Abstract
Objectives
The reported incidence of neoplasia identified at the time of risk-reducing salpingo-oophorectomy (RRSO) in germlineBRCA1/2mutation carriers ranges from 4–12% but long-term outcomes have not been described. We evaluated recurrence and survival outcomes of mutation carriers with neoplastic lesions identified at RRSO.
Methods
We identified BRCA1/2mutation carriers with neoplasia at RRSO at three institutions. Data was collected on clinical variables, adjuvant treatment and follow-up.
Results
We identified 32 mutation carriers with invasive carcinomas (n=15) or high-grade intraepithelial neoplasia (n=17) that were not suspected prior to surgery. 26 occurred in BRCA1 and 6 in BRCA2 mutation carriers. Median and mean age for carcinomas was 50 years and 49.3 respectively, significantly younger than for intraepithelial neoplasm, median 53years, mean 55 years (P=0.04). For the 15 invasive carcinomas, median follow up was 88months (range 45–172 months), 7 recurred (47%), median time to recurrence was 32.5 months and 3 have died of disease; 1 additional patient died of breast cancer. Overall survival was 73%, disease specific overall survival was 80% and disease free survival was 66%. For the 17 high-grade intraepithelial neoplasms, median follow up was 80 months (range 40–150), 4 were treated with chemotherapy. One recurred at 43months and is currently not on therapy with a normal CA125, 16months later. All patients with noninvasive neoplasia are alive.
Conclusions
BRCA1 and BRCA2 mutation carriers with unsuspected invasive carcinoma at RRSO have a relatively high rate of recurrence despite predominantly early stage, small volume disease. High-gradeintraepithelial neoplasms rarely recur as carcinoma and may not require adjuvant chemotherapy.
Introduction
In the last decade, we have learned much about women BRCA1 and BRCA2mutation carriers who undergo risk-reducing salpingo-oophorectomy (RRSO). The incidence of unsuspected neoplasia found at the time of RRSO ranges in these women from 4–12% 1–6. The incidence is higher in older women, in those with a BRCA1 mutation compared to BRCA2, and is more likely to be detected when the fallopian tubes are submitted for comprehensive pathological assessment that includes complete serial sectioning of ovaries and tubes 7–11. The majority of occult neoplasms in high risk women undergoing RRSO occur in the fallopian tubes and not the ovaries, which has led to important insights regarding the cell of origin of “ovarian” carcinoma 6,8,12–20. We and others have recommended a rigorous surgical-pathologic protocol for RRSO in high risk a woman which includes complete resection of the fallopian tubes, collection of intra-peritoneal cytology, and serial sectioning of the fallopian tubes and ovaries in order to optimize detection of occult neoplasm 3,7–9. The majority of these unsuspected neoplasms identified at RRSO are early stage and many are non-invasive high-grade intraepithelial neoplasia, which has also been called serous tubal intraepithelial carcinoma (STIC)11.
Although there is some reference in the literature to the uncommon recurrences of these cancers and to the 1–4% rate of primary peritoneal carcinoma after RRSO, the long-term risk of recurrence of patients with these unsuspected invasive or noninvasive neoplasia has not been the focus of any prior report.5,21–24 Since clinical outcomes have not been clearly described, there are no consistent recommendations for the need for surgical staging or adjuvant chemotherapy 25 and most recently, even the recommendation for removal of the ovaries in addition to the tubes is being reassessed26. This multi-institutional study is the first report focusing on the long-term outcome of BRCA1/2 mutation carriers found to have unsuspected invasive carcinomas or intraepithelial neoplasia/STIC at the time of RRSO.
Methods
We identified individuals with unsuspected or occult invasive carcinoma or high-grade intraepithelial neoplasia diagnosed at the time of RRSO. All subjects were part of the collaborating centers’ long-term prospective databases of women with hereditary gynecologic cancer as approved by each site’s institutional review board. The databases were reviewed from January, 1995 (Center 1 and Center 2) and January 1999 (Center 3) until June 2009. Of the 407 women in the combined databases who had RRSOs, neoplasia was reported in 34 cases. After chart review, we eliminated 2 cases because the surgeon counseled the patient that cancer was likely before surgery based on clinical suspicion and therefore the surgery was not strictly risk reducing (e.g. one case in which patient was scheduled for RRSO, but pre-operative CA125 was 867 IU/ml). The three institutions maintained longitudinal detailed data collection on participants. The cohort were identified with atypical pathology at the time of RRSO and were followed for cancer recurrence until 31st March 2012. All cases were ascertained at the time of RRSO and no additional cases were added at a later date or at the time of the diagnosis of recurrence. The total person years of follow up was 247.75. All surgical specimens were evaluated by a gynecologic pathologist and all cases included complete serial sectioning of ovaries and fallopian tubes with the exception of one invasive cancer from Center 1, and all included peritoneal washings. Our centers were some of the first to identify and report on fallopian tube neoplasm in high risk women. Even prior to publication of these reports, each center had independently established protocols to comprehensively assess the fallopian tubes. Center 1 performed microsectioning on entire fallopian tubes and ovaries in RRSO specimens starting in 2000. The first patient in this study from Center 1 had an RRSO in August 1999. This patient had a left fallopian tube invasive cancer seen at surgery with a peritoneal metastasis. The fallopian tubes were submitted in entirety but microsectioning was not specifically stated, however there were 8 slides of the left fallopian tube and 4 of the right, suggesting more than representative sectioning, and probable comprehensive sectioning on the involved side. For the purpose of this study, this patient was classified as not having microsectioning of the tubes. All subsequent Center 1 cases (#2–5, 16–22) had the entire fallopian tubes and ovaries submitted with 2–3mm cuts. At Center 2, a surgical and pathological protocol was established in 1996 and standardized by 1998, and included complete surgical resection of the ovaries and fallopian tubes up to their insertion into the cornua of the uterus, peritoneal washings as well as biopsies of the pelvic peritoneum, gutter peritoneum, and the omentum.3 The entire ovaries and fallopian tubes are then serially sectioned in 2mm cuts for pathology review. The first patient (patient 6) included in this study had RRSO in February 1998, All Center 2 cases were performed using the complete surgical-pathologic protocol as previously published.3,9 In three cases, (#2,4, 10) the fimbriae were sectioned longitudinally and submitted separately.9 The first patient (#11) in this cohort from Center 3 underwent RRSO in November 1999, this patient and all subsequent Center 3 patients (#10, 12, 13, 14, 15, 27–32) underwent complete microsectioning of the entire fallopian tubes and ovaries with 2–4 mm cuts (Table 1 and 2).29,30,31 Only subjects with invasive carcinoma or with high-grade intraepithelial neoplasia or carcinoma in situ or as classified by some, serous tubal intraepithelial carcinoma (STIC) on the RRSO pathology reports were included in the study. Subjects whose pathology reports described only moderate atypia, dyplasia or focal p53 staining in histologically normal epithelium were excluded. Similarly, terminology from the original pathology report was used to categorize cases, including two cases describing “noninvasive carcinoma in the ovary”, one associated with fallopian tube intraepithelial neoplasia and one associated with fallopian tube carcinoma (FTC). We collected data on patient age at RRSO, mutation status, prior personal history of breast cancer and exposure to chemotherapy and/or tamoxifen, family history of ovarian (OC) or FTC, preoperative serum CA125 level, pre-operative ultrasound indings, histopathology, stagin, and clinical follow up including chemotherapy, date and type of recurrence, and diagnosis or recurrence of other cancers (e.g. breast cancer), disease free survival and overall survival.
