Abstract
Introduction:
Enamel subsurface lesions or white spot lesions (WSLs) are commonly found in orthodontic patients with a prevalence of 5% to 97%.
Aim:
This systematic review aimed to evaluate the efficacy of casein phosphopeptide amorphous calcium phosphate (CPP-ACP) and casein phosphopeptide amorphous calcium phosphate fluoride (CPP-ACPF) for prevention and remineralization of WSLs in orthodontic patients in human randomized controlled clinical trials (RCTs).
Methods:
Relevant articles were retrieved by searching the Web of Science, Scopus, PubMed, and Cochrane Library databases up to November 2018 with no language or date restriction. The collected data included examination method, groups included in each study with number of patients in each group, study design, follow-up period and summary of important findings of each study. The risk of bias of each study was assessed according to the guidelines of the Cochrane Collaboration’s tool.
Results:
Of 213 articles retrieved, 13 RCTs were included in this systematic review (none of them were included in the meta-analysis). Three articles showed superior efficacy of CPP-ACP for remineralization of WSLs while four studies reported the superior clinical efficacy of CPP-ACPF for this purpose.
Conclusion:
Both CPP-ACP and CPP-ACPF can decrease the prevalence and increase the remineralization of WSLs during/after orthodontic treatment.
Keywords: Orthodontics, White spot lesions, Casein phosphopeptide amorphous calcium phosphate, Casein phosphopeptide amorphous calcium phosphate fluoride
1. INTRODUCTION
Enamel subsurface lesions or white spot lesions (WSLs) are commonly found in orthodontic patients with a prevalence of 5% to 97% (1, 2). WSLs can progress and cause further demineralization of enamel (3), which is common during and after orthodontic treatment (4). Even in patients with low prevalence of caries, around 61% of WSLs may progress during orthodontic treatment despite a comprehensive preventive program (5). The newly introduced bioactive agents derived from milk products can increase enamel and dentin remineralization by releasing active ions under cariogenic conditions. Casein phosphopeptide amorphous calcium phosphate (CPP-ACP) is a nano-complex of milk protein (casein phosphopeptide) and amorphous calcium phosphate (6). The CPP-ACP complexes are available in gel, cream or mousse forms and may also be incorporated into chewing gums (7). Tooth Mousse Plus (MI Paste Plus) is a commercial product containing casein phosphopeptide amorphous calcium phosphate fluoride (CPP-ACPF). It is assumed to provide greater therapeutic effects compared to Tooth Mousse (MI Paste), which contains CPP-ACP alone (8). Evidence shows that application of CPP-ACP may prevent demineralization, enhance remineralization or do both (9, 10). In addition, fluoride ions are believed to be highly effective for prevention of dental caries via several mechanisms (11).
2. AIM
This systematic review aimed to evaluate the efficacy of CPP-ACP and CPP-ACPF for prevention and remineralization of WSLs in orthodontic patients in human randomized clinical trials (RCTs).
3. METHODS
Search methodology
This systematic review was performed based on the PRISMA guidelines (12). The study question was: How effective are the products containing CPP-ACP and CPP-ACPF compared to placebo and other treatments? Table 1 shows the selective criteria according to PICOS process.
Table 1. PICOS process. Abbreviations: CPP-ACP: Casein phosphopeptide amorphous calcium phosphate; CPP-ACPF: Casein phosphopeptide amorphous calcium phosphate fluoride; WSL: White spot lesion.
| Component | Description |
|---|---|
| Population | Participants included during and after fixed orthodontic treatment |
| Intervention | Products containing CPP-ACP or CPP-ACPF |
| Comparison |
Use of CPP-ACP vs. control (toothbrushing or fluoridated toothpaste), placebo, or other products (± fluoridated toothpaste) Use of CPP-ACPF vs. control (toothbrushing or fluoridated toothpaste), placebo, or other products (± fluoridated toothpaste) |
| Outcome | Changes in development of new WSLs and improvement of the appearance of the existing WSLs |
| Study design | Human randomized controlled clinical trials |
Relevant articles were retrieved by searching four databases namely the Web of Science, Scopus, PubMed, and Cochrane Library up to November 2018 with no language or date restriction. The search terms were: (orthodontic*) and (“Tooth Mousse” or “MI Paste” or “CPP-ACP” or “Tooth Mousse Plus” or “MI Paste Plus” or “CPP-ACPF”) and (white spot lesion* or WSL* or “demineralization” or “remineralization” or “remineralizing”).
