Abstract
Protein arginine methyltransferases (PRMTs) are enzymes that regulate the evolutionarily conserved process of arginine methylation. It has been reported that PRMTs are involved in many metabolic regulatory pathways. However, until now, their roles in adipocyte function, especially browning and thermogenesis, have not been evaluated. Even though Prmt1 adipocyte-specific–deleted mice (Prmt1fl/flAQcre) appeared normal at basal level, following cold exposure or β-adrenergic stimulation, impaired induction of the thermogenic program was observed in both the interscapular brown adipose tissue and inguinal white adipose tissue of Prmt1fl/flAQcre mice compared with littermate controls. Different splicing variants of Prmt1 have been reported. Among them, PRMT1 variant 1 and PRMT1 variant 2 (PRMT1V2) are well conserved between humans and mice. Both variants contribute to the activation of thermogenic fat, with PRMT1V2 playing a more dominant role. Mechanistic studies using cultured murine and human adipocytes revealed that PRMT1V2 mediates thermogenic fat activation through PGC1α, a transcriptional coactivator that has been shown to play a key role in mitochondrial biogenesis. To our knowledge, our data are the first to demonstrate that PRMT1 plays a regulatory role in thermogenic fat function. These findings suggest that modulating PRMT1 activity may represent new avenues to regulate thermogenic fat and mediate energy homeostasis. This function is conserved in human primary adipocytes, suggesting that further investigation of this pathway may ultimately lead to therapeutic strategies against human obesity and associated metabolic disorders.
It is well recognized that obesity, mainly caused by excessive energy intake and/or decreased energy expenditure, has become a global health epidemic (1). Obesity and various associated metabolic diseases such as insulin resistance, type 2 diabetes, and cardiovascular disease pose a serious burden for public health, ultimately resulting in the present demand for effective therapeutic interventions. White adipose tissue is primarily responsible for the excess energy storage seen in obesity, whereas brown adipose tissue (BAT) controls adaptive thermogenesis through uncoupling protein 1 (UCP1), which can produce heat by dissipating the proton gradient to uncouple fuel oxidation from ATP synthesis (2). Thermogenic beige adipocytes, which are prominent within the subcutaneous white adipose tissue [inguinal white adipose tissue (IWAT), for example], can be activated by cold exposure or sympathetic stimulation (3). The activation of thermogenic brown and beige adipocytes has been increasingly recognized as a promising solution to counteract obesity and has thus attracted considerable attention in the field.
The protein arginine methyltransferase (PRMT) family of enzymes is comprised of 11 family members responsible for catalyzing arginine methylation (4). In mammals, they are subdivided into type I (PRMT1, 2, 3, 4, 6, and 8), type II (PRMT5 and 9), and type III (PRMT7) according to their methylation manner (5). PRMT1, a well-established member of the PRMT family, contributes >85% of all type I PRMT enzyme activity in mammalian cells and tissues (4, 6). PRMT1 has been reported to participate in a variety of biological processes, including gene transcription, signal transduction, and development (5). PRMT1 was further shown to be involved in cancer progression (7) and some metabolic regulations, such as hepatic lipogenesis (8) and insulin signaling (9). However, little is known about how PRMT1 modulates adipose tissue function.
Peroxisome proliferator–activated receptor γ coactivator 1-α (PGC1α) is a transcriptional coactivator that was first identified in brown adipocytes (10). PGC1α functions as a major regulator in multiple metabolic processes, such as mitochondrial biogenesis, hepatic response to fasting, and adaptive thermogenesis (11).
In the current study, we discovered that adipocyte-specific Prmt1 deletion led to impaired thermogenic activation upon cold exposure. Gain-of-function analyses revealed that PRMT1, particularly the isoform encoded by variant 2, activates thermogenic gene expression, at least in part through coactivation of PGC1α. This thermogenic effect of PRMT1 was observed in both murine and human primary adipocytes.
Materials and Methods
Reagents
Insulin (catalog no. I5500), dexamethasone (catalog no. D4902), 3-isobutyl-1-methylxanthine (catalog no. I7018), biotin (catalog no. B4639), d-pantothenic acid hemicalcium salt (catalog no. P5155), CL 316,243 (catalog no. C5976), oligomycin A (catalog no. 75351), fetal bovine serum (FBS; catalog no. F2442), newborn calf serum (catalog no. N4762), horse serum (catalog no. H1270), red blood cell lysing buffer (catalog no. R7757), and Oil Red O (catalog no. O0625) were purchased from Sigma-Aldrich. Rosiglitazone (catalog no. 71740) was obtained from Cayman Chemical. DMEM/F-12 GlutaMAX (catalog no. 10565-042), DMEM (catalog no. 10313-021), DMEM–low glucose (catalog no. 11885-076), MesenPRO RS medium (catalog no. 12746-012), penicillin streptomycin solution (catalog no. 15140122), and Hanks balanced salt solution (HBSS) with calcium and magnesium, no phenol red (catalog no. 14-025-092) were purchased from Life Technologies. Recombinant murine fibroblast growth factor–basic (catalog no. AF-450-33) was obtained from PeproTech. Collagenase type I (catalog no. LS004194) and type IV (catalog no. LS004188) were purchased from Worthington Biochemical. Collagenase D (catalog no. 11088882001), collagenase B (catalog no. 11088831001), and dispase II (catalog no. 04942078001) were purchased from Roche. Polyethylenimine (PEI; linear, molecular mass of 25 kDa; catalog no. 23966-1) was purchased from Polysciences.
Animal studies
Two independent strains of Prmt1fl/fl mice were used in this study. Both of the conditional alleles lead to Cre-mediated deletion of exon 4 and 5 of the Prmt1 gene (12, 13). Similar results were observed in both strains. Adipocyte-specific Prmt1 knockout mice (Prmt1fl/flAQcre) were generated by crossing Prmt1fl/fl with adiponectin-Cre mice (catalog no. JAX 028020; The Jackson Laboratory). The mice were maintained on an ad libitum chow diet (catalog no. 5L0D; PicoLab Laboratory rodent diet) under a 12-hour light/12-hour dark cycle at room temperature (23°C). For cold exposure, mice were singly housed in an environmental chamber kept at 4 or 10°C. For β3-adrenergic agonist treatment, 1 mg/kg/d CL 316,243 in PBS was injected IP for 2 days. Blood glucose levels were measured in tail blood from mice fasted for 4 hours using the OneTouch Ultra glucometer (Lifescan). Body temperature was determined using a RET-3 mouse rectal probe (World Precision Instruments). All animal studies were performed according to procedures approved by the Institutional Animal Care and Use Committee at the University of Michigan. Both male and female mice were used in this study and similar results were observed.
Gene expression
Total RNA was extracted from tissues using TRIzol (Sigma-Aldrich) according to the manufacturer’s instructions. cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (Life Technologies). Quantitative real-time PCR (qPCR) reactions were performed with SYBR Green (Thermo Fisher Scientific) on a QuantStudio 5 real-time PCR system (Thermo Fisher Scientific). Results were analyzed using the 2−ΔΔCT method and normalized to levels of TATA-box binding protein (Tbp). All qPCR primer sequences are listed in an online repository (14).
Mature adipocytes and stromal vascular fraction isolation
The isolation of mature adipocytes and stromal vascular fraction (SVF) was performed as described previously (15). In brief, IWAT and BAT were dissected, minced, and digested in a collagenase solution containing dispase II and collagenase B (BAT) or collagenase D (IWAT) for 20 minutes in a water bath shaker at 37°C, then filtered through a 100-μm cell strainer. The floating mature adipocytes were collected after centrifugation for 5 minutes at 300g to 500g. The resuspended cell pellet was passed through a 40-μm cell strainer and the filtrate was centrifuged to precipitate SVF. RNA from mature adipocytes and SVF was extracted using the GenElute mammalian total RNA Miniprep kit (Sigma-Aldrich).
Ear mesenchymal stem cell isolation, culture, and differentiation
Mouse primary ear mesenchymal stem cells (EMSCs) were isolated, cultured, and differentiated into adipocytes as reported previously (16). Two ears from one mouse were dissected and washed with HBSS containing 1% penicillin-streptomycin. Ears were minced into tiny pieces and digested in collagenase solution (2 mg/mL collagenase I in sterile HBSS) for 1 hour at 37°C in a shaking water bath. Digested tissue was neutralized by DMEM/F-12 GlutaMAX containing 10% FBS and filtered through a 100-μm cell strainer. The filtrate was centrifuged at 500g for 10 minutes to pellet the cells. One milliliter of red blood cell lysing buffer was added to remove erythrocyte contamination for 1 minutes and the mixture was centrifuged at 500g for 10 minutes. The pellet was resuspended in DMEM/F-12 GlutaMAX containing 15% FBS, 10 ng/mL fibroblast growth factor–basic, and 1% penicillin-streptomycin and plated in a 35-mm dish. Cells were split at a ratio of 1:3 or 1:4. For differentiation, confluent cells were stimulated with DMEM/F-12 GlutaMAX containing 10% FBS, 1% penicillin-streptomycin, insulin (0.5 μg/mL), rosiglitazone (1 μM), dexamethasone (5 μM), and 3-isobutyl-1-methylxanthine (0.5 mM). Two days later, the cells were cultured with DMEM/F-12 GlutaMAX containing 10% FBS, 1% penicillin-streptomycin, insulin (0.5 μg/mL), and rosiglitazone (1 μM) for another 4 days. For adenovirus infection experiments, adenovirus was added into the medium on day 3. The cells were harvested on day 6.
