Abstract
Carbapenem-resistant gram-negative bacteria including Enterobacteriaceae as well as nonfermenters, such as Pseudomonas aeruginosa and Acinetobacter baumannii, have emerged as significant global clinical threats. Although new agents have recently been approved, none are active across the entire range of resistance mechanisms presented by carbapenem-resistant gram-negative bacteria. Cefiderocol, a novel siderophore cephalosporin, has been shown in large surveillance programs and independent in vitro studies to be highly active against all key gram-negative causative pathogens isolated from patients with hospital-acquired or ventilator-associated pneumonia, bloodstream infections, or complicated urinary tract infections. The improved structure, the novel mode of entry into bacteria, and its stability against carbapenemases enables cefiderocol to exhibit high potency against isolates that produce carbapenemases of all classes or are resistant due to porin channel mutations and/or efflux pump overexpression. Resistance to cefiderocol is uncommon and appears to be multifactorial.
Keywords: carbapenem-resistant Acinetobacter, carbapenem-resistant Enterobacteriaceae, carbapenem-resistant Pseudomonas, cefiderocol, Stenotrophomonas maltophilia
The alarming spread of antimicrobial-resistant pathogens is a significant public health issue. Most importantly, carbapenem-resistant Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii have been designated by the World Health Organization as high-priority pathogens for which new antimicrobials are urgently needed [1, 2]. In addition, Stenotrophomonas maltophilia, which is now being recognized as more prevalent in immunocompromised patients, is another difficult-to-treat nonfermenting gram-negative species owing to its intrinsic resistance to carbapenems and other β-lactam antibiotics [3–6].
Recently, several new antimicrobials, such as ceftazidime-avibactam, ceftolozane-tazobactam, meropenem-vaborbactam, imipenem-cilastatin-relebactam, plazomicin, and eravacycline, have been approved, and their use will help to address globally increased antimicrobial resistance [7–9]. All of these antimicrobials belong to well-established classes of antibiotics such as β-lactams, aminoglycosides, and tetracyclines, with unique features that overcome some of the resistance mechanisms identified in multidrug-resistant gram-negative pathogens. However, these compounds still have certain gaps in their spectrum of activity against carbapenem-resistant gram-negative bacteria; the new β-lactam–β-lactamase inhibitor (BL-BLI) combinations only restore the activity of the parent antibiotic against some serine-carbapenemases (eg, Klebsiella pneumoniae carbapenemase [KPC], oxacillin carbapenemases [OXA]) but not metallo-β-carbapenemases (eg, New Delhi metallo-β-lactamase [NDM]), and the non-β-lactam compounds have limited activity against the nonfermenters [10]. As reported by Nordmann and Poirel, the incidences of different types of enzymes vary greatly in different geographical locations [11].
Cefiderocol is a novel siderophore cephalosporin in development, with activity against a broad range of carbapenem-resistant gram-negative bacteria. Its broad activity is explained by its distinctive mechanism of penetration using active iron transporters and its high stability against all classes of carbapenemases, including serine-carbapenemases (KPC and OXA) but importantly also metallo-β-lactamases (MBLs) such as NDM, Verona integron-encoded metallo-β-lactamase (VIM), imipenemase metallo-β-lactamase (IMP), and L1 [12–14]. The combination of active transport and stability against all β-lactamases provides a uniquely broad spectrum of activity against gram-negative bacteria, including almost all Enterobacteriaceae and nonfermenter species [15].
MINIMUM INHIBITORY CONCENTRATION DETERMINATION UNDER IRON-DEPLETED CONDITIONS
Cefiderocol mimics the action of natural siderophore molecules and forms a chelate complex with ferric iron at the site of infection, which is followed by binding to the iron transporters embedded into the outer bacterial membrane [12]. The natural bacterial iron transporters are upregulated under iron-depleted conditions that occur during acute infections. Thus, iron concentration needs to be taken into account when determining the in vitro activity of such antibiotics.
The iron concentration in standard culture media (eg, cation-adjusted Mueller-Hinton broth [CAMHB]) is neither controlled nor limited, and it can vary depending on the manufacturer [16]. To test the in vitro activity of siderophore antibiotic conjugates, iron-depleted media are required to provide reproducible minimum inhibitory concentrations (MICs) that predict in vivo efficacy [17, 18]. The Clinical and Laboratory Standards Institute (CLSI) has approved the use of iron-depleted CAMHB to determine cefiderocol MICs. The growth medium is prepared by removing all cations from the Mueller-Hinton broth through incubation with a cation-binding resin, followed by replenishment of Mg2+, Ca2+, and Zn+ [19].
Based on the preclinical in vivo efficacy and pharmacokinetic/pharmacodynamic (PK/PD) analyses using this MIC testing methodology, provisional susceptible, intermediate, and resistant cefiderocol breakpoints of 4, 8, and 16 μg/mL, respectively, have been approved by CLSI for Enterobacteriaceae, P. aeruginosa, A. baumannii, and S. maltophilia [19]. This was the first case of breakpoints being approved by CLSI prior to approval of a new drug based on in vitro activity and preclinical in vivo PK/PD data.