Study selection
One author (MS) electronically searched the titles and abstracts of all identified studies and evaluated them. Another author (MMI) re-assessed the previous search and evaluation. In case of presence of ambiguity regarding the titles or abstracts, full-texts of the articles were retrieved and reviewed. We selected the studies that met our criteria.
Data extraction
Two authors (MS and MMI) extracted the data from each study. The extracted data included the examination method, groups included in each study with number of patients in each group, study design, follow-up period and the summary of important findings of each study. In case of presence of a disagreement between the two authors, it was resolved by discussion.
Quality assessment
The risk of bias of each study was assessed according to the guidelines of the Cochrane Collaboration’s tool (13) by one author (MS).
Statistical analysis
No meta-analysis was carried out due to the heterogeneity between the studies.
4. RESULTS
Selection and exclusion of studies
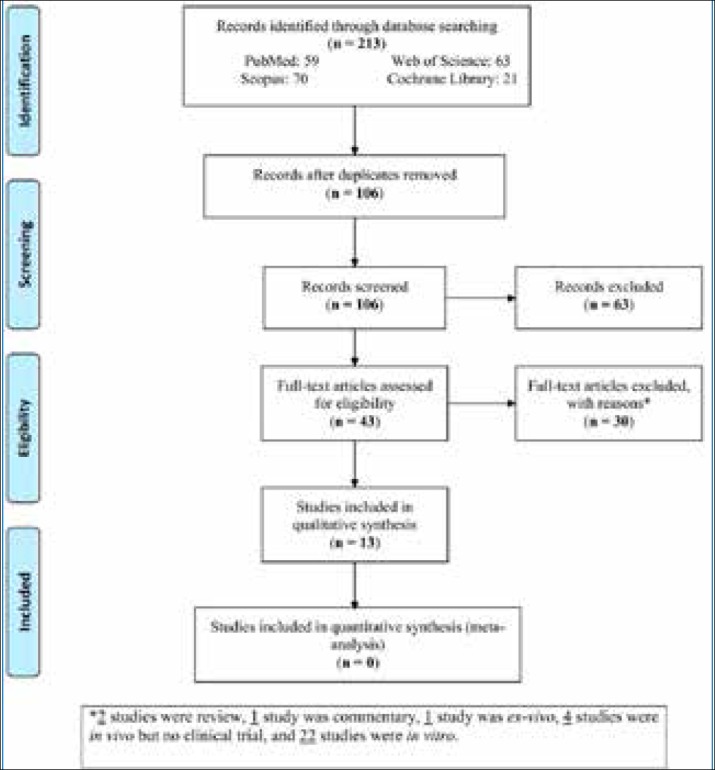
A total of 213 studies were retrieved. After excluding the duplicates and irrelevant studies, the full texts of 43 articles were reviewed for eligibility (Figure 1). Of the articles with available full texts, 30 studies were excluded with reasons: two studies were review articles, one study was commentary article, one study had an ex-vivo design, four studies were in vivo but no clinical trial, and twenty-two studies had an in vitro design. Finally, 13 RCTs were included in this systematic review (none of them were included in the meta-analysis).
Figure 1. PRISMA flow diagram of study search.
