SUMMARY
Volumetric laser endomicroscopy (VLE) uses optical coherence tomography (OCT) for real-time, microscopic cross-sectional imaging. A US-based multi-center registry was constructed to prospectively collect data on patients undergoing upper endoscopy during which a VLE scan was performed. The objective of this registry was to determine usage patterns of VLE in clinical practice and to estimate quantitative and qualitative performance metrics as they are applied to Barrett's esophagus (BE) management. All procedures utilized the NvisionVLE Imaging System (NinePoint Medical, Bedford, MA) which was used by investigators to identify the tissue types present, along with focal areas of concern. Following the VLE procedure, investigators were asked to answer six key questions regarding how VLE impacted each case. Statistical analyses including neoplasia diagnostic yield improvement using VLE was performed. One thousand patients were enrolled across 18 US trial sites from August 2014 through April 2016. In patients with previously diagnosed or suspected BE (894/1000), investigators used VLE and identified areas of concern not seen on white light endoscopy (WLE) in 59% of the procedures. VLE imaging also guided tissue acquisition and treatment in 71% and 54% of procedures, respectively. VLE as an adjunct modality improved the neoplasia diagnostic yield by 55% beyond the standard of care practice. In patients with no prior history of therapy, and without visual findings from other technologies, VLE-guided tissue acquisition increased neoplasia detection over random biopsies by 700%. Registry investigators reported that VLE improved the BE management process when used as an adjunct tissue acquisition and treatment guidance tool. The ability of VLE to image large segments of the esophagus with microscopic cross-sectional detail may provide additional benefits including higher yield biopsies and more efficient tissue acquisition. Clinicaltrials.gov NCT02215291
Keywords: Barrett's esophagus, dysplasia, endomicroscopy, imaging
INTRODUCTION
Barrett's esophagus (BE) is a risk factor for the development of esophageal adenocarcinoma (EAC).1–4 Challenges in the management of patients with BE include detecting areas of dysplasia or superficial cancer and surveillance after endoscopic treatment to evaluate for residual or recurrent disease. Dysplasia in BE may not be apparent during inspection using white light endoscopy (WLE). Therefore, current guidelines recommend endoscopic surveillance of BE with random 4-quadrant biopsy sampling every 1–2 cm (Seattle Protocol), in addition to targeted biopsy sampling of any visible abnormalities.5,6 This imperfect surveillance protocol can result in missed disease, with an estimated 25.3% of EAC procedures occurring within 1 year of a surveillance endoscopy.6
Dysplasia can be treated with endoscopic therapies including endoscopic mucosal resection (EMR),7 radiofrequency ablation (RFA),8 cryotherapy,9 and others.10,11 However, high recurrence rates have been reported, including up to 33% recurrence of intestinal metaplasia or dysplasia at 2 years in the case of RFA.12 Residual disease, particularly at the GEJ, and the existence of disease buried beneath neosquamous epithelium are also sources of concern.13-17
Recently, advanced imaging techniques such as narrow band imaging (NBI) and confocal laser endomicroscopy (CLE) have sought to improve dysplasia detection in BE patients by allowing biopsies to be taken in a targeted rather than random fashion, even when focal abnormalities are absent on WLE inspection.18-22 Volumetric laser endomicroscopy (VLE) utilizes optical coherence tomography (OCT) to produce high-resolution, cross-sectional surface, and subsurface images of the esophageal wall over a long continuous segment (Fig.1).23-27 Studies have examined the efficacy of VLE as applied to dysplasia detection in pre and post-treatment surveillance28-30 as well as informing treatment selection.29 While the safety and feasibility of esophageal VLE imaging has been shown,31 the objective of this registry was to determine usage patterns of VLE in clinical practice and to estimate quantitative and qualitative performance metrics as they are applied to BE management.
Fig. 1.
NinePoint Medical, NvisionVLE Imaging System with Single-Use Optical Probe.