ACTIVITY AGAINST CLINICAL ISOLATES IN MULTINATIONAL STUDIES
The in vitro activity of cefiderocol has been investigated in small independent and large-scale multinational surveillance studies. As part of the preclinical development of cefiderocol, large multinational surveillance studies (ie, SIDERO-WT studies) were initiated in North America and Europe [20–22]. In parallel, carbapenem-resistant isolates collected in Europe, North America, South America, and the Asia-Pacific region are being tested in the SIDERO-CR program [23]. In addition, several independent studies to determine cefiderocol activity have included collections of difficult-to-treat carbapenem-resistant pathogens gathered from various countries. The activity of cefiderocol in these studies was compared with that of the recently approved BL-BLI combinations, such as ceftolozane-tazobactam and ceftazidime-avibactam.
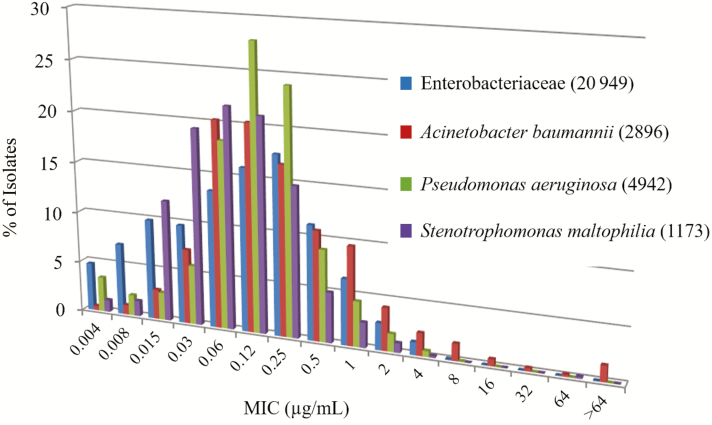
In the SIDERO-WT program [20, 21, 24–26], 3 consecutive multinational surveillance studies tested a total of 9205 gram-negative bacterial clinical isolates in 2014–2015, 8954 in 2015–2016, and 10 470 in 2016–2017. The isolates were randomly collected from approximately 100 hospitals in North America (~50) and Europe (~50). The results showed that cefiderocol MICs were low for a range of gram-negative bacterial species (Table 1). Of note, >99% of isolates had low cefiderocol MIC values in each testing period. For Enterobacteriaceae, the minimum inhibitory concentration required to inhibit the growth of 90% of organisms (MIC90) ranged from 0.25 to 1 μg/mL for Escherichia coli, Klebsiella spp, Citrobacter spp, Enterobacter spp, and Serratia spp. For the nonfermenters, the MIC90 ranged from 0.03 to 1 μg/mL against P. aeruginosa, Burkholderia cepacia, and S. maltophilia, and from 1 to 4 μg/mL against A. baumannii. Importantly, the MIC distribution range of cefiderocol showed peak MIC values between 0.06 μg/mL and 0.12 μg/mL for most of the Enterobacteriaceae, P. aeruginosa, A. baumannii, and S. maltophilia isolates (Figure 1). These results demonstrate that cefiderocol has potent activity against a wide variety of gram-negative bacterial species. The surveillance program also demonstrated that cefiderocol was highly active even against the carbapenem-resistant gram-negative strains of Enterobacteriaceae, P. aeruginosa, and A. baumannii [20–22, 24–40].
Table 1.
Minimum Inhibitory Concentration Required to Inhibit the Growth of 90% of Organisms Against Each Bacterial Species From 3 Annual Consecutive SIDERO-WT Studies
| SIDERO-WT-2014 | SIDERO-WT-2015 | SIDERO-WT-2016 | ||||
|---|---|---|---|---|---|---|
| Bacterial Species | No. of Test Strains | MIC90, μg/mL | No. of Test Strains | MIC90, μg/mL | No. of Test Strains | MIC90, μg/mL |
| Enterobacteriaceae | 6087 | 0.5 | 6013 | 1 | 7019 | 1 |
| Escherichia coli | 1529 | 0.5 | 1830 | 1 | 1780 | 1 |
| Klebsiella pneumoniae | 1526 | 1 | 1528 | 1 | 1573 | 1 |
| Klebsiella oxytoca | 505 | 0.25 | 389 | 0.5 | 540 | 0.25 |
| Citrobacter freundii | 303 | 0.5 | 252 | 1 | 273 | 0.5 |
| Citrobacter koseri | 172 | 0.5 | 169 | 0.5 | 176 | 1 |
| Enterobacter cloacae | 514 | 1 | 594 | 1 | 692 | 1 |
| Enterobacter aerogenes | 442 | 0.5 | 244 | 0.5 | 331 | 0.5 |
| Serratia marcescens | 927 | 0.25 | 776 | 0.5 | 679 | 0.5 |
| Serratia spp | 996 | 0.25 | 794 | 0.5 | 718 | 0.5 |
| Morganella morganii | NT | NT | NT | NT | 195 | 0.25 |
| Proteus vulgaris | NT | NT | NT | NT | 40 | 0.12 |
| Proteus mirabilis | NT | NT | NT | NT | 329 | 0.25 |
| Pseudomonas aeruginosa | 1530 | 0.5 | 1540 | 0.5 | 1872 | 0.5 |
| Acinetobacter baumannii | 1148 | 1 | 837 | 2 | 911 | 4 |
| Burkholderia cepacia | 12 | 1 | 45 | 0.12 | 37 | 0.03 |
| Stenotrophomonas maltophilia | 428 | 0.25 | 340 | 0.5 | 405 | 0.25 |
Source: Adapted from [20, 21, 25, 26].
Abbreviations: MIC90, minimum inhibitory concentration required to inhibit the growth of 90% of organisms; NT, not tested.
Figure 1.
