Abstract
The emergence of antimicrobial resistance is a significant public health issue worldwide, particularly for healthcare-associated infections caused by carbapenem-resistant gram-negative pathogens. Cefiderocol is a novel siderophore cephalosporin targeting gram-negative bacteria, including strains with carbapenem resistance. The structural characteristics of cefiderocol show similarity to both ceftazidime and cefepime, which enable cefiderocol to withstand hydrolysis by β-lactamases. The unique chemical component is the addition of a catechol moiety on the C-3 side chain, which chelates iron and mimics naturally occurring siderophore molecules. Following the chelation of iron, cefiderocol is actively transported across the outer membrane of the bacterial cell to the periplasmic space via specialized iron transporter channels. Furthermore, cefiderocol has demonstrated structural stability against hydrolysis by both serine- and metallo-β-lactamases, including clinically relevant carbapenemases such as Klebsiella pneumoniae carbapenemase, oxacillin carbapenemase-48, and New Delhi metallo-β-lactamase. Cefiderocol has demonstrated promising in vitro antibacterial and bactericidal activity, which correlates with its in vivo efficacy in several animal models. This article reviews the discovery and chemistry of cefiderocol, as well as some of the key microbiological and in vivo findings on cefiderocol from recently conducted investigations.
Keywords: carbapenemase, cephalosporin, gram-negative bacteria, penicillin-binding protein 3, siderophore
Siderophores are natural iron-chelating molecules that are produced and released by nearly all bacterial species to facilitate the transport of iron into the bacterial cell for survival and growth. Siderophore molecules may be covalently linked to chemical moieties with antibacterial activity, and are then known as sideromycins. For example, albomycin is a naturally occurring sideromycin, and contains a ferrichrome siderophore group and a thioribosyl pyrimidine antibiotic [1]. Albomycin is taken up by the bacterial cell via an active transport system through the outer membrane, a mechanism that overcomes the problem of poor permeability of the outer membrane of gram-negative bacteria to antibiotics. Research into sideromycins has revealed that the involvement of an active transport system results in greater susceptibility to antibiotics and lower minimum inhibitory concentrations (MICs) compared with those molecules that are transported through the outer membrane via a passive or other mechanism [1].
Based on these observations, research into synthetic β-lactams conjugated with siderophores was started in the 1980s [2–6]. Several pharmaceutical companies have identified siderophore-conjugated antibiotics with potent in vitro antibacterial activity against gram-negative bacteria, including Pseudomonas aeruginosa. The siderophore moiety of the conjugates sequesters environmental iron, and then the iron–siderophore–antibiotic complex binds to an iron transporter outer membrane protein and is actively taken up [7–10]. This mechanism is called a “Trojan horse” strategy, enabling more efficient penetration by overcoming certain intrinsic or acquired antibiotic resistance mechanisms [7, 8]. Following entry into the periplasmic space of the bacterial cell, the complex needs to dissociate for the antibiotic to exert its antibacterial activity [8].
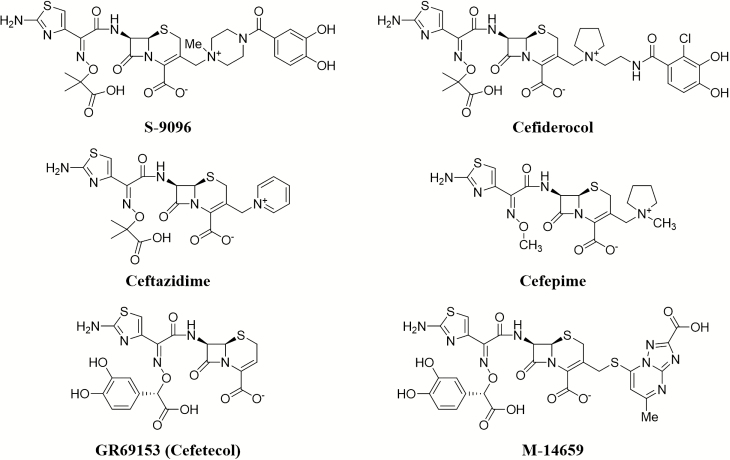
Past efforts to advance selected siderophore cephalosporin candidates, such as GR69153 (cefetecol, a catechol-substituted cephalosporin) and M-14659 (an antipseudomonal cephalosporin) (Figure 1), into clinical-stage development were not successful, despite demonstration of potent activity in vitro [11, 12]. In the early 1990s, Shionogi & Co, Ltd (Osaka, Japan) identified the siderophore cephalosporin S-9096, which had potent antibacterial activity against gram-negative pathogens, including P. aeruginosa [13, 14]. This compound possesses a catechol moiety at the C-3 side chain, resulting in good permeability of the outer membrane of gram-negative bacteria by utilizing ferric iron transport systems. However, S-9096 did not progress to clinical development because of cardiovascular toxicity and low substance stability (unpublished data).