MATERIALS AND METHODS
Patient selection
This is a prospective observational cohort study from August 2014 to April 2016. Patients were eligible
for inclusion in this study if undergoing a clinically-indicated upper endoscopy during which VLE was used for evaluation of the esophagus. Procedures were performed at 18 centers throughout the United States (Table 1). Each site was eligible to enroll up to 100 subjects, with an overall registry enrollment cap of 1000 patients. Investigators were free to recruit patients with a variety of disease states at various stages of clinical management. Patients for whom the VLE device would be in conflict with the manufacturer's Instructions For Use were excluded. This included use in anatomies where catheter deployment would generate significant risk, such as the setting of a tight stricture. The research protocol and informed consent forms were approved by each of the participating institutional review boards, and informed consent was obtained from each participant prior to enrollment.
Table 1.
Enrollment by Registry Site
| Institution | Number of patients enrolled |
|---|---|
| Temple University Hospital | 100 |
| North Shore University Hospital | 100 |
| Ochsner-Kenner Medical Center | 100 |
| West Penn Allegheny Hospital | 100 |
| VA Boston Hospital | 73 |
| Dartmouth-Hitchcock Medical Center | 73 |
| Beth Israel Deaconess Medical Center | 71 |
| Geisinger Medical Center | 54 |
| Weill Cornell Medical Center | 46 |
| UC Irvine Medical Center | 45 |
| University of South Alabama Medical Center | 43 |
| Methodist Dallas Medical Center | 41 |
| Mayo Clinic Florida | 35 |
| Keck Hospital of USC | 30 |
| Florida Hospital | 29 |
| University of Vermont Medical Center | 27 |
| University of Chicago Medical Center | 26 |
| Columbia University Medical Center | 7 |
| Total | 1000 |
Endoscopic procedure and postprocedure questionnaire
All patients underwent standard of care endoscopy including WLE in accordance with their institution's standard procedures followed by VLE examination. Sample VLE features relevant to normal and abnormal structures in the esophagus were used as a general guideline to interpret VLE images in the study (Fig. 2).28,31–33 Investigators were trained on the use of the technology and supported as needed onsite and offsite by technical experts from the sponsor throughout the study. VLE scans were registered longitudinally and rotationally with the WLE image of the esophagus. When a lesion was identified on VLE, the investigator would triangulate the location of the lesion by recording the distance and clockface registered with the WLE orientation. This information then was used to guide the investigator to acquire the tissue using WLE. At the time of the study, this was the method that was available to target a tissue site for sampling. Additional procedure details can be found in Supplementary Material A.
Fig. 2.
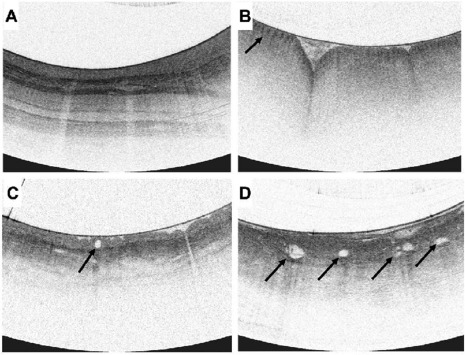
Sample VLE features: (A) Normal squamous epithelium, showing well-defined layers of the esophagus. (B) Gastric cardia identified with gastric rugae and pit-and-crypt architecture (arrow). (C) NDBE seen with an irregular surface, isolated, round, regular, gland in the epithelium (arrow) and a partially effaced layer. (D) dysplastic BE showing complete layer effacement with atypical glands (arrows).
Following VLE, each investigator performed any desired diagnostic or therapeutic actions based on their standard of care according to WLE and advanced imaging findings. Highest grade of disease on the pathology results was recorded for advanced imaging guided tissue acquisition, targeted endoscopic tissue acquisition, and random biopsies. VLE guided tissue acquisition refers to the subgroup of advanced imaging guided tissue biopsy or resection specimens where only VLE imaging was used to identify the areas of interest.
Investigators were given a questionnaire post-procedure (Table 2) and data were collected as to the clinical workflow and utility of the VLE images. The questions included whether VLE guided either their tissue sampling or therapeutic decisions for each patient, and whether VLE identified suspicious areas not seen on WLE or other advanced imaging modalities.
