to receive opioids (AOR = 3.53; 95% CI = 3.20, 3.91). Visits in which cancer-related pain and pain other than cancer diagnosed were more likely to receive opioids (p < .001).
In coprescriptions of both benzodiazepines and opioids, patterns were similar to that of the opioid prescriptions only, with two exceptions. In reason for visit, it was routine chronic problem, not surgery-related visits, which was associated with a greater likelihood of having coprescriptions (AOR = 1.41; 95% CI = 1.15, 1.71). Second, having a diagnosis of anxiety disorder, along with all other pain diagnoses, was associated with a greater likelihood of coprescriptions (AOR = 6.36; 95% CI = 3.82, 10.58).
Discussion
This study investigated national rates, trends, and correlates of coprescribing of benzodiazepines and opioids among older adults in a nationally representative sample of office-based outpatient visits between 2006 and 2015. Prescribing rates of individual drug classes and the coprescribing rate increased monotonically over time. The coprescribing rate is lower than those of previous studies (3,4,11,12), which reported prevalence rates of 2.7% in ED settings (4), 9% among privately insured adults (11), and 27% among male veterans (12). The finding cannot be compared directly to these studies due in part to different populations of interest, settings, data sources (eg, survey-based vs claims-based), and inclusion criteria of medications.
Among demographic factors, while being older was associated with a lower likelihood of coprescribing, being female was associated with a higher likelihood of coprescribing. Among clinical factors, chronic care visits were associated with a higher likelihood of coprescribing. Furthermore, visits in which polypharmacy documented, and anxiety and pain diagnosed were also associated with a higher likelihood of coprescribing of benzodiazepines and opioids. These patterns are similar to previous studies (3,6,26), but direct comparisons could not be made as this study focuses primarily on older adults in office-based outpatient settings.
There are several implications from this study. First, because individual benzodiazepine and opioid classes of medication are increasingly prescribed over time, further pharmaco-vigilance studies are needed to track the coprescribing trends and adverse drug events among older adults. Further, as the Food and Drug Administration issued its warnings on couse of benzodiazepines and opioids in 2016 (27), physicians and other healthcare providers (eg, nurse practitioners and physician assistants) should be more cautious about the use of these medications concomitantly. Evidence-based interventions (eg, academic detailing and computerized prescribing alerts) (28–31) may also be effective in reducing the couse of these medications since these interventions allow healthcare providers to prescribe safer pharmacological alternatives (eg, nonopioid analgesics for pain management).
Second, among visits in which the couse of benzodiazepines and opioids was documented, older adults often take more than six medications concomitantly. While such polypharmacy can be justified by multimorbidities, it may still carry potential harms due to medication burdens in older adults. Thus, older adults with polypharmacy, who are exposed to the couse of benzodiazepines and opioids, may be particularly vulnerable, and therefore, future research is needed to address their quality of care and quality of life.
Several methodological limitations deserve a comment. First, the NAMCS does not capture outpatient visits from hospital-affiliated clinics or ED visits, which account for about 8.5% of all outpatient visits (19). Furthermore, it excludes any prescriptions ordered by phone. Second, NAMCS selected information in a randomly selected visit, and thus, incomplete patient information is inevitable. These factors may underestimate the magnitude of current findings in this study.
Strengths of this study are generalizability using nationally representative data, as NAMCS represents prescribing patterns in office-based care at the national level. Findings from this study lay the foundation for future research to improve better prescribing practices among older adults taking either benzodiazepines or opioids, or both.
Author Contributions
Study concept and design: T.G.R.; Data acquisition and statistical analyses: T.G.R.; Interpretation of data: T.G.R.; Drafting of manuscript: T.G.R.; Critical revision of manuscript for important intellectual content: T.G.R.
Data Access and Responsibility
T.G.R. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
T.G.R. received funding support from the National Institutes of Health (NIH) (#T32AG019134). The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Conflict of Interest
T.G.R. completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and none were reported.
References
- 1. Centers for Disease Control and Prevention. Drug Overdose Death Data 2017; https://www.cdc.gov/drugoverdose/data/statedeaths.html Accessed July 3, 2018.
- 2. Centers for Disease Control and Prevention. Prescription Opioid Data 2017; https://www.cdc.gov/drugoverdose/data/prescribing.html Accessed September 19, 2018.
- 3. Hwang CS, Kang EM, Kornegay CJ, Staffa JA, Jones CM, McAninch JK. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002–2014. Am J Prev Med. 2016;51:151–160. doi: 10.1016/j/amepre.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 4. Kim HS, McCarthy DM, Mark Courtney D, Lank PM, Lambert BL. Benzodiazepine-opioid co-prescribing in a national probability sample of ED encounters. Am J Emerg Med. 2017;35:458–464. doi: 10.1016/j.ajem.2016.11.054 [DOI] [PubMed] [Google Scholar]
- 5. Jann M, Kennedy WK, Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract. 2014;27:5–16. doi: 10.1177/0897190013515001 [DOI] [PubMed] [Google Scholar]
- 6. Ladapo JA, Larochelle MR, Chen A, et al. Physician prescribing of opioids to patients at increased risk of overdose from benzodiazepine use in the United States. JAMA Psychiatry. 2018;75:623–630. doi: 10.1001/jamapsychiatry.2018.0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hernandez I, He M, Brooks MM, Zhang Y. Exposure-response association between concurrent opioid and benzodiazepine use and risk of opioid-related overdose in medicare part D beneficiaries. JAMA Network Open. 2018;1:e180919. doi: 10.1001/jamanetworkopen.2018.0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones JD, Mogali S, Comer SD. Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 2012;125:8–18. doi: 10.1016/j.drugalcdep.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The American Geriatrics Society Beers Criteria Update Expert Panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227–2246. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 10. Rose AJ, Bernson D, Chui KKH, et al. Potentially inappropriate opioid prescribing, overdose, and mortality in Massachusetts, 2011–2015. J Gen Intern Med. 2018;33:1512–1519. doi: 10.1007/s11606-018-4532-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760. doi: 10.1136/bmj.j760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi: 10.1136/bmj.h2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Center for Health Statistics. Ambulatory Health Care Data: Questionnaires, Datasets, and Related Documentation 2017; https://www.cdc.gov/nchs/ahcd/ahcd_questionnaires.htm Accessed June 28, 2018.
