Abstract
Adrenocortical carcinoma (ACC) is a rare orphan disease with a dismal prognosis. Surgery remains the first-line treatment, but most patients eventually develop metastatic disease. Mitotane is often used with chemotherapy with modest success. Little information is available concerning the efficacy of immunotherapy in combination with mitotane. We conducted a retrospective review of our initial six patients with metastatic ACC, for whom mitotane alone or with chemotherapy failed, and who were subsequently treated with a combination of pembrolizumab and mitotane, between July 2016 and March 2019. Imaging was analyzed per Response Evaluation Criteria in Solid Tumours 1.1 criteria. Two patients had a partial response and four patients had stable disease (8 to 19 months). One patient had grade 3 hepatitis and pembrolizumab was discontinued after 8 months. She died with disease progression 16 months after initiating pembrolizumab. One patient developed brain metastasis after 19 months of treatment and was transitioned to hospice. One patient had focal pneumonitis after 18 months of treatment, and pembrolizumab was discontinued. Three remaining patients continue pembrolizumab plus mitotane at the time of this writing. The current standard of care for ACC is a combination of etoposide, doxorubicin, cisplatin, and mitotane with an overall survival of 14.8 months. All six patients lived for at least 16 months after starting pembrolizumab added to mitotane therapy. The therapy appeared to be effective in both microsatellite instability-high and microsatellite stable tumors, suggesting some synergistic effect with mitotane. Combined immunotherapy and mitotane should be considered in future clinical trials in patients with ACC.
Keywords: adrenocortical cancer, immunotherapy, mitotane, pembrolizumab, anti-PD1, PD1
Adrenocortical carcinoma (ACC) is an orphan cancer with an incidence rate of 1.26 per million person-years [1]. The five-year survival is 80% in those with stage I disease, but most patients present with advanced disease and survival is only 13% at five years in those with stage IV disease [2]. Surgery is the primary mode of therapy for nonmetastatic ACC, but many patients have metastatic disease at the time of presentation. Mitotane, an insecticide with adrenolytic properties, the only Food and Drug Administration-approved treatment of ACC, is often poorly tolerated and its effect limited. An international multicenter randomized controlled study demonstrated that a combination of mitotane and chemotherapy consisting of etoposide, doxorubicin, and cisplatin (EDP-mitotane) led to longer median progression-free survival when compared with streptozocin plus mitotane [3]. However, no between-group difference in overall survival (OS) was observed, 14.8 months in the EDP-mitotane group and 12 months in the streptozocin-mitotane group [3]. The National Comprehensive Cancer Network Guidelines and European Society of Endocrinology Clinical Practice Guidelines recommend that the current standard of care for patients with metastatic ACC is EDP-mitotane, streptozocin-mitotane, or mitotane monotherapy [4, 5].
Immunotherapy has changed the landscape of treatment paradigms in various cancer types, including melanoma, lung cancer, and renal cancer [6]. Most have targeted the cytotoxic T-lymphocyte–associated antigen 4 and programmed cell death protein 1 (PD-1) immune checkpoint pathways; here our focus is on anti-PD1 therapy. PD-1 is a receptor that downregulates the immune system and suppresses T cell activity [7]. Pembrolizumab is a PD-1 inhibitor approved for the treatment of many different tumor types. Although limited clinical studies are equivocal of the role of immunotherapy in ACC [8–10], we have suggested preclinical evidence to support the use of PD-1 inhibitors in a subset of patients [11]. Consistent with this hypothesis, we recently generated an ACC patient derived xenograft in a humanized mouse model [11]. Treatment of these mice with pembrolizumab demonstrated antitumor activity. Importantly, we observed an even better partial response (PR) in the parallel patient who received concomitant mitotane [11]. Based our early clinical observations, we report here the effectiveness of pembrolizumab with mitotane on an expanded access program in patients with advanced ACC.
1. Materials and Methods
A. Study Design
This study was approved by the Institutional Review Board of the University of Colorado and all patients gave consent for data collection. A retrospective chart review of patients with ACC who received pembrolizumab and mitotane between July 2016 and March 2019 was performed. Each patient was seen in the Multidisciplinary Adrenal Tumor Program.
Patient information, tumor characteristics, treatment regimens, and clinical outcomes were collected including sex, race, Eastern Cooperative Oncology Group performance status, stage and grade of the tumor, location of the tumor, hormones secreted, sites of metastasis, previous and current treatment regimens, progression of disease, and adverse events. Adverse events were included if they were deemed at least possibly related to the immunotherapy. Data were analyzed using descriptive statistics.