Minimum inhibitory concentration distribution of Enterobacteriaceae, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia combined from 3 annual consecutive SIDERO-WT studies. Adapted from [20, 21, 24–40]. Abbreviation: MIC, minimum inhibitory concentration.
Analysis of the subpopulation of meropenem-nonsusceptible isolates of the SIDERO-WT studies [20–22, 35, 38] showed that, in the 3 sequential collection periods, cefiderocol was also active against meropenem-nonsusceptible strains of Enterobacteriaceae with MIC90 values of 1/4/4 μg/mL, with MIC90 values of 0.5/1/1 μg/mL against strains of P. aeruginosa, and with MIC90 values of 1/2/4 μg/mL against strains of A. baumannii, respectively. Furthermore, cefiderocol demonstrated activity against meropenem-susceptible strains of Enterobacteriaceae with MIC90 values of 0.5/1/1 µg/mL, with MIC90 values of 0.5/0.5/0.5 µg/mL against strains of P. aeruginosa, and with MIC90 values of 1/2/4 µg/mL against strains of A. baumannii, respectively in the same periods. These results showed that no significant increase of MIC was observed in the presence of meropenem resistance. Of note, cefiderocol suppressed the growth of 97.0%/99.6%/98.7% of meropenem-nonsusceptible strains of Enterobacteriaceae, 100%/99.7%/100% of P. aeruginosa, 96.9%/96.1%/91.0% of A. baumannii, and 100%/99.4%/100% of S. maltophilia at ≤4 μg/mL in the 3 consecutive collection periods [20, 21, 24, 35, 38, 40].
The SIDERO-CR-2014/2016 program collected carbapenem-nonsusceptible clinical isolates, including carbapenem-nonsusceptible Enterobacteriaceae, multidrug-resistant (MDR) A. baumannii, MDR P. aeruginosa, and S. maltophilia, from 52 countries (in North America, South America, Europe, Asia-Pacific, and Africa) between 2014 and 2016 [23, 41, 42]. The studies showed that cefiderocol suppressed the growth of 96.2% of these isolates at ≤4 μg/mL [23]. Against 1022 strains of carbapenem-nonsusceptible Enterobacteriaceae, including 23.0% ceftazidime-avibactam–resistant strains and 22.2% colistin-resistant isolates, cefiderocol MIC90 was 4 μg/mL and it suppressed the growth of 97.0% of the isolates at ≤4 μg/mL [23, 41]. Against 368 MDR A. baumannii, 262 MDR P. aeruginosa, and 217 S. maltophilia isolates, cefiderocol MIC90 values were 8, 1, and 0.25 μg/mL, respectively, and it inhibited the growth of 90.9%, 99.2%, and 100% of the isolates at ≤4 μg/mL, respectively [23]. It should be noted that 71.4% of MDR P. aeruginosa strains were ceftolozane-tazobactam resistant and 5.4% of MDR A. baumannii strains were colistin resistant. The susceptibility ratios of cefiderocol against these isolates were significantly larger than those of the comparators, such as ceftazidime-avibactam, ceftolozane-tazobactam, and colistin (Table 2) [23].
Table 2.
Susceptibility Ratio to Cefiderocol and Comparators of Carbapenem-resistant Isolates From the SIDERO-CR-2014/2016 Study
| Ratio of Susceptible Strainsa, (%) | |||||
|---|---|---|---|---|---|
| Species (No. of Strains) | Cefiderocol | Ceftazidime-avibactam | Ceftolozane-tazobactam | Ciprofloxacin | Colistin |
| Carbapenem-nonsusceptible strainsb | |||||
| Enterobacteriaceae (1022) | 97.0 | 77.0 | 1.7 | 11.5 | 77.8c |
| Pseudomonas aeruginosa (262) | 99.2 | 36.3 | 24.1 | 1.2 | 99.6 |
| Acinetobacter baumannii (368) | 90.9 | NA | NA | 0 | 94.6 |
| Stenotrophomonas maltophilia (217) | 100e | NA | NA | NA | NA |
Source: Adapted from [23].
Abbreviation: NA, susceptibility breakpoints not available.
aRatio of susceptible strains (%) was calculated using the following minimum inhibitory concentration (MIC) criteria: cefiderocol MIC ≤4 µg/mL; ceftazidime-avibactam MIC ≤8 µg/mL; ceftolozane-tazobactam MIC ≤2 µg/mL for Enterobacteriaceae, ≤4 µg/mL for nonfermenters; ciprofloxacin MIC ≤1 µg/mL; colistin MIC ≤2 µg/mL.
bCarbapenem-nonsusceptible strain was defined as meropenem MIC ≥2 µg/mL for Enterobacteriaceae, ≥4 µg/mL for nonfermenters.
cIncludes 39 Serratia species that are intrinsically resistant to colistin.
In vitro activity of cefiderocol has been demonstrated in a multinational randomized phase 2 clinical study that enrolled patients with complicated urinary tract infections, and a small proportion with acute uncomplicated pyelonephritis [43]. The majority of causative pathogens were Enterobacteriaceae spp, although a small proportion of patients were infected with P. aeruginosa. All species had cefiderocol MIC90 values of ≤4 μg/mL, and only a small number of K. pneumoniae had a cefiderocol MIC of 8 μg/mL, suggesting a very high susceptibility rate among clinically relevant pathogens of urinary tract infections [43].