Table 1:
Characteristics of Unsuspected Invasive Neoplastic Lesions of the Ovary and Fallopian Tube Found On Pathological Examination Of 15 BRCA1 and BRCA2 Mutation Carriers Undergoing Prophylactic RRSO
| Center | Patient | Age RRSO | BRCA1/2 Mutation | CA125 | USS | Site of Lesion | Cytology | Path: 2–4mm | Complete Staging including nodes | Invasive stage | 1ry Disease CXT | Cycles | Recurrence | Presentation | Site of Recurrence | DFI | Status | OS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OV | FT | ||||||||||||||||||
| 1 | 1 | 50 | BRCA1 | 12 | NR | √ | negative | cuts No | Yes | 2c | C/T | 6 | Yes | Routine CT | Liver metastases | 34 | DECEASED | 57 | |
| 2 | 59 | BRCA1 | 10 | NR | √ | √ | negative | Yes | Yes | 1c | C/T | 6 | 0 | 86 | DECEASED | 86 | |||
| 3 | 52 | BRCA1 | ND | 3cm simple cyst | √ | positive | Yes | Yes | 3c | RT | 0 | NED | 148 | ||||||
| 4 | 48 | BRCA1 | ND | ND | √ | negative | Yes | Yes | 2c | C/T | 3 | Yes | Patient noted mass | Isolated groin mass | 29 | AWD | 105 | ||
| 5 | 43 | BRCA1 | 54 | 2 cm cyst | √ | √ | positive | Yes | No | 2c | C/T | 6 | 0 | NED | 77 | ||||
| 2 | 6 | 50 | BRCA2 | 12 | 7cm L cyst1 | √ | negative | Yes | No | 1a | C/T | 3 | 0 | NED | 172 | ||||
| 7 | 53 | BRCA2 | 7 | NAD | √ | negative | Yes | Yes | 1a | C/T | 6 | 0 | NED | 88 | |||||
| 8 | 52 | BRCA1 | 9 | NAD | √ | positive | Yes | Yes | 1c | C/T | 6 | 0 | NAD | 101 | |||||
| 9 | 49 | BRCA1 | 8 | 1.5 cm L 1.4 cm R cyst | √ | negative | Yes | No | 1a | NT | Yes | Serial Ca125 elevated | Omental deposits | 83 | AWD | 143 | |||
| 10 | 44 | BRCA1 | 17 | 2.3 cm L cyst | √ | √ | positive | Yes | Yes | 3c | C/T | 6 | 0 | NED | 45 | ||||
| 3 | 11 | 63 | BRCA1 | 5 | NAD | √ | positive | Yes | No | 1c2 | C/T | 6 | Yes | Serial Ca125 elevated | Pelvis | 17 | DECEASED | 150 | |
| 12 | 49 | BRCA1 | 8503 | NAD | √ | √ | positive | Yes | Yes | 3c | C/T/LD | 6 | Yes | Serial Ca125 elevated | Unknown | 31 | DECEASED | 121 | |
| 13 | 39 | BRCA1 | 4 | NAD | √ | negative | Yes | No | 1a | C/T | 6 | Yes | Bloating, early satiety, nausea | Peritoneal secondaries, positive nodes and ascites | 47 | AWD | 71 | ||
| 14 | 50 | BRCA1 | 27 | Questionable solid lesion L ovary | √ | negative | Yes | Yes | 1a | C/T | 6 | Yes | Serial Ca125 elevated | Isolated periaortic lymph node | 24 | NED | 66 | ||
| 15 | 39 | BRCA1 | 15 | ND | √ | negative | Yes | Yes4 | 1a | C/T | 3 | 0 | NED | 59 | |||||
Key: RRSO risk reducing salpingo-oophorectomy NAD no abnormality detected NR no record ND not done CXT chemotherapy C carboplatin T taxane NT not treated DFI disease free interval [months] OS overall survival [months] AWD alive with disease NED no evidence of disease ND not done Path: Yes = entire fallopian tubes and ovaries submitted for 2–4 millimeter cross-sectional cuts of specimen, No: entire tubes were submitted but 8 slides were performed on left and 4 on right, with no note of microsectioning.
Neoplasia on opposite side from ultrasound finding
Staged after 3 cycles of CXT
CA125 result obtained day after RRSO
Staged after 3 cycles of CXT
Table 2:
Characteristics of Unsuspected Noninvasive Neoplastic Lesions of the Ovary and Fallopian Tube Found On Pathological Examination Of 17 BRCA1 and BRCA2 Mutation Carriers Undergoing Prophylactic RRSO
| Center | Patient | Age RRSO | BRCA1/2 mutation | CA125 | USS | Location of Lesion | Cytology | Path 2–4mm | Staging1 | Stage | 1ry Disease | Cycles | Recurrence | Presentation | Site of Recurrence | DFI | Status | OS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OV | FT | ||||||||||||||||||
| 1 | 16 | 61 | BRCA1 | 26 | NAD | √ | Negative | cutts Yes | Yes | 0 | CXT NT | 0 | NED | 99 | |||||
| 17 | 73 | BRCA1 | 8 | NAD | √ | Negative | Yes | No | 0 | NT | 0 | NED | 133 | ||||||
| 18 | 58 | BRCA1 | NAD | NAD | √ | Negative | Yes | Yes | 0 | NT | 0 | NED | 78 | ||||||
| 19 | 76 | BRCA1 | NAD | NAD | √ | Negative | Yes | Partial | 0 | NT | 0 | NED | 75 | ||||||
| 20 | 54 | BRCA1 | 7 | NAD | √ | Negative | Yes | No | 0 | NT | 0 | NED | 40 | ||||||
| 21 | 49 | BRCA1 | 25 | 2 cm simple cyst | √ | Negative | Yes | No | 0 | NT | 1 | Serial CA125 elevated | Omental deposits | 43 | NED | 54 | |||
| 2 | 22 | 52 | BRCA1 | 5 | NAD | √ | Positive | Yes | Partial | 0 | NT | 0 | NED | 101 | |||||
| 23 | 59 | BRCA1 | 5 | Dermoid3 | √ | √ | Negative | Yes | Partial | 0 | NT | 0 | NED | 143 | |||||
| 24 | 50 | BRCA1 | 34 | NAD | √ | Negative | Yes | Partial | 0 | NT | 0 | NED | 64 | ||||||
| 25 | 43 | BRCA2 | 10 | 3 cm R cyst | √ | Negative | Yes | Partial | 0 | NT | 0 | NED | 47 | ||||||
| 26 | 51 | BRCA1 | 11 | NAD | √ | Negative | Yes | Partial | 0 | NT | 0 | NED | 88 | ||||||
| 3 | 27 | 47 | BRCA1 | ND | NAD | √ | Positive | Yes | Partial | 0 | C/T | 6 | 0 | NED | 150 | ||||
| 28 | 65 | BRCA2 | 6 | R 1.8cm hydrosalphinx | √ | Negative | Yes | No | 0 | C/T | 3 | 0 | NED | 138 | |||||
| 29 | 53 | BRCA1 | ND | ND | √ | Negative | Yes | No | 0 | NT | 0 | NED | 99 | ||||||
| 30 | 45 | BRCA1 | 16 | ND | √ | Negative | Yes | No | 0 | NT | 0 | NED | 80 | ||||||
| 31 | 62 | BRCA1 | 18 | NAD | √ | Positive | Yes | Yes2 | 0 | C/T | 6 | 0 | NED | 75 | |||||
| 32 | 46 | BRCA2 | 10 | NAD | √ | Negative | Yes | No | 0 | C/T | 3 | 0 | NED | 58 | |||||
Key: RRSO risk reducing salpingo-oophorectomy NAD no abnormality detected NR no record ND not done CXT chemotherapy C carboplatin T taxane NT not treated DFI disease free interval [months] OS overall survival [months] AWD alive with disease NED no evidence of disease ND not done Path: Yes = entire fallopian tubes and ovaries submitted for 2–3 millimeter cross-sectional cuts of specimen.