Table 2.
Post-procedure questionnaire and results
| Question | % Responding ‘Yes’ | |
|---|---|---|
| 1 | Suspicious areas/disease identified on VLE by the physician? | 77% (689/894) |
| 2 | Did you see any suspicious areas on VLE that you did NOT see on WLE? | 59% (526/894) |
| 3 | Did you see any suspicious areas on VLE that you did NOT see using advanced imaging (i.e. NBI, FICE, i-Scan, chromatography, or CLE)? | 56% (401/710) |
| 4 | Did VLE guide tissue acquisition? | 72% (515/714) |
| 5 | Did findings on VLE guide treatment at the current visit? | 52% (182/352) |
| 6 | Was either the depth or extent of disease identified on VLE used to determine treatment modality? | 40% (140/353) |
CLE, confocal laser endomicroscopy; NBI, narrow band imaging; VLE, volumetric laser endomicroscopy; WLE, white light endoscopy.
Data analysis
Descriptive statistics were used for quantitative analyses in the study. In light of the vast majority of registry patients having suspected or confirmed BE, the investigators elected to focus initial analysis on this group and to assess potential roles of VLE in BE management. Suspected BE refers to patients with no prior histologic confirmation of BE who had salmon colored mucosa found on endoscopic examination with WLE. The analysis focused on the incremental diagnostic yield improvement of VLE as an adjunct modality on top of the standard of care practice. Procedures with confirmed neoplasia (defined as high grade dysplasia [HGD], intramucosal carcinoma [IMC], and esophageal adenocarcinoma [EAC]) were included in the analysis. The procedures were divided into subgroups according to whether the tissue acquisition method was VLE targeted. Dysplasia diagnostic yields were calculated using the number of procedures in each subgroup and total number of procedures in patients with previously diagnosed or suspected BE. Negative predictive value (NPV) analysis in patients with prior BE treatment evaluated the utility of VLE on top of the standard of care (SoC) surveillance to predict when there is no dysplasia present. Procedures with negative endoscopy findings and negative VLE findings but with tissue acquisition performed were included in the analysis and NPVs for both SoC and SoC + VLE were calculated.
The primary evaluation focused on HGD and cancer since the recommended image interpretation criteria were validated for detecting BE-related neoplasia,28 and treatment is recommended for patients with neoplasia per existing guidelines.34,35
RESULTS
From August 2014 through April 2016, 1000 patients were enrolled across 18 trial sites (Table 1). The majority of patients were male (734), with a mean age of 64 years (range: 21–89). A total of 894 patients had suspected or confirmed BE at the time of enrollment including 103 patients with suspected BE and 791 patients with prior histological confirmation. Of the confirmed BE patients, 368 had BE with neoplasia, 170 had BE with low grade dysplasia (LGD), 49 had BE indefinite for dysplasia (IND), and 204 had non-dysplastic BE (NDBE). A total of 56% of patients had undergone prior endoscopic or surgical interventions for BE including RFA, Cryo, and EMR (Table 3).
Table 3.
Demographics and patient history
| Overall | Previously diagnosed or suspected BE | |
|---|---|---|
| Number of patients | 1000 patients | 894 patients |
| Median age (range) | 64 years (21–89) | 65 years (22–89) |
| Male (%) | 734 (73%) | 679 (76%) |
| Prior highest grade of pathology | 845 patients | 791 patients |
| Invasive Adenocarcinoma | 34 (4.1%) | 30 (3.8%) |
| BE with IMC | 82 (10%) | 82 (10.4%) |
| BE with HGD | 258 (31%) | 256 (32.3%) |
| BE with LGD | 170 (20%) | 170 (21.5%) |
| BE with IND | 50 (6%) | 49 (6.2%) |
| NDBE | 204 (24%) | 204 (25.8%) |
| Squamous dysplasia | 20 (2%) | N/A |
| Other | 27 (3%) | N/A |
| Prior treatment† | 549 patients | 501 patients |
| RFA | 381 (69.4%) | 369 (73.6%) |
| Cryo | 90 (16.4%) | 85 (17%) |
| EMR | 197 (35.9%) | 192 (38.3%) |
| Other | 67 (12.2%) | 41 (8.2%) |
†Some patients had more than one esophageal intervention.