Quality assessment
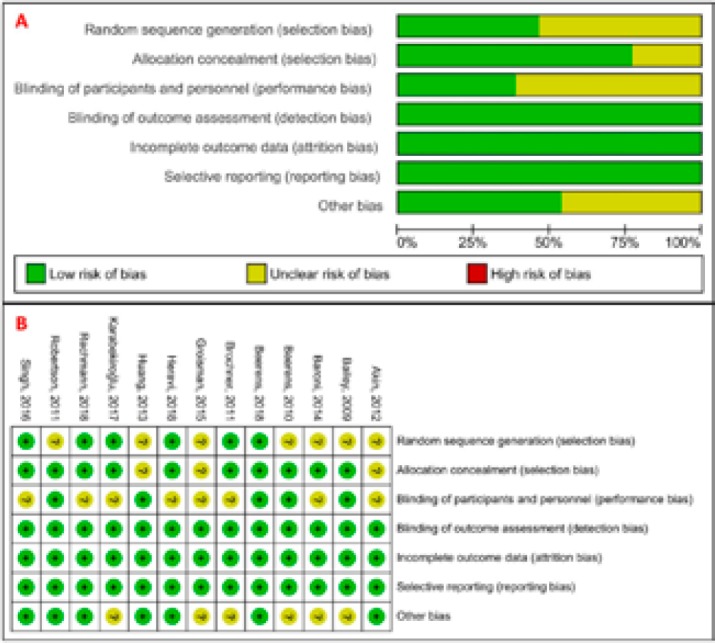
The most obvious risk of bias in RCTs was blinding of participants and personnel (performance bias) followed by random sequence generation, other bias, and allocation concealment (Figure 2). These biases can be due to different outcomes, treatment procedures and examination methods of RCTs in this systematic review. We did not observe the risk of attrition bias, detection bias, and reporting bias in studies. The overall risk of all types of bias in RCTs was generally low to unclear.
Figure 2: Cochrane risk of bias of the studies included in this systematic review: (A) graph and (B) summary.
Study characteristics
Thirteen relevant studies were found published from 2009 to 2018. Five studies (8, 14-17) were reported from Asia, four studies (1, 18-20) from America, three (9, 21, 22) from Europe, and one study (23) from Australia (Table 2). Examination methods used in this study included clinical examination, quantitative light-induced fluorescence, digital photography, scanning electron microscopy (SEM), and DIAGNOdent pen; each study used one or more of them. There were five studies (9, 14, 16, 17, 23) that had one test group with a product containing CPP-ACP; eight studies (1, 8, 15, 18-22) had one test group with a product containing CPP-ACPF. Totally, the CPP-ACP group included 80 and the CPP-ACPF group included 182 participants. Both groups were compared with one or more other groups such as placebo, control (± fluoridated toothpaste), fluoride varnish, fluoride rinse, micro-abrasion, and Remin Pro. All studies were RCTs with/without blinding. The follow-up period ranged from 3 to 36 months.
Table 2. Characteristics of RCTs included in this systematic review (n=13) Abbreviations: CPP-ACP: Casein phosphopeptide amorphous calcium phosphate; CPP-ACFP: Casein phosphopeptide amorphous calcium phosphate fluoride.
