Abstract
Objectives
Methanogenic archaea have been found to make up part of the bioaerosols in pig, cattle, and poultry farms. So far no attempts have been made to determine how season, farm type, and farm characteristics may affect workers’ exposure to archaea.
Methods
Personal filter samples from 327 farmers working on 89 Danish farms were analysed for the number of 16S rRNA gene copies from archaea and bacteria and for their dust and endotoxin content. The farms were visited during summer and winter. Information on farm type and stable characteristics were collected using self-reported activity diaries and walk-through surveys. Differences in archaea and bacteria levels with farm type and stable characteristics and correlations with dust and endotoxin levels were examined.
Results
Personal archaea exposure was documented in all farm types including, for the first time, during mink farming. At 7.3*104 gene copies m−3 the archaea levels were around two orders of magnitude lower than bacteria levels at 5.7*106 gene copies m−3. At 1.7*105 gene copies m−3 among pig farmers and 1.9*104 gene copies m−3 among cattle farmers the archaea levels differed with farm type (P < 0.0005). The archaea and bacteria levels correlated weakly with a Pearson correlation coefficient of 0.17. Neither archaea nor bacteria levels differed by season. In pig farms the archaea levels differed by type of ventilation and by wetness of the floor.
Conclusions
Archaea levels were not neglible and appeared to vary greatly between farm types. In pig farms they varied with some farm characteristics. Archaea levels appeared to depend on factors that differed from those of bacteria.
Keywords: agriculture, archaea, bacteria, bioaerosols, endotoxin, exposure assessment, personal sampling, ventilation
Introduction
Bioaerosol exposure is considered an important determinant of occupational health among workers in livestock farms. Bioaerosols are composed of numerous microorganisms and related compounds.
Methanogenic archaea, obligate anaerobic microorganisms most commonly associated with extreme environments such as submarine sediments (Niemann et al., 2006), have been found to make up a part of the bioaerosols in Canadian pig and dairy farms (Nehmé et al., 2009; Blais Lecours et al., 2012). Although archaea are not known to be pathogenic to humans, the dead organisms or products from their degradation can become aerosolized. In a murine model, methanogenic archaea were found to cause pulmonary inflammation by accumulation of leukocytes and induction of histopathological changes (Blais Lecours et al., 2011). As the adverse effects of exposure to bioaerosols in livestock farms have not been fully explained by other components it is possible that archaea contribute to adverse reactions by inhalation. The mechanisms could be similar to those of other microbial associated molecular patterns (Sigsgaard et al., 2008) independent of the pathogenicity of the microbes in bioaerosols.
Endotoxins from gram-negative (G−) bacteria, peptidoglycans from gram-positive (G+) bacteria, and β-glucans from moulds are the immunogenic microbial membrane compounds that have been most studied in farm bioaerosols. In addition, membrane lipids from archaea are known to have immunogenic properties (Sprott et al., 1997) and can be used as adjuvants to potentiate immune responses to vaccines by intravenous as well as intranasal administration (Patel et al., 2010). Archaea have been sparsely studied in environments occupied by humans but have been found in urban aerosols (Brodie et al., 2007) in addition to aerosols from pig, dairy and poultry farms (Just et al., 2013).
Endotoxins are found at extremely high concentrations during work in pig and poultry farms (Létourneau et al., 2010; Basinas et al., 2012; O’shaughnessy et al., 2012). Although endotoxins are very important contributors to the health effects of livestock farm aerosols, they do not act alone. Health effects of bioaerosols are rarely explained by a single component but rather by a diversity of microbial components (Poole and Romberger, 2012). Effects of these aerosols range from episodes of flu-like cough and fever over worsening of existing airway disease to reduced pulmonary function (May et al., 2012). Prolonged exposure may result in chronic obstructive pulmonary disease and asthma but may also be protective against allergic asthma (Wunschel and Poole, 2016). As many farms have become large concentrated animal farming operations in recent decades full-time work inside animal houses has become more common resulting in prolonged exposure to the environment among some workers. Hence, it is important to identify working conditions that can be improved. In Canadian swine confinement buildings, higher levels of bioaerosols including archaea have been observed during winter than during summer (Nehmé et al., 2009).
As an extension of the SUS (Sund Stald, i.e. healthy stables) study of Danish farmers (Elholm et al., 2010) we therefore applied molecular methods (Blais Lecours et al., 2012) for the identification of archaea and bacteria in bioaerosols from personal exposure samples among farmers.
Our aim was to investigate how personal exposure to archaea varies with type and characteristics of farms and of tasks conducted on these farms. Further, we wanted to identify the numerically most important bacteria and archaea and to compare their concentrations with previous studies.
We hypothesized that in addition to the well-known richness in G+ and G− bacteria, methanogenic archaea would also be abundant in the air breathed by Danish pig and cattle farmers and that they could be identified in other types of animal farming too. We hypothesized that the levels would be higher during winter than during summer.