Human adipose stem cell culture and differentiation
Human adipose stem cells (hASCs) were isolated from subcutaneous adipose tissue of healthy adults undergoing liposuction (a gift from Dr. Jeffrey M. Gimble, Tulane University, New Orleans, LA). All specimens were collected under the protocols reviewed and approved by the Western Institutional Review Board (Puyallup, WA) or the University of Michigan Medical School Institutional Review Board (Ann Arbor, MI). Cells were cultured with MesenPRO RS medium supplemented with 1% penicillin-streptomycin solution on collagen-coated plates. For differentiation, the cells were stimulated with DMEM/F-12 GlutaMAX, supplemented with 10% FBS, insulin (0.5 μg/mL), rosiglitazone (5 μM), dexamethasone (5 μM), 3-isobutyl-1-methylxanthine (0.5 mM), biotin (33 μM) and d-pantothenic acid (17 μM) for 4 days. After 4 days of stimulation, cells were maintained in maintenance medium (DMEM/F-12 GlutaMAX containing 10% FBS, 0.5 μg/mL insulin, 5 μM dexamethasone, 33 μM biotin, and 17 μM d-pantothenic acid) until they were fully differentiated.
C2C12 cell culture and differentiation
C2C12 myoblasts were cultured and differentiated as previously described (17). Briefly, cells were cultured in DMEM containing 20% newborn calf serum, 1% penicillin-streptomycin. At near confluence, cells were induced to differentiate by growth in differentiation medium (DMEM supplemented with 2% horse serum, 1% penicillin-streptomycin). After growing in differentiation medium for 2 days, C2C12 cells were infected with adenoviruses overexpressing green fluorescent protein (GFP), PRMT1 variant 2 (PRMT1V2), or PGC1α. The cells were harvested 48 hours after transduction.
Primary hepatocyte isolation and culture
Mouse primary hepatocytes were isolated and cultured as previously reported (18). Briefly, the liver was perfused with washing buffer [HBSS buffer containing 0.5 mM EGTA, 25 mM HEPES (pH 7.4)] and then with digestion medium (DMEM–low glucose containing 200 mg/L CaCl2, 1% penicillin-streptomycin, 15 mM HEPES, and 100 U/mL collagenase IV) via the inferior vena cava. Dispersed cells were pelleted after filtering the suspension using a BD Biosciences disposable Falcon tube nylon filter. Hepatocytes were washed twice and seeded on collagen-coated culture plates at 3×105 cells/mL with the isolation medium (DMEM/F-12 GlutaMAX, supplemented with 10% FBS, 1% penicillin-streptomycin, 1 μM dexamethasone, and 0.1 μM insulin). After 4 hours, the hepatocytes were maintained in culture medium (DMEM–low glucose containing 10% FBS, 1% penicillin-streptomycin, 0.1 μM dexamethasone, and 1 nM insulin) and infected with adenoviruses overexpressing GFP, PRMT1V2, or PGC1α overnight. The infected hepatocytes were maintained in culture medium without FBS for an additional 12 hours before harvesting.
Immunoblotting
Adipose tissue was homogenized using a handheld homogenizer (Cole Parmer) in radioimmunoprecipitation assay buffer [150 mM NaCl, 50 mM Tris-HCl (pH 8.0), 5 mM EDTA, 0.5% sodium deoxycholate, 0.1% SDS, 1% Nonidet P-40], which was supplemented with protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitors [10 mM NaF, 60 mM β-glycerolphosphate (pH 7.5), 2 mM sodium orthovanadate, and 10 mM sodium pyrophosphate]. Protein concentration was determined using DC protein assay reagents (Bio-Rad Laboratories) on a SpectraMax M3 multi-mode microplate reader (Molecular Devices). Protein extracts were subjected to SDS-PAGE electrophoresis and transferred onto nitrocellulose membranes followed by incubation with blocking buffer (5% nonfat milk in 1% TBS with Tween 20) for 1 hour. The membranes were probed with primary anti-UCP1 (R&D Systems; catalog no. MAB6158-SP) (19), anti-PRMT1 (Cell Signaling Technology; catalog no. 2449S) (20), anti–Asymmetric Di-Methyl Arginine Motif (Cell Signaling Technology; catalog no.13522) (21), anti-histone H3 (Active Motif; catalog no. 39763) (22), anti–α-tubulin (Cell Signaling Technology; catalog no. 2144) (23), anti-PPARγ (Santa Cruz Biotechnology; catalog no. sc-7196) (24), anti-HSP90 (Cell Signaling Technology; catalog no. 4874S) (25), or anti–β-actin (Cell Signaling Technology; catalog no. 8457S) (26) antibody. Anti-mouse or anti-rabbit horseradish peroxidase secondary antibody was diluted in TBS with Tween 20 containing 5% milk and incubated for 90 minutes at room temperature. The blots were developed with ECL (Fisher Scientific; catalog no. 45-000-875). UCP1 protein levels were quantified using ImageJ software (National Institutes of Health).
Histology
Adipose tissues were fixed in 10% formalin at 4°C overnight. Paraffin embedding and hematoxylin and eosin (H&E) staining were performed by the University of Michigan Comprehensive Cancer Center Research Histology and Immunoperoxidase Laboratory. Images were captured using a Nikon E800. Inguinal adipocyte size was analyzed from H&E-stained sections of Prmt1fl/fl and Prmt1fl/flAQcre mice (n = 3 per genotype) using automated software (ImageJ-Adiposoft) according to the described methods (27). Adipocyte size was determined in at least 100 adipocytes from each animal. The measurements from three mice were pooled to calculate adipocyte size distribution.
Tissue triglyceride levels
Triglyceride levels were analyzed in homogenates of BAT using a triglyceride colorimetric assay kit (Cayman Chemical) according to the manufacturer’s instructions and were normalized with protein amount.
Mitochondrial DNA content
Genomic DNA was isolated from BAT. DNA extraction was performed by a conventional phenol-chloroform method using TRIzol (Sigma-Aldrich) according to the manufacturer’s instructions. Mitochondrial DNA copy number was determined by qPCR and analyzed by normalizing the mitochondrial-specific gene CoxI with the nuclear marker gene Hk2.
Tissue respiration
Tissue respiration was performed as previously described (15). BAT was freshly isolated and minced in respiration buffer [120 mM NaCl, 4.5 mM KCl, 2.5 mM glucose, 5 mM pyruvate, 2.5 mM malate, 0.7 mM Na2HPO4, 1.5 mM NaH2PO4, 0.5 mM MgCl2 (pH 7.4)]. Basal and uncoupled oxygen consumption was recorded for ∼1 minute for each stage using a Clark electrode (Strathkelvin Instruments). The uncoupled oxygen consumption was determined by adding 4 mg/mL oligomycin into the respiration chamber. The data were presented by normalizing the oxygen consumption rate with tissue weight.
Oil Red O staining
Cells were washed with PBS three times after removing medium. Formalin (10%) was added and incubated for 30 minutes to fix the cells. Cells were washed with PBS and then 60% isopropanol. Next, the cells were incubated with freshly prepared Oil Red O working solution (0.5% Oil Red O in isopropanol was diluted with distilled H2O at a ratio of 3:2 freshly and passed through a 0.45-µm syringe filter) for 10 minutes. Oil Red O retained in the cells was eluted with 100% isopropanol, and optical absorbance was measured at 500 nm. Images were captured using a Leica DM IRB inverted microscope and a Diagnostic Instruments spot camera.
Coimmunoprecipitation
Hemagglutinin (HA)-PRMT1 and PGC1α plasmids were described previously (17, 28). pcDNA3-HA-PRMT1V2 plasmid was generated from pcDNA3-HA-PRMT1 variant 1 (PRMT1V1) by self-ligation after amplification with 5′ phosphorylated primers 5′-GTGTCCTGTGGCCAGGCGGAAA-3′ and 5′-CTCCATGATGCAGTTCGCGGCC-3′ to delete the exon 2. The GFP control vector, pcDNA3-HA-PRMT1V2, and pcDNA3-PGC1α or both pcDNA3-HA-PRMT1V2 and pcDNA3-PGC1α were transfected into 293T cells. A total of 4 μg of DNA for each condition was transfected using the PEI method into 90% confluent 293T cells in 10-cm dishes. At 48 hours following transfection, cells were washed with cold PBS to remove the medium and lysed with 1 mL of prechilled coimmunoprecipitation (CoIP) buffer [20 mM Tris-HCl (pH 8.0), 137 mM NaCl, 1% Nonidet P-40, 2 mM EDTA] on a shaker at 4°C for 30 minutes. The lysate was centrifuged at 14,000g for 15 minutes at 4°C to pellet debris and the supernatant was placed into a fresh Eppendorf tube. After quantification by DC assay, 1 mg of protein for each group was incubated with anti-HA antibody (Cell Signaling Technology; catalog no. 3724) (29) overnight at 4°C with rotation. Five percent of the lysate was saved as input. Thirty microliters of protein A–agarose (Santa Cruz Biotechnology; catalog no. sc-2001) (30) was washed with CoIP buffer three times and then was added into protein lysate following rotation at 4°C for 3 hours. The beads were pelleted by quick spin and washed three times with CoIP buffer. After discarding the supernatant, 30 µL of 2× sample buffer was added to elute the immunoprecipitated proteins followed by boiling at 98°C for 5 minutes. Immunoprecipitated protein was subjected to immunoblotting as above, using primary anti-PRMT1 antibody (Cell Signaling Technology; catalog no. 2449S) (20) and anti-PGC1α antibody (Millipore; catalog no. ST1204) (31).