BE, Barrett's esophagus; IMC, intramucosal carcinoma; HGD, high grade dysplasia; LGD, low grade dysplasia; IND, indefinite for dysplasia; NDBE, non-dysplastic BE; RFA, radiofrequency ablation; Cryo, Cryoablation; EMR, Endoscopic Mucosal Resection.
Post-procedure questionnaires were completed for all procedures in patients with previously diagnosed or suspected BE (Table 2). VLE identified focal areas of concern in 77% of BE procedures. In over half of the procedures, investigators identified areas of concern not seen on either WLE or other advanced imaging modalities. Both VLE and endoscopic BE treatment were performed in 352 procedures. VLE guided the intervention in 52% of these procedures. In 40% of procedures, the depth or extent of disease identified on VLE aided the selection of a treatment modality.
Neoplasia (43 HGD, 12 IMC, and 21 EAC) was confirmed on tissue sampling performed in 76 procedures within the cohort of patients with previously diagnosed or suspected BE (Fig. 3). Among these procedures, VLE-guided tissue acquisition alone found neoplasia in 26 procedures (34%), with an additional case where HGD on random forceps biopsy was upstaged to IMC on VLE-targeted sampling. Histology from these procedures included 16 HGD, 5 IMC, and 6 EAC. Thus, VLE-guided tissue acquisition as an adjunct to standard practice detected neoplasia in an additional 3% (26/894) of the entire cohort of patients with previously diagnosed or suspected BE, and improved the diagnostic yield by at least 55% (27 patients with neoplasia found on VLE/49 patients with neoplasia found on standard of care imaging) (Fig. 3).
Fig. 3.
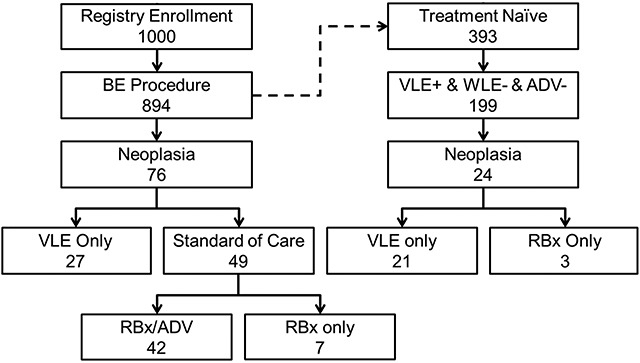
Flow chart describing the diagnostic yield improvement analysis. ADV, advanced imaging modality other than VLE; ADV-: no lesion or suspicious area was identified using advanced imaging other than VLE; RBx: random biopsy; VLE, volumetric laser endomicroscopy; WLE, white light endoscopy.
Of the 894 BE patients, 393 (44%) had no prior history of esophageal therapy. Mean Prague classification score for this cohort were C = 2.3 cm (range: 0–17 cm), M = 4.1 cm (range: 0.5–18 cm). In 199 (51%) of these treatment naïve patients, VLE identified at least one focally suspicious area not appreciated during either WLE or other advanced imaging evaluation. Neoplasia was confirmed on histology in 24 procedures (Fig. 3). In (20/24) of these procedures, VLE alone identified neoplasia as all random biopsies for these patients were negative. Additionally, one case where HGD was found on random forceps biopsy was upstaged to IMC on VLE-targeted sampling. In this group, VLE-guided tissue acquisition increased neoplasia detection by 700% (21/3) (Table 4).
Table 4.