B. Medication Protocol
At the University of Colorado ACC clinic mitotane was initiated at 500 mg daily, increased every 3 days by 500 mg, titrated up to 1.5 g twice daily, and then adjusted based upon the serum mitotane levels checked monthly. Patients were instructed to take mitotane with one tablespoon of flaxseed oil. A proton pump inhibitor as well as ondansetron were administered twice daily before breakfast and dinner. The target mitotane level was 14 to 20 μg/mL. In all patients, glucocorticoid deficiency developed at 6 to 16 weeks and twice daily prednisone was added with meals. In some cases, mineralocorticoid deficiency was detected and twice daily fludrocortisone with meals was added. The regimen was designed for twice daily therapy to increase compliance and quality of life for patients. Side effects of mitotane including central hypothyroidism, adrenal insufficiency, and/or neurotoxicity, were treated with adjustment of medications. Pembrolizumab was administered, after mitotane only (three patients) or mitotane plus chemotherapy (three patients) regimen failed, as either 200 mg IV or 2 mg/kg IV every 21 days at the University of Colorado Hospital or with the patient’s local oncologist.
C. Pathology Evaluation
Tumor histology was reviewed, and tumor samples were sent for Foundation One genomic profiling including microsatellite instability (MSI) status. Microsatellite instability was reported to be high (MSI-H), indeterminate (MSI-I), or stable (MSS). The diagnosis of ACC was confirmed on hematoxylin and eosin stained sections based on established histologic features such as increased or atypical mitoses, venous invasion, severe nuclear atypia, and tumor necrosis as well as positivity for MelanA, alpha-inhibin, and synaptophysin stains. Tumors were classified as low or high grade based on mitotic KI-67 activity. Low-grade tumors had ≤20 mitoses per 50 high-power fields, and high-grade tumors had ≥20 mitoses per 50 high-power fields.
D. Radiologic Evaluation
All radiologic images were centrally reviewed and analyzed in Philips IntelliSpace PACS Radiology (version 4.4.516.42, Koninklijke Philips N.V., Amsterdam, Netherlands). Target lesions were established per Response Evaluation Criteria in Solid Tumours 1.1 criteria on a baseline contrast-enhanced CT. Lesions were reassessed on subsequent contrast-enhanced CTs and two-dimensional area relative to baseline and area percent decrease relative to baseline were computed. Most CTs were performed every three cycles of pembrolizumab; some were performed at longer time intervals because of practice variation.
2. Results
A. Patient Baseline Characteristics
Over 2.5 years (July 2016 to March 2019), six patients received pembrolizumab added to mitotane therapy; two treated at the University of Colorado Cancer Center and four by their local oncologists with the guidance of the University of Colorado Adrenal Tumor Program physicians. Endocrine faculty coordinated the mitotane dosing. Patient baseline characteristics are outlined in Table 1. Two patients were stage IV at the time of diagnosis whereas four patients had recurrent metastatic disease. Three were previously treated with chemotherapy. All were currently receiving mitotane. Three patients had hormonally active tumors secreting cortisol and/or adrenal androgens. Prior to treatment with immunotherapy, three patients had progressed on mitotane alone and three patients progressed on mitotane plus chemotherapy. Duration of mitotane therapy prior to adding pembrolizumab varied between 3 and 46 months, and total duration on mitotane was 14 to 62 months.
Table 1.