- 14. Rhee TG, Rosenheck RA. Initiation of new psychotropic prescriptions without a psychiatric diagnosis among US adults: rates, correlates, and national trends from 2006 to 2015. Health Serv Res. 2018. doi: 10.1111/1475-6773.13072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375:1596–1597. doi: 10.1056/NEJMc1610830 [DOI] [PubMed] [Google Scholar]
- 16. Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23:63–75. doi: 10.1097/HRP.0000000000000075 [DOI] [PubMed] [Google Scholar]
- 17. Rhee TG, Capistrant BD, Schommer JC, Hadsall RS, Uden DL. Effects of the 2009 USPSTF depression screening recommendation on diagnosing and treating mental health conditions in older adults: a difference-in-differences analysis. J Manag Care Spec Pharm. 2018;24:769–776. doi: 10.18553/jmcp.2018.24.8.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rhee TG, Choi YC, Ouellet GM, Ross JS. National prescribing trends for high-risk anticholinergic medications in older adults. J Am Geriatr Soc. 2018;66:1382–1387. doi: 10.1111/jgs.15357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rhee TG, Mohamed S, Rosenheck RA. Antipsychotic prescriptions among adults with major depressive disorder in office-based outpatient settings: national trends from 2006 to 2015. J Clin Psychiatry. 2018;79:17m11970. doi: 10.4088/JCP.17m11970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rhee TG, Schommer JC, Capistrant BD, Hadsall RL, Uden DL. Potentially inappropriate antidepressant prescriptions among older adults in office-based outpatient settings: national trends from 2002 to 2012. Adm Policy Ment Health. 2018;45:224–235. doi: 10.1007/s10488-017-0817-y [DOI] [PubMed] [Google Scholar]
- 21. Rhee TG, Capistrant BD, Schommer JC, Hadsall RS, Uden DL. Effects of depression screening on diagnosing and treating mood disorders among older adults in office-based primary care outpatient settings: an instrumental variable analysis. Prev Med. 2017;100:101–111. doi: 10.1016/j.ypmed.2017.04.015 [DOI] [PubMed] [Google Scholar]
- 22. United States Census Bureau. Metropolitan and Micropolitan 2018; https://www.census.gov/programs-surveys/metro-micro/about.html Accessed September 18, 2018.
- 23. Van Ness PH, Charpentier PA, Ip EH, et al. Gerontologic biostatistics: the statistical challenges of clinical research with older study participants. J Am Geriatr Soc. 2010;58:1386–1392. doi: 10.1111/j.1532-5415.2010.02926.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stata Statistical Software. Release 15 [computer program]. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 25. SAS 9.4 Product Documentation [computer program]. Cary, NC: SAS Institute Inc.; 2018. [Google Scholar]
- 26. Hirschtritt ME, Delucchi KL, Olfson M. Outpatient, combined use of opioid and benzodiazepine medications in the United States, 1993–2014. Prev Med Rep. 2018;9:49–54. doi: 10.1016/j.pmedr.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use 2016; https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Accessed December 28, 2018.
- 28. Cochella S, Bateman K. Provider detailing: an intervention to decrease prescription opioid deaths in Utah. Pain Med. 2011;12(Suppl 2):S73–S76. doi: 10.1111/j.1526-4637.2011.01125.x [DOI] [PubMed] [Google Scholar]
- 29. Simon SR, Smith DH, Feldstein AC, et al. Computerized prescribing alerts and group academic detailing to reduce the use of potentially inappropriate medications in older people. J Am Geriatr Soc. 2006;54:963–968. doi: 10.1111/j.1532-5415.2006.00734.x [DOI] [PubMed] [Google Scholar]
- 30. Raebel MA, Charles J, Dugan J, et al. Randomized trial to improve prescribing safety in ambulatory elderly patients. J Am Geriatr Soc. 2007;55:977–985. doi: 10.1111/j.1532-5415.2007.01202.x [DOI] [PubMed] [Google Scholar]
- 31. Terrell KM, Perkins AJ, Dexter PR, Hui SL, Callahan CM, Miller DK. Computerized decision support to reduce potentially inappropriate prescribing to older emergency department patients: a randomized, controlled trial. J Am Geriatr Soc. 2009;57:1388–1394. doi: 10.1111/j.1532-5415.2009.02352.x [DOI] [PubMed] [Google Scholar]