Staging: Yes: complete with nodes, Partial: without nodes
Center 3 Pt 31 had complete staging with nodes after 6 cycles of chemotherapy
Cyst on the opposite site from the neoplastic lesion
Statistical Analysis
Data were assembled into 2 × 2 tables and either t test or Fisher’s exact were used to test for significant (at p<0.05) associations between outcomes and factors described by the tables. All analyses were carried out using the SISA online statistical tool.
Results
Thirty-two subjects (7.9% of the total patients undergoing RRSO) were diagnosed with noninvasive (n=17) or invasive neoplasms (15), including 5 ovarian, 22 fallopian tube (FT), and 5 with synchronous ovarian and FT lesions. There were two additional unsuspected endometrial carcinomas diagnosed that were not included in the follow up cohort. Characteristics of the 15 invasive and 17 non-invasive neoplastic FT/OC cases are shown in Tables 1 and 2 respectively and summarized in Table 3. Occult neoplasms were identified exclusively in women with deleterious BRCA1 or BRCA2 mutations; no neoplasms occurred in women with negative genetic testing or variants of uncertain significance. Subjects were more likely to have BRCA1 mutations than BRCA2 (26 vs. 6). Fourteen of 32 subjects (44%), had a prior personal history of breast cancer, including 6 patients in the invasive and 8 in the intraepithelial neoplasia cohort (Table 3). Interestingly, the mean age of women with invasive carcinoma was younger than the age of women with intraepithelial neoplasm (49.3y versus 55.5y p = 0.04 at 95% CI). There was also a non-significant trend for those with noninvasive lesions to have a history of primary breast cancer at a later age (39.3y versus 49.6y p = 0.1, at 95% CI).
Table 3.
Characteristics of 32 patients with unsuspected Ov and/or FT lesions found at RRSO
| RRSO disease n =32 | Family history of Ov/FT cancers | AJ mutation | BRCA1 mutation | BC diagnosis before RRSO | Mean age [y] BC diagnosis before RRSO | Mean age [y] RRSO occult disease | Time [y] between BC diagnosis and RRSO | Primary BC after RRSO | BC recurrence after RRSO | RRSO disease in FT only |
|---|---|---|---|---|---|---|---|---|---|---|
| Invasive n = 15 | 8 | 7 | 13 | 6 | 39.3 | 49.3 | 12.1 | 1 | 1 | 7 |
| Non Invasive n = 17 | 9 | 8 | 13 | 8 | 49.6 | 55.5 | 9.3 | 1 | 0 | 16 |
| P value | NS | NS | NS | NS | 0.1* | 0.04* | NS | NS | NS | 0.004** |
RRSO – Risk reducing salpingo-oophorectomy
BC – Breast cancer
t test 95% CI
Fisher exact test
Invasive neoplasia
Of the 15 patients who had invasive carcinoma at the time of RRSO, 13 had BRCA1 mutations at a median age of 49 (range 39–59) and 2 had BRCA2, median age 51.5 (range 50–53, Table 1). Despite having normal exams and preoperative testing, three women had stage IIC (20%) and three women had stage IIIC (20%) disease at the time of RRSO. The remaining 9 invasive cases (60%) were stage IA or IC. Twelve had a CA125 recorded pre-operatively, of these, 11 were in the normal range (less than 35 U/ml). In Case 5, CA125 was elevated at 54 U/ml; pre-operative transvaginal ultrasound demonstrated only a 2 cm simple cyst; histopathology revealed a stage IIC ovarian carcinoma and complete staging was otherwise negative. Case 12 did not undergo pre-operative imaging or CA125 measurement and was identified to have visible small volume implants on the ovarian surface and pelvic peritoneum. She underwent full surgical staging and was identified on final pathology with multiple metastases in pelvic and para-aortic lymph nodes and invasive involvement in the fallopian tube consistent with stage IIIC serous FTC. CA125 was measured one day after surgery and was 850U/ml. Presumably, that subject would have had an elevated CA125 if checked prior to surgery, but since neoplasm was unsuspected prior to surgery, she met our inclusion criteria and was included in this series. The median pre-operative CA125 of all invasive carcinomas was 11 U/ml (range 4-54 SD13.7).
Pre-operative transvaginal ultrasound was available in 10/15 (4 unknown, 1 not performed) patients with invasive neoplasms. Of these, 5 were reported as normal. Five were reported as showing the presence of one or more simple cysts, none suggested a neoplastic lesion or ascites, nor accurately predicted the site of the invasive lesion[s].