Patients Characteristics
| Patient ID | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age at diagnosis, y | 24 | 44 | 54 | 29 | 46 | 65 |
| Sex | F | F | F | F | F | F |
| Race | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian |
| ECOG performance status just prior to starting pembrolizumab | 1 | 1 | 1 | 0 | 1 | 1 |
| Tumor size at time of diagnosis, cm | 4 | 20 | 15 | 9.5 | 8.2 | 19 |
| Side of tumor | Left | Left | Left | Left | Left | Left |
| Tumor grade | High | Low | High | High | High | High |
| Stage at diagnosis | 1a | 3 | 4 | 4 | 3 | 3 |
| Tumor rupture | Yes | No | No | No | Yes | No |
| Lynch syndrome | Yes (MSH2) | No | Yes (MSH2) | No | No | No |
| Tumor MSI-H | Yes | No | Indeterminate | No | No | No |
| Hormones secreted by tumor | None | None | Cortisol,androgens | Cortisol,b androgens | None | Cortisol |
| Sites of metastasis | Liver, lung, peritoneum, ovary | Liver, lung, kidney, spleen | Lung, peritoneum, renal vein, IVC, intracardiac, brain | Liver, lung, pulmonary vein, intracardiac, kidney, spleen, uterus, retroperitoneum | Lung | Lung, renal vein |
| Prior chemotherapy | Mitotane | Mitotane, | Mitotane | Mitotane, | Mitotane | Mitotane |
| EDP | adriamycin, | Etoposide | ||||
| cyclophosphamide, | ||||||
| and etopisode | ||||||
| Best response to chemotherapy | N/A | Progression after 2 cycles | N/A | Progression | Stable disease | N/A |
| Number of surgeries | 4 | 1 | 3 | 2 | 3 | 1 |
| Type of surgery | Laparoscopic | Open | Open | Open | Open | Open |
| Prior radiation | Yes | None | None | None | Yes | No |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; IMRT, intensity modulated radiation therapy; SBRT, stereotactic body radiation therapy; XRT, radiation therapy.
Lymph nodes not examined, had recurrence 7 mo later.
Recurrence of Cushing with ACC metastasis; started on ketoconazole (2 mo).
B. Clinical Course
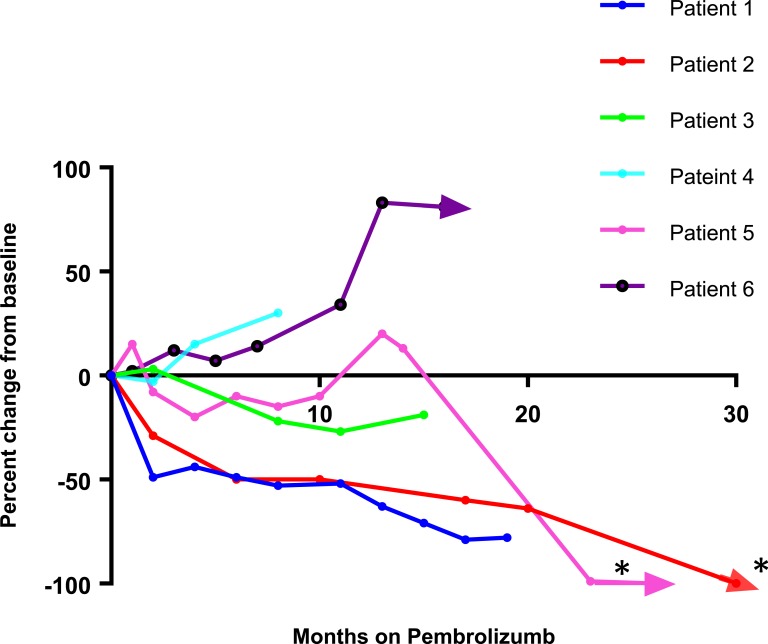
The therapeutic regimens for each patient are outlined in Table 2 and response to immunotherapy added to mitotane is outlined in Figure 1. While on pembrolizumab and mitotane therapy, two patients had PR and the other four patients had stable disease (SD) at 19, 8, 15, and 11 months. In addition, pembrolizumab and mitotane combination allowed two patients (patient 2 and 5), to undergo additional localized therapy to achieve complete remission (Fig. 1). Patient 1 had Lynch syndrome with an MSI-H tumor; she achieved a PR for 18 months until she developed focal pneumonitis after 27 cycles of pembrolizumab. She continues to have SD after 6-plus months off pembrolizumab with continued mitotane therapy. Patient 2 had a PR at 21 months and is still receiving therapy after 31 months. Prior to starting pembrolizumab, she had more than 20 liver metastasis. While taking pembrolizumab, all but three lesions disappeared. She subsequently had microwave ablation of these lesions, and now has no evidence of disease. Patient 3 had Lynch syndrome with an indeterminate MSI tumor. She had an unresectable inferior vena cava (IVC) tumor and a right atrium tumor at presentation. She had SD on combination therapy and eventually had partial debulking of her IVC clot and right atrium lesions. She later had a stroke and was found to have brain metastasis after 19 months and was transitioned to hospice. She had a progression-free survival (PFS) of 19 months and OS of 21 months. Patient 4 had SD but developed grade 3 hepatitis after 8 months of treatment with pembrolizumab, and 2 months taking ketoconazole and mitotane requiring discontinuation of therapy. She died of disease progression 8 months later. She had a PFS of 8 months and OS of 16 months. Patient 5 had SD after 15 months of therapy. She recently had surgical resection of residual disease, and currently has no evidence of disease at 26 months and is still undergoing therapy. Patient 6 had SD initially. She had progression of disease after 11 months, elected to continue treatment, and was still undergoing therapy after 16 months.