Virus construction and production
HA-PRMT1V1 and V2 constructs were cloned from pcDNA3-HA-PRMT1V1 andV2, respectively, with primers 5′-CCTAAGCTTATGGCTTACCCGTACGAC-3′ and 5′-GATATCTTATCAGCGCATCCGGTAGT-3′. The pAdTrack-CMV plasmid was amplified using primers 5′-GATGCGCTGATAAGATATCC GATCCACCGG-3′ and 5′-GTAAGCCATAAGCTTAGGCTCGAGCG-3′. pAdTrack-PRMT1V1 and V2 were assembled using the Gibson assembly method (New England BioLabs, E2611), and adenovirus was generated using the AdEasy system as described previously (32). Adenoviruses that overexpress GFP, PGC1α (17), PRDM16 (33), and adenoviral–oxygen-dependent degradation domain (ODD) luciferase–based thermogenic activity measurement (OLTAM) (34) were generated as described previously.
Luciferase reporter assays
Plasmids expressing PPARγ or PRDM16 were described previously (33). The Ucp1 luciferase reporter was constructed by cloning the promoter fragment containing 3.1 kb of the 5′ flanking region of the mouse Ucp1 gene into the pGL3 basic vector (Promega) upstream of the firefly luciferase–encoding region. 293T cells were plated onto a 12-well plate and transiently transfected using the PEI method with plasmid expressing PRMT1V2 (100 ng) or empty vector (100 ng) and PPARγ (100 ng) and PGC1α (100 ng) or PRDM16 (100 ng) in cotransfection assays together with 250 ng of Ucp1 promoter luciferase reporter construct. Cells were lysed 48 hours after transfection. Luciferase activity was measured by the a luciferase assay kit (Promega) according to the manufacturer’s recommendations, using a PerkinElmer EnSpire 2300 multilabel microplate reader.
ODD-luciferase (adenoviral-OLTAM) activity
ODD-luciferase activity was determined as previously described (34). Briefly, differentiated mouse primary EMSCs were infected with adenoviruses expressing adenoviral-OLTAM and GFP, PRMT1V2, or PGC1α. Cells were lysed at 3 days after infection and luciferase activity was measured by a luciferase assay kit (Promega) according to the manufacturer’s recommendations, using a PerkinElmer EnSpire 2300 multilabel microplate reader.
Statistical analysis
All data are reported as mean ± SEM. P values are presented as *P < 0.05, **P < 0.01, and ***P < 0.001. Variance normality was tested using Shapiro–Wilk tests. For normally distributed data, homogeneity of variances was assessed using a Levene test. For the data that were normally distributed and had equal variance, two-tailed Student t tests were used to test the difference between two groups. For the data that were not normally distributed or did not have equal variance, Mann–Whitney U tests were used. All analyses were performed using SPSS (IBM).
Results
Loss of PRMT1 in adipocytes reduces adaptive thermogenic capacity in brown and beige fat
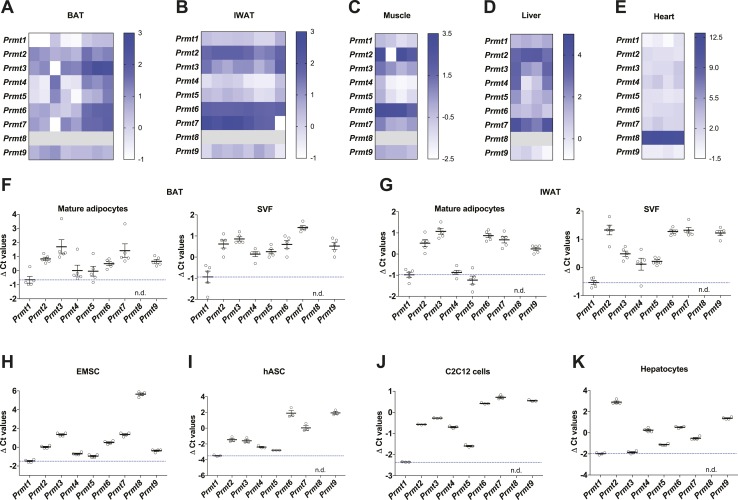
It has been previously reported that PRMT1 is involved in various cellular processes in metabolic organs such as muscle, liver, and heart (8, 35, 36). However, its role in thermogenic fat has not been elucidated yet. Prmt1 was identified as one of the top expressing Prmts in thermogenic brown and inguinal adipose tissues, as well as major metabolic organs including the skeletal muscle, liver, and heart from wild-type (WT) mice [Fig. 1A–1E; online repository (14)]. Adipose tissue is comprised of mature adipocytes and SVF containing preadipocytes, immune cells, endothelial cells, and other cell types. Prmt1 was the major form of type I Prmt in mouse primary mature adipocytes of BAT and IWAT (Fig. 1F and 1G). Prmt1 was also expressed at significant levels in many cultured cells, including adipocytes differentiated from murine EMSCs and hASCs that have thermogenic capacity, in addition to mouse C2C12 myocytes and primary hepatocytes (Fig. 1H–1K). These results suggest that PRMT1 may play a key role in metabolic regulation, including modulating thermogenic fat function.
Figure 1.
Prmt expression profiles in metabolic tissues. (A–E) Heat maps of mRNA expression levels of Prmts in major metabolic tissues, including (A) brown fat (n = 8), (B) inguinal fat (n = 8), (C) skeletal muscle (n = 4), (D) liver (n = 4), and (E) heart (n = 4) of WT mice. The Ct value was obtained by qPCR analyses and that of target genes for each sample was normalized with the expression of reference gene Tbp (ΔCt value). A low ΔCt value indicated with white means high mRNA expression levels. Each row and column corresponds to each Prmt subtype and a different sample, respectively. Prmt8 was not detectable in BAT, IWAT, muscle, and liver, and it was expressed at very low levels in the heart. (F and G) qPCR analyses of Prmts mRNA expression in mature adipocytes and SVF fractionated from (F) BAT (n =5) or (G) IWAT (n=5) of WT mice. (H–K) qPCR analyses of Prmts mRNA expression in different metabolic cell types, including differentiated (H) mouse primary EMSCs (n = 4), (I) human primary ASCs (n = 3), (J) C2C12 mouse muscle cells (n = 3), and (K) mouse primary hepatocytes (n = 4). Data are presented as mean ± SEM. n.d., not detected.