| First author, year | Country | Examination methods | Groups (n) | Study design | Follow-up period |
|---|---|---|---|---|---|
| Bailey, 2009 (23) | Australia | Clinical examination; quantitative light-induced fluorescence; digital photographs | Placebo (22), CPP-ACP (23) | Double-blind, parallel-group, randomized controlled trial | 3 months |
| Beerens, 2010 (21) | Netherlands | Clinical examination; quantitative light-induced fluorescence | Control (27), CPP-ACPF (27) | Double-blind prospective, randomized controlled trial | 3 months |
| Brochner, 2011 (9) | Denmark | Clinical examination; quantitative light-induced fluorescence; digital photographs | Control (25), CPP-ACP (25) | Single-blind, randomized controlled trial | One month |
| Robertson, 2011 (18) | USA | Clinical examination; digital photographs | Placebo (26), CPP-ACPF (26) | Double-blind, prospective, randomized controlled trial | 3 months |
| Akin, 2012 (14) | Turkey | Clinical examination; digital photographs | Control (20), fluoride rinse (20), CPP-ACP (20), micro-abrasion (20) | Parallel-group, prospective randomized controlled trial | 6 months |
| Huang, 2013 (1) | USA | Digital photographs | Control (41), CPP-ACPF (34), fluoride varnish (40) | Parallel-group, single-blind, active-controlled, randomized trial | 6 months |
| Baroni, 2014 (15) | Italy | Scanning electron microscopy (SEM) | Control (10), CPP-ACPF (10) | Single-blind, prospective, randomized controlled trial | 6 months |
| Groisman, 2015 (19) | Brazil | Clinical examination; digital photographs | CPP-ACPF (12) | Randomized controlled trial | 12 months |
| Singh, 2016 (16) | India | Clinical examination; DIAGNOdent pen | Control (15), fluoride varnish (15), CPP-ACP (15) | Single-blind, randomized controlled trial | 6 months |
| Karabekiroğlu, 2017 (17) | Turkey | Clinical examination; DIAGNOdent pen | Control (12), CPP-ACP (12) | Single blinded-randomized controlled trial | 36 months |
| Beerens, 2018 (22) | Netherlands | Clinical examination; quantitative light-induced fluorescence | Control (27), CPP-ACPF (27) | Double-blinded, prospective, placebo-controlled randomized clinical trial | 3 months |
| Heravi, 2018 (8) | Iran | Quantitative light-induced fluorescence | Control (12), CPP-ACFP (12), Remin Pro (12) | Single-blind, parallel-group, randomized, controlled trial | 3 months |
| Rechmann, 2018 (20) | USA | Clinical examination; quantitative light-induced fluorescence; digital photographs | Fluoride rinse (18), CPP-ACPF (19) | Single-blind, prospective, randomized controlled trial | 12 months |
In addition, Table 3 shows the summary of the findings of each study.
Table 3. Summary of the findings of each study included in this systematic review (n=13). Abbreviations: CPP-ACP: Casein phosphopeptide amorphous calcium phosphate; CPP-ACPF: Casein phosphopeptide amorphous calcium phosphate fluoride, WSL: White spot lesion.
| First author, year | Summary of findings |
|---|---|
| Bailey, 2009 (23) | WSLs had a significantly greater chance of regression at 12 weeks following twice a day application of a remineralizing cream containing CPP-ACP compared with a placebo cream (P<0.05). |
| Beerens, 2010 (21) | There was no clinical advantage for use of CPP-ACPF compared to a fluoride-free control paste over a 12-week period. |
| Brochner, 2011 (9) | Topical treatment with CPP-ACP significantly decreased the change in fluorescence values (∆F) and area of the lesions after 4 weeks (P<0.05), but the improvement was not superior compared to daily use of fluoride toothpaste (control). |
| Robertson, 2011 (18) | CPP-ACPF prevented the development and decreased the number of WSLs, but the placebo had no preventive action. |
| Akin, 2012 (14) | CPP-ACP increased the remineralization of demineralized enamel more than fluoride rinse and control groups (P<0.05), but not the micro-abrasion group. |
| Huang, 2013 (1) | CPP-ACPF did not appear to be more effective than normal home or fluoride varnish care for improving the appearance of WSLs over an 8-week period. |
| Baroni, 2014 (15) | CPP-ACPF had positive in vivo effects on enamel surfaces. Significant changes in surface roughness occurred after a 3-week period of application of CPP-ACPF compared to control. |