Table 2.
Pembrolizumab and Mitotane Therapy Details
| Patient ID | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Time from diagnosis until starting pembrolizumab, mo | 95 | 7 | 5 | 76 | 58 | 38 |
| Cycles of pembrolizumab received until March 2019 | 27 | 42 | 25 | 11 | 35 | 25 |
| Months on pembrolizumab until March 2019 | 18 | 31 | 19 | 8 | 26 | 16 |
| Number of pembrolizumab cycle delays | 1 | 3 | 1 | None | 1 | None |
| Discontinuation of pembrolizumab because of side effects | Yes | No | No | Yes | No | No |
| Duration of mitotane therapy prior to starting pembrolizumab, mo | 46 | 3 | 5 | 10 | 56 | 37 |
| Mean mitotane level during treatment with pembrolizumab, μg/mL | 18.2 | 11.3 | 11.2 | 2.6 | 13.3 | 16.6 |
| Range of mitotane levels during treatment with pembrolizumab, μg/mL | 15.1–25.2 | 9.8–12.1 | 2.9–25.6 | 0–6 | 10.0–15.0 | 14.9–17.6 |
| Time spent off mitotane while on pembrolizumab, mo | 0 | 0 | 2 | 4 | 2 | 0 |
| Total duration of mitotane therapy, mo | 62 | 26 | 22 | 14 | 72 | 45 |
Figure 1.
Tumor response in six patients with ACC using Response Evaluation Criteria in Solid Tumours 1.1 criteria on pembrolizumab plus mitotane regiment. Arrows denote patients that currently remain on pembrolizumab. *, Denotes two patients who were able to undergo additional localized therapy and presently have no evidence of disease.
C. Adverse Effects
The immune-related adverse events are outlined in Table 3. There were four grade 3 adverse events during treatment. One patient developed elevated liver enzymes, and was diagnosed with autoimmune hepatitis, necessitating cessation of therapy. The other three grade 3 events did not require treatment cessation or dose reduction. One patient had one episode of adrenal insufficiency requiring rehydration and antiemetics, but this did not recur subsequently. Another patient developed mouth sores associated with weight loss, but this resolved with topical treatment. One patient had diarrhea that was initially grade 3; colonoscopy was negative for colitis and the diarrhea improved to grade 1 while on therapy. One patient developed grade 2 focal lung pneumonitis found incidentally on CT scan, confirmed by bronchoscopy and she was treated with steroids and immunotherapy was discontinued. Another patient discontinued therapy because of development of brain metastasis and transition to hospice, not caused by the development of immune-related adverse events.
Table 3.
Immunotherapy-Related Adverse Events
| Any Grade Adverse Events That Occurred in ≥2 Patients | N (%) |
|---|---|
| Fatigue | 4 (66.7) |
| Pruritis | 2 (33.3) |
| Rash | 3 (50) |
| Decreased appetite | 2 (33.3) |
| Headaches | 2 (33.3) |
| Neuropathy | 2 (33.3) |
| Dyspnea (pneumonitis ruled out) | 2 (33.3) |
| Nausea | 4 (66.7) |
| Diarrhea (colitis ruled out) | 3 (50) |
| Rectal bleeding | 2 (33.3) |
| Dehydration | 2 (33.3) |
| Grade ≥3 adverse events that occurred in ≥1 patient | N, (%) |
| Hepatitis (grade 3) | 1 (16.7) |
| Nausea (grade 3) | 1 (16.7) |
| Mouth sores (grade 3) | 1 (16.7) |
| Diarrhea (colitis ruled out) (grade 3) | 1 (16.7) |
| Adverse events of special interest | |
| Focal pneumonitis (grade 2) | 1 (16.7) |
All six patients had central hypothyroidism; five had the diagnosis related to mitotane therapy prior to therapy with pembrolizumab. One patient who developed hypothyroidism during treatment with pembrolizumab developed central hypothyroidism also thought to be caused by mitotane. Hypothyroidism in all patients was managed with oral levothyroxine.