Materials and methods
Selection of farms and farmers
Details on the aims and design of the original SUS study of all students from farming schools in Denmark in 1992–1994 and the 15-year follow-up can be found elsewhere (Sigsgaard et al., 1997; Elholm et al., 2010). So can the details on the selection of farms and on the exposure assessment for this study (Basinas et al., 2012). A simplified table of farm characteristics is provided as Supplementary Table 1 (available at Annals of Occupational Hygiene online). In brief we identified 423 subjects from the original SUS cohort who were still full-time employed in farming. Of these 75 pig, 33 cattle, and 3 mink farmers were randomly selected and approached if they were still full-time employed in the region of Jutland, in a primary pig, mink or cattle farm. Fifty-four pig farms, 26 cattle farms, and 3 mink farms of which one combined pig, cattle, and mink and three combined pig and cattle were enrolled. Sampling was performed separately during work in each of these types of farming if possible on every farm. In addition, field work was performed on 16 of these farms and separate samples were taken during this activity. In addition to the farms on which participants from the SUS study worked, three poultry farms with eight workers (two layer and one broiler farm) were included from the Danish Agricultural Advisory Service. All workers on the enrolled farms including those who were not from the original SUS-cohort were invited to participate in this study. If the farm owner and the participant were different, the farm owner was also asked to give consent. Twenty-eight farmers were either excluded (e.g. due to health, employment, or contacting problems), were unable to, or refused to participate. The resulting number of 327 participating workers included more than 90% of the total workforce in the 85 farms that were visited (Basinas et al., 2012, 2013).
Farm visits
Information on the farms (e.g. number of employees and units, type of production, locations, number and type of animals) was obtained during interviews. Two visits were scheduled for all the selected pig and cattle farms. Summer visits took place between 1 May and 1 October and winter visits between 17 November and 3 April. During each farm visit detailed walk-though surveys were performed. For every compartment of the farm general farm and production and management characteristics (e.g. ventilation type, flooring type, applied feeding, bedding and manure handling practices) were registered. The researchers also evaluated the general hygienic condition present on the compartment including the level of wetness of the floor (dry versus wet) and the accumulation of manure (low, medium, high) present. Farm characteristics were considered present when farmers spent more than a certain proportion of their in-stable time working in their presence (Basinas et al., 2013). Cutoff levels were >80% for dry and wet feed, >60% for mechanical ventilation, >50% for full slatted or mostly slatted floors, and >80% for wet or dry floor. Almost all farms combined animal with crop production. All workers on the selected farms were included in the measurements. Sampling for those working on pig, cattle, mixed, and poultry layer farms was performed during their whole work-shift including both field and stable work. Mink farms were visited during the breeding, whelping, furring and pelting phases and the broiler farm during the preparation of the stables and when the chicks aged 1–2 days, 21–22 days, and 1–7 days before being harvested. Measurements in broiler farms were task-based performed only when workers were involved in the corresponding production activity of interest. In all cases but two, only one or two personal inhalable dust samples were obtained. Two of the five workers on broiler farms contributed each three and five personal inhalable dust samples. All measurements were performed on randomly chosen workdays in 2008 and 2009.
Dust and endotoxin analyses
As published previously in (Basinas et al., 2012) personal inhalable dust samples were collected on glass fibre filters at a flow rate of 3.5 l min−1. The amount of collected dust on the filters was determined gravimetrically on a scale with a 0.0001 mg precision after a 24-h desiccation period. Results were calculated as mg m−3. Following gravimetric analysis the samples were stored at −20°C. Filter extraction was performed in pyrogen-free water with 0.05% (v/v) Tween-20. The sample tubes were quaked vigorously and centrifuged for 15 min at 1.000 g at room temperature. The concentration of endotoxin in the extracts was determined using a quantitative kinetic chromogenic Limulus Amboecyte Lysate test. The extracts were analysed in duplicates in a 1:200 dilution and a 12-point standard curve obtained from an Escherichia coli reference with a range of 0.01–25 EU ml−1 used to determine the concentration of endotoxin.
DNA extraction, quantitative real-time polymerase chain reaction (PCR), denaturing gradient gel electrophoresis (DGGE), sequencing, and phylogenetic analysis
Detailed descriptions of these methods have been published in (Nehmé et al., 2009) and slightly modified in (Blais Lecours et al., 2012) and (Just et al., 2013). In brief a Qiagen QIAamp DNA extraction kit (Qiagen, Mississauga, ON, Canada) was used for total DNA extraction from airborne dust according to the manufacturer’s instructions with the required modification for bacteria. PCR amplifications were carried out on a DNA Engine Opticon 2 (Bio-Rad Laboratories, Mississauga, ON, Canada). PCR for archaeal 16S rRNA genes was optimized for archaea-only amplification without bias for any archaeal phylum and was conducted using 0.5 µM A751F and A976R primers. Tenfold serial dilutions of methanogenic archaea Methanosarcina mazei DNA (ATCC BAA-159D) were used for the standard curve. PCR for bacterial 16S rRNA genes was conducted using the primers, probe, and amplification program used by Bach et al. (2002) with the iQ Supermix (Bio-Rad Laboratories, Hercules, CA, USA). Tenfold serial DNA dilutions of plasmid containing (ATCC 25922) 16S rRNA gene sequences were used for the standard curve. Field blanks and negative controls were included to detect PCR reagent contamination. Amplification programs were as described in (Blais Lecours et al., 2012). Data were acquired using the Opticon monitor software (Bio-Rad, version 2.02.24). The threshold was determined by the software with a standard deviation of one. Results are expressed as absolute 16S rRNA copy (n m−3).