Figure 1.
Structures of synthetic siderophore-conjugated β-lactams and cephalosporins.
DISCOVERY AND CHEMISTRY OF CEFIDEROCOL
After more than 15 years, Shionogi resumed the search for novel siderophore cephalosporins. At this time, reports of carbapenem resistance had threatened the effectiveness of available treatment options and thus represented a great and growing unmet medical need [15]. Thus, the research focused on chemical compositions that could provide improved stability to clinically important and prevalent β-lactamases, including metallo-β-lactamases (MBLs). In addition, it was important that their in vitro potency could be translated into in vivo efficacy in animal infection models and supported by advantageous pharmacokinetic/pharmacodynamic (PK/PD) relationship. Shionogi’s focus on the development of a siderophore cephalosporin with a catechol moiety contrasted with the research efforts in monobactams, which used a hydroxypyridone siderophore moiety (BAL30072, MB-1, SMC-3176) [7, 8, 16–18]. Of note, the natural siderophores enterobactin and pyoverdine produced by Escherichia coli and P. aeruginosa, respectively, are also catechol substituted molecules [19]. The investigations by Shionogi led to the identification of cefiderocol (formerly S-649266, GSK2696266) [19]. The structure–activity relationship of numerous chemical compositions related to cefiderocol has been described in detail by Aoki et al [19]. Cefiderocol is similar to cefepime in that it has a pyrrolidinium group on the C-3 side chain, which improves antibacterial activity and stability against β-lactamases (Figure 2). Additionally, similarly to ceftazidime, cefiderocol has a carboxypropanoxyimino group on the C-7 side chain, which improves transport across the outer membrane (Figure 2) [19]. The major difference in the chemical structure of cefiderocol and ceftazidime or cefepime is the addition of a chlorocatechol group on the end of the C-3 side chain, which confers the siderophore activity. Of note, the methoxy form of the chlorocatechol group has reduced antibacterial activity because it is unable to chelate iron, thus it is not transported efficiently into bacteria [20]. Investigation of the structure–activity relationship has revealed that modifications on both the C-7 and C-3 side chains contribute to the increased potency against drug-resistant gram-negative bacteria by conferring stability against β-lactamases, including carbapenemases, while maintaining high affinity to the molecular target, the penicillin-binding proteins (PBPs) [19, 21, 22].
Figure 2.
Structure–activity relationships for cefiderocol. Abbreviation: PBP, penicillin-binding protein.
MECHANISM OF ACTION
As with other β-lactam antibiotics, cefiderocol exerts a primary effect on cell wall synthesis by inhibiting PBPs, which results in cell death [22]. Similarly to other cephalosporin antibiotics such as ceftazidime, cefiderocol showed potent in vitro bactericidal activity in time-kill curve experiments against 4 main bacterial pathogens—Klebsiella pneumoniae, E. coli, P. aeruginosa, and Acinetobacter baumannii—as a result of the high binding affinities, primarily to PBP3 [22].
Carbapenem-resistant strains of gram-negative bacteria are genetically diverse and may harbor multiple resistance mechanisms, such as carbapenemase production and lower outer membrane permeability due to reduced expression or conformational mutations of porin channels and/or the upregulation of efflux pumps [23]. However, the abovementioned key features have enabled cefiderocol to overcome these resistance mechanisms.