| Groisman, 2015 (19) | Commercially available CPP-ACPF paste was successful in enamel remineralization of WSLs during a 12-month follow-up of orthodontic patients, even when used for only for 4 weeks. |
| Singh, 2016 (16) | The use of fluoride varnish and CPP-ACPF cream had no superior efficacy compared to daily use of fluoride toothpaste alone (control) in reducing the severity of WSLs. |
| Karabekiroğlu, 2017 (17) | Daily use of CPP-ACP did not appear to be more effective than 1,450 ppm fluoridated toothpaste (control) for improving the appearance of WSLs after 36 months. |
| Beerens, 2018 (22) | Additional use of CPP-ACPF in patients with subsurface enamel lesions after fixed orthodontic treatment did not improve these lesions during one year after debonding. |
| Heravi, 2018 (8) | (I) Application of CPP-ACPF caused a significant decrease in the area of WSLs, which was significantly greater than that of the control group (P<0.05). (II) The mineral content of WSLs was enhanced in all groups throughout the experiment, but the degree of enhancement was significantly greater in CPP-ACPF than the control group. (III) A significantly greater improvement in the appearance of WSLs occurred following the use of CPP-ACPF as compared to patients receiving the usual home care (P<0.05). |
| Rechmann, 2018 (20) | (I) Daily application of CPP-ACPF resulted in no statistically significant difference in the sum of enamel decalcification index and international caries detection and assessment system scores. (II) Daily application of CPP-ACPF did not appear to significantly decrease the incidence of WSLs during fixed orthodontic treatment. |
Effect of products containing CPP-ACP on WSLs
Of four studies having a CPP-ACP group, one study compared this group with a placebo group (23), while three studies compared this group with controls (9, 14, 17), and one study (4) compared this group with fluoride rinse or micro-abrasion group. Among these studies, three studies (9, 14, 23) showed superior efficacy of CPP-ACP for remineralization of WSLs, while one study (17) did not report a superior effect compared to other groups.
Effect of products containing CPP-ACPF on WSLs
Of nine studies with a CPP-ACPF group, seven studies compared this group with controls (1, 8, 15, 16, 21, 22), one study (18) compared this group with a placebo group, two studies (1, 16) compared this group with fluoride varnish group, one study (20) compared this group with fluoride rinse group, one study (8) compared this group with Remin Pro group, and one study (19) made no comparison with any other group. Among these studies, four studies (8, 15, 18, 19) reported superior clinical advantage of CPP-ACPF for prevention and remineralization of WSLs, while the remaining five studies (1, 16, 20-22) did not report remineralization of WSLs compared to other groups.
5. DISCUSSION
This study evaluated the efficacy of products containing CPP-ACP and CPP-ACPF for remineralization of WSLs in orthodontic patients by reviewing RCTs. CPP-ACP stabilizes calcium and phosphate ions on the tooth surface in a bioavailable form and it can promote remineralization of subsurface enamel lesions in situ, even in presence of fluoride (24, 25). One RCT showed the significant effect of a remineralizing cream containing CPP-ACP plus fluoridated toothpaste compared with a placebo cream plus fluoridated toothpaste after 3 months on regression of WSLs (23). Also, daily topical use of CPP-ACP plus fluoridated toothpaste significantly decreased changes in fluorescence values (∆F) and area of the lesions after one month but it was not superior to daily use of fluoridated toothpaste (control) in another RCT (9). Another study showed that the effect of CPP-ACP plus fluoridated toothpaste was significantly greater than that of fluoride rinse and control (brushing alone), but not the micro-abrasion group after six months (14). Daily use of CPP-ACP plus fluoridated toothpaste was not more effective than 1,450 ppm fluoridated toothpaste applied twice a day (control group) after 36 months for improving the appearance of WSLs; 1,450 ppm fluoridated toothpaste was found to be effective for decreasing only the lesion severity and activity during this time period in a RCT (17). In addition, the majority of in vitro, animal, and in situ studies have demonstrated that CPP-ACP can promote the remineralization of subsurface enamel lesions (7, 26-32).