3. Discussion
The scarcity of data on effectiveness of immunotherapy in ACC has been largely because of disease rarity and limited number of preclinical models to test response to new therapeutic combinations. However, with the creation of new ACC cell lines, patient-derived xenograft models, and humanized xenograft models, there is now preclinical evidence to support the use of pembrolizumab in adrenocortical carcinoma [11].
During the period of our study, two phase 2 of the use of single-agent anti-PD1 therapy in ACC have been reported with mixed results [12, 13]. Carneiro et al. [12] reported that of 10 patients with ACC who were treated with nivolumab, 1 had PR, 2 had SD and 6 progression of disease, with median follow up of 4.5 months. Habra et al. [13] reported that of 14 patients with ACC who were treated with pembrolizumab, 2 had PR, 7 had SD, and 5 had progressive disease. In contrast to our current study, the patients in previous two clinical studies were not treated concomitantly with therapeutic levels of mitotane.
Although our initial experience is based on a limited number of patients, we hypothesize that the combination of pembrolizumab with mitotane may be important for a clinical response in this aggressive disease. In our cohort, all six patients derived clinical benefit with two PR and four SD. Two patients had documented progression on pembrolizumab, two discontinued pembrolizumab secondary to immune-related toxicities, and two had additional treatment (surgery and microablation) and now have no evidence of residual disease. The PFS for those who progressed were 8 and 11 months. Those who died had OS of 21 and 16 months. Those who are still taking pembrolizumab have been on therapy for more than 16 to 31 months.
Lynch syndrome, associated with a deficiency in the DNA mismatch repair mechanism, is a genetic predisposition to a variety of cancers. Approximately 3.2% of patients with ACC have Lynch syndrome [14]. Patients with Lynch syndrome associated colon cancer often have MSI-H tumors [15]. However, one study found that in their cohort of four patients with ACC and Lynch syndrome, all patients were MSS, but had deficient mismatch repair [14]. A recent case report on two patients with Lynch syndrome and metastatic ACC treated with pembrolizumab monotherapy found that one patient had a long-term complete response, whereas another patient did not respond [10].
In our two patients who had ACC and Lynch syndrome (patients 1 and 3), one tumor was MSI-H and one was MSI-I. Patient 1 had a germline mutation in MSH2 with loss of the MSH2 protein in the tumor, whereas patient 3 had a germline mutation in MSH2 with loss of the MSH2 and MutS protein homolog 6 proteins. Both responded to the addition of immunotherapy (PR and SD), indicating that it may be the absence of the protein, not necessarily the presence of instability, that drives response. Patient 2 had a tumor mutation in MutS protein homolog 6 and was MSS per Foundation One testing. She achieved PR. Taken together, these data suggest that mismatch repair protein testing may be more reliable than MSI and may be predictive markers for response to immunotherapy and mitotane.
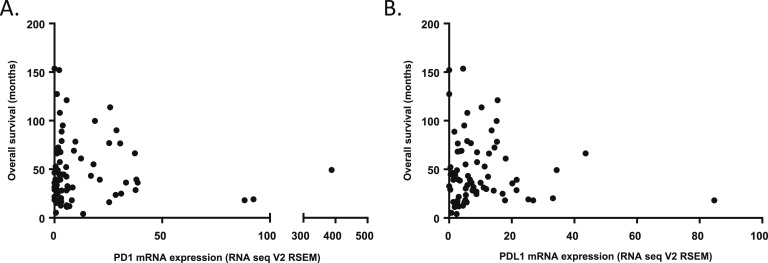
The remaining three patients (patients 4, 5, and 6) did not have Lynch syndrome, MSI, or mismatch repair protein mutation, yet had clinical benefit. Their best response was SD (for 8, 26, and 16 months, respectively). One possible explanation is the expression levels of programmed cell death ligand (PD-L1). In other tumor types, pembrolizumab is effective in cancers with a high level of PD-L1 expression [16]. Higher levels of PD-L1 expression were reported in 11% of all ACC tumors [17]. Examination of The Cancer Genome Atlas Program RNA Seq data expression reported [18, 19] similar results as shown in Figure 2. PDL1 testing was not available in our patients but may be a biomarker to track in future studies.
Figure 2.
mRNA expression in ACC The Cancer Genome Atlas Program tumors of (A) PD-1 and (B) PDL-1 immune checkpoints in comparison with overall survival (mo).