All but 1of the 15 patients with invasive neoplasia had partial staging with omental biopsies or peritoneal biopsies, 8 patients had comprehensive initial staging including pelvic and paraortic lymph nodes, and 2 additional patients had complete staging after completing 3 or 6 cycles of chemotherapy respectively. The median follow up was 88 months (range 45–172months).Fourteen received chemotherapy immediately after the diagnosis of carcinoma (Table 1). The one patient, case 9, not initially treated had a stage IA occult invasive ovarian carcinoma and initial chemotherapy was not recommended. She had a recurrence 7 years after RRSO. She had been followed with biannual serial CA125 measurements. These were in the normal range until August 2005 when her CA125 was elevated at 40. In September 2006 the CA125 was 28 but in March 2007 was measured at 508, and a CT scan showed multiple omental implants. Of the 14 ovarian and tubal carcinomas treated immediately after RRSO all received platinum and taxane chemotherapy. One patient with Stage IIICFTC had progressive disease on carboplatin and paclitaxel, and subsequently had a complete response to topotecan, but died of recurrent disease at 121 months.
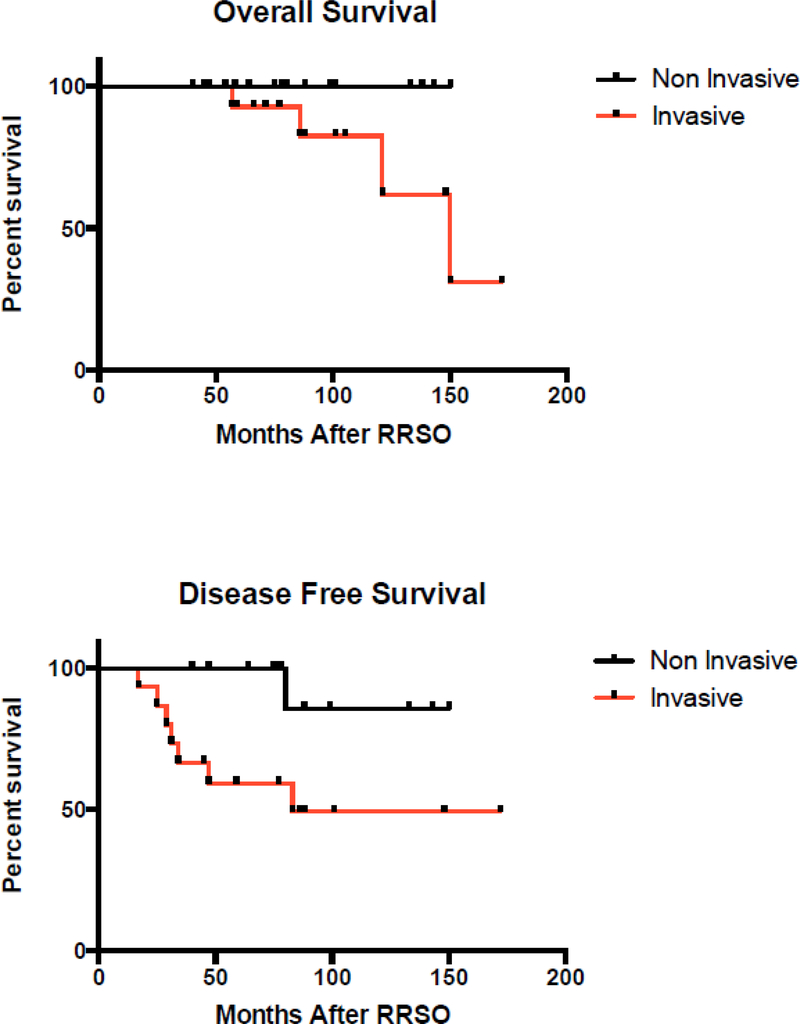
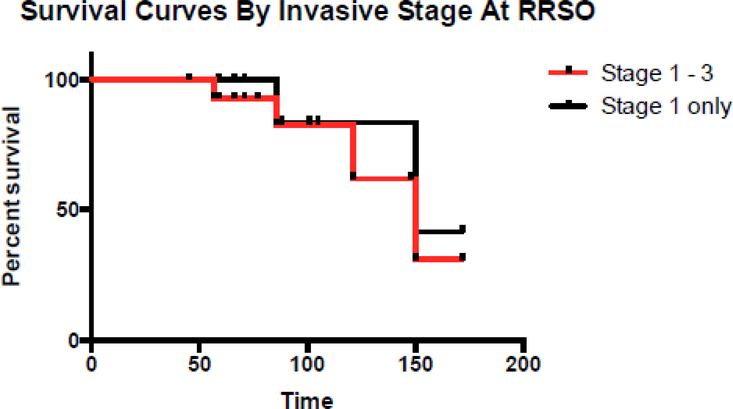
Seven of 15 patients with OC or FTC recurred (Table 1, and Figure 2 and 3 K-M curves), and all had BRCA1 mutations. Of the seven women who recurred, four women have died of disease (27%), three are AWD (20%), and only one is disease free. Of the seven patients who recurred; median disease free interval was 32.5 months (range 25–83 months). All received multiple chemotherapy regimens after recurrence. There were a total of 121 years of follow up for the group with invasive carcinomas with a recurrence rate of 0.06 per person-year. The one patient, case 14, who is now disease free had Stage IA ovarian carcinoma. Following primary treatment with 6 cycles of carboplatin and paclitaxel, the disease recurred after 25 months after RRSO in an isolated 3.5 cm para-aortic lymph node. She underwent surgical resection, followed by carboplatin and gemcitabine for 6 cycles and then intensity modulated radiation therapy to the para-aortic lymph node bed. She is currently disease free at 66 months.
Fig. 2.
Survival after detection of occult lesions in BRCA positive women undergoing rrso.
Fig. 3.
Survival curves by invasive stage at RRSO.
Noninvasive neoplasia
Of the 17 patients with noninvasive neoplasms, the median age of 13 BRCA1 carriers was 53 years (range 45–73), and the median age of 4 BRCA2 carriers was 55.5 (range 43–76). Fifteen of 17 patients who had noninvasive neoplasms on RRSO had a pre-operative CA125 recorded; all were in the normal range (2 reported as normal without a numeric value) with a median value of 10 U/ml (range 5–34, SD 9.3). Pre-operative transvaginal ultrasound was done in 15/17 patients with noninvasive neoplasms, and was normal in 11; three women were noted to have incidental benign cysts (a 6 cm dermoid in the opposite ovary, a 2cm simple cyst and a 3cm serous cyst) and one was identified with findings consistent with a hydrosalpinx on the side of a FT intraepithelial neoplasm.
Eight patients had hysterectomies at the time of RRSO, and another four had a subsequent surgery to remove the uterus after the discovery of tubal neoplasia. Ten patients had partial or complete staging, two patients underwent complete surgical staging at the time of initial surgery, including lymph node dissection, and one underwent complete staging after chemotherapy, five patients had omental biopsies and random peritoneal biopsies and two additional women had omental biopsies only. Peritoneal cytology was available on all 17 cases, and cytology was positive in 3 cases. All omental biopsies and subsequent staging procedures were negative for neoplasia. The median follow up was 80 months (range 40–150 months), all are alive. Four patients were treated with chemotherapy initially, including three with positive cytology. Two patients had 3 cycles and 2 had 6 cycles of carboplatin and paclitaxel. All treated patients with noninvasive neoplasia at RRSO came from a single center [Center 3]. Two of these 4 had positive cytology in addition to the noninvasive neoplasm. Another patient from Center 2 who also had positive cytology in addition to STIC did not receive chemotherapy and is disease free at 101 months after RRSO.