PCR DGGE was used to separate different archaeal sequences and for reamplification and sequencing. Archaeal 16S rDNA amplification was conducted using 0.5 µM A333F and A751R(GC) primers, 3.5 mM MgCl2, 100 µM dNTP, 5% (v/v) dimethyl sulfoxide, 2.5 U Taq (Promega) polymerase, and 2 µl DNA template in a 50-µl reaction mixture. The amplification programs were performed as previously described (Gilbert et al., 2010; Blais Lecours et al., 2012). Following 1% agarose gel electrophoresis, DNA was quantified by comparing band intensities to the Bio-Rad molecular mass ladder (Bio-Rad Laboratories, Mississauga, ON, Canada) measured with GeneTools software (Syngene, Cambridge, UK). A total of 100 ng of amplified DNA was loaded in 8% polyacrylamide gels with 25 to 65% denaturing gradient gels. Electrophoresis and DNA fragment staining were carried out. For the sequencing analysis DNA from gel bands was excised using a micropipette tip and put into PCR mix for reamplification. Amplicons were sequenced and proofread with FinchTV 1.4.0 software (Geospiza; PerkinElmer, Seattle, WA, USA). Amplicons that did not result in high-quality sequences were cloned into Qiagen pDrive cloning vector using the Qiagen PCR cloning kit. The DNA constructions were transformed into homemade E. coli DH5α competent cells with the electroporation method using the Bio-Rad Gene Pulser apparatus (Bio-Rad Laboratories) equipped with a Bio-Rad pulse controller as described by the manufacturer (Bio-Rad Laboratories, La Jolla, CA, USA).
Each DNA sequence obtained was compared to sequences available in databases by using basic local alignment search tool nucleotide from the National Center of Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/). Sequences’ affiliations to known genera or species were assessed regarding their similarity.
Statistical analyses
All exposure data including archaeal and bacterial RNA data showed a log-normal distribution and LN-transformation was applied. A total of six values of endotoxin and dust were below the detection limit and were replaced by multiple imputation. Geometric means (GM) and geometric standard deviations (GSD) were calculated for statistical analyses. Pearson correlation tests were applied to the exposure data. One-way analysis of variance tests were performed to test for effects of the season, type of farm work and farm characteristics on the exposure levels. As sampling for most of the farmers included work inside several stables, for the purpose of the analyses farm characteristics were allocated following an analysis of the proportion of time spent by the farmers in presence of each type of characteristic as previously described (Basinas et al., 2013). The general linear model test was used for two-way analyses with Bonferroni correction for post hoc tests of effects of farm characteristics on archaea and bacteria levels. P-values < 0.05 were considered statistically significant. IBM SPSS Statistics version 25 was used to perform the statistical analyses.
Results
A total of 327 workers from 89 farms contributed 507 personal measurement samples; collected with a mean (SD) sampling time of 339 (102) min. Of these 476 archaeal rRNA and 491 bacterial rRNA measurements were of sufficient quality. Both archaea and bacteria were detected in all types of farm work. Methanobrevibacter species predominated among both pig and cattle farmers as listed in Table 1. There were statistically significant positive correlations between archaea and bacteria, dust, and endotoxin levels as shown in Table 2. The correlation between archaea and bacteria could only be observed during winter (data not shown). The weakest correlation (r = 0.17) was observed between archaea and bacteria. Correlations of similar magnitudes were observed during both seasons.
Table 1.
Archaeal species identified on farms in the study, listed in order of frequency.
| Species | Farm types |
|---|---|
| Methanobrevibacter sp. | pigs, cattle |
| Methanobrevibacter smithii | pigs |
| Methanogenic archaeon | pigs, cattle, chicken |
| Methanosarcina sp. | cattle, chicken |
| Halorubrum sp. | pigs, cattle |
| Archaeon 26-a134 | pigs |
| Archaeon 26-5a1 | pigs |
| Archaeon 26-4a6 | pigs |
Table 2.
Pearson correlations between log concentrations of archaea 16S rRNA (n m−3), bacteria 16S rRNA (n m−3), dust (mg m−3), and endotoxin (EU m−3) in the study.
| Exposure | Archaea | Bacteria | Dust | Endotoxin |
|---|---|---|---|---|
| Archaea | 0.17* | 0.34* | 0.22* | |
| Bacteria | 0.20* | 0.17* | ||
| Dust | 0.63* | |||
| Endotoxin |
N ranges from 476 to 507.
