Abstract
Testicular adrenal rest tumors (TARTs) are a common cause of male infertility in patients with classic congenital adrenal hyperplasia (CAH). These tumors are located in the rete testis and can lead to impaired blood flow and functional impairment of seminiferous tubules. We describe restoration of fertility in a man with CAH and bilateral TARTs with use of lower-dose glucocorticoid therapy than previously described. A 28-year-old man with classic salt-wasting CAH presented with impaired fertility. Biochemical evaluation showed poor CAH control despite reported compliance with prednisone 5 mg every morning and fludrocortisone 50 μg twice daily. Semen analysis showed azoospermia. Testicular ultrasonography showed TARTs occupying 16% of total testicular volume. After 5 months of dexamethasone 250 μg at bedtime, total TART volume decreased 90%, biochemical control improved, and semen analysis showed a sperm count of 132 × 106 million per milliliter. The patient’s wife was confirmed to be pregnant 9 months after the initial visit and delivered a healthy full-term baby girl. The patient’s glucocorticoid therapy was changed to prednisone 3 mg twice daily, and 2 years later he continues to show adequate CAH control, stable TART volume, and normal semen analysis, and his wife is pregnant again. Management of CAH in men with TARTs needs to be individualized, and high-dose dexamethasone may not be indicated. The use of a long-acting glucocorticoid at typical recommended dosages can decrease TART size and reverse male infertility. Prednisone given once daily does not adequately control the ACTH-driven complications of CAH.
Reduced fecundity is common in men with classic congenital adrenal hyperplasia (CAH) [1]. Pathological semen analysis was reported in all men with CAH in a cross-sectional study in Germany [1]. Common causes of infertility include the presence of testicular adrenal rest tumor (TART) and hypogonadotropic hypogonadism due to excess adrenal sex steroid production. Semen analysis, although imperfect, is the cornerstone for evaluating male infertility. The World Health Organization reference values for human semen characteristics suggest that sperm characteristics lower than reference limits identify men who may need infertility treatment [2]. Clinically, sperm concentration, poor sperm motility, and abnormal sperm morphology are considered the most important parameters.
The prevalence of TARTs among men with classic CAH is estimated to be ∼40% (range 14% to 89%) [3]. TARTs are thought to originate from adrenocortical primordial cells displaced and nested within the gonadal primordium and descend with the gonads during embryonic development. Morphologically and functionally, these tumors are similar to adrenal cortical tissue [4]. TARTs are typically located bilaterally near the mediastinum (rete) testes and result in mechanical obstruction of the seminiferous tubules and blood flow. Biopsies of residual testicular tissue in patients with long-standing TARTs show a decrease in tubular diameter, peritubular fibrosis, and tubular hyalinization, indicative of severe testicular damage [5]. In addition, excess adrenal androgen production in men with classic CAH can lead to hypogonadotropic hypogonadism by negative feedback.
Previous reports have described successful induction of fertility with high doses of dexamethasone of 0.75 to 1 mg daily [6, 7]. We describe successful induction of fertility with low-dose dexamethasone and subsequent twice-daily prednisone at typical recommended daily dosages, underscoring the significance of individualizing the treatment plan.
1. Case Description
A 28-year-old man with classic salt-wasting CAH due to 21-hydroxylase deficiency presented with subfertility. For the past 6 months, he described a minimal coital frequency of 2 or 3 times per week without the use of contraception. He had been receiving prednisone 5 mg each morning and fludrocortisone 50 μg twice daily over the last decade. He had known bilateral TARTs, first noted at age 18 and occupying 1.2% of his total testicular volume (0.38 mL of 30.68 mL) (Fig. 1). A recent testicular ultrasound showed increased TART volume, now occupying 16% of his total testicular volume (5.01 mL of 32.30 mL testicular volume), and semen analysis showed azoospermia. On examination, the patient was short-statured, with a standing height of 153.4 cm (z score: −3.1); midparental height was 174.1 cm (z score: −0.4). Testicular volume was 25 mL bilaterally. Biochemical evaluation at 0800 before medication showed 17-hydroxyprogesterone 13,060 ng/dL (reference: 13 to 120 ng/dL), androstenedione (A4) 1025 ng/dL (reference: 26 to 125 ng/dL), ACTH 866 pg/mL (reference: 5 to 46 pg/mL), plasma renin activity (PRA) 7.1 ng/mL/h (reference: 0.6 to 4.3 ng/mL/h), FSH 1.7 U/L (reference: 1 to 11 U/L), LH 1.3 U/L (reference: 1 to 8 U/L), and total testosterone (T) 473 ng/ dL (reference: 240 to 950 ng/dL). His A4/T ratio of 2.2 (>0.5) with low LH and FSH was suggestive of a mostly adrenal origin of his T. His glucocorticoid therapy was switched from prednisone 5 mg daily to dexamethasone 250 μg at bedtime to suppress the nocturnal ACTH surge.