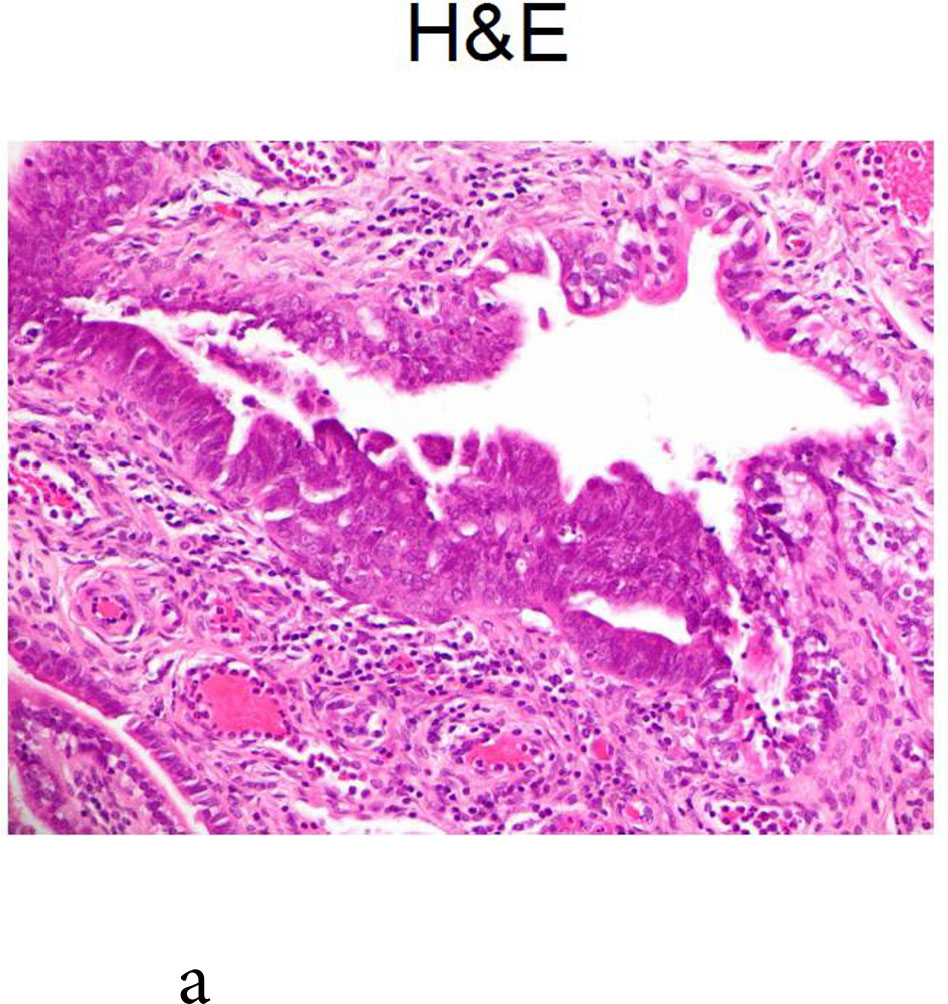
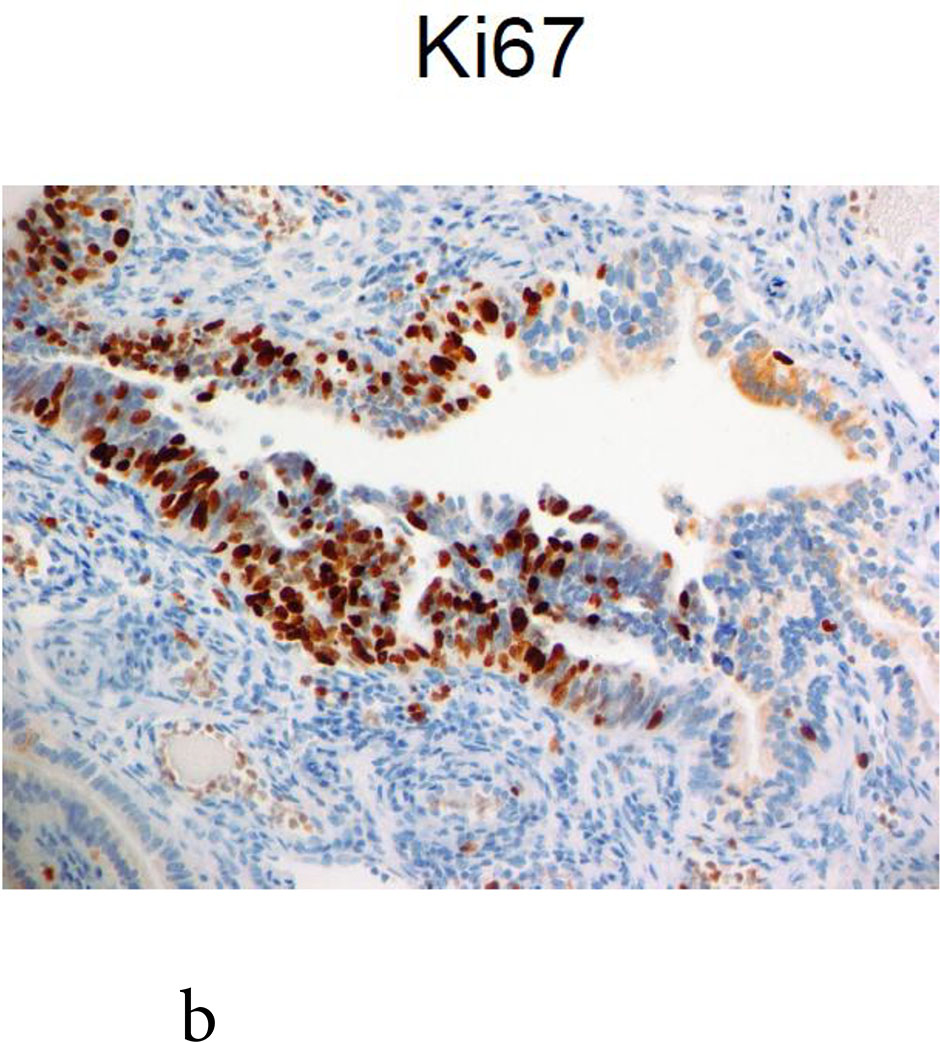
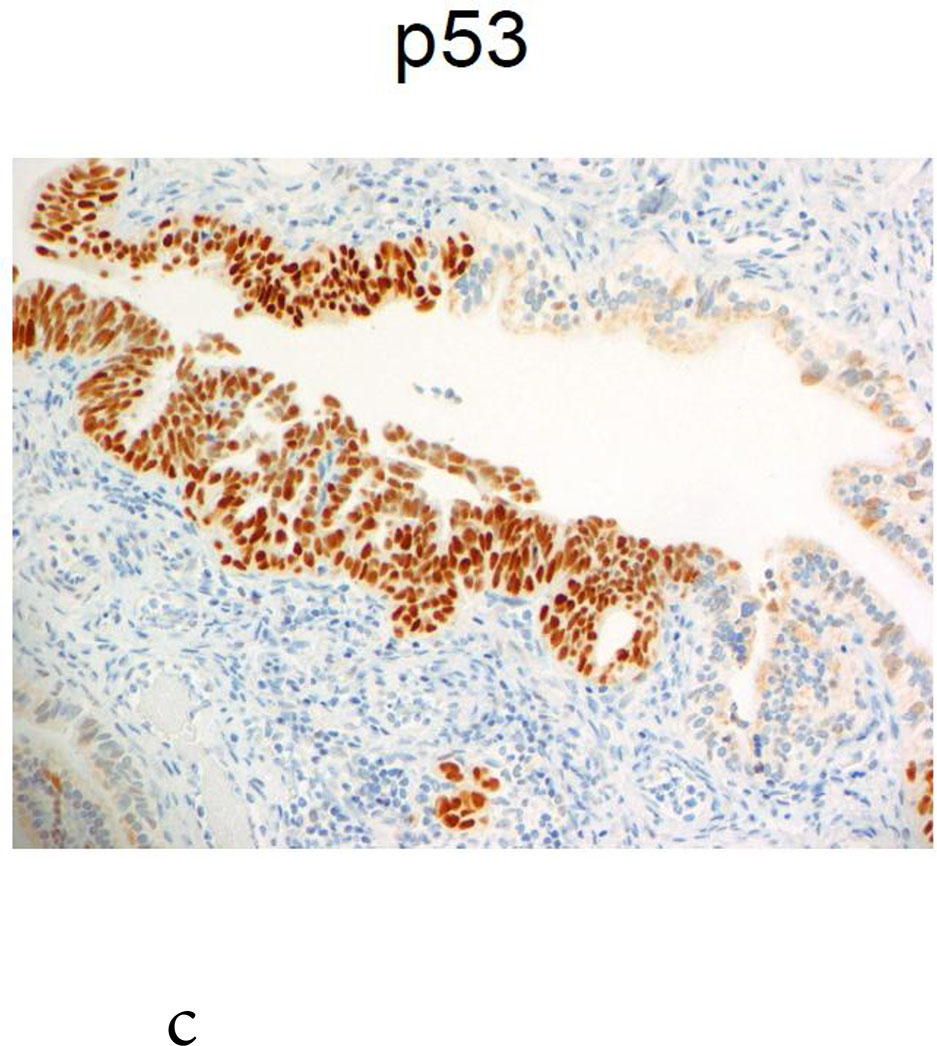
One BRCA1 positive patient, case 21, age 49 at RRSO, with high-grade fallopian tube intraepithelial neoplasia recurred at 43 months. The initial pathology report described: “The epithelial dysplasia at the fimbriated end of the left fallopian tube is high-grade virtually in situ serous carcinoma” and stained positive for Ki-67 and P53. The recurrence was detected by elevated CA125 on routine surveillance and she was found at surgery to have omental metastases. She was treated with surgical cytoreduction followed by platinum and paclitaxel for 8 cycles, was last seen 16months from the recurrence, without clinical evidence of disease and a CA125 of 11 at that time. Because the recurrence was so unexpected, the initial pathology, shown in Figure 1, was subjected to multiple expert gynecologic pathology reviews, both within the institution and independently at two other academic institutions. Consensus review confirmed the noninvasive neoplasia but no invasion in the RRSO specimen.
Fig. 1.
Case 21: shown is the noninvasive lesion in the fallopian tube.
There was a total of 127 years of follow up for the patients with unsuspected FT intraepithelial neoplasms diagnosed at RRSO, the recurrence rate for invasive disease in this group was 0.008 per person-year. After surgery there was one new breast carcinoma in both groups and one recurrent breast cancer in a BRCA1 positive patient who died of this disease at 82 months after RRSO for invasive stage ICFTC.
Discussion
In recent years, there have been multiple reports documenting the incidence of neoplasia at RRSO and describing the previously unrecognized noninvasive neoplastic lesions in the fallopian tubes5,21–24. The majority of the unsuspected neoplasia being of fallopian tube origin has lead to a significant new understanding of the precursor of papillary serous ovarian carcinoma in the tubal fimbria. However, this is the first report to describe the clinical behavior of these neoplasms and the long-term outcomes of women found to have these unsuspected neoplasms at RRSO.