Additional investigation into the mechanism of action of cefiderocol has revealed that the specific outer membrane iron transporters CirA and Fiu in E. coli and PiuA in P. aeruginosa are involved in the active transport of cefiderocol [20, 22]. Strains with mutations that result in deficiency in the activity of these transporter molecules showed significantly increased MICs (ie, ≥16-fold). Other siderophore-conjugated β-lactams, such as GR69153, BMS180680, MC-1, and SMC-3176, showed similarly reduced effects against mutant strains, with the exception of cefiderocol activity to P. aeruginosa, which is unaffected by the deficiency of PirA [11, 18, 22, 24–26]. The active transport process of the cefiderocol–ferric iron complex is not fully understood, and other transport determinants specific for cefiderocol might exist. This active transport mechanism contributes to not only to deliver cefiderocol efficiently into the periplasmic space where the target PBPs are located but also overcomes permeability-related drug resistance due to porin channel loss and overexpression of multidrug efflux pumps. Further investigation has revealed that cefiderocol potency for K. pneumoniae mutants with defects in the porin genes ompK35 and/or ompK36 resulted in only a 2- to 4-fold increase in MIC, whereas simultaneous mutations in these porin channels increased the MIC for meropenem 8-fold compared with that of the parent strain [22, 27, 28]. Similarly, against P. aeruginosa, cefiderocol MIC was little affected by the defect (ie, Tn insertion mutation) in carbapenem permeation porin protein oprD or the overproduction of MexA-MexB-OprM multidrug efflux pump due to a defect in the mexR or nalD regulator gene [22]. Additionally, the change in cefiderocol MIC values may result from the deficiency of the components of the efflux pump compared with that of the PAO1 parent strain, regardless of the absence or presence of iron in the media [22]. The data currently suggest that the influence of efflux pumps and porin channels on the in vitro activity of cefiderocol is insignificant.
Another key feature of cefiderocol is the intrinsic structural stability against a wide range of serine- and metallo-β-lactamases such as K. pneumoniae carbapenemase (KPC), oxacillin carbapenemase (OXA), New Delhi metallo-β-lactamase (NDM), and Verona integron-encoded metallo-β-lactamase (VIM) carbapenemase [21, 29]. The catalytic efficiencies (kcat/Km) of MBLs of VIM-2, imipenemase metallo-β-lactamase (IMP) 1, and L1 for cefiderocol were quite low, ≥260-fold lower than those for meropenem, and only a low rate of hydrolysis of cefiderocol by KPC-3 was observed [21]. The relative hydrolysis velocity of cefiderocol by NDM-1 was approximately 3–10 times lower than that of meropenem, ceftazidime, or cefepime [21]. Additionally, no or very weak hydrolysis of cefiderocol was observed by class D serine-carbapenemases such as OXA-48, OXA-40, and OXA-23 [29]. Regarding the class C β-lactamases (noncarbapenemase enzymes, which could lead to carbapenem resistance when overproduced), cefiderocol had 40-fold and >940-fold lower affinity to ampicillin chromosomal cephalosporinase (AmpC) β-lactamases of P. aeruginosa SR24-12 and Enterobacter cloacae P99, respectively, compared with ceftazidime [30]. The MIC of cefiderocol was not elevated against P. aeruginosa that overproduced AmpC due to mutation of the dacB gene (the gene coding d-alanyl-d-alanine carboxypeptidase) [30]. Cefiderocol also has a low propensity of AmpC induction in both bacterial species, similar to other oxyimino-β-lactams such as ceftazidime and cefepime, which are known as weak AmpC inducers [30, 31]. The AmpC-inducing ability of β-lactams could lead to limited choices for their combination use with β-lactamase inhibitors because overproduction of AmpC results in resistance to a variety of β-lactam antibiotics, including third- and fourth-generation cephalosporins.
MECHANISM OF RESISTANCE
Understanding how resistance to new antibiotics develops is an important aspect of drug development because many antibiotics lose their potency with increased and widespread clinical use. Previous investigation comparing the frequency of resistance at drug concentrations of 4 × and 10 × the MIC in P. aeruginosa PAO1 strain and detected spontaneous mutation rates of 2.9 × 10−8 and <7.1 × 10−9 to cefiderocol, and higher rates of 3.1 × 10−7 and 3.4 × 10−7 to ceftazidime, respectively [20]. Mutations in the bacterial iron uptake systems associated with a 4-fold elevated cefiderocol MIC (from 0.5 to 2 μg/mL) were identified in this study, although none of the mutations appeared in the responsible iron transporter gene piuA [32]. The mutations were identified in the upstream regions of pvdS (a gene regulating pyoverdine synthesis), and/or fecI (a gene regulating the ferric citrate transporter, FecA). These mutations resulted in an overexpression of pyoverdine and FecA protein, respectively, and conferred a 32-fold increase in cefiderocol MICs, whereas ceftazidime MICs were not affected [32]. In another study, the spontaneous mutation rates for 7 strains (ie, 2 multidrug-resistant [MDR] P. aeruginosa [MDRP; 1 IMP-1 producer, 1 ceftazidime resistant], 2 KPC-producing K. pneumoniae, and 3 Enterobacteriaceae [susceptible to ceftazidime-avibactam]) ranged from <1.4 × 10−8 to 1.6 × 10−6 for cefiderocol and from <1.0 × 10−8 to 2.2 × 10−5 for ceftazidime at drug concentrations of 10 × the MIC [33]. No resistant colonies appeared with 4 strains including 1 MDR P. aeruginosa and 2 KPC-producing K. pneumoniae when tested with cefiderocol. The colonies with ≥8-fold elevated cefiderocol MICs were recovered only from IMP-1–producing MDRP that had the mutations in the upstream region of pvdS [33]. However, these resistant mutants did not appear during 72 hours’ exposure in an in vitro PD model that simulated human drug exposure of 2 g, over a 3-hour infusion, every 8 hours, implying that the risk of resistance development during treatment with cefiderocol against this strain is low [33]. Resistance acquisition in experiments of daily serial passage for 10 days with 1 MDR P. aeruginosa (IMP-1 producer) and 2 KPC-producing K. pneumoniae were also studied, and MICs of these 3 strains increased up to 4-fold for cefiderocol [34].