Pathology in treatment-naïve patients with neoplasia when positive VLE but negative WLE or other advanced imaging findings
| Pathology | Random biopsies only | VLE-guided only | Total |
|---|---|---|---|
| EAC | 0 | 3 | 3 |
| IMC | 0 | 5 | 5 |
| HGD | 3 | 13 | 16 |
Note: There were no cases where both random and VLE-guided biopsies found neoplasia in the same patient.
EAC, esophageal adenocarcinoma; HGD, high grade dysplasia; IMC, intramucosal carcinoma; VLE, volumetric laser endomicroscopy.
For these untreated BE patients, VLE-guided tissue acquisition as an adjunct to standard practice detected neoplasia in an additional 5.3% of procedures (21/393). The number needed to test with VLE to identify neoplasia not detected with standard of care technique was 18.7. An average of 1.7 additional sites per patient required targeted tissue acquisition when suspected regions were identified using VLE compared to an average of 11 random biopsies per patient.
A sub-analysis was conducted in the 238 patients with prior BE treatment and either no visible BE (C0M0) or irregular z-line. From this group, 82% (211/238) had no focally suspicious findings on WLE examination, where two procedures were subsequently diagnosed with neoplasia (1 HGD and 1 EAC). Thus, the NPV for WLE was 99% (209/211, CI = [96.2%, 99.8%]) for neoplasia. When combining WLE/NBI with VLE as an adjunct, we found that 49% (103/211) of the post-treatment procedures had no suspicious WLE or VLE findings. Neoplasia was found in none of these procedures, corresponding to a negative predictive value of 100% (CI = [95.5%, 100%]) (Fig. 4).
Fig. 4.
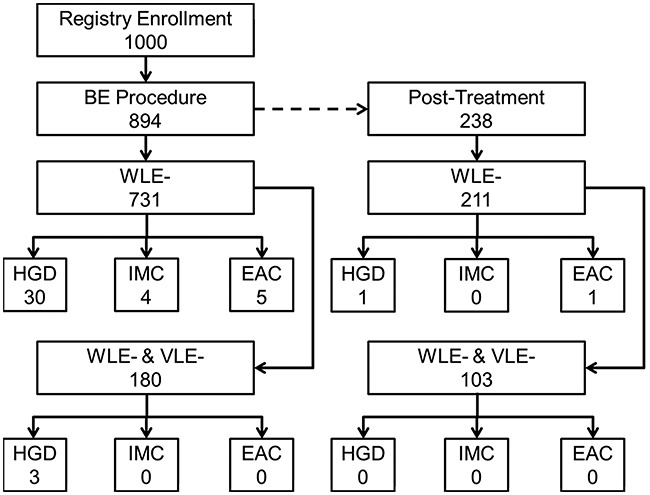
Flow chart describing the negative predictive value analysis. EAC, esophageal adenocarcinoma; HGD, high grade dysplasia; IMC, intramucosal carcinoma; VLE, volumetric laser endomicroscopy; WLE, white light endoscopy.
DISCUSSION
Advanced imaging techniques including high definition-WLE, NBI, CLE, and chromoendoscopy have continued to improve the evaluation of Barrett's esophagus. However, these provide only superficial epithelial evaluation. VLE breaks this boundary by imaging the mucosa, submucosa, and frequently, down to the muscularis propria. It does so while evaluating a large tissue area in a short period of time without sacrificing resolution.
This 1000-patient multi-center registry assessed the clinical utility of VLE for the management of esophageal disorders and has demonstrated its potential as an adjunct tool for detecting disease. Abnormalities were found on VLE which were not seen with other imaging in over half of the procedures. Endoscopists using VLE in this study felt that it guided tissue acquisition in over 70% of procedures and BE treatment in the majority of procedures where interventions were performed. VLE visualization of subsurface tissue structures allows comprehensive morphological evaluation, resulting in physicians reporting suspicious areas only seen on VLE when other advanced imaging modalities were also used in more than half of procedures. Although subjective, these results still provide useful insight into the physicians’ perception of the technology.