*P < 0.0005.
Correlations between bioaerosol components within the different types of farm work are shown in Table 3. Archaea and bacteria 16S rRNA gene levels correlated most strongly during work with broilers, field work, and on cattle farms, but did not correlate in pig farms. During field work positive correlations were observed between all components. Positive but non-significant associations between all three components were found during work with mink (n = 7).
Table 3.
Pearson correlations between log concentrations of archaea 16S rRNA (n m−3), bacteria 16S rRNA (n m−3), dust (mg m−3), and endotoxin (EU m−3) across different types of farm work involved.
| Exposure | Archaea | Bacteria | Dust | Endotoxin |
|---|---|---|---|---|
| Pig farming | ||||
| Archaea | 0.11 | 0.27*** | −0.11 | |
| Bacteria | 0.13* | 0.06 | ||
| Dust | 0.48*** | |||
| Endotoxin | ||||
| Cattle farming | ||||
| Archaea | 0.26** | −0.12 | 0.02 | |
| Bacteria | 0.35*** | 0.39*** | ||
| Dust | 0.51*** | |||
| Endotoxin | ||||
| Field work | ||||
| Archaea | 0.54* | 0.74** | 0.53* | |
| Bacteria | 0.48* | 0.46* | ||
| Dust | 0.61*** | |||
| Endotoxin | ||||
| Mink farming | ||||
| Archaea | 0.37 | 0.27 | 0.18 | |
| Bacteria | 0.45 | 0.72 | ||
| Dust | 0.49 | |||
| Endotoxin | ||||
| Broiler work | ||||
| Archaea | 0.63* | −0.20 | −0.17 | |
| Bacteria | 0.31 | −0.32 | ||
| Dust | 0.83* | |||
| Endotoxin |
N in pig farming 320–340; in cattle farming 113–122; in field work 15–20; in mink farming 7; and in broiler work 11.
***P < 0.0005, **P < 0.005, *P < 0.05.
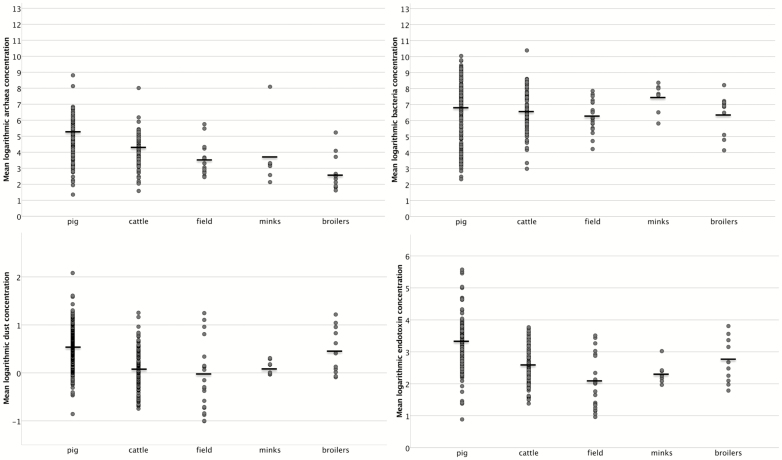
The concentrations (GM) of archaeal and bacterial 16S rRNA gene copies, endotoxin and dust during the different types of farm work are shown in the fig. 1. Neither the archaeal nor the bacterial gene levels differed with season. The mean archaeal gene level was approximately 100-fold lower than the bacteria level (P < 0.0005). This ratio between archaea and bacterial 16S rRNA gene copy concentrations varied greatly between types of farm work, being highest (0.024) in pig farming, intermediate (0.005) in cattle farming, and lowest in mink and broiler farming (0.0002).
Figure 1.
Personal inhalable logarithmic and geometric mean concentrations of archaea RNA (n m−3), bacteria RNA (n m−3), dust (mg m−3), and endotoxin (EU m−3) by type of farm work.
Archaeal 16S rRNA gene levels varied significantly (P < 0.0005) with the type of farm work. This variation with type of work was similar during summer and winter (data not shown). The archaea concentrations were highest during work with pigs, followed by work with layers (n = 3) and cattle. Work with broilers was associated with the lowest archaea exposure. In contrast, bacterial 16S rRNA gene levels did not vary much between the types of farm work, being highest during mink handling. There were several fold variations between individual samples in both the archaea and bacteria levels within all types of farm work.
As shown in Table 4, among pig farm workers archaea, but not bacteria concentrations, differed by type of ventilation (P = 0.004) and by floor conditions (P < 0.0005), but not by feed type or floor construction. Archaea concentrations were higher with neutral pressure ventilation than with mixed types (including natural) and negative pressure ventilation. The wetness of the animal house floor also affected archaea with concentrations being higher when working during mixed dry and wet conditions than during wet floor conditions.