The combination of CPP-ACP and fluoride can have a synergistic effect on enamel remineralization due to the formation of stabilized ACPF (25). Beerens et al. (33) reported that CPP-ACPF has the same potential as CPP-ACP plus the additional benefits of supplemental fluoride. Four human RCTs (8, 15, 18, 19) reported significant effect of CPP-ACPF on remineralization of WSLs (1, 16, 20-22). In other RCTs, although there was no significant difference between CPP-ACPF group and other groups, it had a generally positive effect on remineralization of WSLs. Controversy in the results of studies can be due to different outcome measures of WSLs. Two studies (29, 34) reported that the fluoride content of MI Paste Plus has a synergistic effect with CPP-ACP, and increases its remineralizing potential. Heravi et al. (8) reported that the mineral content of WSLs was slightly greater in Remin Pro than MI Paste Plus (16% and 22% increase compared to baseline, respectively); this difference can be due to the more efficient delivery of calcium and phosphate ions by hydroxyapatite. One possible limitation affecting the results of in vivo studies is preservation of CPP-ACPF in MI Paste Plus, which can explain the positive results found in vitro and in situ (22). Some researchers believe that concentrated fluoride products should not be used for treatment of demineralized enamel immediately after removal of appliances, since they cause fluoride precipitation on the enamel surface of the tooth with less effect on subsurface lesions, and therefore, the appearance of the demineralized enamel would not improve (1, 35). It should be noted that none of the RCTs made a comparison between CPP-ACP and CPP-ACPF that can be used for future research.
This systematic review had several limitations: (I) There was no standard protocol or system for using CPP-ACP and CPP-ACPF. (II) The visual impact of decalcification (scoring of the enamel decalcification index and international caries detection and assessment) can be different for each observer. (III) Lack of blinding of patients and the supplier because commercial products were used in some studies. (IV) Some studies had a small sample size. (V) Heterogeneity of the outcomes of RCTs; therefore, we could not perform a meta-analysis on the outcomes.
6. CONCLUSION
Review of the findings of human RCTs revealed that both CPP-ACP and CPP-ACPF can have a positive impact on enhancing remineralization or decreasing the prevalence of WSLs during/after orthodontic treatment. This effect was significant in a number of studies compared to other groups. But due to the small number of homogeneous studies, future studies are required to select the same outcomes and groups to draw a more accurate conclusion regarding the efficacy of CPP-ACP and CPP-ACPF for remineralization of WSLs caused by orthodontic treatment.
Acknowledgments:
This work was performed in partial fulfillment of the requirements for the degree of general dentistry by “Aida Afnaniesfandabad” at Faculty of Dentistry, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Author contributions:
MMI designed the study. MS contributed to the conception of the study and drafted the manuscript. MS, AA, HM, AG, RS, & HRM reviewed the information of the articles. MMI & MS critically edited the manuscript. All authors have read and approved the final paper prior to its submission.
Conflicts of Interest:
There are no conflicts of interest.
Financial support and sponsorship:
The authors gratefully acknowledge the Research Council of Kermanshah University of Medical Sciences for the financial support (Code: 97780).
REFERENCES
- 1.Barkley RA. New York: Guilford Press; 1998. Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment. [Google Scholar]
- 2.Ogrim G, Hestad K. Effects of Neurofeedback Versus Stimulant Medication in Attention-Deficit/Hyperactivity Disorder: A Randomized Pilot Study. Journal of Child Adolescent Psychopharmacology. 2013;23(7):448–457. doi: 10.1089/cap.2012.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Classi P, Ward S, Sarsour Kh, Johnston J. Social and emotional difficulties in children with ADHD and the impact on school attendance and healthcare utilization. Child and Adolescent Psychiatry and Mental Health. 2012;6(33) doi: 10.1186/1753-2000-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biederman J, Petty CR, Dolan C, Hughes S, Mick E, Monuteaux MC, et al. The long-term longitudinal course of oppositional defiant disorder and conduct disorder in ADHD boys: findings from a controlled 10-year prospective longitudinal follow-up study. Psychol Med. 2008;38(7):1027–1036. doi: 10.1017/S0033291707002668. [DOI] [PubMed] [Google Scholar]