Importantly, though unsuspected at the time of preventive surgery, six of 15 cases with invasive neoplasia (40%) were stage IIC or IIIC at diagnosis. There were a total of 8 recurrences in 32 women (25%), 7 occurring in women with invasive FT or OC (7/15, 47%) and one occurring in an individual with noninvasive tubal neoplasia; all patients with recurrences had BRCA1 mutations. Compared to women with invasive carcinoma, the recurrence rate was significantly lower in women with non-invasive neoplasia or STIC (1/17, 6%, p = 0.01). Perhaps surprisingly the recurrence rate was similar in the 9 women with Stage I carcinoma (4/9, 44%) and advanced carcinoma (3/6, 50%). One explanation could be that some of the Stage 1 carcinomas did not have complete staging with lymphadenectomies performed and therefore could have been occult Stage 3. For patients with invasive FTC and OC found at RRSO, nearly all received adjuvant chemotherapy and the overall survival of 11/15 (73%), disease specific survival 12/15 (80%) is high with a median follow up of 88 months. This overall favorable survival, despite the high recurrence rates probably reflects the known improved survival in BRCA1/2 associated ovarian cancinoma28. However, three additional women are alive with disease and likely to ultimately succumb, and the recurrence rate of 7/15 or 47% is relatively high when considering the relatively early stage distribution and near universal use of adjuvant chemotherapy. Eliminating one individual with stage IIIC FTC, who likely would have been detected pre-operatively if CA125 had been assessed, still results in a recurrence rate of 43%. These data demonstrate that even small occult invasive carcinomas in BRCA1/2 mutation carriers may recur. Therefore, for invasive high-grade serous ovarian or tubal carcinomas identified at RRSO, we recommend complete surgical staging and a full six cycles of taxane and platinum chemotherapy. Given the high recurrence rate it would also be reasonable to consider more aggressive treatment regimens used in advanced ovarian carcinoma such as dose dense paclitaxel in combination with carboplatin or intraperitoneal chemotherapy. Some centers recommend CA125 every 6 months after RRSO in cases of occult neoplasia to detect recurrence or primary peritoneal carcinoma. Indeed in this series, recurrent carcinoma was detected in 5 of 8 cases by routine CA125 post RRSO.