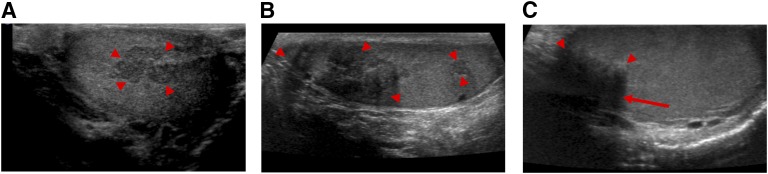
Figure 1.
Testicular ultrasonography. (A) Longitudinal sonographic image of right testis at age 18 demonstrates a heterogeneous hypoechoic lesion along the mediastinum testis consistent with an adrenal rest tumor (arrowheads). (B) Longitudinal sonographic image of right testis 10 y later demonstrates multiple heterogeneous, hypoechoic lesions along the mediastinum testis with overall increased number and volume (arrowheads). (C) Longitudinal sonographic image of right testis at 5-mo follow-up after dexamethasone initiation demonstrates reduced size of the TART (arrowheads) with new posterior shadowing (arrow).
At his 5-month follow-up visit, he reported a weight gain of 5 kg and insomnia. Physical examination revealed supraclavicular fullness with predominantly central weight gain and the presence of abdominal striae, not present on previous exam. Testicular volume was stable at 25 mL. Repeat ultrasound showed 91% decrease in total TART volume (0.47 mL) (Fig. 1). Follow-up semen analysis showed a sperm count of 132 × 106/mL (reference range: >14 × 106/mL), with 7 × 106/mL being morphologically normal (reference range: >3 × 106/mL) and 35% motile (normal >39%). Biochemical evaluation showed significant improvement in CAH control, with 0800 evaluation before medication revealing 17-hydroxyprogesterone 66 ng/dL, A4 16 ng/dL, ACTH 13.5 pg/mL, PRA 11 ng/mL/h, FSH 5.4 U/L, LH 1 U/L, and T 287 ng/dL (Table 1). The patient’s wife was confirmed to be pregnant 9 months after the initiation of dexamethasone and delivered a healthy full-term baby girl. CYP21A2 genotyping was performed. Our patient had a 30-kb deletion (CH-1 chimera subtype) in one allele and IVS2-13A/C>G (In2G) mutation in the second allele; his wife was negative for any known CYP21A2 variants, and his daughter was found to carry a 30-kb deletion (CH-1 chimera subtype) in one allele. Given his weight gain and insomnia, the patient’s therapy was switched to prednisone 3 mg twice daily. He lost 3 kg, and his insomnia resolved. His TART volume, parameters of semen analysis, and biochemical markers of CAH control have remained stable 2 and a half years hence, and he is currently expecting his second child.
Table 1.