Table 4.
Personal inhalable concentrations of archaea (n m−3) and bacteria 16S rRNA (n m−3) among pig farmers by characteristics of the farm.
| Archaea n m−3 | Bacteria n m−3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Farm characteristics | n | GM | GSD | Range | P | GM | GSD | Range | P | ||
| Ventilation |
|||||||||||
| Neutral | 15 | 5.2E+05 | 2.7E+00 | 1.0E+05 | 2.6E+06 | 0.004 | 4.1E+06 | 9.8E+01 | 6.3E+03 | 2.8E+09 | 0.81 |
| Mixed type | 37 | 7.0E+04 | 2.1E+01 | 8.6E+01 | 1.3E+08 | 8.8E+06 | 3.5E+01 | 2.5E+03 | 1.7E+09 | ||
| Negative pressure | 271 | 1.9E+05 | 7.3E+00 | 2.2E+01 | 6.4E+08 | 7.4E+06 | 4.5E+01 | 2.1E+02 | 1.1E+10 | ||
| Feed type |
|||||||||||
| Dry | 170 | 1.7E+05 | 7.4E+00 | 2.2E+01 | 6.9E+06 | 0.49 | 6.7E+06 | 5.2E+01 | 1.5E+03 | 1.1E+10 | 0.71 |
| Dry and wet | 69 | 1.4E+05 | 1.6E+01 | 8.6E+01 | 1.3E+08 | 5.4E+06 | 3.4E+01 | 2.1E+02 | 1.7E+09 | ||
| Wet | 84 | 2.1E+05 | 5.8E+00 | 3.0E+03 | 6.4E+08 | 9.8E+06 | 3.6E+01 | 9.7E+02 | 2.8E+09 | ||
| Floor condition |
|||||||||||
| Dry floor | 156 | 2.4E+05 | 8.4E+00 | 2.2E+01 | 6.4E+08 | <0.0005 | 8.1E+06 | 4.5E+01 | 2.1E+02 | 5.9E+09 | 0.69 |
| Mixed conditions | 81 | 3.1E+05 | 5.3E+00 | 7.5E+02 | 1.3E+08 | 8.2E+06 | 4.6E+01 | 1.9E+03 | 1.8E+09 | ||
| Wet floor | 86 | 5.7E+04 | 8.3E+00 | 1.4E+02 | 2.3E+06 | 5.3E+06 | 4.6E+01 | 3.1E+02 | 1.1E+10 | ||
| Floor construction | |||||||||||
| Full slatted | 37 | 1.7E+05 | 6.8E+00 | 2.9E+02 | 2.4E+06 | 0.28 | 7.3E+06 | 3.3E+01 | 9.7E+02 | 1.1E+10 | 0.84 |
| Mostly slatted | 108 | 2.3E+05 | 6.9E+00 | 1.4E+02 | 6.4E+08 | 8.7E+06 | 4.7E+01 | 3.1E+02 | 5.5E+09 | ||
| Mostly concrete | 178 | 1.5E+05 | 9.7E+00 | 2.2E+01 | 1.3E+08 | 6.6E+06 | 4.8E+01 | 2.1E+02 | 5.9E+09 |
In pairwise two-way analyses between ventilation and feed type or between ventilation and floor condition there was a significant interaction. Ventilation alone did not remain significantly associated with 16S rRNA archaea concentrations in these models. The lowest archaea concentrations were observed during work in houses with mixed/natural ventilation when the feed was mixed dry and wet and during work with mixed/natural ventilation in houses with mostly dry floors. Wet floors remained significantly associated with lower archaea concentrations in two-way models with type of feed and type of floor. Wetness of the floor significantly interacted with type of feed so that archaea concentrations were lower during work in houses with dry floors and mixed dry and wed feed than when using other types of feed. No interactions appeared in two-way analyses of 16S rRNA bacteria concentrations and the farm characteristics.
The effects of ventilation and floor conditions on the archaea levels were of similar directions and magnitude during both summer and winter (data not shown). The effect of ventilation on the archaea levels was only statistically significant during winter. Furthermore, floor construction appeared to be of importance to bacterial 16S gene concentration during winter (P = 0.017) with a similar tendency (P = 0.083) for archaea; levels being higher with mixed mostly slatted floors.
Broiler production stage did not systematically affect the concentration of archaea or bacteria (data not shown).
Discussion
This study confirmed findings from Canada that archaea are common in modern farming environments at levels below those of bacteria (Blais Lecours et al., 2012). Personal sampling was used to study archaea concentrations in several different types of farm work and it was shown that farmers are exposed to archaea also during field work and work with mink. Although exposures to archaea in these work tasks were lower than in pig and cattle farming, the highest observed exposures in mink farming did reach levels comparable with cattle farming. As DNA sequencing methods develop at a high pace the methods presented are to be considered outdated. Yet, for our primary aim of studying the effects of farm and job characteristics on archaea levels the applied methods were suitable.