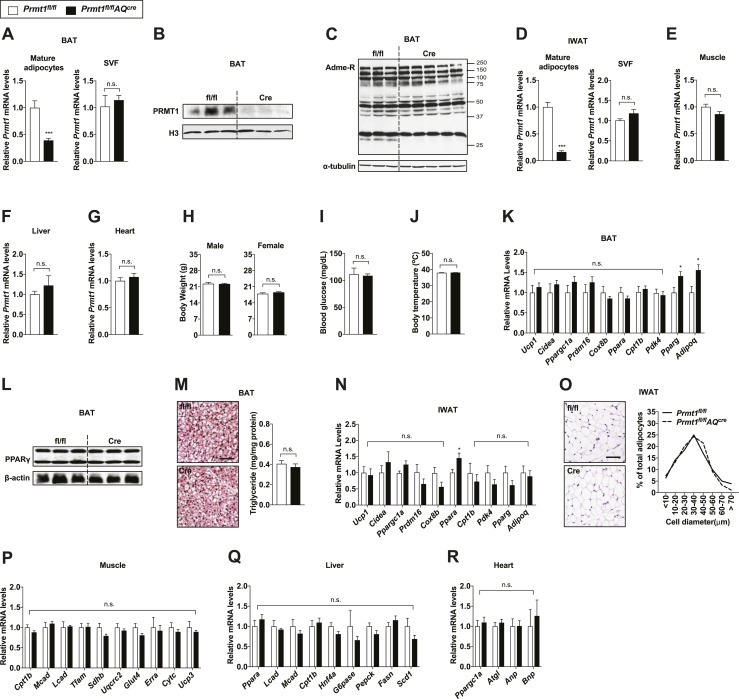
To examine the role of PRMT1 in adipose tissue, we generated mice in which the Prmt1 gene was deleted only in adipocytes (Prmt1fl/flAQcre) with no impact on Prmt1 expression levels in other metabolic organs such as skeletal muscle, liver, and heart [Fig. 2A–2G; online repository (14)]. It has been shown that type I PRMTs, including PRMT1, catalyze the formation of asymmetric arginine dimethylation (4). Minimal effects of Prmt1 deletion were observed on global asymmetric arginine demethylation profiles in both BAT and IWAT of Prmt1fl/flAQcre mice, presumably due to compensating effects from the other type I PRMTs, particularly Prmt6 [Fig. 2C; online repository (14)] (35, 37). In contrast to the more severe defects observed in other tissue-specific Prmt1 knockout mice, such as the central nervous system–specific Prmt1-deficient mice that suffer growth retardation and early death (38), no gross abnormalities were observed in Prmt1fl/flAQcre mice when housed at room temperature (23°C). Similar body weights, fasting blood glucose levels, and body temperature were observed between the two genotypes (Fig. 2H–2J). Thermogenic gene expression in BAT of Prmt1fl/flAQcre mice was comparable to their littermate Prmt1fl/fl controls (Fig. 2K). Even though the mRNA expression of adipogenic genes (Pparg and Adipoq) was slightly higher in BAT of Prmt1fl/flAQcre mice than that of the controls, we did not see differences in PPARγ protein levels and triglyceride levels in BAT between the two genotypes, suggesting minimal effects of PRMT1 in adipogenesis (Fig. 2L and 2M). Similarly, thermogenic and adipogenic gene expression was largely unaffected by adipose Prmt1 deletion in IWAT. A mild induction of Ppara mRNA levels was observed; however, expression levels of known PPARα target genes (Ucp1, Cidea, Cpt1b, and Pdk4) were not affected, indicating that the slight increase in Ppara mRNA by itself may not be of functional significance in this context (Fig. 2N) (39, 40). This is consistent with the observation that similar morphology and adipocyte size distribution in IWAT from Prmt1fl/fl and Prmt1fl/flAQcre mice was observed [Fig. 2O; online repository (14)]. We further assessed potential secondary impacts of adipose Prmt1 ablation in major metabolic tissues, such as skeletal muscle, liver, and heart, via determination of thermogenesis-related metabolic gene expression. No differences were seen in the mRNA levels of shivering genes (Cpt1b, Mcad, Lcad, Tfam, Sdhb, Uqcrc2, Glut4, Erra, Cytc, and Ucp3) in the skeletal muscle, glucose, and lipid metabolism genes (Ppara, Lcad, Mcad, Cpt1b, Hnf4a, G6pase, Pepck, Fasn, and Scd1) in the liver and lipolysis and cardiac hormone markers (Ppargc1a, Atgl, Anp, and Bnp) in the heart (Fig. 2P–2R).
Figure 2.
Adipocyte-specific Prmt1-deficient mice show normal metabolic phenotypes at the basal condition. (A) qPCR analyses of Prmt1 in mature adipocytes and SVF isolated from BAT of Prmt1fl/fl and Prmt1fl/flAQcre mice housed at room temperature (n = 5 per genotype). (B and C) Immunoblot analyses of (B) PRMT1 and (C) asymmetric-dimethylated arginine (Adme-R) in BAT from Prmt1fl/fl and Prmt1fl/flAQcre mice. Histone H3 and α-tubulin were used as loading controls. (D) qPCR analyses of Prmt1 in mature adipocytes and SVF isolated from IWAT of Prmt1fl/fl and Prmt1fl/flAQcre mice (n = 5 per genotype). (E–G) qPCR analyses of Prmt1 mRNA expression in (E) the skeletal muscle, (F) liver, and (G) heart of Prmt1fl/fl and Prmt1fl/flAQcre mice (n = 4 for genotype). (H) Body weight of 8-wk-old male (n = 6 for fl/fl, n = 7 for Cre) and female (n = 7 for fl/fl, n = 6 for Cre) Prmt1fl/fl and Prmt1fl/flAQcre mice. (I) Blood glucose levels of Prmt1fl/fl (n = 6) and Prmt1fl/flAQcre (n = 15) mice fasted for 4 h. (J) Rectal body temperature of Prmt1fl/fl (n = 6) and Prmt1fl/flAQcre (n = 15) mice housed at room temperature. (K) qPCR analyses of thermogenic markers in BAT of Prmt1fl/fl (n = 8) and Prmt1fl/flAQcre (n = 9) mice. (L) Immunoblot analyses of PPARγ in BAT of Prmt1fl/fl and Prmt1fl/flAQcre mice. β-Actin served as a loading control. (M) H&E-stained images (left) and triglyceride levels of BAT (right; n = 10 for fl/fl, n = 14 for Cre) from Prmt1fl/fl and Prmt1fl/flAQcre mice. Scale bar, 50 μm. (N) qPCR analyses of thermogenic gene expression in IWAT of Prmt1fl/fl (n = 7) and Prmt1fl/flAQcre (n = 8) mice. (O) H&E-stained images (left) and distribution of adipocyte size (right) of IWAT from Prmt1fl/fl and Prmt1fl/flAQcre mice. Scale bar, 50 μm. (P–R) qPCR analyses of shivering-, glucose/lipid metabolism-, and thermogenesis-related genes in the (P) skeletal muscle (n = 3 per genotype), (Q) liver (n = 4 for fl/fl, n = 8 for Cre), and (R) heart (n = 5 per genotype), respectively. Data are presented as mean ± SEM. *P < 0.05; ***P < 0.001. n.s., not significant (P > 0.05).
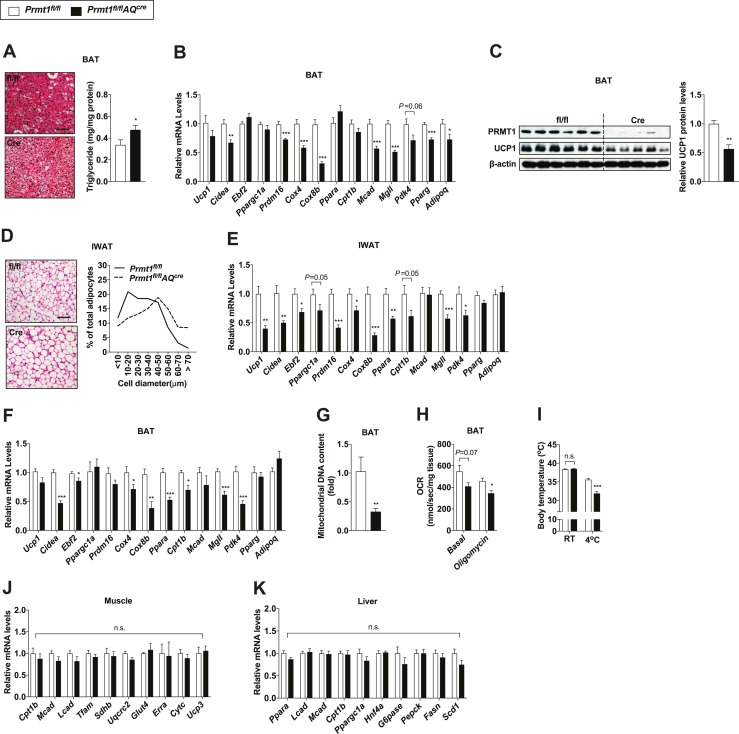
To investigate the potential function of PRMT1 in adaptive thermogenesis, we subjected age- and body weight–matched Prmt1fl/flAQcre mice and their littermate controls to cold exposure, which is known to activate adaptive thermogenesis in both brown and beige fat. Immunoblot analyses demonstrated that adipocyte PRMT1 was deleted in IWAT as well as BAT of Prmt1fl/flAQcre mice exposed to cold (14). Histological analysis revealed that after cold exposure, BAT of Prmt1fl/flAQcre mice contained more lipid droplets in comparison with that of Prmt1fl/fl mice (Fig. 3A). In contrast to that no differences between the two genotypes were observed at room temperature (23°C), which constitutes a mild cold stress (41), gene expression analysis revealed that cold-induced activation of the thermogenic program was defective in BAT from Prmt1fl/flAQcre mice when housed at a more challenging cold environment (10°C), including thermogenic genes (Ucp1 and Cidea), transcriptional regulators that have been shown to be important in thermogenic fat activation (Ebf2, Ppargc1a, and Prdm16), mitochondrial genes (Cox4 and Cox8b), fatty acid oxidation-related genes (Ppara, Cpt1b, and Mcad), and genes involved in regulation of lipid metabolism (Mgll and Pdk4) (Fig. 3B). Less induction of UCP1 protein in BAT of Prmt1fl/flAQcre mice was detected after cold exposure (Fig. 3C), even though Ucp1 mRNA levels were trending lower in BAT of Prmt1fl/flAQcre mice but did not reach statistical significance. Likewise, cold-exposed Prmt1fl/flAQcre mice had a lower proportion of small adipocytes but a greater proportion of large adipocytes in IWAT (Fig. 3D). Induction of thermogenic gene transcription was lower in IWAT of Prmt1fl/flAQcre mice in comparison with control tissues (Fig. 3E).
Figure 3.