From these data, the potential to develop resistance to cefiderocol is considered to be similar to or lower than that of ceftazidime, regardless of the presence of carbapenemase enzymes. Although information is currently limited on resistance mechanisms to cefiderocol, recent studies identified mechanisms specific to genes related to iron acquisition, and no cross-resistance was observed between cefiderocol and ceftazidime [35]. Further investigations are ongoing to fully understand the resistance mechanisms behind elevated cefiderocol MIC values.
MICROBIOLOGY
The susceptibility testing method for cefiderocol by broth microdilution involves the use of iron-depleted cation-adjusted Mueller-Hinton broth (ID-CAMHB), which was approved by the Clinical and Laboratory Standards Institute (CLSI) in January 2016 [36]. The purpose of using an iron-depleted medium is to mimic the hypoferric condition that emerges in the host during infection. By using the ID-CAMHB medium for cefiderocol susceptibility testing, highly reproducible (94.2% within ±1 dilution) MIC results were obtained [37]. Additionally, a high correlation between in vitro susceptibility and in vivo efficacy was achieved when using ID-CAMHB. Depletion of iron in the medium represents a very low free iron concentration of ≤0.03 μg/mL, which is normally prepared by the removal of divalent cations using a cation-binding resin followed by replenishment of Mg2+, Ca2+, and Zn+ [38, 39]. The Quality Control MIC range of 0.06 μg/mL to 0.5 μg/mL for both E. coli American Type Culture Collection (ATCC) 25922 and P. aeruginosa ATCC 27853 have also been approved by the CLSI [38, 40].
Cefiderocol has a unique antibacterial spectrum against a wide variety of clinically relevant aerobic gram-negative bacteria, including not only Enterobacteriaceae spp such as Enterobacter spp, Klebsiella spp, Proteus spp, Serratia marcescens, Shigella flexneri, Salmonella spp, Vibrio spp, and Yersinia spp, but also nonfermenting bacterial species such as Acinetobacter spp, Pseudomonas spp, Burkholderia spp, and Stenotrophomonas maltophilia [22]. Cefiderocol also has in vitro activity against causative pathogens of respiratory tract infections, such as Haemophilus spp, Moraxella catarrhalis, and Bordetella parapertussis, but shows relatively high MICs (>4 μg/mL) against Campylobacter jejuni and ceftriaxone-resistant Neisseria gonorrhoeae among the gram-negative bacteria tested [22]. Importantly, cefiderocol shows high MICs of ≥32 μg/mL against prevalent gram-positive bacteria such as Staphylococcus aureus and Enterococcus faecalis. Against anaerobic gram-negative or gram-positive bacteria, cefiderocol shows much variation in MICs within genera, from ≤0.031 to >32 μg/mL, which are higher than MICs of cefepime or meropenem. This variation in the susceptibility of anaerobes may be partly explained by a lower reliance on the siderophore-iron transporter systems for growth under anaerobic conditions [22].