The maximum concentration of archaeal 16S rRNA n m−3 during work in pig farms of 6.6*108 was similar to the level of ≊108 originally observed in Canadian pig farms (Nehmé et al., 2009). We found a wider range in the 16S archaea gene concentration during work in pig farms than observed in Canada. This is not surprising as the personal sampling applied is likely to capture greater variation in exposure than the stationary samplers used by Nehmé et al.
Compared with the findings in cattle farms of averages of 8.5*10E+05 archaeal and 1.5*10E+08 bacterial 16S genes m−3 (Blais Lecours et al., 2012) our GM levels of 1.9*10E+04 archaeal and 3.6*10E+06 bacterial 16S genes m−3 were lower. However, arithmetic mean concentrations (not shown) were approximately one order of magnitude higher for both archaea and bacteria and thus almost similar to Canadian findings in both pig and dairy farms. Similarly, our range of exposure in cattle farms was wider than what was reported by Blais Lecours and included maximum levels corresponding to or exceeding their findings.
The observed GM archaeal 16S gene levels of 5.1*10E+02 n m−3 in broiler and 3.3*10E+04 n m−3 in layer operations were two orders of magnitude lower than the 2.6*10E+04 n m−3 (floor-housed, i.e. broiler) and 6.5*10E+05 n m−3 (cage-housed) archaea m−3 levels in Canadian poultry operations (Just et al., 2013). Similar to our study personal samplers were used in that study. Just et al. presented mean concentrations of the raw data rather than GM concentrations. Our arithmetic mean concentration of archaea among layer operators was similar to our GM (data not shown). Among broiler operators in our study the mean archaea concentration of archaea was 1.7*10E+04 n m−3, i.e. almost two orders of magnitude higher than the GM, thus deviating little from that reported by Just et al. In broiler operations we observed greater variation but similar maximum levels as did Just et al. Our observed endotoxin concentration of 6*10E+02 EU m−3 was lower than the approximately 3*10E+03 EU m−3 shown in their study.
The concentrations of the 16S gene that we present cannot be directly translated into concentrations of archaea or bacteria in the air. The 16S gene is present in more than one copy in many bacteria and archaea (Louca et al., 2018) and only if the composition of these microorganisms and the numbers of 16S genes in each of these are known in detail can 16S gene numbers be translated into number of organisms. Tools exist that attempt to correct for these differences in 16S gene number copies. With increasing phylogenetic distance between the species in a sampled microbiome these tools loose accuracy and would be expected to be inaccurate for natural environmental samples such as ours (Louca et al., 2018). In the study by (Just et al., 2013) the 16S gene copy number was multiplied by 1.775 in order to correct for the difference in gene copy number between M. mazei and ‘all archaea’. If we had done the same, we would have observed archaea concentrations almost similar to those observed in Canadian poultry operations.
Similar to what was observed in the Canadian farms methanobrevibacter species were the most commonly observed among cattle farmers (Blais Lecours et al., 2012). Species such as halorubrum and methanosarcina that have both previously been found in the work environment of dairy farmers were also identified. Methanosphaera stadtmanii which dominated samples from Canadian pig farms was not found in our samples, whereas Methanobrevibacter smithii was identified in pig farms as observed in Canada (Nehmé et al., 2009). Among our poultry operators we identified two of the three species observed in Canadian poultry operators (Just et al., 2013) though we did not speciate these with the same level of detail. The identification of archaeal species depends on the 16S rRNA gene clones libraries available. We did not attempt to identify and speciate every archaea (or bacteria). Thus, the list of microorganisms encountered during farm work in Denmark reported in this study is not complete. The relatively high degree of similarity in the species between studies suggests that the phylogenetic distance between our study and previous studies is small enough for meaningful comparisons of the 16S gene copy numbers.
In order to identify factors that determine archaeal and bacterial exposure levels in farming, we investigated the effects of season, farm and work characteristics. In contrast to dust and endotoxin levels (Basinas et al., 2013) neither bacterial nor archaeal 16S gene concentrations varied with season. In Canada lower archaeal 16S gene levels in pig farming were observed during summer (Nehmé et al., 2009). A plausible explanation for this is that differences in ventilation rates between seasons are larger in the continental climate of Québec than in the coastal climate of Denmark. Whether this difference between archaea and bacteria stem from differences in how the microorganisms grow and are released into the air remains to be explored.