Trends in Biochemical, Sperm, and Sonographic Characteristics in a 28-Y-Old Man With Classic Salt-Wasting 21-Hydroxylase Deficiency and Testicular Adrenal Test Tumors
| Patient Variable | Baseline | Follow-Up at 5 Mo | Follow-Up at 24 Mo |
|---|---|---|---|
| Medication | Prednisone 5 mg daily | Dexamethasone 250 μg daily | Prednisone 3/3 mg twice daily |
| Fludrocortisone 50/50 μg twice daily | Fludrocortisone 50/50 μg twice daily | Fludrocortisone 100/50 μg twice daily | |
| 17-hydroxyprogesterone (13–120 ng/dL) | 13,060 | 66 | 160 |
| A4 (26–125 ng/dL) | 1025 | 16 | 30 |
| ACTH (5–46 pg/mL) | 866 | 13.5 | 45.1 |
| PRA (0.6–4.3 ng/mL/h) | 7.1 | 11 | 3.5 |
| FSH (1–11 U/L) | 1.7 | 5.4 | 4.3 |
| LH (1–8 U/L) | 1.3 | 1.0 | 3.0 |
| T (total) (240–950 ng/dL) | 473 | 287 | 355 |
| Dehydroepiandrosterone sulfate (0.80–5.60 μg/mL) | 0.50 | 0.23 | 0.27 |
| Estradiol (10–40 pg/mL) | 27.8 | 11.7 | N/A |
| A4/T ratio | 2.2 | 0.06 | 0.08 |
| Sperm count (>14 × 106/ mL) | 0 | 132 | 60 |
| % Motile sperm (>39%) | 0 | 35% | 50 |
| TART volume, mL | 5.01 | 0.47 | 0.59 |
| % Functional testicular volume | 84% | 99% | 99% |
| Weight, kg | 63.3 | 68.8 | 65.7 |
2. Discussion
This case illustrates the significance of individualizing therapy for induction of fertility in patients with CAH complicated by TARTs. Despite the use of a lower dosage of dexamethasone (250 μg daily) compared with previous reports [6, 7], our patient experienced reversal of his infertility with dosing aimed at reducing the nighttime ACTH surge but experienced significant weight gain and insomnia. This result reflects the considerable interindividual variability in glucocorticoid metabolism and the ability of long-acting glucocorticoids, especially dexamethasone, to suppress the hypothalamic-pituitary-adrenal axis [8] and underscores the need to individualize disease management. Physicians should aim to use the lowest glucocorticoid dosage possible and evaluate for symptoms and signs of hypercortisolism irrespective of the glucocorticoid dosage.
In the patient described, the TARTs showed reduction in volume with intensification of his glucocorticoid regimen. However, posterior shadowing noted on follow-up ultrasonography suggested fibrosis. Induction of fibrosis within the tumor does not occur until the end stages of TART development. Peritubular fibrosis of surrounding testicular tissue indicates early damage, potentially leading to irreversible destruction of residual parenchyma. Intensification of the glucocorticoid regimen is ineffective in shrinking the tumor size once fibrosis has occurred, because adrenal rest cells may dedifferentiate with loss of ACTH and angiotensin II dependency. Despite this evidence of potential testicular damage due to long-standing TARTs, our patient’s infertility was reversed by optimizing medical management of his glucocorticoid regimen.
Patients with the severe salt-wasting form of the disease have been described to be at greater risk of TART formation, presumably because of exposure to higher concentrations of ACTH [9]. However, TARTs have been rarely described in men with nonclassic CAH [9]. Our patient’s genotype and phenotype are consistent with the salt-wasting form. His short stature and biochemical profile at presentation suggest inadequate control of CAH during childhood and in the recent past. Poor CAH control has been described as a predictor of TART formation in some studies, although these findings have not been consistent [10, 11]. The presence of TART may remain occult because patients can remain asymptomatic and because these tumors are commonly not palpable until they are >2 cm because of their central location. The current Endocrine Society guideline for the management of CAH recommends screening for TARTs beginning in adolescence, with ongoing periodic monitoring to assess for development of TARTs [12].
TARTs should be differentiated from Leydig cell tumors, the most common neoplasm of the testicular interstitial compartment, because the two share many common histological features, and misdiagnosis can result in unnecessary orchiectomy. Clinically, a history of CAH or a hormonal profile consistent with the disease, presence of bilaterality (83% in TARTs vs 3% in Leydig cell tumors), presence of Reinke crystalloids on histology, tumor shrinkage in response to intensive glucocorticoid therapy, and lack of malignant features have been used to distinguish the two.
Although biochemical profile reflects adequacy of CAH control at a snapshot in time, improvement in semen analysis parameters demonstrates adequacy of CAH control in the preceding 70 to 80 days, the time duration necessary for spermatogenesis. Initiation of dexamethasone may necessitate adjustments of fludrocortisone dosing because of the lack of mineralocorticoid activity of dexamethasone. Continued stability of the patient’s biochemical, semen, and sonographic parameters on prednisone 3 mg twice daily suggests that once-daily prednisone is inadequate in controlling the ACTH-driven comorbidities in CAH, but a twice-daily prednisone regimen can improve control, probably by decreasing the nighttime ACTH surge.