Cefiderocol displays potent in vitro activity with relatively low MICs against the drug-resistant gram-negative pathogens producing multiple β-lactamases, including extended-spectrum β-lactamases, AmpC, and both serine- and metallo-carbapenemases [22]. The MICs of cefiderocol were ≤2 μg/mL against almost all of the clinical isolates producing genetically diverse β-lactamases (ie, CTX-M-14/-15/-27, TEM-10/-26, CMY-2, NDM-1, or IMP-1 in E. coli; SHV-5/-18, GES-4, CMY-8/-17, DHA, KPC-2/-3, OXA-48, or NDM-1 in K. pneumoniae; VIM-1/-2/-6 or IMP-1 in P. aeruginosa; OXA-23/-58 in A. baumannii) [22]. Additionally, cefiderocol demonstrated potent activity, with an MIC90 (the lowest concentration of the antibiotic at which the growth of 90% of the isolates is inhibited) of 1 μg/mL, against Enterobacteriaceae strains producing IMP-6, a subtype of IMP MBL conferring a paradoxical phenotype of imipenem susceptibility but meropenem resistance [41].
Recently, the emergence of E. coli isolates that carry a PBP3 mutation, namely an insertion of 4 amino acids “YRIN” or “YRIK,” has been reported [42]. This insertion in the PBP3 gene confers reduced susceptibility to a broad range of β-lactams (eg, ceftazidime, cefepime, and aztreonam), but not to carbapenems. However, cefiderocol susceptibility is reported to be little affected by the YRIN(K) insertion in E. coli isolates [43].
Conversely, cefiderocol MICs were affected by a D179Y mutation in the Ω-loop of KPC β-lactamases, which are resistant to ceftazidime-avibactam, in K. pneumoniae clinical strains as well as E. coli laboratory strains [44].
IN VIVO EFFICACY
Previous siderophore monobactams (eg, MB-1 or SMC-3176) have not advanced into clinical studies because their in vivo efficacy in preclinical infection models did not correlate with their high in vitro activity [16, 18]. This was partially explained by the presence or emergence of adaptive resistance, described in detail in the article by Page [45], which may be due to downregulation of iron transporter receptors, in P. aeruginosa clinical isolates used in the in vivo infection models treated with either MB-1 or SMC-3176 [16, 18]. In contrast to these findings, cefiderocol treatment demonstrated consistent in vivo efficacy against the same strains of P. aeruginosa [46]. Although the siderophore monobactams did not demonstrate the expected in vivo efficacy based on their in vitro potency in the same study [46], a high degree of correlation was noted with cefiderocol using a dose replicating the human pharmacokinetics of cefiderocol [47]. Additionally, the magnitude of % fraction of time over MIC (ƒT/MIC) associated with 1-log10 reduction in bacterial load was 81.9% ± 18.3% in a neutropenic murine thigh infection model using P. aeruginosa strains (n = 8), which had previously displayed variable in vivo efficacy against MB-1 and SMC-3176 [47].
Furthermore, several in vivo mouse models have demonstrated that cefiderocol is efficacious against a number of pathogenic gram-negative bacteria, including carbapenem-resistant strains. In mouse lung infection models infected with carbapenem-resistant P. aeruginosa (CRPA), carbapenem-resistant A. baumannii (CRAB), KPC-producing K. pneumoniae, or S. maltophilia, cefiderocol treatment, in contrast to meropenem treatment, proved to be bactericidal [48, 49]. Cefiderocol is also efficacious in other infection models such as the systemic (intraperitoneal) infection model with CRPA, CRAB, S. maltophilia, or Burkholderia cepacia, or the urinary tract infection model with Enterobacteriaceae spp, and the subcutaneous abscess model with K. pneumoniae or A. baumannii [50]. Overall, cefiderocol time-dependent in vivo efficacy in various preclinical infection models has been established for carbapenem-resistant pathogens, which was predicted by its in vitro potency and supported by a reliable PK/PD profile. Safety information on cefiderocol is discussed by Katsube et al [51] and Echols et al [52] in this supplement.
CONCLUSIONS
Cefiderocol is a novel parenteral cephalosporin antibiotic with siderophore–iron binding properties as well as intrinsic structural stability to a wide range of serine- and metallo-β-lactamases, including clinically relevant carbapenemases. Several in vitro and in vivo studies have demonstrated that this innovative drug has the potential to overcome all 3 mechanisms of β-lactam resistance, including carbapenem resistance increasingly observed in gram-negative bacterial infections. The emergence of resistance to cefiderocol does not appear to be higher than that of other β-lactam antibiotics, and because of its multiple characteristics described above, resistance may be less of a threat to the utility of cefiderocol. Robust preclinical studies in infection models, including urinary tract and systemic infections, established the in vivo efficacy of cefiderocol, which was not demonstrated with earlier monobactam conjugates. With the likely continued emergence and spread of β-lactam resistance mechanisms, cefiderocol could play an important role in future antibacterial therapy against problematic gram-negative bacteria, particularly MBL-producing carbapenem-resistant Enterobacteriaceae, as well as MDR or carbapenem-resistant P. aeruginosa and MDR or carbapenem-resistant A. baumannii.