In pig farms we observed that archaea 16S gene concentrations partly depended on farm characteristics that did not affect bacterial levels. Probably the anaerobic archaea are only released from the pigs’ stools and the variation in the levels in the aerosols depends largely on the housing conditions that affect their aerosolization. In contrast bacteria, of which most are less strict anaerobic than archaea, could also stem from other sources in the pig houses than the pigs themselves—such that have better conditions during the warm and humid summer months. If true, one would expect poorer correlation between archaea and bacteria levels during summer than during winter and this is indeed what we observed. The number of other farm types than pig farms was not sufficient for meaningful analyses on differences in archaea and bacteria levels related to farm characteristics. Lee et al. identified a limited number of archaea at the phylum level in bedroom dust in a US farming population only among those with crop and animal farming (Lee et al., 2018). By use of stationary samplers one study has investigated how archaea 16S gene concentrations varied with building characteristics of homes (Pakpour et al., 2016). Higher archaea to bacteria rates of 0.2–0.6 were observed in homes compared with the ratios of 0.0002–0.02 that we found in personal samples. Similar to our study, archaea concentrations were found to depend on factors that included ventilation and surface materials. The huge differences in archaea to bacteria ratios between different types of farm task support the idea that the conditions that favour exposure to archaea differ from those that favour exposure to bacteria.
So far our understanding of the possible health effects (if any) associated with inhalation of archaea during farm work is extremely limited. Archaea may pose little risk of adverse effects compared with the more abundant bacteria and constituents thereof such as endotoxins. However, archaea have been shown to be able to induce immune responses after intranasal installations (Patel et al., 2010; Blais Lecours et al., 2011). A more recent study demonstrated that Methanosphaera stadtmanae is able to induce a type IV hypersensitivity response in mice and that M. smithii was also able to induce a weak inflammatory response, albeit at higher concentrations. In addition to these archaea-specific effects, cocktail effects could be imagined in which archaea in combination with other components from microorganisms induce immune reactions, thereby affecting health. Like bacteria, archaea including M. smithii are present in the human digestive system where they may influence on inflammatory bowel diseases (Blais Lecours et al., 2014). The size distribution previously observed (Blais Lecours et al., 2012) showed that archaea are more common in the larger particle fractions (above 2 µm) at least in dairy farms, suggesting that they reach the alveoli of the lungs only to a limited extent.
This article adds to our understanding of what determines the exposures of workers to archaea during farm work. If it turns out that occupational archaea exposures affects health, more studies of remediation are needed. Archaea resist degradation well (Albers et al., 2000).
Strengths of this study are the size, the use of personal rather than stationary measurements, and the fact that it investigated several different farming environments with similar methods. Despite this, and as discussed elsewhere (Basinas et al., 2012), our study does not capture all the likely variation in exposure. The grouping into different types of farms does not fully capture the variation between different tasks within type of farms. Thus the study does not allow for precise quantification and identification of archaea (or bacteria) between different types of work within farms. We aimed at performing task-based personal measurements in the different farm types visited. It was unavoidable that workers spent a varying amount of time outside their main task. The fraction of time spent outside the main tasks may have differed between types of farms and thus have affected the sample concentrations to different degrees, rendering comparisons of the concentrations of, e.g. archaea between farm types less valid. Furthermore the concentrations among mink and broiler workers stem from a limited number of measurements and workers (each worker in some cases contributing more than two samples). Thus, the means from these types of farm work must be interpreted with greater caution. The quantification of archaea and bacteria by PCR is known to capture more of the exposures than would quantification by other methods. Still the method does not allow for precise quantification of the concentration of microorganisms, dead or viable, in the breathing zone of the workers but only the number of gene copies.
Conclusion
By use of personal measurements we observed greater variation of archaea than in previous studies in pig, cattle, and poultry farms and observed for the first time the presence of methanogenic and other archaea in field and mink farm aerosols. In addition, we were able to confirm the dominance of M. smithii archaea in pig and cattle farms previously shown in farms of Eastern Canada. Plausibly archaea are abundant under similar work conditions elsewhere in the world. Further studies are needed to elucidate the role these organisms could play in occupational lung disease encountered by workers in animal houses and to determine their concentrations and determinants thereof in other farms and regions of the world.
Ethics approval
The study was approved by the Central Denmark Region Committees on Biomedical Research Ethics (M-20070074).
Funding
The SUS study was supported from the Danish Agency for Science Technology and Innovation, the Danish Medical Research Council, the Danish Agricultural Research Council, Helsefonden, the PC Petersen Foundation, the Danish Working Environment Research Fund, the Danish Research Council, Aarhus University and the Danish Lung Association. C.D. was supported by National Sciences and Engineering Research Council of Canada Discovery Granta.
Disclaimer
Funding for this project was provided by public and private funds without any commercial interest in the subject. The authors declare no conflict of interest relating to the material presented in this article. Its contents, including any opinions and/or conclusions expressed, are solely those of the authors.
Supplementary Material
Acknowledgement
The authors would like to thank the participating farmers and farm owners for making the present work possible and Melissa Marcoux-Voiselle for her skilful laboratory work with the samples.