Adipocyte-specific Prmt1-deficient mice display impaired adaptive thermogenesis. (A) H&E-stained images (left) and triglyceride levels of BAT (right; n = 8 for fl/fl, n = 12 for Cre) from Prmt1fl/fl and Prmt1fl/flAQcre mice exposed to cold (10°C) for 2 d. Scale bar, 50 μm. (B) qPCR analyses of thermogenic markers in BAT of Prmt1fl/fl (n = 8) and Prmt1fl/flAQcre (n = 7) mice after 2-d cold exposure (CE) (10°C). (C) Immunoblot analyses of PRMT1 and UCP1 (left) and quantification (right; n = 6 for fl/fl, n = 5 for Cre) of UCP1 levels in BAT of Prmt1fl/fl and Prmt1fl/flAQcre mice after 2-d CE (10°C). β-Actin was used as a loading control. (D) H&E-stained images (left) and distribution of adipocyte size (right) of IWAT from Prmt1fl/fl and Prmt1fl/flAQcre mice following 2-d CE (10°C). Scale bar, 50 μm. (E) qPCR analyses of thermogenic markers in IWAT of Prmt1fl/fl (n = 8) and Prmt1fl/flAQcre mice (n = 7) after 2-d CE (10°C). (F and G) qPCR analyses of (F) thermogenic gene expression (n = 6 for fl/fl, n = 7 for Cre) and (G) mitochondrial DNA copy number (n = 7 for fl/fl, n = 9 for Cre) in BAT from Prmt1fl/fl and Prmt1fl/flAQcre mice treated with 1 mg/kg/d CL 316,243 (CL) for 2 d at room temperature. Tissues were harvested 48 h after the first injection. (H) Oxygen consumption rate (OCR) in homogenates of BAT from Prmt1fl/fl and Prmt1fl/flAQcre mice pretreated with CL for 2 d and exposed to 4°C for 1 h (n = 6 per genotype). (I) Rectal body temperature of Prmt1fl/fl (n = 6) and Prmt1fl/flAQcre (n = 8) mice pretreated with CL for 2 d and exposed to 4°C for 1 h. (J and K) qPCR analyses of shivering- and lipid metabolism-related genes in (J) the skeletal muscle (n = 4 per genotype) and (K) liver (n = 9 for fl/fl, n = 7 for Cre), respectively, from Prmt1fl/fl and Prmt1fl/flAQcre mice exposed to cold (10°C) for 2 d. Data are presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.005. n.s., not significant (P > 0.05).
Similar defective thermogenic gene activation was also observed in both BAT and IWAT of Prmt1fl/flAQcre mice when we injected Prmt1fl/flAQcre mice and littermate control Prmt1fl/fl mice with CL 316,243, a β3-adrenergic receptor agonist [Fig. 3F; online repository (14)]. Consistent with these results, mitochondrial DNA copy number was lower in BAT of Prmt1fl/flAQcre mice than that of control animals after β-adrenergic stimulation (Fig. 3G). This defective thermogenic gene regulation in the absence of adipose Prmt1 led to impaired oxygen consumption rate in BAT of Prmt1fl/flAQcre mice and rendered them less capable of maintaining body temperature during acute cold stimulation (Fig. 3H and 3I). Shivering in skeletal muscle has been well reported to play a key role in the adaptive response to acute cold exposure (2). It has recently been reported that liver-derived lipids may influence brown fat function during cold exposure (42). No compensatory effects were observed on the transcriptional levels of thermogenesis-related metabolic genes in the skeletal muscle and liver from Prmt1fl/flAQcre mice after cold (Fig. 3J and 3K). Taken together, these data reveal an important role for PRMT1 in modulating thermogenic activation in fat.
PRMT1 interacts with PGC1α and stimulates thermogenic activation in a cell-autonomous manner
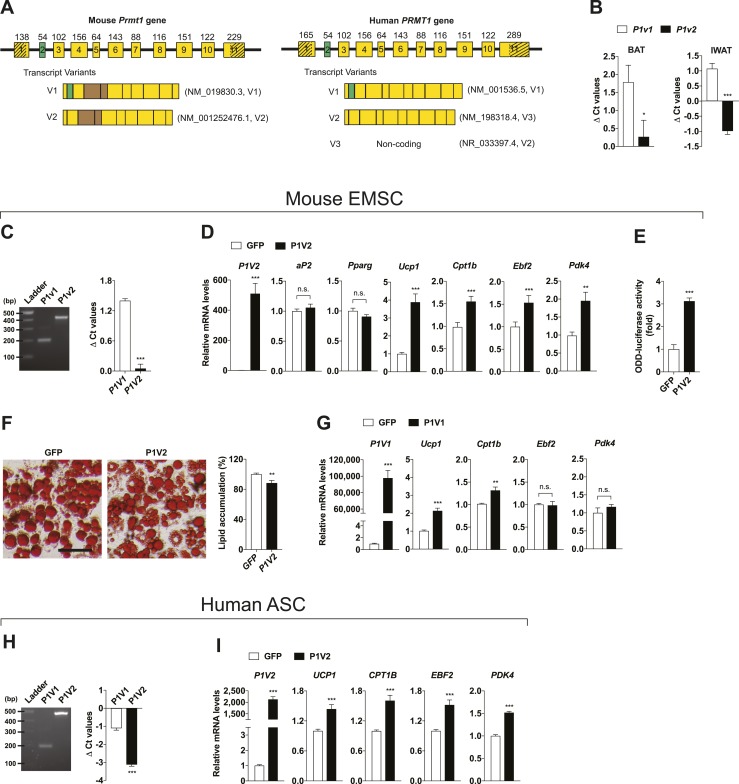
It is known that Prmt1 has different splicing variants that yield alternative isoforms with diverse enzyme activity, substrate specificity, and subcellular localization (43). Among them, PRMT1V1 and PRMT1V2 are well conserved between humans and mice. They are structurally parallel but differ from each other by a CRM1-dependent nuclear export sequence (NES). The CRM1-dependent NES is encoded by exon 2 (Fig. 4A). Both variants expressed at significant levels in mouse BAT and IWAT in which Prmt1v2 expression was relatively higher than Prmt1v1 (Fig. 4B). We decided to study how each isoform of PRMT1 functions in fat individually. We first tested PRMT1V2 using differentiated mouse primary EMSCs because it lacks an NES and has been shown to primarily localize within the nucleus (43). We first confirmed that the expression pattern of Prmt1v1 and Prmt1v2 in EMSCs was similar to that in thermogenic adipose tissues (Fig. 4C). We infected EMSCs isolated from Prmt1 adipocyte-specific deficient mice with adenovirus expressing GFP or PRMT1V2. PRMT1V2 overexpression did not affect adipogenesis (aP2 and Pparg) per se, but it significantly increased thermogenic gene expression (Fig. 4D). We adopted the OLTAM system that monitors intracellular hypoxia levels and thereby indicates cellular thermogenic activity. An induction of thermogenic gene expression by PRMT1V2 overexpression aligns well with the increased cellular thermogenic activity, which was indicated by higher ODD-luciferase activity in PRMT1V2 overexpressing cells (Fig. 4E), leading to reduced lipid accumulation due to increased fuel burning (Fig. 4F). Overexpression of PRMT1V1 in EMSCs isolated from Prmt1 adipocyte-specific deleted mice also increased thermogenic gene expression, albeit to a lesser extent in comparison with PRMT1V2 (Fig. 4G). We next studied whether the effect of PRMT1 is conserved in humans. Differentiated hASCs expressed PRMT1V1 and PRMT1V2 at significant levels in which PRMT1V2 expression was relatively higher than PRMT1V1 (Fig. 4H). We infected hASCs with adenoviruses expressing GFP or PRMT1V2 and found that thermogenic effects of PRMT1V2 were also observed in human subcutaneous adipocytes (Fig. 4I).
Figure 4.
PRMT1V2 (P1V2) stimulates thermogenic gene expression. (A) Schematic of mouse and human PRMT1 gene genomic structure. Exons are indicated as numbered boxes; the size (in bp) of the respective exons is indicated; 5′ untranslated region (UTR) and 3′ UTR are indicated as shadowed parts. In both of the Prmt1fl/flAQcre mice used in this study, exons 4 and 5 (in brown color) of Prmt1 are deleted in adipocytes. National Center for Biotechnology Information (NCBI) sequence reference numbers of each variants were as listed. It is of note that the “human PRMT1V2” in this study is annotated as transcript variant 3 at NCBI [NM_198318.4 (V3)], as the transcript variant 2 of human PRMT1 annotated at NCBI is noncoding [NR_033397.4 (V2)]. The alignment of murine and human amino acid sequences of PRMT1 reveals 99% identity between these two species. All of the plasmids related with PRMT1 that we used in this study were cloned from the human PRMT1 open reading frame. (B) qPCR analyses of Prmt1v1 (P1v1) and P1v2 mRNA expression in BAT and IWAT of WT mice (n = 5 per group). The Ct value of P1v1 or P1v2 for each sample was normalized with the expression of reference gene Tbp (ΔCt values). (C) qPCR analyses of P1v1 and P1v2 mRNA expression in differentiated mouse primary EMSCs (n = 4 per group). These indicate that the variants express at significant levels in murine adipocytes. Clear, single bands with predicted sizes were observed following amplification of the variants by qPCR and agarose gel electrophoresis, confirming the specificity of the qPCR primers. (D) qPCR analyses of P1V2, adipogenic and thermogenic markers after infection with adenoviruses overexpressing GFP or P1V2 in differentiated mouse primary EMSCs isolated from Prmt1fl/flAQcre mice (n = 6 per group). (E) ODD-luciferase activity in differentiated mouse primary EMSCs infected with adenoviruses overexpressing GFP or P1V2 after transduction of adenoviral-OLTAM (n = 6 per group). (F) Oil Red O staining (left) and its quantification (right; n = 16 per group) in differentiated EMSCs isolated from Prmt1fl/flAQcre mice after infection with adenoviruses overexpressing GFP or P1V2. Scale bar, 50 μm. (G) qPCR analyses of P1V1 and thermogenic markers in differentiated mouse primary EMSCs transduced with adenoviruses overexpressing GFP or P1V1 (n = 6 per group). (H) qPCR analyses of P1V1 and P1V2 mRNA expression in differentiated human primary ASCs (n = 4 per group). The specificity of the qPCR primers was confirmed by agarose gel electrophoresis using qPCR products. (I) qPCR analyses of thermogenic gene expression in differentiated human primary ASCs after infection with adenoviruses overexpressing GFP or P1V2 (n = 6 per group). Data are presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. n.s., not significant (P > 0.05).