ACTIVITY AGAINST CARBAPENEMASE PRODUCERS
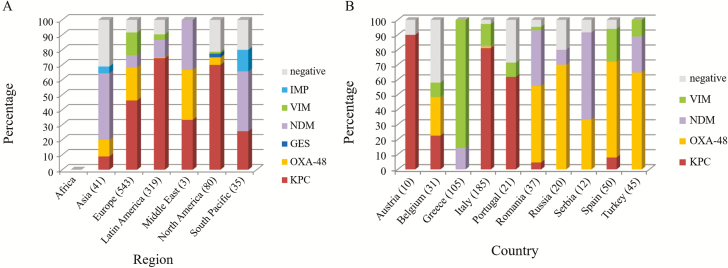
The recently approved BL-BLI combination drugs, such as ceftazidime-avibactam, meropenem-vaborbactam, and imipenem-cilastatin-relebactam, have been shown to be active against only KPC and/or OXA-48 producers, and not against other carbapenemase-producing organisms, suggesting that rapid diagnosis of specific carbapenemase enzymes using molecular methods will be important in guiding antibiotic selection. Investigation of the carbapenemase production profile of the isolates from the SIDERO-CR-2014/2016 study showed some variation in the carbapenemase enzymes between regions (Figure 2A) and countries (Figure 2B) [38, 44].
Figure 2.
Distribution of carbapenemases produced by carbapenem-resistant Enterobacteriaceae, from the SIDERO-CR study between regions (A) and countries (B). Adapted from [38, 44]. Abbreviations: GES, Guiana extended-spectrum β-lactamase; IMP, imipenemase metallo-β-lactamase; KPC, Klebsiella pneumoniae carbapenemase; NDM, New Delhi metallo-β-lactamase; OXA, oxacillin carbapenemase; VIM, Verona integron-encoded metallo-β-lactamase.
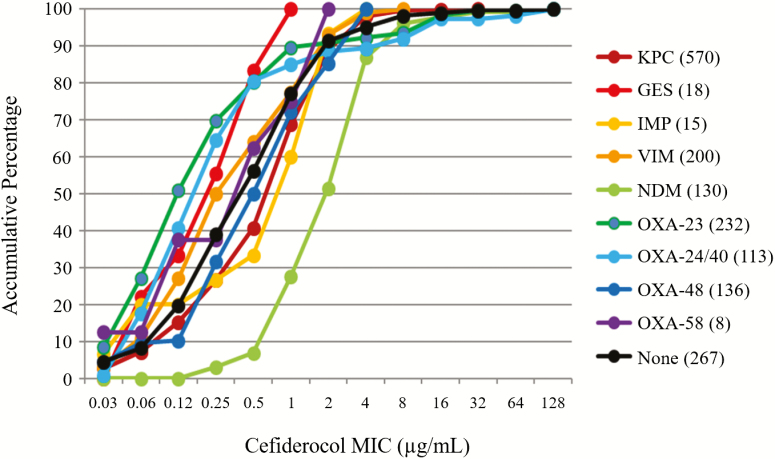
These new results from the SIDERO-CR study [44] showed that cefiderocol had potent activity against each carbapenemase-producing organism, irrespective of the bacterial species and the carbapenemase molecular types (class A such as KPC and Guiana extended-spectrum β-lactamase [GES]; class B such as VIM, NDM, and IMP; and class D such as OXA-23, -24/40, -48, and -58) with MIC90 values of 0.5–8 μg/mL (Figure 3). One feature was that cefiderocol was active against class B MBL producers such as NDM, VIM, and IMP; this finding is in contrast to the profile of the recently approved BL-BLI combination drugs [22]. The activity of cefiderocol against a broad range of pathogens harboring carbapenemases could be due to the unique mode of action of cefiderocol, which is described in detail by Sato and Yamawaki [45].
Figure 3.
In vitro activity of cefiderocol against various carbapenemase producers from the SIDERO-CR study. Adapted from [23, 44]. Abbreviations: GES, Guiana extended-spectrum β-lactamase; IMP, imipenemase metallo-β-lactamase; KPC, Klebsiella pneumoniae carbapenemase; MIC, minimum inhibitory concentration; NDM, New Delhi metallo-β-lactamase; OXA, oxacillin carbapenemase; VIM, Verona integron-encoded metallo-β-lactamase.
ACTIVITY AGAINST COLLECTIONS OF DIFFICULT-TO-TREAT PATHOGENS
Among multiple independent studies, Rolston et al reported that cefiderocol showed activity against 478 gram-negative clinical isolates from the MD Anderson Cancer Center [46]. In this study, composed mostly of blood culture isolates, 97% had a cefiderocol MIC of ≤4 μg/mL. Cefiderocol was shown to be active against less common pathogens such as Achromobacter spp. (MIC90 of 0.125 µg/mL), as well as MDR P. aeruginosa, Acinetobacter spp, and S. maltophilia, with MIC90 values of 1, 4, and 0.25 μg/mL, respectively [46]. The authors also found that cefiderocol inhibited the growth at ≤8 µg/mL against clinical isolates of Pantoea spp, Sphingomonas paucimobilis, Rhizobium radiobacter, and Elizabethkingia meningoseptica. Separately, Robertson et al reported that cefiderocol was active against 185 clinical isolates of a biothreat pathogen, Burkholderia pseudomallei, which was isolated in Northern Australia, with an MIC90 of 0.125 μg/mL [47]. These results show that cefiderocol has in vitro activity against a wide variety of gram-negative bacteria including carbapenem-resistant Enterobacteriaceae and nonfermenters.