References
- Albers SV, van de Vossenberg JL, Driessen AJ et al. (2000) Adaptations of the archaeal cell membrane to heat stress. Front Biosci; 5: D813–20. [DOI] [PubMed] [Google Scholar]
- Bach HJ, Tomanova J, Schloter M et al. (2002) Enumeration of total bacteria and bacteria with genes for proteolytic activity in pure cultures and in environmental samples by quantitative PCR mediated amplification. J Microbiol Methods; 49: 235–45. [DOI] [PubMed] [Google Scholar]
- Basinas I, Schlünssen V, Takai H et al. (2013) Exposure to inhalable dust and endotoxin among Danish pig farmers affected by work tasks and stable characteristics. Ann Occup Hyg; 57: 1005–19. [DOI] [PubMed] [Google Scholar]
- Basinas I, Sigsgaard T, Heederik D et al. (2012) Exposure to inhalable dust and endotoxin among Danish livestock farmers: results from the SUS cohort study. J Environ Monit; 14: 604–14. [DOI] [PubMed] [Google Scholar]
- Blais Lecours P, Duchaine C, Taillefer M et al. (2011) Immunogenic properties of archaeal species found in bioaerosols. PLoS One; 6: e23326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais Lecours P, Marsolais D, Cormier Y et al. (2014) Increased prevalence of Methanosphaera stadtmanae in inflammatory bowel diseases. PLoS One; 9: e87734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais Lecours P, Veillette M, Marsolais D et al. (2012) Characterization of bioaerosols from dairy barns: reconstructing the puzzle of occupational respiratory diseases by using molecular approaches. Appl Environ Microbiol; 78: 3242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie EL, DeSantis TZ, Parker JP et al. (2007) Urban aerosols harbor diverse and dynamic bacterial populations. Proc Natl Acad Sci U S A; 104: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elholm G, Omland O, Schlünssen V et al. (2010) The cohort of young Danish farmers - A longitudinal study of the health effects of farming exposure. Clin Epidemiol; 2: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert Y, Veillette M, Duchaine C (2010) Metalworking fluids biodiversity characterization. J Appl Microbiol; 108: 437–49. [DOI] [PubMed] [Google Scholar]
- Just N, Blais Lecours P, Marcoux-Voiselle M et al. (2013) Archaeal characterization of bioaerosols from cage-housed and floor-housed poultry operations. Can J Microbiol; 59: 46–50. [DOI] [PubMed] [Google Scholar]
- Lee MK, Carnes MU, Butz N et al. (2018) Exposures related to house dust microbiota in a U.S. farming population. Environ Health Perspect; 126: 067001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létourneau V, Nehmé B, Mériaux A et al. (2010) Impact of production systems on swine confinement buildings bioaerosols. J Occup Environ Hyg; 7: 94–102. [DOI] [PubMed] [Google Scholar]
- Louca S, Doebeli M, Parfrey LW (2018) Correcting for 16S rRNA gene copy numbers in microbiome surveys remains an unsolved problem. Microbiome; 6: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May S, Romberger DJ, Poole JA (2012) Respiratory health effects of large animal farming environments. J Toxicol Environ Health B Crit Rev; 15: 524–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehmé B, Gilbert Y, Létourneau V et al. (2009) Culture-independent characterization of archaeal biodiversity in swine confinement building bioaerosols. Appl Environ Microbiol; 75: 5445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann H, Lösekann T, de Beer D et al. (2006) Novel microbial communities of the Haakon Mosby mud volcano and their role as a methane sink. Nature; 443: 854–8. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy P, Peters T, Donham K et al. (2012) Assessment of swine worker exposures to dust and endotoxin during hog load-out and power washing. Ann Occup Hyg; 56: 843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakpour S, Scott JA, Turvey SE et al. (2016) Presence of archaea in the indoor environment and their relationships with housing characteristics. Microb Ecol; 72: 305–12. [DOI] [PubMed] [Google Scholar]
- Patel GB, Zhou H, Ponce A et al. (2010) Intranasal immunization with an archaeal lipid mucosal vaccine adjuvant and delivery formulation protects against a respiratory pathogen challenge. PLoS One; 5: e15574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Romberger DJ (2012) Immunological and inflammatory responses to organic dust in agriculture. Curr Opin Allergy Clin Immunol; 12: 126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigsgaard T, Hjort C, Omland, et al. (1997) Respiratory health and allergy among young farmers and non-farming rural males in Denmark. The SUS study. J Agromedicine; 4: 63–78. [PubMed] [Google Scholar]
- Sigsgaard T, Hoffmann HJ, Thorne PS (2008) The role of innate immunity in occupational allergy: recent findings. Curr Opin Allergy Clin Immunol; 8: 120–5. [DOI] [PubMed] [Google Scholar]
- Sprott GD, Tolson DL, Patel GB (1997) Archaeosomes as novel antigen delivery systems. FEMS Microbiol Lett; 154: 17–22. [DOI] [PubMed] [Google Scholar]
- Wunschel J, Poole JA (2016) Occupational agriculture organic dust exposure and its relationship to asthma and airway inflammation in adults. J Asthma; 53: 471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.