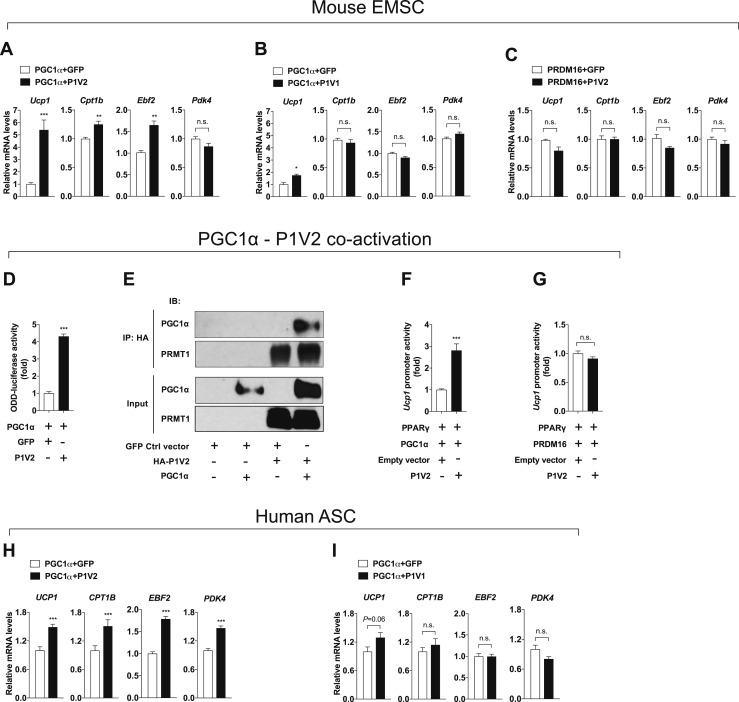
PGC1α is a transcriptional cofactor that regulates mitochondrial biogenesis and has many physiological functions in metabolism (11). It has been reported that PRMT1 modifies PGC1α and activates PGC1α downstream gene expression (44). However, whether PRMT1 can drive adipocyte-autonomous thermogenesis through PGC1α is not known. As expected, overexpression of PGC1α significantly increased thermogenic gene expression in EMSCs (14). This thermogenic activation was further enhanced when PRMT1V2 was co-overexpressed with PGC1α, whereas coactivation of PRMT1V1/PGC1α showed minimal effects, with a slight induction of Ucp1 (Fig. 5A and 5B). In contrast, no synergistic effects were observed between PRMT1V2 and PRDM16, another transcriptional regulator that controls thermogenic fat function [Fig. 5C; online repository (14)] (33). Induction of ODD-luciferase activity supported the finding that upregulated thermogenic gene expression by PRMT1V2/PGC1α coactivation indeed led to the activation of thermogenic activity in adipocytes (Fig. 5D). PRMT1 and PGC1α physically interact in cells, as shown by CoIP (Fig. 5E). The specific coactivation effects of PGC1α and PRMT1V2 were further confirmed using a reporter construct driven by the Ucp1 promoter (Fig. 5F and 5G). Consistent with the results in EMSCs, further effects of PGC1α were observed upon overexpression of PRMT1V2, but not upon overexpression of PRMT1V1, in hASCs (Fig. 5H and 5I). These data collectively support the notion that PRMT1, particularly PRMT1V2, stimulates thermogenic gene expression in adipocytes, at least in part through coactivation of PGC1α.
Figure 5.
PRMT1V2 (P1V2) interacts with PGC1α to modulate thermogenic activation. (A–C) qPCR analyses of thermogenic markers after infection with adenoviral vectors overexpressing GFP, PRMT1V1 (P1V1), or P1V2 along with adenoviral delivery of transcriptional regulators including (A and B) PGC1α or (C) PRDM16 in differentiated mouse primary EMSCs isolated from Prmt1fl/flAQcre mice (n = 6 per group). (D) ODD-luciferase activity in differentiated mouse primary EMSCs coinfected with adenoviral vectors overexpressing PGC1α and GFP or P1V2 after transduction of adenoviral-OLTAM (n = 6 per group). (E) CoIP of P1V2 with PGC1α. 293T cells were transfected with plasmids as indicated. Lysates were immunoprecipitated with an anti-HA antibody. Both input and immunoprecipitates were analyzed by immunoblotting as indicated. (F and G) Ucp1 promoter activity in 293T cells transiently transfected with Ucp1 promoter luciferase-reporter plasmid and vectors encoding transcriptional regulators PPARγ, (F) PGC1α or (G) PRDM16, and empty vector or P1V2 as indicated (n = 6 per group). (H and I) qPCR analyses of thermogenic gene expression after coinfection with adenoviral vectors overexpressing PGC1α and GFP, (H) P1V2 or (I) P1V1 in differentiated human primary ASCs (n = 6 per group). Data are presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. n.s., not significant (P > 0.05). IB, immunoblot.
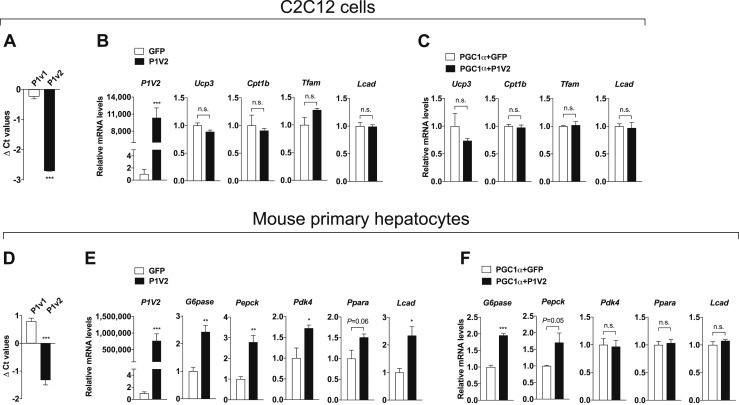
Because we observed that Prmt1 expresses at significant levels in the skeletal muscle and liver (Fig. 1), we further evaluated the effects of PRMT1V2/PGC1α coactivation in mouse C2C12 myocytes and primary hepatocytes. Similar to what we saw in thermogenic fat cell types, relatively higher levels of Prmt1v2 were detected compared with Prmt1v1 (Fig. 6A). However, the mRNA expression of PGC1α-dependent shivering genes was unchanged by overexpression of PRMT1V2 in C2C12 myocytes (Fig. 6B). Additionally, coactivation with PGC1α did not affect shivering gene expression in PRMT1V2-overexpressed C2C12 myocytes [Fig. 6C; online repository (14)]. In contrast to the myocytes, hepatocytes exhibited effects mediated by PRMT1V2/PGC1α coactivation. We first validated endogenous Prmt1v1 and Prmt1v2 expression patterns in isolated mouse primary hepatocytes (Fig. 6D). Key glucose (G6pase, Pepck, and Pdk4) and lipid metabolism (Ppara and Lcad) markers mediated by PGC1α were significantly upregulated in PRMT1V2 overexpressed primary mouse hepatocytes (Fig. 6E). Among them, gluconeogenic G6pase and Pepck were further induced by coactivation with PRMT1V2/PGC1α in comparison with overexpression of PGC1α alone [Fig. 6F; online repository (14)]. These data demonstrate cell type–specific effects of PRMT1V2 or PRMT1V2/PGC1α coactivation, suggesting that future therapeutic attempts targeting PRMT1 activity should take all affected metabolic organs into account.
Figure 6.