The potent activity of cefiderocol against carbapenem-resistant gram-negative bacteria including various carbapenemase producers was also demonstrated separately by external investigators who conducted multiple independent studies using a collection of difficult-to-treat carbapenem-resistant pathogens. Cefiderocol was shown to be effective against a collection of carbapenem-resistant gram-negative pathogens from Greek hospitals. Activity against A. baumannii (n = 107), P. aeruginosa (n = 82), K. pneumoniae (n = 244), and Enterobacter cloacae (n = 14) was demonstrated by MIC90 values of 0.5, 0.5, 1, and 1 μg/mL, respectively [48]. In a study that investigated a worldwide collection of isolates from hospitalized patients, cefiderocol was shown to be active with an MIC90 of 2 μg/mL against 753 MDR gram-negative bacteria, including 164 E. coli, 298 K. pneumoniae, 159 Enterobacter species, 45 P. aeruginosa, and 87 A. baumannii isolates. These also included 127 KPC-producing Enterobacteriaceae, 154 OXA-48 producing Enterobacteriaceae, 134 MBL-producing Enterobacteriaceae (NDM, VIM, or IMP), 74 colistin-resistant Enterobacteriaceae (15 mobilized colistin resistance [MCR]-1 producers), 30 carbapenemase-producing P. aeruginosa (IMP, KPC, VIM, São Paulo metallo-β-lactamase [SPM], or German imipenemase [GIM]), and 85 OXA-producing A. baumannii (OXA-23, -40, -58, or -72). Cefiderocol suppressed the growth of 733 of 753 isolates at concentrations of ≤4 μg/mL [49].
The in vitro activity of cefiderocol against carbapenem-resistant gram-negative pathogens was also investigated by the Antibacterial Resistance Leadership Group. The results of this independent study showed that the MIC90 values against A. baumannii, S. maltophilia, and P. aeruginosa were 1, 0.25, and 0.5 μg/mL, respectively [50]. For Enterobacteriaceae, the MIC90 was 1 μg/mL in isolates harboring OXA-48 carbapenemase, 2 μg/mL in isolates with KPC-3, and 8 μg/mL for the group of isolates harboring Temoneira class A extended-spectrum β-lactamase (TEM)/sulfhydryl variant of the TEM enzyme extended-spectrum β-lactamase (SHV), NDM, and KPC-2 [50].
A recent study conducted by Public Health England investigated the activity of cefiderocol against 210 carbapenem-resistant nonfermenting clinical isolates from the United Kingdom with diverse carbapenemase production profiles [51]. Against 111 carbapenem-resistant P. aeruginosa clinical isolates from the United Kingdom, cefiderocol inhibited 86.5% of all isolates at ≤4 μg/mL, except for those with NDM (72.7%) and Pseudomonas extended resistant (PER) (73.3%) β-lactamases. Against 99 carbapenem-resistant A. baumannii clinical isolates, cefiderocol inhibited 88.9% of all isolates at ≤4 μg/mL, except for those with NDM (80.0%) [51]. Cefiderocol was also shown to be effective against well-characterized carbapenem-resistant Enterobacteriaceae, including the isolates with the mutation in KPC genes involving the Ω-loop insertion, which has been reported to occur in patients during treatment with ceftazidime-avibactam. In this collection of isolates, cefiderocol showed an MIC90 of 4 μg/mL and an 8% resistance rate, whereas ceftazidime-avibactam showed an MIC90 of >8 μg/mL and a 14% resistance rate [52].
In summary, cefiderocol has been shown to have potent antimicrobial activity against a wide variety of carbapenemase-producing carbapenem-resistant bacterial species that were collected globally or from selected countries. The acquisition of resistance to cefiderocol mediated by specific carbapenemase production has not been observed, although the susceptibility rate was lower for NDM producers than for other carbapenemase-producing organisms.
RESISTANCE MECHANISMS TO CEFIDEROCOL
In the ongoing SIDERO-WT surveillance program, a low proportion of cefiderocol-nonsusceptible isolates, with an MIC of >4 μg/mL, was observed (ie, 0.4% in 2014–2015; 0.6% in 2015–2016; and 0.7% in 2016–2017, respectively). Among a total of 161 isolates with a cefiderocol MIC of >4 μg/mL, the most frequent species was A. baumannii (n = 127), followed by Enterobacteriaceae (n = 28) [20–22]. In the SIDERO-CR program (N = 1873 isolates), 3.8% (n = 72 isolates) were cefiderocol-nonsusceptible, and the most frequent species were A. baumannii (n = 38) and Enterobacteriaceae (n = 31) [23].
Whole genome nucleotide sequence analysis of 111 isolates with cefiderocol MIC >4 μg/mL from the SIDERO-WT-2014 [20] and SIDERO-CR [23] studies showed that the most common isolates were 57 PER-producing A. baumannii (most frequently in Russia [n = 33], and Turkey [n = 12]) and 25 NDM-producing Enterobacteriaceae (including E. cloacae and K. pneumoniae; 8 from Guatemala, 5 from Turkey) [27, 53], and interestingly, these isolates were found only in specific countries.
Further investigations have revealed that cefiderocol resistance could be reverted in the presence of BLIs, suggesting that the production of β-lactamases might have been responsible for the elevated cefiderocol MICs in almost all cases. In MBL-producing organisms, including NDM-producing Enterobacteriaceae, the elevated cefiderocol MIC was not decreased in the presence of metallo-β-lactamase inhibitors, although the MIC was decreased when both metallo- and serine-β-lactamases were inhibited. These results suggest that not only NDM production but also the simultaneous production of NDM and some serine-β-lactamases could lead to cefiderocol resistance.