- 5.Lahey BB, Pelham WE, Stein MA, Loney J, Trapani C, Nugent K, et al. Validity of DSM-IV attention-deficit/hyperactivity disorder for younger children. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37(7):695–702. doi: 10.1097/00004583-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Claude D. The development of ADHD boys: a 12-year follow-up. Canadian Journal of Behavioral Science. 1995;27:226–249. P F. [Google Scholar]
- 7.AD/HD Nrco. Complementary and Alternative Treatments: Neurofeedback (EEG Biofeedback) and ADHD CHADD: ADHD NrCo. 2012. (cited 2018)
- 8.Monastra VJ, Lynn S, Linden M, Lubar JF, Gruzelier J, La Vaque TJ. Electroencephalographic biofeedback in the treatment of attention-deficit/hyperactivity disorder. Applied Psychophysiology and Biofeedback. 2005;30(2):95–114. doi: 10.1007/s10484-005-4305-x. [DOI] [PubMed] [Google Scholar]
- 9.Moreno-García I, Delgado-Pardo G, Camacho-Vara de Rey C, Meneres-Sancho S, Servera-Barceló M. Neurofeedback, pharmacological treatment and behavioral therapy in hyperactivity: Multilevel analysis of treatment effects on electroencephalography. International Journal of Clinical and Health Psychology. 2015;15(3):217–225. doi: 10.1016/j.ijchp.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tinius TP. The Integrated Visual and Auditory Continuous Performance Test as a neuropsychological measure. Archives of Clinical Neuropsychology. 2003;18(5):439–454. [PubMed] [Google Scholar]
- 11.Sanford J, Turner A. UK: Brain Train Inc; 2004. IVA+ Plus: Integrated visual and auditory continuous performance test interpretation manual. [Google Scholar]
- 12.San Francisco: University of California; 2010. An introduction to effectiveness, dissemination and implementation research: A Resource Manuals and Guides to Community-Engaged Research Retrieved January. D S. Clinical Translational Science Institute Community Engagement Program. [Google Scholar]
- 13.Duric NS, Assmus J, Gundersen D. Neurofeedback for the treatment of children and adolescents with ADHD: a randomized and controlled clinical trial using parental reports. BMC psychiatry. 2012;12(1):107. doi: 10.1186/1471-244X-12-107. IB E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellison K. Study may show whether neurofeedback helps people with ADHD and other disorders: The Washington Post. 2009. [2018]. Available from: www.washingtonpost.com Health.
- 15.Gevensleben H, Holl B, Albrecht B, Vogel C, Schlamp D, Kratz O. Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. J Child Psychol Psyc. 2009;50(7):780–789. doi: 10.1111/j.1469-7610.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 16.Karami M. Clinical Decision Support Systems and Medical Imaging. Radiol Manage. 2015;37(2):25–32. [PubMed] [Google Scholar]
- 17.Vogl T, Mangis J, Rigler A, Zink W, Alkon D. Accelerating the convergence of the back-propagation method. Biological Cybernetics. 1988;59(4-5): 257–263. [Google Scholar]
- 18.Richardson M. UK: University of Oxford; 2009. Principal Component Analysis. [Google Scholar]
- 19.Karami M, Hafizi N. E-game in Healthcare: As an E-intervention to Promote Public Health. Iranian Journal of Public Health. 2016;45(12):1662–1664. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JL, Jin GL, Yuan ZG. Artificial neural network predicts hemorrhagic contusions following decompressive craniotomy in traumatic brain injury. J Neurosurg Sci. 2017 Sep 07; doi: 10.23736/S0390-5616.17.04123-6. [DOI] [PubMed] [Google Scholar]
- 21.Skoch J, Tahir R, Abruzzo T, Taylor JM, Zuccarello M, Vadivelu S. Predicting symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage with an artificial neural network in a pediatric population. Childs Nerv Syst. 2017 Aug 29; doi: 10.1007/s00381-017-3573-0. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandran N, Chiong HS, Sime MJ, Wilson GA. Diabetic retinopathy screening using deep neural network. Clin Exp Ophthalmol. 2017 Sep 07; doi: 10.1111/ceo.13056. [DOI] [PubMed] [Google Scholar]