For patients with high-grade fallopian tube intraepithelial neoplasm survival is 100%, with a median follow up of 80 months. Noninvasive neoplasia did not recur in 16/17 during the follow up period despite few cases receiving adjuvant chemotherapy. There is concern that the patient with the recurrence has been followed for only 16 months after relapse, too short a period to evaluate her long term survival. Currently there is no standard of care for women found to have tubal intraepithelial neoplasm and our data allow some cautious recommendations. Since most cases are only identified post-operatively on the final pathology report, the necessity of returning to the operating room for surgical staging is debatable. In a recent review of 31 FT high grade intraepithelial neoplasia/STIC lesions reported in the literature, Manchanda et al28advocate for full staging in these patients if cytology is positive, although complete staging was not clearly done on the majority of cases in their review and there were no positive findings of metastasis reported. In our series, none of six patients who underwent partial or complete surgical staging had positive biopsies and patients without surgical staging had an equally high survival of 100%. Our review of the literature revealed no case of noninvasive neoplasia with positive staging findings other than cytology 2,8,10,28–36. Our documented recurrence was in a patient with noninvasive neoplasia at RRSO who had an initial hysterectomy and negative cytology in addition to RRSO but no formal staging. Since her recurrence was 43 months later, it seems unlikely that there would have been disease found if initial staging had been performed. We therefore feel that surgical staging can safely be omitted in the majority of noninvasive neoplasms. However, we and others 28,37continue to recommend obtaining intraperitoneal cytology at the time of RRSO, which may provide prognostic information. Whether hysterectomy can be safely omitted in women identified with FT intraepithelial neoplasm remains uncertain, since 12 out of 16 of our cases did have hysterectomy performed concurrently with RRSO or immediately after RRSO pathology reporting. The low recurrence seen in patients with intraepithelial neoplasm would suggest hysterectomy is not necessary.
The role of adjuvant chemotherapy for noninvasive neoplasms is also controversial. Only four of the seventeen women received chemotherapy but the outcome was excellent for all women with intraepithelial neoplasm irrespective of whether they had chemotherapy or not. Chemotherapy can likely be safely omitted, at least in cases with negative cytology. BRCA1/2 mutation carriers are at increased risk of peritoneal primary carcinoma of up to 4.3% after RRSO.36The risk of cancer after unsuspected noninvasive neoplasia is identified at RRSO is low. The one case from our study is the only recurrence after a noninvasive neoplasm seen at RRSO that was found in the 40 cases reported 2,3,8,10,27–31,33–38. Manchanda refers to another unpublished case of a primary peritoneal cancer occurring 4 years after a documented STIC lesion at RRSO 28. This suggests that the detection of high-grade intraepithelial neoplasm in the tubes at RRSO does not place a patient at excess risk of primary peritoneal carcinoma above the patient with no intraepithelial neoplasm at RRSO and that adjuvant chemotherapy would not be justified to reduce this risk.
Whether chemotherapy contributed to the excellent outcome in the subset of patients with noninvasive neoplasm and positive cytology remains uncertain at this time. Of our three cases with positive peritoneal cytology, two received chemotherapy. Manchanda et al28,37 reported 10 cases of STIC in the literature with positive cytology, 5 treated with chemotherapy and 5 not, with no recurrences. Landon et al36 has also reported on 128 RRSO cases in which cytology was performed, three were atypical and one was malignant, none were associated with FT intraepithelial neoplasm, and only the case with malignant cytology was treated with chemotherapy. None of the four recurred. Nine additional patients had atypical findings of the tubes or ovaries. All had negative cytology and there were no recurrences of cancer during follow up. We agree with Manchanda et al37that clarity on these questions would be greatly aided by an international registry of BRCA1/2 positive RRSO patients.
A counterintuitive and intriguing finding in our study is that the mean age of women with invasive carcinomas was younger than that of women with noninvasive neoplasms (P=0.04). The prevailing hypothesis that FT intraepithelial neoplasm (STIC) is a precursor lesion to invasive ovarian and tubal carcinoma would predict that the age of women with noninvasive neoplasms would be younger than for women with invasive carcinoma. The small numbers in our study preclude a firm conclusion and this needs to be confirmed in larger registries. Our long-term survival data suggest that unsuspected invasive and noninvasive lesions in BRCA1/2 positive women may have a different natural history. Interestingly, there was also a non-significant trend for women with noninvasive neoplasms to have had breast cancer (8/17) at older age than those women who had invasive neoplasms (6/15) (39.3 v 49.6 years). There has been debate in the literature as to whether prior exposure to chemotherapy or tamoxifen for breast cancer might be a factor protecting against invasive tubal or ovarian carcinoma23,39,40,, although in this series prior exposure to chemotherapy and tamoxifen was the same in both groups.
A weakness of this study is that there was no centralized pathology review. However this was an ‘intention to treat’ study reflecting best practice at each institution at the time of diagnosis of these unsuspected neoplasms, which all included review of cases by an experienced gynecological pathologist. Ruling out invasion is probably the key factor to safely omit chemotherapy. Since invasion can be focal, we recommend examining multiple serial sections before a noninvasive neoplasm is confirmed. This study was also not an attempt to compare survival of patients with unsuspected neoplasia at RRSO with BRCA1/2 carriers with clinically identified ovarian, tubal or peritoneal carcinoma or with patients with RRSO with benign findings but rather to describe the outcomes of these unsuspected neoplasms to inform clinical care.
In summary, we have found that women with early stage invasive carcinoma identified at RRSO have a high recurrence rate on long term follow up and should be treated aggressively with state of the art chemotherapy. In contrast, women with high grade intraepithelial neoplasms (including STIC) have an excellent outcome and may not require surgical staging or adjuvant chemotherapy when invasion has been thoroughly excluded by rigorous pathological sampling.
Highlights.
Longterm outcomes of 32 patients with unsuspected noninvasive and invasive neoplasia found at RRSO are reported.
45% recurrence rate of the 15 invasive lesions was reported in the median 88mth follow up.
The first documented case of a recurrence at 43 months, after a noninvasive neoplasm in the fallopian tube is reported.