Against all non-NDM/VIM producers, mainly PER-producing A. baumannii, cefiderocol resistance was suppressed by the addition of avibactam. This suggests that combination with avibactam could be effective against cefiderocol-resistant isolates without MBL production. However, cefiderocol showed an MIC90 of 1 μg/mL against other PER-producing bacteria, suggesting that PER expression alone might not be the cause of cefiderocol resistance.
From these results, cefiderocol resistance in clinical isolates was mainly due to the production of β-lactamases, and PER and NDM could be important factors responsible for cefiderocol resistance, although NDM or PER production alone might not be sufficient to cause cefiderocol resistance [27, 53].
CONCLUSIONS
Cefiderocol shows potent activity against both carbapenem-susceptible and nonsusceptible/resistant gram-negative bacteria, almost irrespective of the type of carbapenemases, although some NDM producers showed an elevated MIC. The enhanced activity of cefiderocol against gram-negative bacteria could be due to the combination of rapid penetration via iron transport channels and high stability to both serine- and metallo-carbapenemases. The ongoing SIDERO-WT and SIDERO-CR surveillance studies will continue to monitor resistance rates to cefiderocol globally.
Notes
Acknowledgments. The authors thank M. Hackel, D. F. Sahm, K. M. Kazmierczak, and colleagues, International Health Management Associates Inc (Schaumburg, Illinois), for their ongoing contribution to the SIDERO surveillance programs. Editorial support was provided by Highfield (Oxford, United Kingdom), sponsored by Shionogi Inc (Florham Park, New Jersey).
Financial support. This review article is sponsored by Shionogi & Co, Ltd (Osaka, Japan).
Supplement sponsorship. This supplement is sponsored by Shionogi & Co., Ltd.
Potential conflicts of interest. Y. Y. is an employee of Shionogi & Co, Ltd. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics 2017. Available at: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf. Accessed 1 February 2019.
- 2. Tacconelli E, Carrara E, Savoldi A, et al. . WHO Pathogens Priority List Working Group Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18:318–27. [DOI] [PubMed] [Google Scholar]
- 3. Looney WJ, Narita M, Mühlemann K. Stenotrophomonas maltophilia: an emerging opportunist human pathogen. Lancet Infect Dis 2009; 9:312–23. [DOI] [PubMed] [Google Scholar]
- 4. Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 2012; 25:2–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang YT, Lin CY, Chen YH, Hsueh PR. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol 2015; 6:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. A novel algorithm to analyze epidemiology and outcomes of carbapenem resistance among patients with hospital-acquired and ventilator-associated pneumonia: a retrospective cohort study. Chest 2019; 155:1119–30. [DOI] [PubMed] [Google Scholar]
- 7. Wright H, Bonomo RA, Paterson DL. New agents for the treatment of infections with gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect 2017; 23:704–12. [DOI] [PubMed] [Google Scholar]
- 8. Kish T. New antibiotics in development target highly resistant gram-negative organisms. P T 2018; 43:116–20. [PMC free article] [PubMed] [Google Scholar]
- 9. Doi Y. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin Infect Dis 2019; 69(Suppl 7):559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watkins RR, Van Duin D. Current trends in the treatment of pneumonia due to multidrug-resistant gram-negative bacteria. F1000Res 2019; 8. doi: 10.12688/f1000research.16517.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin Infect Dis 2019; 69(Suppl 7):521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ito A, Nishikawa T, Matsumoto S, et al. . Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2016; 60:7396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ito-Horiyama T, Ishii Y, Ito A, et al. . Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother 2016; 60:4384–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poirel L, Kieffer N, Nordmann P. Stability of cefiderocol against clinically significant broad-spectrum oxacillinases. Int J Antimicrob Agents 2018; 52:866–7. [DOI] [PubMed] [Google Scholar]
- 15. Zhanel GG, Golden AR, Zelenitsky S, et al. . Cefiderocol: a siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant gram-negative bacilli. Drugs 2019; 79:271–89. [DOI] [PubMed] [Google Scholar]
- 16. Girardello R, Bispo PJ, Yamanaka TM, Gales AC. Cation concentration variability of four distinct Mueller-Hinton agar brands influences polymyxin B susceptibility results. J Clin Microbiol 2012; 50:2414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakamura R, Toba S, Ito A, Tsuji M, Yamano Y, Shimada J. S-649962, a novel siderophore cephalosporin: V. Pharmacodynamic assessment in murine thigh infection models. Poster presented at: 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5–9 September 2014. Poster F-1559. [Google Scholar]
- 18. Ghazi IM, Monogue ML, Tsuji M, Nicolau DP. Humanized exposures of cefiderocol, a siderophore cephalosporin, display sustained in vivo activity against siderophore-resistant Pseudomonas aeruginosa. Pharmacology 2018; 101:278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 29th informational supplement. CLSI supplement M100–S29. Wayne, PA: CLSI, 2019. [Google Scholar]
