Abstract
Cefiderocol, a novel parenteral siderophore cephalosporin, exhibits potent in vitro activity and in vivo efficacy against most gram-negative bacteria, including carbapenem-resistant strains of Enterobacteriaceae, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia. In phase 1 studies, cefiderocol demonstrated linear pharmacokinetics, primarily urinary excretion, an elimination half-life of 2–3 hours, and a protein binding of 58% in human plasma. Cefiderocol is a time-dependent cephalosporin; the probability of a target attainment at ≥75% of the dosing interval during which the free drug concentration exceeds the minimum inhibitory concentration (ƒT/MIC) for bacterial strains with an MIC of ≤4 μg/mL is likely to be achieved at the therapeutic dose of 2 g over 3-hour infusion every 8 hours in most patients. As expected, renal function markers were the most influential covariates for the pharmacokinetics of cefiderocol for patients with renal impairment or augmented renal clearance (ARC). Dose adjustment is recommended for patients with impaired renal function, and additionally, in ARC patients with creatinine clearance >120 mL/minute, a more frequent dosing regimen (ie, 2 g every 6 hours) was predicted to achieve the target fT > MIC. The single and multiple doses of cefiderocol tested were well tolerated in both healthy subjects and those with renal impairment. Furthermore, neither QT interval prolongation nor drug–drug interaction via organic anion transporters was demonstrated in healthy subjects. Cefiderocol is being investigated in phase 3 clinical studies for the treatment of infections caused by carbapenem-resistant bacteria.
Keywords: carbapenem resistance, cefiderocol, dose adjustment, linear pharmacokinetics, time-dependent cephalosporin
The prevalence of carbapenem-resistant nonfermenting gram-negative bacteria and Enterobacteriaceae worldwide has reached an alarming level and represents a great challenge in all types of infection [1, 2]. In response to the increasing levels of antimicrobial resistance, the World Health Organization categorized these pathogens as high priority to prompt pharmaceutical companies to urgently develop new antibiotics [3, 4]. Although many new antibiotics that have recently been approved or are still under development have demonstrated improved in vitro activity against Ambler class A, C, and D carbapenemase enzymes, they do not have activity against gram-negative bacteria with metallo-β-lactamases or many resistant nonfermenters [2, 5]. Patients with such infections are frequently critically ill or septic, or have multiple comorbidities, and such conditions have a major influence on the pharmacokinetic/pharmacodynamic (PK/PD) profiles of most antibiotics that are currently used in clinical practice [1]. Thus, an improved in vitro potency in addition to a well-characterized favorable PK/PD profile are crucial to achieve both adequate exposure to the antibiotic over the minimum inhibitory concentration (MIC) of the pathogen and clinical cure in patients infected with drug-resistant pathogens [1, 6]. PK/PD profiling of new antibiotics is therefore essential in both preclinical animal models and clinical investigation to understand which pharmacodynamic parameter best correlates with in vivo efficacy and predicts clinical success.
PHARMACOKINETIC ASSESSMENT OF CEFIDEROCOL IN ANIMAL MODELS
β-lactam antibiotics, such as carbapenems and cephalosporins, are well known to exert time-dependent bactericidal activity [6, 7]. Based on available clinical evidence, when β-lactam antibiotics are selected for the treatment of critically ill patients, extended or continuous infusion, with a starting loading dose and therapeutic drug monitoring, are often recommended instead of rapid intravenous boluses to achieve high clinical cure rates [2, 7, 8].
Cefiderocol, a novel parenteral siderophore cephalosporin discovered and developed by Shionogi & Co, Ltd, exhibits potent in vitro activity against most gram-negative bacteria, including carbapenem-resistant strains of Enterobacteriaceae and nonfermenters Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia [9–12]. This has been described in detail in the article by Yamano in this supplement [13].
In an extensive preclinical investigation, the results of the studies conducted in neutropenic murine infection models suggest that for cefiderocol the key pharmacodynamic parameter that most closely correlated with bactericidal efficacy was the fraction of the dosing interval during which the free drug concentration exceeds the MIC (% fT > MIC) [14–17]. The in vivo neutropenic murine thigh and lung infection models, using carbapenem-susceptible and -resistant strains of Enterobacteriaceae, P. aeruginosa, A. baumannii, and S. maltophilia, demonstrated a bacteriostatic effect at approximately 40% to 70% fT > MIC and a bactericidal effect (≥1 log10 reduction) at approximately 55% to 88% fT > MIC [14–18]. Furthermore, the in vivo efficacy of cefiderocol was similar in all investigated infection types (ie, systemic, urinary, lung, or subcutaneous) [12, 18–21]. In immunocompetent rat lung infection models, infected with P. aeruginosa, A. baumannii, Escherichia coli, Klebsiella pneumoniae, or S. maltophilia, rats receiving humanized cefiderocol doses over a 3-hour infusion period vs a 1-hour infusion period achieved greater efficacy [22–25]. In additional studies, the humanized exposures of cefiderocol in neutropenic murine thigh infection models produced a similar reduction in bacterial density for most of the test pathogens with MICs of ≤4 μg/mL [26]. Thus, bacterial stasis or ≥1 log10 reduction was observed in 75.0%, 81.8%, 85.0%, and 87.5% of 20 K. pneumoniae, 11 E. coli, 20 P. aeruginosa, and 16 A. baumannii isolates, respectively, although only 2 of 28 strains with MICs of ≥8 μg/mL displayed bacterial stasis or ≥1 log10 reduction [26]. These data provide the delineation of susceptibility breakpoints for these gram-negative pathogens, identifying an MIC of 4 μg/mL for cefiderocol as determined in iron-depleted cation-adjusted Mueller-Hinton broth medium.