Notes
Acknowledgments. The authors thank all members of the Shionogi & Co, Ltd S-649266 project, especially K. Masuda, M. Kinoshita, M. Tsuji, Y. Yamano, and R. Echols for their valuable suggestions and contribution to the discussions. Editorial support was provided by Highfield (Oxford, United Kingdom), sponsored by Shionogi Inc (Florham Park, New Jersey).
Financial support. This review article is sponsored by Shionogi & Co, Ltd (Osaka, Japan).
Supplement sponsorship. This supplement is sponsored by Shionogi & Co., Ltd.
Potential conflicts of interest. T. S. and K. Y. are employees of Shionogi & Co, Ltd. K. Y. has an approved patent application on cefiderocol. Both authors report no potential conflicts. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Braun V, Pramanik A, Gwinner T, Köberle M, Bohn E. Sideromycins: tools and antibiotics. Biometals 2009; 22:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brochu A, Brochu N, Nicas TI, et al. Modes of action and inhibitory activities of new siderophore-beta-lactam conjugates that use specific iron uptake pathways for entry into bacteria. Antimicrob Agents Chemother 1992; 36:2166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diarra MS, Lavoie MC, Jacques M, et al. Species selectivity of new siderophore-drug conjugates that use specific iron uptake for entry into bacteria. Antimicrob Agents Chemother 1996; 40:2610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghosh A, Ghosh M, Niu C, Malouin F, Moellmann U, Miller MJ. Iron transport-mediated drug delivery using mixed-ligand siderophore-beta-lactam conjugates. Chem Biol 1996; 3:1011–9. [DOI] [PubMed] [Google Scholar]
- 5. Miyakawa S, Noto T, Okazaki H. Mechanisms of resistance in Escherichia coli to the sideromycin antibiotic no. 216: isolation and characterization of the resistant mutants. Microbiol Immunol 1982; 26:885–95. [DOI] [PubMed] [Google Scholar]
- 6. Verbist L, Verhaegen J. GR-20263: a new aminothiazolyl cephalosporin with high activity against Pseudomonas and Enterobacteriaceae. Antimicrob Agents Chemother 1980; 17:807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ji C, Juárez-Hernández RE, Miller MJ. Exploiting bacterial iron acquisition: siderophore conjugates. Future Med Chem 2012; 4:297–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Page MG. Siderophore conjugates. Ann N Y Acad Sci 2013; 1277:115–26. [DOI] [PubMed] [Google Scholar]
- 9. Wencewicz TA, Miller MJ. Sideromycins as pathogen-targeted antibiotics. chapter in topics in medicinal chemistry May 2017. In: Fisher J, Mobashery S, Miller M, eds. Antibacterials. Topics in medicinal chemistry. Vol. 26 Cham: Springer, 2017. Available at: https://rd.springer.com/chapter/10.1007/7355_2017_19. Accessed 4 March 2019. [Google Scholar]
- 10. Wilson BR, Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y. Siderophores in iron metabolism: from mechanism to therapy potential. Trends Mol Med 2016; 22:1077–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silley P, Griffiths JW, Monsey D, Harris AM. Mode of action of GR69153, a novel catechol-substituted cephalosporin, and its interaction with the tonB-dependent iron transport system. Antimicrob Agents Chemother 1990; 34:1806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mochizuki H, Yamada H, Oikawa Y, et al. Bactericidal activity of M14659 enhanced in low-iron environments. Antimicrob Agents Chemother 1988; 32:1648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Onoue H, Konoike T, Ishitobi H. EP0416410A1. Piperaziniocephalosporins Application, 3 March 1991. Available at: https://register.epo.org/application?number=EP90116330. Accessed 4 March 2019.