- 20. Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant gram-negative bacilli from North America and Europe, including carbapenem-nonsusceptible isolates (SIDERO-WT-2014 Study). Antimicrob Agents Chemother 2017; 61:e00093–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karlowsky JA, Hackel MA, Tsuji M, Yamano Y, Echols R, Sahm DF. In vitro activity of cefiderocol, a siderophore cephalosporin, against gram-negative bacilli isolated by clinical laboratories in North America and Europe in 2015–2016: SIDERO-WT-2015. Int J Antimicrob Agents 2019; 53:456–66. [DOI] [PubMed] [Google Scholar]
- 22. Kazmierczak KM, Tsuji M, Wise MG, et al. . In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int J Antimicrob Agents 2019; 53:177–84. [DOI] [PubMed] [Google Scholar]
- 23. Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother 2018; 62:e01968–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hackel M, Tsuji M, Echols R, Sahm D. In vitro antibacterial activity of cefiderocol (S-649266) against gram-negative clinical strains collected in North America and Europe (SIDERO-WT-2014 study). Poster presented at: IDWeek 2016,New Orleans, LA, 26–30 October 2016. Poster 1828. [Google Scholar]
- 25. Tsuji M, Hackel M, Yamano Y, Echols R, Sahm D. Cefiderocol in vitro activity against gram-negative clinical isolates collected in Europe: result from three SIDERO-WT surveillance studies between 2014–2017. Poster presented at: 29th European Congress of Clinical Microbiology and Infectious DiseasesAmsterdam, Netherlands, 13–16 April 2019. Poster 1852. [Google Scholar]
- 26. Tsuji M, Hackel M, Echols R, Yamano Y, Sahm D. In vitro antibacterial activity of cefiderocol against gram-negative clinical strains collected in North America and Europe, SIDERO-WT-2016. Poster presented at: American Society of Microbiology Annual Meeting (ASM-Microbe), San Francisco, CA, 20–24 June 2019. Poster AAR-767. [Google Scholar]
- 27. Ito A, Kuroiwa M, Ishioka Y, et al. . Characterization of isolates showing high MICs to cefiderocol from global surveillance study SIDERO-WT-2014. Poster presented at: American Society of Microbiology Annual Meeting (ASM-Microbe), San Francisco, CA, 20–24 June 2019. Poster AAR-774. [Google Scholar]
- 28. Nguyen S, Hackel M, Hayes J, et al. . In vitro antibacterial activity of cefiderocol against an international collection of carbapenem-non-susceptible gram-negative bacteria isolated from respiratory, blood, skin/soft tissue and urinary sources of Infection: SIDERO-WT-2014–2016. Poster presented at: 29th European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, Netherlands, 3–16 April 2019. Poster 1855. [Google Scholar]
- 29. Nguyen S, Hackel M, Hayes J, Sahm DF, Echols R. In vitro antibacterial activity of cefiderocol against carbapenem-non-susceptible gram-negative bacteria from hospitalized patients in the United States: SIDERO-WT-2014–2017. Poster presented at: American Society of Microbiology Annual Meeting, San Francisco, CA, 20–24 June 2019. Poster AAR-764. [Google Scholar]
- 30. Tsuji M, Hackel M, Echols R, Yamano Y, Sahm D. Global surveillance of cefiderocol (S-649266) against gram-negative clinical strains collected in North America: SIDERO-WT-2014. Poster presented at: American Society of Microbiology Annual Meeting (ASM-Microbe), New Orleans, LA, 1–5 June 2017. Poster 110. [Google Scholar]
- 31. Tsuji M, Hackel M, Echols R, Yamano Y, Sahm D. In vitro activity of cefiderocol against gram-negative clinical isolates collected in North America from urinary tract source: SIDERO-WT-2014/SIDERO-WT-2015. Poster presented at: IDWeek 2017,San Diego, CA, 4–8 October 2017. Poster 1229. [Google Scholar]
- 32. Tsuji M, Hackel M, Yamano Y, Echols R, Sahm D. In vitro antibacterial activity of cefiderocol (S-649266) against gram-negative clinical strains collected in North America and Europe: SIDERO-WT-2015. Poster presented at: American Society of Microbiology Annual Meeting (ASM-Microbe), New Orleans, LA, 1–5 June 2017. Poster 11. [Google Scholar]
- 33. Tsuji M, Hackel M, Yamano Y, Echols R, Sahm D. Surveillance of cefiderocol in vitro activity against gram-negative clinical isolates collected in Europe: SIDERO-WT-2014. Poster presented at: 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 22–25 April 2017. Poster 1314. [Google Scholar]
- 34. Tsuji M, Hackel M, Yamano Y, Echols R, Sahm D. The in vitro activity of cefiderocol, a novel siderophore cephalosporin, against a global collection of Stenotrophomonas maltophilia. Poster presented at: 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 22–25 April 2017. Poster 1313. [Google Scholar]
- 35. Tsuji M, Kazmierczak K, Hackel M, Echols R, Yamano Y, Sahm D. Cefiderocol (S649266) susceptibility against globally isolated meropenem non-susceptible gram-negative bacteria containing serine- and metallo-carbapenemase genes. Poster presented at: American Society of Microbiology Annual Meeting (ASM-Microbe), New Orleans, LA, 1–5 June 2017. Poster 25. [Google Scholar]
- 36. Tsuji M, Hackel M, Echols R, Yamano Y, Sahm D. Surveillance of cefiderocol against gram-negative clinical strains collected in North America: SIDERO-WT-2015. Poster presented at: IDWeek 2018,San Francisco, CA, 3–7 October 2018. Poster 1349. [Google Scholar]