This study found that VLE as an adjunct modality increased neoplasia diagnosis by 3%, and improved the neoplasia diagnostic yield by 55% over standard practice and other advanced imaging modalities. For a treatment naïve population with no focally suspicious regions found on WLE, VLE-guided tissue acquisition improved neoplastic diagnostic yield by 700%. This finding is impressive, particularly as these procedures were performed prior to the release of a real-time laser marking system.30 Laser marking has since been evaluated by Alshelleh et al., who found a statistically significant improvement of neoplasia (14% vs. 1%, P = 0.001) yield using the VLE laser marking system compared to the standard Seattle protocol.36
In this registry, an additional 2.3 sites per patient on average required guided biopsy or resection when suspected regions were identified using VLE, while an average of 15.8 random biopsies per patient were performed in the cohort of patients with previously diagnosed or suspected BE (894/1000). In general, higher tissue sampling density leads to an increased chance of detecting dysplasia due to its focal nature, therefore taking additional biopsies should increase the diagnostic yield. However, the potential for advanced imaging such as VLE to provide targeted, high yield biopsies could reduce the total number of biopsies necessary to adequately evaluate the diseased mucosa with the Seattle protocol.
The combination of a focally unremarkable WLE and VLE examination provided a negative predictive value of 100% for neoplasia in post-treatment population. Although not reaching statistical significance due to limited sample size, these early results provide promise for the utility of VLE to better predict when there is no disease present, i.e. a ‘clean scan.’ Such a tool could then potentially allow for extended surveillance intervals reducing the number of endoscopies to manage the patient's needs.
The utility of this analysis is subject to several limitations. As a post-market registry study, there was no defined protocol for imaging, image interpretation and tissue acquisition, and there was no control group for matched population comparisons. The early experience of users on VLE image interpretation may have resulted in overcalling areas of concern. Abnormalities located deeper in the esophageal wall could be targeted with forceps biopsies at one site, while other sites would utilize endoscopic resection techniques that are more likely to remove the target. All of these discrepancies could affect any calculations regarding the adjunctive yield of VLE-targeted sampling. Further analysis of the global detection rate of dysplasia by site did not reveal any statistical difference.
At the time of this study, image interpretation was performed using previously published guidelines for detection of neoplasia in Barrett's esophagus with OCT.28 Challenges with histopathological diagnosis of LGD limited the development of VLE criteria for LGD. As such, the analyses in this study focused on neoplasia. Current guidelines suggest that treatment of LGD is acceptable35 so detection of LGD with VLE should be addressed in a future study.
Additionally, the characteristic image features that maximize sensitivity and specificity of confirmatory biopsies must be optimized. Recently, Leggett et al. established an updated step-wise diagnostic algorithm to detect dysplasia based on similar VLE features used in this study.32 This diagnostic algorithm achieved 86% sensitivity, 88% specificity, and 87% diagnostic accuracy to detect BE dysplasia with almost perfect interobserver agreement among three raters (kappa = 0.86).32 Further optimization of VLE image features for identifying dysplasia and neoplasia are ongoing (Clinicaltrials.gov NCT02864043).
Other limitations of the study include the lack of central pathology for interpretation of specimens, which could affect (positively or negatively) the reported benefit of VLE in finding dysplasia. However, this manuscript focuses on neoplasia where there is less interobserver variability compared to low-grade dysplasia. Finally, as a non-randomized study conducted mostly at large BE referral centers with possibly higher pre-test probability of neoplasias, it is plausible that their validity in a community setting is limited. However, the large sample size, its heterogeneity, plus variation in technique by site likely restore at least some of the external validity of the findings.
This registry-based study demonstrates the potential for VLE to fill clinically relevant gaps in our ability to evaluate and manage BE. Physicians perceived significant value of VLE across the BE surveillance and treatment paradigm. Biopsy confirmation demonstrated benefits of VLE for both treatment naïve and posttreatment surveillance, although pathology results did not always align with physician perception, most likely due to limitations of the technology and image criteria at the time of study. Given expected refinement and validation of image interpretation, and the availability of laser-marking for more accurate biopsy targeting, VLE is well positioned to enhance our ability to identify and target advanced disease and enable a more efficient endoscopic examination with higher yield of tissue acquisition.