- 14. Yamano Y, Nishikawa T, Komatsu Y. Ferric iron transport system of Pseudomonas aeruginosa PAO1 that functions as the uptake pathway of a novel catechol-substituted cephalosporin, S-9096. Appl Microbiol Biotechnol 1994; 40:892–7. [Google Scholar]
- 15. Iovleva A, Doi Y. Carbapenem-resistant Enterobacteriaceae. Clin Lab Med 2017; 37:303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomaras AP, Crandon JL, McPherson CJ, et al. Adaptation-based resistance to siderophore-conjugated antibacterial agents by Pseudomonas aeruginosa. Antimicrob Agents Chemother 2013; 57:4197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mushtaq S, Woodford N, Hope R, Adkin R, Livermore DM. Activity of BAL30072 alone or combined with β-lactamase inhibitors or with meropenem against carbapenem-resistant Enterobacteriaceae and non-fermenters. J Antimicrob Chemother 2013; 68:1601–8. [DOI] [PubMed] [Google Scholar]
- 18. Kim A, Kutschke A, Ehmann DE, et al. Pharmacodynamic profiling of a siderophore-conjugated monocarbam in Pseudomonas aeruginosa: assessing the risk for resistance and attenuated efficacy. Antimicrob Agents Chemother 2015; 59:7743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aoki T, Yoshizawa H, Yamawaki K, et al. Cefiderocol (S-649266), A new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other gram-negative pathogens including multi-drug resistant bacteria: structure activity relationship. Eur J Med Chem 2018; 155:847–68. [DOI] [PubMed] [Google Scholar]
- 20. Ito A, Nishikawa T, Matsumoto S, et al. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2016; 60:7396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ito-Horiyama T, Ishii Y, Ito A, et al. Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother 2016; 60:4384–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ito A, Sato T, Ota M, et al. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against gram-negative bacteria. Antimicrob Agents Chemother 2017; 62:e01454–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruppé É, Woerther PL, Barbier F. Mechanisms of antimicrobial resistance in gram-negative bacilli. Ann Intensive Care 2015; 5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fung-Tomc J, Bush K, Minassian B, et al. Antibacterial activity of BMS-180680, a new catechol-containing monobactam. Antimicrob Agents Chemother 1997; 41:1010–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McPherson CJ, Aschenbrenner LM, Lacey BM, et al. Clinically relevant gram-negative resistance mechanisms have no effect on the efficacy of MC-1, a novel siderophore-conjugated monocarbam. Antimicrob Agents Chemother 2012; 56:6334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moynié L, Luscher A, Rolo D, et al. Structure and function of the PiuA and PirA siderophore–drug receptors from Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob Agents Chemother 2017; 61:e02531–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsai YK, Fung CP, Lin JC, et al. Klebsiella pneumoniae outer membrane porins OmpK35 and OmpK36 play roles in both antimicrobial resistance and virulence. Antimicrob Agents Chemother 2011; 55:1485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsai YK, Liou CH, Fung CP, Lin JC, Siu LK. Single or in combination antimicrobial resistance mechanisms of Klebsiella pneumoniae contribute to varied susceptibility to different carbapenems. PLoS One 2013; 8:e79640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poirel L, Kieffer N, Nordmann P. Stability of cefiderocol against clinically significant broad-spectrum oxacillinases. Int J Antimicrob Agents 2018; 52:866–7. [DOI] [PubMed] [Google Scholar]
- 30. Ito A, Nishikawa T, Ota M, et al. Stability and low induction propensity of cefiderocol against chromosomal AmpC β-lactamases of Pseudomonas aeruginosa and Enterobacter cloacae. J Antimicrob Chemother 2018; 73:3049–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev 2009; 22:161–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ito A, Nishikawa T, Ishii R, et al. Mechanism of cefiderocol high MIC mutants obtained in non-clinical FoR studies. Poster presented at: IDWeek 2018,San Francisco, CA, 3–7 October 2018. Poster 696. [Google Scholar]
- 33. Kohira N, Ito A, Ota M, et al. Frequency of resistance acquisition and resistance mechanisms to cefiderocol. Poster presented at: American Society of Microbiology Annual Meeting,Atlanta, GA, 6–11 June 2018. Poster Saturday-619. [Google Scholar]
- 34. Kohira N, Nakamura R, Ito A, et al. Resistance acquisition studies of cefiderocol by serial passage and in vitro pharmacodynamic model under human simulated exposure. Poster presented at: American Society of Microbiology Annual Meeting,Atlanta, GA, 6–11 June 2018. Poster Saturday-623. [Google Scholar]