3. Conclusion
In summary, this case illustrates successful induction of fertility in a man with TARTs by use of low-dose dexamethasone (250 μg daily) and subsequent twice-daily prednisone and underscores the importance of personalizing treatment of each patient to minimize the potential harms of glucocorticoid excess.
Acknowledgments
We express our sincere gratitude to the patient and his family for their participation and support and to the staff at Outpatient Clinic 9, NIH Clinical Center.
Financial Support: This research was supported by the Division of Intramural Research of the National Institutes of Health Clinical Center. D.P.M. received unrelated research funds from Diurnal Limited and Spruce Biosciences through the National Institutes of Health Cooperative Research and Development Agreement and is a commissioned officer in the US Public Health Service.
Glossary
Abbreviations:
- A4
androstenedione
- CAH
congenital adrenal hyperplasia
- PRA
plasma renin activity
- T
testosterone
- TART
testicular adrenal rest tumor
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References and Notes
- 1. Reisch N, Flade L, Scherr M, Rottenkolber M, Pedrosa Gil F, Bidlingmaier M, Wolff H, Schwarz HP, Quinkler M, Beuschlein F, Reincke M. High prevalence of reduced fecundity in men with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2009;94(5):1665–1670. [DOI] [PubMed] [Google Scholar]
- 2. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. [DOI] [PubMed] [Google Scholar]
- 3. Engels M, Span PN, van Herwaarden AE, Sweep FCGJ, Stikkelbroeck NMML, Claahsen-van der Grinten HL. Testicular adrenal rest tumors: current insights on prevalence, characteristics, origin, and treatment. Endocr Rev. 2019;40(4):973–987. [DOI] [PubMed] [Google Scholar]
- 4. Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Span PN, Ross HA, Meuleman EJ, Hermus AR. Testicular tumors in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency show functional features of adrenocortical tissue. J Clin Endocrinol Metab. 2007;92(9):3674–3680. [DOI] [PubMed] [Google Scholar]
- 5. Claahsen-van der Grinten HL, Otten BJ, Hermus AR, Sweep FC, Hulsbergen-van de Kaa CA. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia can cause severe testicular damage. Fertil Steril. 2008;89(3):597–601. [DOI] [PubMed] [Google Scholar]
- 6. Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Hermus AR. Repeated successful induction of fertility after replacing hydrocortisone with dexamethasone in a patient with congenital adrenal hyperplasia and testicular adrenal rest tumors. Fertil Steril. 2007;88(3):705.e5–705.e8. [DOI] [PubMed] [Google Scholar]
- 7. Collet TH, Pralong FP. Reversal of primary male infertility and testicular adrenal rest tumors in salt-wasting congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2010;95(5):2013–2014. [DOI] [PubMed] [Google Scholar]
- 8. Whittle E, Falhammar H. Glucocorticoid regimens in the treatment of congenital adrenal hyperplasia: a systematic review and meta-analysis. J Endocr Soc. 2019;3(6):1227–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falhammar H, Nyström HF, Ekström U, Granberg S, Wedell A, Thorén M. Fertility, sexuality and testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. 2012;166(3):441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reisch N, Rottenkolber M, Greifenstein A, Krone N, Schmidt H, Reincke M, Schwarz HP, Beuschlein F. Testicular adrenal rest tumors develop independently of long-term disease control: a longitudinal analysis of 50 adult men with congenital adrenal hyperplasia due to classic 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2013;98(11):E1820–E1826. [DOI] [PubMed] [Google Scholar]
- 11. Mendes-Dos-Santos CT, Martins DL, Guerra-Júnior G, Baptista MTM, de-Mello MP, de Oliveira LC, Morcillo AM, Lemos-Marini SHV. Prevalence of testicular adrenal rest tumor and factors associated with its development in congenital adrenal hyperplasia. Horm Res Paediatr. 2018;90(3):161–168. [DOI] [PubMed] [Google Scholar]
- 12. Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, Meyer-Bahlburg HFL, Miller WL, Murad MH, Oberfield SE, White PC. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(11):4043–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]