Acknowledgments
This work was supported by NIH grants R01CA131965, the Wendy Feuer Ovarian Cancer Research Fund
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stratton JF, Buckley CH, Lowe D, et al. : Comparison of prophylactic oophorectomy specimens from carriers and noncarriers of a BRCA1 or BRCA2 gene mutation. United Kingdom Coordinating Committee on Cancer Research (UKCCCR) Familial Ovarian Cancer Study Group. J Natl Cancer Inst 91:626–8, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Leeper K, Garcia R, Swisher E, et al. : Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol Oncol 87:52–6, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Powell CB, Kenley E, Chen LM, et al. : Risk-reducing salpingo-oophorectomy in BRCA mutation carriers: role of serial sectioning in the detection of occult malignancy. J Clin Oncol 23:127–32, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Finch A, Shaw P, Rosen B, et al. : Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol 100:58–64, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Menon U, Kalsi J, Jacobs I, et al. : International Conference on Ovarian Cancer Screening: 29th-30th November 2011, Royal College of Physicians, London. Foreword. Int J Gynecol Cancer 22 Suppl 1:S1, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Callahan MJ, Crum CP, Medeiros F, et al. : Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol 25:3985–90, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Deligdisch L, Gil J, Kerner H, et al. : Ovarian dysplasia in prophylactic oophorectomy specimens: cytogenetic and morphometric correlations. Cancer 86:1544–50, 1999 [PubMed] [Google Scholar]
- 8.Medeiros F, Muto MG, Lee Y, et al. : The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol 30:230–6, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Rabban JT, Krasik E, Chen LM, et al. : Multistep level sections to detect occult fallopian tube carcinoma in risk-reducing salpingo-oophorectomies from women with BRCA mutations: implications for defining an optimal specimen dissection protocol. Am J Surg Pathol 33:1878–85, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Lamb JD, Garcia RL, Goff BA, et al. : Predictors of occult neoplasia in women undergoing risk-reducing salpingo-oophorectomy. Am J Obstet Gynecol 194:1702–9, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Medeiros F, Kindelberger D, et al. : Advances in the recognition of tubal intraepithelial carcinoma: applications to cancer screening and the pathogenesis of ovarian cancer. Adv Anat Pathol 13:1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Crum CP, Drapkin R, Kindelberger D, et al. : Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res 5:35–44, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson J, Roh MH, Chang MC, et al. : Recent advances in the understanding of the pathogenesis of serous carcinoma: the concept of low- and high-grade disease and the role of the fallopian tube. Diagn Histopathol (Oxf) 14:352–365, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y, Miron A, Drapkin R, et al. : A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol 211:26–35, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Kurman RJ, Shih Ie M: The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 34:433–43, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carcangiu ML, Radice P, Manoukian S, et al. : Atypical epithelial proliferation in fallopian tubes in prophylactic salpingo-oophorectomy specimens from BRCA1 and BRCA2 germline mutation carriers. Int J Gynecol Pathol 23:35–40, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Salvador S, Gilks B, Kobel M, et al. : The fallopian tube: primary site of most pelvic high-grade serous carcinomas. Int J Gynecol Cancer 19:58–64, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Carlson JW, Miron A, Jarboe EA, et al. : Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol 26:4160–5, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kindelberger DW, Lee Y, Miron A, et al. : Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol 31:161–9, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Levanon K, Crum C, Drapkin R: New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol 26:5284–93, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domchek SM, Rebbeck TR: Prophylactic oophorectomy in women at increased cancer risk. Curr Opin Obstet Gynecol 19:27–30, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Rebbeck TR, Kauff ND, Domchek SM: Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst 101:80–7, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domchek SM, Friebel TM, Singer CF, et al. : Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 304:967–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olivier RI, van Beurden M, Lubsen MA, et al. : Clinical outcome of prophylactic oophorectomy in BRCA1/BRCA2 mutation carriers and events during follow-up. Br J Cancer 90:1492–7, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivier RI, Lubsen-Brandsma LA, van Boven H, et al. : Additional salpingectomy after previous prophylactic oophorectomy in high-risk women: sense or nonsense? Gynecol Oncol 96:439–43, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Greene MH, Mai PL, Schwartz PE: Does bilateral salpingectomy with ovarian retention warrant consideration as a temporary bridge to risk-reducing bilateral oophorectomy in BRCA1/2 mutation carriers? Am J Obstet Gynecol 204:19 e1–6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chetrit A, Hirsh-Yechezkel G, Ben-David Y, et al. : Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: the national Israeli study of ovarian cancer. J Clin Oncol 26:20–5, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Manchanda R, Abdelraheim A, Johnson M, et al. : Outcome of risk-reducing salpingo-oophorectomy in BRCA carriers and women of unknown mutation status. BJOG 118:814–24, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Paley PJ, Swisher EM, Garcia RL, et al. : Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: a case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol 80:176–80, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Colgan TJ, Boerner SL, Murphy J, et al. : Peritoneal lavage cytology: an assessment of its value during prophylactic oophorectomy. Gynecol Oncol 85:397–403, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Agoff SN, Garcia RL, Goff B, et al. : Follow-up of in situ and early-stage fallopian tube carcinoma in patients undergoing prophylactic surgery for proven or suspected BRCA-1 or BRCA-2 mutations. Am J Surg Pathol 28:1112–4, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Agoff SN, Mendelin JE, Grieco VS, et al. : Unexpected gynecologic neoplasms in patients with proven or suspected BRCA-1 or −2 mutations: implications for gross examination, cytology, and clinical follow-up. Am J Surg Pathol 26:171–8, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Powell CB, Chen LM, McLennan J, et al. : Risk-reducing salpingo-oophorectomy (RRSO) in BRCA mutation carriers: experience with a consecutive series of 111 patients using a standardized surgical-pathological protocol. Int J Gynecol Cancer 21:846–51, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Finch A, Beiner M, Lubinski J, et al. : Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. Jama 296:185–92, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Carcangiu ML, Peissel B, Pasini B, et al. : Incidental carcinomas in prophylactic specimens in BRCA1 and BRCA2 germ-line mutation carriers, with emphasis on fallopian tube lesions: report of 6 cases and review of the literature. Am J Surg Pathol 30:1222–30, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Landon G, Stewart J, Deavers M, et al. : Peritoneal washing cytology in patients with BRCA1 or BRCA2 mutations undergoing risk-reducing salpingo-oophorectomies: a 10-year experience and reappraisal of its clinical utility. Gynecol Oncol 125:683–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manchanda R, Drapkin R, Jacobs I, et al. : The role of peritoneal cytology at risk-reducing salpingo-oophorectomy (RRSO) in women at increased risk of familial ovarian/tubal cancer. Gynecol Oncol 124:185–91, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Reitsma W, de Bock GH, Oosterwijk JC, et al. : Support of the “fallopian tube hypothesis” in a prospective series of risk-reduing salpingo-oophrectomy specimens. Eur J Cancer. 2012. (avail on line) [DOI] [PubMed] [Google Scholar]
- 39.Eisen A, Lubinski J, Klijn J, et al. : Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol 23:7491–6, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Rebbeck TR, Friebel T, Wagner T, et al. : Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 23:7804–10, 2005 [DOI] [PubMed] [Google Scholar]