Metabolic potential of the coactivation through PRMT1V2 (P1V2)-PGC1α in muscle cells and hepatocytes. (A) qPCR analyses of Prmt1v1 (P1v1) and P1v2 mRNA expression in differentiated C2C12 mouse muscle cells (n = 3). (B) qPCR analyses of P1V2 and shivering-related genes in differentiated C2C12 cells transduced with adenoviral GFP or P1V2 (n = 3 per group). (C) qPCR analyses of shivering genes in differentiated C2C12 cells coinfected with adenoviral vectors overexpressing PGC1α and GFP or P1V2 (n = 4 per group). (D) qPCR analyses of P1v1 and P1v2 mRNA expression in mouse primary hepatocytes (n = 4). (E) qPCR analyses of P1V2 and metabolic genes in mouse primary hepatocytes transduced with adenoviral GFP or P1V2 (n = 4 per group). (F) qPCR analyses of glucose/lipid metabolism genes in mouse primary hepatocytes coinfected with adenovirus overexpressing PGC1α and GFP or P1V2 (n = 4 per group). Data are presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. n.s., not significant (P > 0.05).
Discussion
PRMT1 is a highly conserved PRMT, and germline deletion of Prmt1 leads to embryonic lethality, indicating that PRMT1 is essential during development (12). In this study, we revealed that loss of PRMT1 in mature adipocytes did not affect adipose tissue function at basal level. Note that it has been reported that PRMT1 regulates monocyte to macrophage differentiation in a PPARγ-dependent mechanism (13). In our study, loss of PRMT1 or overexpression of PRMT1 did not affect adipogenesis in fat cells, which was confirmed by analyses of mRNA and protein expression levels of adipogenic markers and overall comparable adipose morphology and lipid content in the fat of Prmt1fl/fl and Prmt1fl/flAQcre mice at the basal condition.
Our Prmt expression profiles demonstrated that Prmt1 is highly expressed in major metabolic organs and cell types, including thermogenic fat tissue and adipocytes. Unexpectedly, even though comparable mRNA levels of Prmt1 were observed in BAT and IWAT of WT mice, its protein levels were higher in IWAT than BAT, which could be explained by higher heterogeneous characteristics of IWAT compared with BAT (many nonfat cell types express high levels of PRMT1) (13, 45, 46) or the involvement of tissue-specific regulators modulating PRMT1 stability (14, 28, 47). This may also contribute to the fact that only a modest reduction in PRMT1 protein levels was observed in IWAT of Prmt1fl/flAQcre mice at the basal conditions (14). It is of note that PRMT1 protein levels in IWAT of Prmt1fl/flAQcre mice exposed to cold were significantly reduced compared with those of controls, likely due to tissue remodeling in IWAT through significant emergence of thermogenic fat upon cold (14, 48).
Mechanistically, we found that PRMT1 interacted with PGC1α in an adipocyte-autonomous manner to stimulate thermogenic gene expression. PGC1α is induced and activated in thermogenic fat during cold exposure and during β3-adrenergic receptor agonist administration, both transcriptionally and through posttranslational modifications (11). Upon cold exposure, the absence of PRMT1 (and thus absence of the PRMT1/PGC1α coactivation complex) rendered thermogenic activation defective in the BAT and IWAT of Prmt1fl/flAQcre mice. This synergistic effect was not observed between PRMT1 and PRDM16, another key regulator of thermogenic fat function. However, it is conceivable that other coregulators/partners besides PGC1α may work with PRMT1 in modulating its thermogenic function in fat. For example, Twist-1, a regulator of PRMT1 (49), has been reported to participate in thermogenic processes through an interaction with PGC1α in adipose tissue (50). Therefore, coactivation between Twist-1 and PRMT1 or among Twist-1, PGC1α, and PRMT1 may also influence PRMT1-dependent thermogenesis in fat. Consistent with this notion, PRMT1V1, the isoform that did not demonstrate significant coactivation with PGC1α, does lead to low levels of thermogenic gene induction when ectopically expressed in cultured adipocytes, but less so than PRMT1V2.
It was reported that PRMT1 regulates hepatic inflammation and lipogenesis through PGC1α-associated mechanisms (8). Recent studies using tissue-specific ablation of Prmt1 showed that arginine methylation through this molecule plays a key role in skeletal muscle and heart function (35, 36). Our further investigation using other metabolic cell types revealed that the coactivation between PRMT1 and PGC1α functions in a cell type–specific fashion and may have broader metabolic impacts. Although shivering-dependent thermogenic gene transcription was not affected by PGC1α/PRMT1 coactivation in myocytes, the regulation of key hepatic gluconeogenic gene transcription was dependent on the coactivation. Thus, PGC1α/PRMT1 coactivation may represent additional mechanisms for PRMT1-mediated hepatic gluconeogenesis, as reported previously (51, 52). It is of note that specific functions of PRMT1 vary in different metabolic contexts. On the one hand, decreased activities of PRMT1 in skeletal muscle, heart, and thermogenic fat lead to muscle atrophy, dilated cardiomyopathy, and defective thermogenesis respectively (Fig. 3) (35, 36). On the other hand, increased PRMT1 signaling may contribute to cancer progression (7, 53), increased hepatic gluconeogenesis (Fig. 6) (52), lipogenesis (8), and β-cell dysfunction (54). Deeper understanding of the regulation of PRMT1 signaling in different cell types is warranted before insights of PRMT1 function can be translated to fruitful clinical use.
In conclusion, our study provides evidence that Prmt1 ablation in adipocytes impairs thermogenic activation induced by cold exposure or β3-adrenergic stimulation. PRMT1 synergistically works with PGC1α, and the function of this PRMT1/PGC1α complex is conserved in both murine and human adipocytes. Insights gained from our study may ultimately lead to novel strategies for modulating thermogenic fat activity and energy homeostasis in humans.
Acknowledgments
We thank Dr. Jiandie Lin (Life Sciences Institute, University of Michigan) for providing the Ucp1 promoter luciferase-reporter plasmids and all of the colleagues in J.W.’s laboratory for helpful discussions.
Financial Support: This work was supported by National Institutes of Health Grant R01DK107583 and American Diabetes Association Grant 1-18-IBS-281 (to J.W.), American Heart Association Postdoctoral Fellowship 17POST33060001 (to D.K.), National Research Foundation Grant NRF-2019R1C1C1007040 (funded by the Ministry of Science and ICT of Korea; to D.K.), a Michigan Life Sciences Fellowship (to A.J.K.), and Chinese Scholarship Council Fellowships 201606100214 (to X.Q.) and 201806370290 (to Y.M.).
Author Contributions: X.Q., D.K., H.J., Y.M., A.J.K., M.-J.P., K.Z., J.H.L., and J.L. performed experiments. S.R. and S.A.W. provided Prmt1fl/fl mice. X.Q., D.K., H.J., and J.W. analyzed data. Y.L. provided research assistance. X.Q., D.K., H.J., and J.W. wrote the manuscript. J.W. oversaw the study.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- ASC
adipose stem cell
- BAT
brown adipose tissue
- CoIP
coimmunoprecipitation
- EMSC
ear mesenchymal stem cells
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- HA
hemagglutinin
- hASC
human adipose stem cell
- HBSS
Hanks balanced salt solution
- H&E
hematoxylin and eosin
- IWAT
inguinal white adipose tissue
- NES
nuclear export sequence
- ODD
oxygen-dependent degradation domain
- OLTAM
oxygen-dependent degradation domain luciferase–based thermogenic activity measurement
- PEI
polyethylenimine
- PGC1α
peroxisome proliferator–activated receptor γ coactivator 1-α
- PRMT
protein arginine methyltransferase
- PRMT1V1
protein arginine methyltransferase 1 variant 1
- PRMT1V2
protein arginine methyltransferase 1 variant 2
- qPCR
quantitative real-time PCR
- SVF
stromal vascular fraction
- Tbp
TATA-box binding protein
- UCP1
uncoupling protein 1
- WT
wild-type
References and Notes
- 1. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. [DOI] [PubMed] [Google Scholar]
- 2. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. [DOI] [PubMed] [Google Scholar]
- 3. Wu J, Jun H, McDermott JR. Formation and activation of thermogenic fat. Trends Genet. 2015;31(5):232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blanc RS, Richard S. Arginine methylation: the coming of age. Mol Cell. 2017;65(1):8–24. [DOI] [PubMed] [Google Scholar]
- 5. Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell. 2009;33(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang J, Frankel A, Cook RJ, Kim S, Paik WK, Williams KR, Clarke S, Herschman HR. PRMT1 is the predominant type I protein arginine methyltransferase in mammalian cells. J Biol Chem. 2000;275(11):7723–7730. [DOI] [PubMed] [Google Scholar]
- 7. Yang Y, Bedford MT. Protein arginine methyltransferases and cancer. Nat Rev Cancer. 2013;13(1):37–50. [DOI] [PubMed] [Google Scholar]
- 8. Park MJ, Kim DI, Lim SK, Choi JH, Kim JC, Yoon KC, Lee JB, Lee JH, Han HJ, Choi IP, Kim HC, Park SH. Thioredoxin-interacting protein mediates hepatic lipogenesis and inflammation via PRMT1 and PGC-1α regulation in vitro and in vivo. J Hepatol. 2014;61(5):1151–1157. [DOI] [PubMed] [Google Scholar]
- 9. Iwasaki H, Yada T. Protein arginine methylation regulates insulin signaling in L6 skeletal muscle cells. Biochem Biophys Res Commun. 2007;364(4):1015–1021. [DOI] [PubMed] [Google Scholar]
- 10. Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. [DOI] [PubMed] [Google Scholar]
- 11. Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–370. [DOI] [PubMed] [Google Scholar]
- 12. Yu Z, Chen T, Hébert J, Li E, Richard S. A mouse PRMT1 null allele defines an essential role for arginine methylation in genome maintenance and cell proliferation [published correction appears in Mol Cell Biol. 2017;37(17):e00298-17]. Mol Cell Biol. 2009;29(11):2982–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tikhanovich I, Zhao J, Olson J, Adams A, Taylor R, Bridges B, Marshall L, Roberts B, Weinman SA. Protein arginine methyltransferase 1 modulates innate immune responses through regulation of peroxisome proliferator-activated receptor γ-dependent macrophage differentiation. J Biol Chem. 2017;292(17):6882–6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qiao X, Kim D, Jun H, Ma Y, Knights AJ, Park MJ, Zhu K, Lipinski JH, Liao J, Li Y, Richard S, Weinman SA, Wu J. Data from: Protein arginine methyltransferase 1 interacts with PGC1α and modulates thermogenic fat activation. figshare 2019. Deposited 27 August 2019 10.6084/m9.figshare.8829227. [DOI] [PMC free article] [PubMed]
- 15. Jun H, Yu H, Gong J, Jiang J, Qiao X, Perkey E, Kim DI, Emont MP, Zestos AG, Cho JS, Liu J, Kennedy RT, Maillard I, Xu XZS, Wu J. An immune-beige adipocyte communication via nicotinic acetylcholine receptor signaling. Nat Med. 2018;24(6):814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rim JS, Mynatt RL, Gawronska-Kozak B. Mesenchymal stem cells from the outer ear: a novel adult stem cell model system for the study of adipogenesis. FASEB J. 2005;19(9):1205–1207. [DOI] [PubMed] [Google Scholar]
- 17. Wu J, Ruas JL, Estall JL, Rasbach KA, Choi JH, Ye L, Boström P, Tyra HM, Crawford RW, Campbell KP, Rutkowski DT, Kaufman RJ, Spiegelman BM. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1α/ATF6α complex. Cell Metab. 2011;13(2):160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α null mice. Cell. 2004;119(1):121–135. [DOI] [PubMed] [Google Scholar]
- 19. RRID:AB_10572490, https://scicrunch.org/resolver/AB_10572490.