- 35. Zhanel GG, Golden AR, Zelenitsky S, et al. Cefiderocol: a siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant gram-negative bacilli. Drugs 2019; 79:271–89. [DOI] [PubMed] [Google Scholar]
- 36. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 28th informational supplement, M100-S28. Wayne, PA: CLSI, 2018. [Google Scholar]
- 37. Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. Reproducibility of broth microdilution MICs for the novel siderophore cephalosporin, cefiderocol, determined using iron-depleted cation-adjusted Mueller-Hinton broth. Diagn Microbiol Infect Dis 2019; 94:321–5. [DOI] [PubMed] [Google Scholar]
- 38. Huband MD, Ito A, Tsuji M, Sader HS, Fedler KA, Flamm RK. Cefiderocol MIC quality control ranges in iron-depleted cation-adjusted Mueller-Hinton broth using a CLSI M23-A4 multi-laboratory study design. Diagn Microbiol Infect Dis 2017; 88:198–200. [DOI] [PubMed] [Google Scholar]
- 39. Nakamura R, Ito-Horiyama T, Takemura M, et al. In vivo pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh infection and lung infection models. Antimicrob Agents Chemother 2019; 63: pii:e02031–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 29th informational supplement, M100-S29. Wayne, PA: CLSI, 2019. [Google Scholar]
- 41. Kanazawa S, Sato T, Kohira N, Ito-Horiyama T, Tsuji M, Yamano Y. Susceptibility of imipenem-susceptible but meropenem-resistant blaIMP-6-carrying Enterobacteriaceae to various antibacterials, including the siderophore cephalosporin cefiderocol. Antimicrob Agents Chemother 2017; 61:e00576–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alm RA, Johnstone MR, Lahiri SD. Characterization of Escherichia coli NDM isolates with decreased susceptibility to aztreonam/avibactam: role of a novel insertion in PBP3. J Antimicrob Chemother 2015; 70:1420–8. [DOI] [PubMed] [Google Scholar]
- 43. Sato T, Ito A, Matsumoto S, et al. In vitro activity of siderophore cephalosporin cefiderocol against YRIN(K) PBP3 insertion-carrying Escherichia coli. Poster presented at: American Society of Microbiology Annual Meeting,Atlanta, GA, 6–11 June 2018. Poster Saturday-621. [Google Scholar]
- 44. Shields RK, Kline EG, Jones CE, et al. Cefiderocol minimum inhibitory concentrations against ceftazidime-avibactam susceptible and resistant carbapenem-resistant Enterobacteriaceae. Poster presented at: American Society of Microbiology Annual Meeting,Atlanta, GA, 6–11 June 2018. Poster Saturday-620. [Google Scholar]
- 45. Page MPG. The role of iron and siderophores in infection and the development of siderophore antibiotics. Clin Infect Dis 2019; 69(Suppl 7):529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ghazi IM, Monogue ML, Tsuji M, Nicolau DP. Humanized exposures of cefiderocol, a siderophore cephalosporin, display sustained in vivo activity against siderophore-resistant Pseudomonas aeruginosa. Pharmacology 2018; 101:278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ghazi IM, Monogue ML, Tsuji M, Nicolau DP. Pharmacodynamics of cefiderocol, a novel siderophore cephalosporin, in a Pseudomonas aeruginosa neutropenic murine thigh model. Int J Antimicrob Agents 2018; 51:206–12. [DOI] [PubMed] [Google Scholar]
- 48. Tsuji M, Horiyama T, Toba S, Nakamura R, Yamano Y. S-649266, a novel siderophore cephalosporin: in vivo efficacy in murine infection model caused by multidrug-resistant gram-negative bacteria. Poster presented at: 25th European Congress of Clinical Microbiology and Infectious Diseases, Copenhagen, Denmark, 25–28 April 2015. Poster 0253. [Google Scholar]
- 49. Takemura M, Nakamura R, Sato T, Tsuji M, Yamano Y. In vivo pharmacokinetic/pharmacodynamic (PK/PD) assessment of cefiderocol against Stenotrophomonas maltophilia in a neutropenic murine lung infection model. Poster presented at: 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain, 21–24 April 2018. Poster 0189. [Google Scholar]
- 50. Nakamura R, Toba S, Tsuji M, Yamano Y, Shimada J. S-649266, a novel siderophore cephalosporin: IV. In vivo efficacy in various murine infection models. Poster presented at: 54th Interscience Congress on Antimicrobial Agents and Chemotherapy 2014,Washington, DC, 5–9 September 2014. Poster F-1558. [Google Scholar]
- 51. Katsube T, Echols R, Wajima T. Pharmacokinetic and pharmacodynamic profiles of cefiderocol, a novel siderophore cephalosporin. Clin Infect Dis 2019; 69(Suppl 7): 552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Echols R, Ariyasu M, Den Nagata T. Pathogen-focused clinical development to address unmet medical need: cefiderocol targeting carbapenem resistance. Clin Infect Dis 2019; 69(Suppl 7):559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]