- 37. Tsuji M, Hackel M, Yamano Y, Echols R, Sahm D. Cefiderocol, a novel siderophore cephalosporin: in vitro activity against global isolates of Stenotrophomonas maltophilia isolated globally. Poster presented at: 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain, 21–24 April 2018. Poster 0184. [Google Scholar]
- 38. Tsuji M, Kazmierczak KM, Hackel M, Echols R, Yamano Y, Sahm D. Cefiderocol (S649266) susceptibility against globally isolated meropenem non-susceptible gram-negative bacteria containing serine- and metallo-carbapenemase genes. Poster presented at: American Society of Microbiology Annual Meeting (ASM-Microbe), Atlanta, GA, 6–11 June 2018. Poster 622. [Google Scholar]
- 39. Yamano Y, Tsuji M, Echols R, Hackel M, Sahm D. In vitro activity of cefiderocol against gram-negative clinical isolates from respiratory specimens: SIDERO-WT-2014. Poster presented at: American Thoracic Society International Conference, San Diego, CA, 18–23 May 2018. Poster A2613/1001. [Google Scholar]
- 40. Tsuji M, Kazmierczak KM, Wise M, Hackel M, Echols R, Yamano Y. Cefiderocol susceptibility against globally isolates meropenem non-susceptible gram-negative bacteria containing serine- and metallo-carbapenemase genes: SIDERO-WT-2014 and 2015. Poster presented at: American Society of Microbiology Annual Meeting (ASM-Microbe), San Francisco, CA, 20–24 June 2019. Poster AAR-765. [Google Scholar]
- 41. Yamano Y, Tsuji M, Echols R, Hackel M, Sahm D. In vitro activity of cefiderocol against globally collected carbapenem resistant gram-negative bacteria including isolates resistant to ceftazidime/avibactam, ceftolozane/tazobactam and colistin: SIDERO-CR-2014/2016 study. Poster presented at: 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 22–25 April 2017. Poster 1316. [Google Scholar]
- 42. Tsuji M, Hackel M, Echols R, Yamano Y, Sahm D. In vitro activity of cefiderocol against globally collected carbapenem-resistant gram-negative bacteria isolated from urinary tract source: SIDERO-CR-2014/2016. Poster presented at: IDWeek 2017,San Diego, CA, 4–8 October 2017. Poster 1199. [Google Scholar]
- 43. Portsmouth S, van Veenhuyzen D, Echols R, et al. . Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2018; 18:1319–28. [DOI] [PubMed] [Google Scholar]
- 44. Tsuji M, Kazmierczak KM, Hackel M, et al. . Cefiderocol susceptibility profiling against a global collection of gram-negative bacteria containing serine- and metallo-carbapenemase genes. Poster presented at: 29th European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, Netherlands, 13–16 April 2019. Poster 1853. [Google Scholar]
- 45. Sato T, Yamawaki K. Cefiderocol: discovery, chemistry, and pharmacological profiles of a novel siderophore cephalosporin. Clin Infect Dis 2019; 69(Suppl 7): 538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rolston KVI, Gerges B, Raad I, Aitken SL, Reitzel R, Prince R. In-vitro activity of cefiderocol and comparator agents against gram-negative isolates from cancer patients. Poster presented at: IDWeek 2018,San Francisco, CA, 3–7 October 2018. Poster 1375. [Google Scholar]
- 47. Robertson GJ, Henderson A, Paterson DL, Harris PNA. In vitro activity of cefiderocol (S-649266) against clinical isolates of Burkholderia pseudomallei. Poster presented at: 29th European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, Netherlands, 13–16 April 2019. Poster 1858. [Google Scholar]
- 48. Falagas ME, Skalidis T, Vardakas KZ, Legakis NJ; Hellenic Cefiderocol Study Group Activity of cefiderocol (S-649266) against carbapenem-resistant gram-negative bacteria collected from inpatients in Greek hospitals. J Antimicrob Chemother 2017; 72:1704–8. [DOI] [PubMed] [Google Scholar]
- 49. Dobias J, Dénervaud-Tendon V, Poirel L, Nordmann P. Activity of the novel siderophore cephalosporin cefiderocol against multidrug-resistant gram-negative pathogens. Eur J Clin Microbiol Infect Dis 2017; 36:2319–27. [DOI] [PubMed] [Google Scholar]
- 50. Jacobs MR, Abdelhamed AM, Good CE, et al. . ARGONAUT-I: activity of cefiderocol (S-649266), a siderophore cephalosporin, against gram-negative bacteria, including carbapenem-resistant nonfermenters and Enterobacteriaceae with defined extended-spectrum β-lactamases and carbapenemases. Antimicrob Agents Chemother 2018; 63:e01801–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mushtaq S, Sadouki Z, Vickers A, Livermore DM, Woodford N. In vitro activity of cefiderocol against extensively drug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii from the UK. Poster presented at: 29th European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, Netherlands, 13–16 April 2019. Poster 1860. [Google Scholar]
- 52. Shields RK, Kline EG, Jones CE, et al. . Cefiderocol minimum inhibitory concentrations against ceftazidime–avibactam susceptible and resistant carbapenem-resistant Enterobacteriaceae. Poster presented at: American Society of Microbiology Annual Meeting, Atlanta, GA, 6–11 June 2018. Poster 620. [Google Scholar]
- 53. Ito A, Hackel M, Sahm D, Tsuji M, Yamano Y. Characterization of isolates showing high MICs to cefiderocol from global surveillance study SIDERO-CR-2014/2016. Poster presented at: 29th European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, Netherlands, 13–16 April 2019. Poster 1857. [Google Scholar]