Acknowledgments
The authors thank NinePoint Medical along with each site's study coordinators, for their assistance in facilitating protocol approval, site initiation, patient enrollment, and data collection and analysis.
Specific author contributions: Dr. Smith contributed to the concept and design of the study. All authors contributed to the acquisition of data followed by its analysis and interpretation. Drs. Cash, Diehl, Ganguly, Joshi, Konda, Mashimo, Smith, and Trindade contributed to the draft of the manuscript. Drs. Cash, Chang, DeMeester, Joshi, Konda, Mashimo, Smith, Trindade, and Wallace gave critical revisions to the manuscript for important intellectual content. All authors had final review and approval of the final submission.
References
- 1. Drewitz D J, Sampliner R E, Garewal H S. The incidence of adenocarcinoma in Barrett's esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol 1997; 92: 212–5. [PubMed] [Google Scholar]
- 2. Sharma P, Falk G W, Weston A P, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2006; 4: 566–72. [DOI] [PubMed] [Google Scholar]
- 3. Rastogi A, Puli S, El-Serag H B, et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 2008; 67: 394–8. [DOI] [PubMed] [Google Scholar]
- 4. Belghazi K, Bergman J, Pouw R E. Endoscopic resection and radiofrequency ablation for early esophageal neoplasia. Dig Dis 2016; 34: 469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wani S, Puli S R, Shaheen N J, et al. Esophageal adenocarcinoma in Barrett's esophagus after endoscopic ablative therapy: a meta-analysis and systematic review. Am J Gastroenterol 2009; 104: 502–13. [DOI] [PubMed] [Google Scholar]
- 6. Visrodia K, Singh S, Krishnamoorthi R, et al. Magnitude of missed esophageal adenocarcinoma after Barrett's esophagus diagnosis: a systematic review and meta-analysis. Gastroenterology 2016; 150: 599–607.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's esophagus. Gastroenterology 2000; 118: 670–7. [DOI] [PubMed] [Google Scholar]
- 8. Shaheen N J, Sharma P, Overholt B F, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009; 360: 2277–88. [DOI] [PubMed] [Google Scholar]
- 9. Shaheen N J, Greenwald B D, Peery A F, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc 2010; 71: 680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pereira-Lima J C, Busnello J V, Saul C, et al. High power setting argon plasma coagulation for the eradication of Barrett's esophagus. Am J Gastroenterol 2000; 95: 1661–8. [DOI] [PubMed] [Google Scholar]
- 11. Yoshinaga S, Gotoda T, Kusano C, et al. Clinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junction. Gastrointest Endosc 2008; 67: 202–9. [DOI] [PubMed] [Google Scholar]
- 12. Gupta M, Iyer P G, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett's esophagus: results from a US multicenter consortium. Gastroenterology 2013; 145: 79–86.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stier M W, Konda V J, Hart J, et al. Postablation surveillance in Barrett's esophagus: a review of the literature. World J Gastroenterol 2016; 22: 4297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gray N A, Odze R D, Spechler S J. Buried metaplasia after endoscopic ablation of Barrett's esophagus: a systematic review. Am J Gastroenterol 2011; 106: 1899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Titi M, Overhiser A, Ulusarac O, et al. Development of subsquamous high-grade dysplasia and adenocarcinoma after successful radiofrequency ablation of Barrett's esophagus. Gastroenterology 2012; 143: 564–566.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anders M, Lucks Y, El-Masry M A, et al. Subsquamous extension of intestinal metaplasia is detected in 98% of cases of neoplastic Barrett's esophagus. Clin Gastroenterol Hepatol 2014; 12: 405–10. [DOI] [PubMed] [Google Scholar]
- 17. Spechler S J, Souza R F. Barrett's esophagus. N Engl J Med 2014; 371: 836–45. [DOI] [PubMed] [Google Scholar]
- 18. Joshi V, Brewer S, Egger A. A comparison of high definition white light endoscopy (HD WLE) versus high definition microscopy in patients diagnosed with Barrett's esophagus: a retrospective review. United Eur Gastroenterol J 2015; 3: 1769. [Google Scholar]