- 23.Roshanov PS, Misra S, Gerstein HC, Garg AX, Sebaldt RJ, Mackay JA, et al. Computerized clinical decision support systems for chronic disease management: a decision-maker-researcher partnership systematic review. Implement Sci. 2011;6:92. doi: 10.1186/1748-5908-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahota N, Lloyd R, Ramakrishna A, Mackay JA, Prorok JC, Weise-Kelly L, et al. Computerized clinical decision support systems for acute care management: a decision-maker-researcher partnership systematic review of effects on process of care and patient outcomes. Implement Sci. 2011;6:91. doi: 10.1186/1748-5908-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaspers MW, Smeulers M, Vermeulen H, Peute LW. Effects of clinical decision-support systems on practitioner performance and patient outcomes: a synthesis of high-quality systematic review findings. J Am Med Inform Assoc. 2011 May 1;18(3):327–334. doi: 10.1136/amiajnl-2011-000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieuwlaat R, Connolly SJ, Mackay JA, Weise-Kelly L, Navarro T, Wilczynski NL, et al. Computerized clinical decision support systems for therapeutic drug monitoring and dosing: a decision-maker-researcher partnership systematic review. Implement Sci. 2011;6:90. doi: 10.1186/1748-5908-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemens BJ, Holbrook A, Tonkin M, Mackay JA, Weise-Kelly L, Navarro T, et al. Computerized clinical decision support systems for drug prescribing and management: a decision-maker-researcher partnership systematic review. Implement Sci. 2011;6:89. doi: 10.1186/1748-5908-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Souza NM, Sebaldt RJ, Mackay JA, Prorok JC, Weise-Kelly L, Navarro T, et al. Computerized clinical decision support systems for primary preventive care: a decision-maker-researcher partnership systematic review of effects on process of care and patient outcomes. Implement Sci. 2011;6:87. doi: 10.1186/1748-5908-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin HC, Wu HC, Chang CH, Li TC, Liang WM, Wang JY. Development of a real-time clinical decision support system upon the Web MVC-based architecture for prostate cancer treatment. BMC Med Inform Decis Mak. 2011;11:16. doi: 10.1186/1472-6947-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang YJ, Tsai CY, Yeh ML, Li YC. Assessing the impact of user interface to the usability of a clinical decision support system. AMIA Annu Symp Proc. 2003;808 [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roshanov PS, You JJ, Dhaliwal J, Koff D, Mackay JA, Weise-Kelly L, et al. Can computerized clinical decision support systems improve practitioners’ diagnostic test ordering behavior? A decision-maker-researcher partnership systematic review. Implement Sci. 2011;6:88. doi: 10.1186/1748-5908-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trafton JA, Martins SB, Michel MC, Wang D, Tu SW, Clark DJ, et al. Designing an automated clinical decision support system to match clinical practice guidelines for opioid therapy for chronic pain. Implement Sci. 2010;5:26. doi: 10.1186/1748-5908-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campion TR, Jr, Waitman LR, May AK, Ozdas A, Lorenzi NM, Gadd CS. Social, organizational, and contextual characteristics of clinical decision support systems for intensive insulin therapy: a literature review and case study. Int J Med Inform. 2010;79(1):31–43. doi: 10.1016/j.ijmedinf.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Field TS, Rochon P, Lee M, Gavendo L, Subramanian S, Hoover S, et al. Costs associated with developing and implementing a computerized clinical decision support system for medication dosing for patients with renal insufficiency in the long-term care setting. J Am Med Inform Assoc. 2008;15(4):466–472. doi: 10.1197/jamia.M2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu S, Reddy M, Yen J, DeFlitch C. SRCAST-Diagnosis: understanding how different members of a patient-care team interact with clinical decision support system. AMIA Annu Symp Proc. 2011;2011:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 37.Denekamp Y. Clinical decision support systems for addressing information needs of physicians. Isr Med Assoc J. 2007;9(11):771–776. [PubMed] [Google Scholar]
- 38.Nazari A, Karami M, Safdari R, Yaghoubi Ashrafi M. Optimizing Disease Management with Data Warehousing. Life Science Journal. 2013;10(4):929–932. [Google Scholar]