- 20. RRID:AB_2237696, https://scicrunch.org/resolver/AB_2237696.
- 21. RRID:AB_2665370, https://scicrunch.org/resolver/AB_2665370.
- 22. RRID:AB_2650522, https://scicrunch.org/resolver/AB_2650522.
- 23. RRID:AB_2210548, https://scicrunch.org/resolver/AB_2210548.
- 24. RRID:AB_654710, https://scicrunch.org/resolver/AB_654710.
- 25. RRID:AB_2121214, https://scicrunch.org/resolver/AB_2121214.
- 26. RRID:AB_10950489, https://scicrunch.org/resolver/AB_10950489.
- 27. Galarraga M, Campión J, Muñoz-Barrutia A, Boqué N, Moreno H, Martínez JA, Milagro F, Ortiz-de-Solórzano C. Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. J Lipid Res. 2012;53(12):2791–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim D, Lim S, Park M, Choi J, Kim J, Han H, Yoon K, Kim K, Lim J, Park S. Ubiquitination-dependent CARM1 degradation facilitates Notch1-mediated podocyte apoptosis in diabetic nephropathy. Cell Signal. 2014;26(9):1774–1782. [DOI] [PubMed] [Google Scholar]
- 29. RRID:AB_1549585, https://scicrunch.org/resolver/AB_1549585.
- 30. RRID:AB_10201241, https://scicrunch.org/resolver/AB_10201241.
- 31. RRID:AB_10807905, https://scicrunch.org/resolver/AB_10807905.
- 32. Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, Vogelstein B, He TC. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2(5):1236–1247. [DOI] [PubMed] [Google Scholar]
- 33. Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6(1):38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim DI, Liao J, Emont MP, Park MJ, Jun H, Ramakrishnan SK, Lin JD, Shah YM, Omary MB, Wu J. An OLTAM system for analysis of brown/beige fat thermogenic activity. Int J Obes. 2018;42(4):939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choi S, Jeong HJ, Kim H, Choi D, Cho SC, Seong JK, Koo SH, Kang JS. Skeletal muscle-specific Prmt1 deletion causes muscle atrophy via deregulation of the PRMT6-FOXO3 axis. Autophagy. 2019;15(6):1069–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pyun JH, Kim HJ, Jeong MH, Ahn BY, Vuong TA, Lee DI, Choi S, Koo SH, Cho H, Kang JS. Cardiac specific PRMT1 ablation causes heart failure through CaMKII dysregulation. Nat Commun. 2018;9(1):5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dhar S, Vemulapalli V, Patananan AN, Huang GL, Di Lorenzo A, Richard S, Comb MJ, Guo A, Clarke SG, Bedford MT. Loss of the major type I arginine methyltransferase PRMT1 causes substrate scavenging by other PRMTs. Sci Rep. 2013;3(1):1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hashimoto M, Murata K, Ishida J, Kanou A, Kasuya Y, Fukamizu A. Severe hypomyelination and developmental defects are caused in mice lacking protein arginine methyltransferase 1 (PRMT1) in the central nervous system. J Biol Chem. 2016;291(5):2237–2245.All data generated or analyzed during this study are included in this published article or in the data repositories listed in References. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rakhshandehroo M, Knoch B, Müller M, Kersten S. doi: 10.1155/2010/612089. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res . 2010; 2010 :612089. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barberá MJ, Schluter A, Pedraza N, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor α activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem. 2001;276(2):1486–1493. [DOI] [PubMed] [Google Scholar]
- 41. Fischer AW, Cannon B, Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Mol Metab. 2018;7:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Simcox J, Geoghegan G, Maschek JA, Bensard CL, Pasquali M, Miao R, Lee S, Jiang L, Huck I, Kershaw EE, Donato AJ, Apte U, Longo N, Rutter J, Schreiber R, Zechner R, Cox J, Villanueva CJ. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab. 2017;26(3):509–522.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goulet I, Gauvin G, Boisvenue S, Côté J. Alternative splicing yields protein arginine methyltransferase 1 isoforms with distinct activity, substrate specificity, and subcellular localization. J Biol Chem. 2007;282(45):33009–33021. [DOI] [PubMed] [Google Scholar]
- 44. Teyssier C, Ma H, Emter R, Kralli A, Stallcup MR. Activation of nuclear receptor coactivator PGC-1α by arginine methylation. Genes Dev. 2005;19(12):1466–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fan Z, Li J, Li P, Ye Q, Xu H, Wu X, Xu Y. Protein arginine methyltransferase 1 (PRMT1) represses MHC II transcription in macrophages by methylating CIITA. Sci Rep. 2017;7(1):40531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ishimaru T, Ishida J, Kim JD, Mizukami H, Hara K, Hashimoto M, Yagami KI, Sugiyama F, Fukamizu A. Angiodysplasia in embryo lacking protein arginine methyltransferase 1 in vascular endothelial cells. J Biochem. 2017;161(3):255–258. [DOI] [PubMed] [Google Scholar]
- 47. Lai Y, Li J, Li X, Zou C. Lipopolysaccharide modulates p300 and Sirt1 to promote PRMT1 stability via an SCFFbxl17-recognized acetyldegron. J Cell Sci. 2017;130(20):3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19(10):1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Avasarala S, Van Scoyk M, Karuppusamy Rathinam MK, Zerayesus S, Zhao X, Zhang W, Pergande MR, Borgia JA, DeGregori J, Port JD, Winn RA, Bikkavilli RK. PRMT1 is a novel regulator of epithelial-mesenchymal-transition in non-small cell lung cancer. J Biol Chem. 2015;290(21):13479–13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pan D, Fujimoto M, Lopes A, Wang YX. Twist-1 is a PPARδ-inducible, negative-feedback regulator of PGC-1α in brown fat metabolism. Cell. 2009;137(1):73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yamagata K, Daitoku H, Takahashi Y, Namiki K, Hisatake K, Kako K, Mukai H, Kasuya Y, Fukamizu A. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol Cell. 2008;32(2):221–231. [DOI] [PubMed] [Google Scholar]
- 52. Choi D, Oh KJ, Han HS, Yoon YS, Jung CY, Kim ST, Koo SH. Protein arginine methyltransferase 1 regulates hepatic glucose production in a FoxO1-dependent manner. Hepatology. 2012;56(4):1546–1556. [DOI] [PubMed] [Google Scholar]
- 53. Choucair A, Pham TH, Omarjee S, Jacquemetton J, Kassem L, Trédan O, Rambaud J, Marangoni E, Corbo L, Treilleux I, Le Romancer M. The arginine methyltransferase PRMT1 regulates IGF-1 signaling in breast cancer. Oncogene. 2019;38(21):4015–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lv L, Chen H, Sun J, Lu D, Chen C, Liu D. PRMT1 promotes glucose toxicity-induced β cell dysfunction by regulating the nucleo-cytoplasmic trafficking of PDX-1 in a FOXO1-dependent manner in INS-1 cells. Endocrine. 2015;49(3):669–682. [DOI] [PubMed] [Google Scholar]