- 19. Pai S G, Fuloria J, Joshi V. Single-center early experience of optical coherence tomography (OCT) for evaluation of high-grade dysplasia or early cancer in Barrett's esophagus. J Clin Oncol 2015; 33: 56–56. [Google Scholar]
- 20. Cash B D, Joshi V, Wolfsen H C, et al. Volumetric laser endomicroscopy improves detection of persistent or recurrent Barrett's esophagus, dysplasia and neoplasia following endoscopic treatment. Gastrointest Endosc 2016; 83: AB550. [Google Scholar]
- 21. Dunbar K B, Okolo P, 3rd, Montgomery E, et al. Confocal laser endomicroscopy in Barrett's esophagus and endoscopically inapparent Barrett's neoplasia: a prospective, randomized, double-blind, controlled, crossover trial. Gastrointest Endosc 2009; 70: 645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wallace M B, Sharma P, Lightdale C, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett's esophagus with probe-based confocal laser endomicroscopy. Gastrointest Endosc 2010; 72: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qumseya B J, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett's esophagus: a meta-analysis and systematic review. Clin Gastroenterol Hepatol 2013; 11: 1562–1570.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang D, Swanson E A, Lin C P, et al. Optical coherence tomography. Science 1991; 254: 1178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suter M J, Gora M J, Lauwers G Y, et al. Esophageal-guided biopsy with volumetric laser endomicroscopy and laser cautery marking: a pilot clinical study. Gastrointest Endosc 2014; 79: 886–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yun S H, Tearney G J, Vakoc B J, et al. Comprehensive volumetric optical microscopy in vivo. Nat Med 2006; 12: 1429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vakoc B J, Shishko M, Yun S H, et al. Comprehensive esophageal microscopy by using optical frequency-domain imaging (with video). Gastrointest Endosc 2007; 65: 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Evans J A, Poneros J M, Bouma B E, et al. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett's esophagus. Clin Gastroenterol Hepatol 2006; 4: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsai T-H, Zhou C, Tao Y K, et al. Structural markers observed with endoscopic 3-dimensional optical coherence tomography correlating with Barrett's esophagus radiofrequency ablation treatment response (with videos). Gastrointest Endosc 2012; 76: 1104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swager A F, de Groof A J, Meijer S L, et al. Feasibility of laser marking in Barrett's esophagus with volumetric laser endomicroscopy: first-in-man pilot study. Gastrointest Endosc 2017; 86: 464–72. [DOI] [PubMed] [Google Scholar]
- 31. Wolfsen H C, Sharma P, Wallace M B, et al. Safety and feasibility of volumetric laser endomicroscopy in patients with Barrett's esophagus (with videos). Gastrointest Endosc 2015; 82: 631–40. [DOI] [PubMed] [Google Scholar]
- 32. Leggett C L, Gorospe E C, Chan D K, et al. Comparative diagnostic performance of volumetric laser endomicroscopy and confocal laser endomicroscopy in the detection of dysplasia associated with Barrett's esophagus. Gastrointest Endosc 2016; 83: 880–888.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trindade A J, George B J, Berkowitz J, et al. Volumetric laser endomicroscopy can target neoplasia not detected by conventional endoscopic measures in long segment Barrett's esophagus. Endosc Int Open 2016; 4: E318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spechler S J, Sharma P, Souza R F, et al. American gastroenterological association technical review on the management of Barrett's esophagus. Gastroenterology 2011; 140: e18–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaheen N J, Falk G W, Iyer P G, et al. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol 2016; 111: 30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alshelleh M, Inamdar S, McKinley M, et al. Incremental yield of dysplasia detection in Barrett's esophagus using volumetric laser endomicroscopy with and without laser marking compared with a standardized random biopsy protocol. Gastrointest Endosc 2018; 88: 35–42. [DOI] [PubMed] [Google Scholar]