Our results suggest that the combination of mitotane with the immunotherapy may be synergistic. Patient 4 had the lowest and often nontherapeutic mitotane levels during treatment and she had the most rapid progression. In contrast, patient 1 had consistently therapeutic mitotane levels and she had a partial, long-lasting response. The JAVELIN study evaluated 50 patients with metastatic ACC who had prior platinum-based chemotherapy and received the anti-PDL1 agent, avelumab rather than anti-PD1 [8]. Half of their patients were receiving mitotane, and 42% had SD. There may have been a slightly better response rate to immunotherapy in the patients receiving mitotane, although mitotane levels were not reported. Ongoing studies of the mechanisms of mitotane and immunotherapy are underway in our ACC-patient-derived xenograft humanized mouse models to better predict who may respond to this combination of therapy.
Cortisol secretion has been associated with more aggressive ACC tumors and potentially less response to immunotherapy [20]. Patients 3, 4, and 6 had cortisol secreting tumors, and they all had SD on pembrolizumab and mitotane. Patients 3 and 4 both died, which may have been partially the result of the more aggressive nature their tumors as well as a decrease in the rate of response to immunotherapy. A recent case report of a patient with a cortisol-secreting ACC and a mutation in MSH2 reported progression after two cycles of pembrolizumab [9]. This patient was also treated with mitotane, although it was discontinued midway through treatment because of transaminitis. In our case series, patient 3 had a cortisol-secreting tumor and MSH2 mutation, and she has had SD.
There was one grade 3 adverse event (hepatitis) that required cessation of therapy. Autoimmune hepatitis occurs in 0.7% of patients on pembrolizumab [21]. Of note, this patient was also receiving ketoconazole and mitotane at the time, which both can cause elevated liver function tests. One patient had grade 3 diarrhea, but colitis was ruled out and it self-resolved, so we suspect it was not related to immunotherapy. Another patient had grade 2 focal pneumonitis. The adverse events that occurred in our ACC patients were consistent with the most common adverse events in patients treated with pembrolizumab for other tumor types [21].
Limitations of the study include its retrospective design in a limited number of patients. Trends in the data suggest a benefit of pembrolizumab in combination with mitotane, but no conclusions can be made because of the small sample size. The variability in total duration of mitotane treatment, tumor grade, and hormone production reflects disease heterogeneity that could also contribute to patient outcomes. It is also important to note that our primary focus on PD-1 checkpoint inhibition was because of the availability of PD-1 inhibitor, and the efficacy of other checkpoint inhibitors, such as anti-CTLA-4, need to be evaluated in future studies. In addition, future prospective clinical trials comparing pembrolizumab-mitotane to EDP-mitotane are needed to clarify the effectiveness of this approach to patients with this deadly disease.
Acknowledgments
We would like to thank the patients for their contributions to this research.
Financial Support: This work was supported by National Institutes of Health; National Cancer Institute K08CA222620 (to K.K.-V.), Cancer League of Colorado Award (to K.K.-V. and S.L.), Doris Duke CU-FSRC (to K.K.-V.), and Veterans Affairs Merit Review Award 001 (to M.E.W.). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
Glossary
Abbreviations:
- ACC
adrenocortical carcinoma
- EDP-mitotane
etoposide, doxorubicin, and cisplatin
- IVC
inferior vena cava
- MSI
microsatellite instability
- MSI-H
microsatellite instability high
- MSI-I
microsatellite instability indeterminate
- MSS
microsatellite instability stable
- OS
overall survival
- PD-1
cell death protein 1
- PDL-1
programmed cell death ligand
- PFS
progression-free survival
- PR
partial response
- SD
stable disease
Contributor Information
Katja Kiseljak-Vassiliades, Email: Katja.Kiseljak-Vassiliades@cuanschutz.edu.
Stephen Leong, Email: Stephen.Leong@cuanschutz.edu.
Additional Information
Disclosure Statement: The authors declare no potential conflicts of interest.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References and Notes
- 1. James BC, Aschebrook-Kilfoy B, Cipriani N, Kaplan EL, Angelos P, Grogan RH. The incidence and survival of rare cancers of the thyroid, parathyroid, adrenal, and pancreas. Ann Surg Oncol. 2016;23(2):424–433. [DOI] [PubMed] [Google Scholar]
- 2. Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, Mueller HH, Hahner S, Allolio B; German Adrenocortical Carcinoma Registry Group; European Network for the Study of Adrenal Tumors. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115(2):243–250. [DOI] [PubMed] [Google Scholar]
- 3. Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de la Fouchardière C, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Müller HH, Skogseid B; FIRM-ACT Study Group. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–2197. [DOI] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network. Neuroendocrine and adrenal tumors (version 1.2019) Available at: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. Accessed 18 April 2019.
- 5. Fassnacht M, Dekkers O, Else T, Baudin E, Berruti A, de Krijger R, Haak HR, Mihai R, Assie G, Terzolo M. European Society of Endocrinology Clinical Practice Guidelines on the Management of Adrenocortical Carcinoma in Adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):G1–G46.30299884 [Google Scholar]
- 6. Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11(1):24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le Tourneau C, Hoimes C, Zarwan C, Wong DJ, Bauer S, Claus R, Wermke M, Hariharan S, von Heydebreck A, Kasturi V, Chand V, Gulley JL. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer. 2018;6(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casey RT, Giger O, Seetho I, Marker A, Pitfield D, Boyle LH, Gurnell M, Shaw A, Tischkowitz M, Maher ER, Chatterjee VK, Janowitz T, Mells G, Corrie P, Challis BG. Rapid disease progression in a patient with mismatch repair-deficient and cortisol secreting adrenocortical carcinoma treated with pembrolizumab. Semin Oncol. 2018;45(3):151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mota JM, Sousa LG, Braghiroli MI, Siqueira LT, Neto JEB, Chapchap P, Hoff AAO, Hoff PM. Pembrolizumab for metastatic adrenocortical carcinoma with high mutational burden: two case reports. Medicine (Baltimore). 2018;97(52):e13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lang J, Capasso A, Jordan KR, French JD, Kar A, Bagby SM, Barbee J, Yacob BW, Head LS, Tompkins KD, Freed BM, Somerset H, Clark TJ, Pitts TM, Messersmith WA, Eckhardt SG, Wierman ME, Leong S, Kiseljak-Vassiliades K. Development of an adrenocortical cancer humanized mouse model to characterize anti-PD1 effects on tumor microenvironment [published online ahead of print 12 September 2019]. J Clin Endocrinol Metab. doi.org/10.1210/clinem/dgz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carneiro BA, Konda B, Costa RB, Costa RLB, Sagar V, Gursel DB, Kirschner LS, Chae YK, Abdulkadir SA, Rademaker A, Mahalingam D, Shah MH, Giles FJ. Nivolumab in metastatic adrenocortical carcinoma: results of a phase II trial [published online ahead of print 5 July 2019]. J Clin Endocrinol Metab. doi.org/10.1210/jc.2019-00600. [DOI] [PubMed] [Google Scholar]
- 13. Habra MA, Stephen B, Campbell M, Hess K, Tapia C, Xu M, Rodon Ahnert J, Jimenez C, Lee JE, Perrier ND, Boraddus RR, Pant S, Subbiah V, Hong DS, Zarifa A, Fu S, Karp DD, Meric-Bernstam F, Naing A. Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J Immunother Cancer. 2019;7(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raymond VM, Everett JN, Furtado LV, Gustafson SL, Jungbluth CR, Gruber SB, Hammer GD, Stoffel EM, Greenson JK, Giordano TJ, Else T. Adrenocortical carcinoma is a lynch syndrome-associated cancer. J Clin Oncol. 2013;31(24):3012–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nayak SS, Roy P, Arora N, Arun I, Roy MK, Banerjee S, Mallick I, Mallath MK. Prevalence estimation of microsatellite instability in colorectal cancers using tissue microarray based methods—A tertiary care center experience. Indian J Pathol Microbiol. 2018;61(4):520–525. [DOI] [PubMed] [Google Scholar]
- 16. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fay AP, Signoretti S, Callea M, Telό GH, McKay RR, Song J, Carvo I, Lampron ME, Kaymakcalan MD, Poli-de-Figueiredo CE, Bellmunt J, Hodi FS, Freeman GJ, Elfiky A, Choueiri TK. Programmed death ligand-1 expression in adrenocortical carcinoma: an exploratory biomarker study. J Immunother Cancer. 2015;3(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puglisi S, Perotti P, Pia A, Reimondo G, Terzolo M. Adrenocortical carcinoma with Hypercortisolism. Endocrinol Metab Clin North Am. 2018;47(2):395–407. [DOI] [PubMed] [Google Scholar]
- 21. Merck. Highlights of prescribing information. Available at: https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Accessed 1 November 2019. [Google Scholar]