PHARMACOKINETIC AND SAFETY ASSESSMENTS OF CEFIDEROCOL IN HUMANS
The pharmacokinetics, safety, and tolerability of cefiderocol were evaluated in phase 1 single- and multiple-dose studies in healthy subjects [27–29] and in uninfected subjects with renal impairment [30]. Additionally, a thorough QT/QTc study was conducted to assess the potential effects of a 2 g (therapeutic) and 4 g (supratherapeutic) dose of cefiderocol on the QT interval [31], and a drug-interaction study evaluated the inhibitory effects of cefiderocol on organic anion drug transporters (OAT) [32]. Pharmacokinetics and safety of cefiderocol were evaluated in patients with complicated urinary tract infection (cUTIs) enrolled into the phase 2 APEKS (Acinetobacter, Pseudomonas, Escherichia, Klebsiella, Stenotrophomonas)-cUTI clinical study [33, 34].
The population pharmacokinetic analysis was initially performed based on plasma and urine concentrations from healthy subjects and plasma concentration data from subjects with varying renal function [35]. The second population pharmacokinetic analysis was performed including plasma concentration data from patients with cUTI with or without pyelonephritis or acute uncomplicated pyelonephritis (AUP) [36].
To support the dose rationale of cefiderocol, Monte Carlo simulations were performed to calculate the probability of target attainment (PTA) for each renal function group, including augmented renal clearance (ARC) [37]. The presence of ARC resulting from a hyperdynamic cardiovascular state as a consequence of a systemic inflammatory response results in an increased glomerular filtration rate (GFR), and has been reported in critically ill patients [37]. This increased clearance may lead to reduced exposure to cefiderocol, possibly resulting in decreased efficacy. Therefore, a shortening of the dosing interval may be warranted for patients with ARC.
PHARMACOKINETICS, SAFETY, AND TOLERABILITY IN HEALTHY SUBJECTS
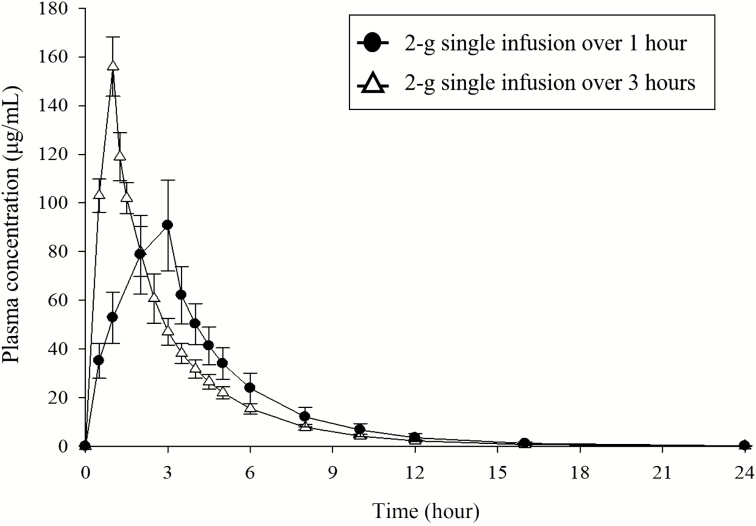
In a phase 1 study, both the maximum plasma concentration (Cmax) and the area under the plasma concentration–time curve (AUC) of cefiderocol increased in a dose-proportional manner following administration of single ascending doses within the range from 100 to 2000 mg [27]. Elimination half-life ranged from 1.98 to 2.74 hours [27]. The protein binding was approximately 58% in humans [25]. Cefiderocol is mainly excreted unchanged via the kidneys. With every 8-hour dosing up to 10 days, the steady state is attained within 1 day during multiple-dose administration and there is only a slight accumulation of cefiderocol in plasma [27]. The pharmacokinetic parameters of cefiderocol following intravenous infusion of single or multiple (2 g every 8 hours) doses for 1 or 3 hours are presented in Table 1. The plasma concentration–time curves of cefiderocol following a single dose of 2000 mg infused over 1 hour or 3 hours are shown in Figure 1.
Table 1.
Pharmacokinetic Parameters of Cefiderocol Following Intravenous Infusions of Cefiderocol 2 g for 1 or 3 Hours in Healthy Subjects
| 2000 mg as a 1-h Infusiona | 2000 mg as a 3-h Infusiona | ||
|---|---|---|---|
| Single Dose | Multiple Dosesb, Day 10 | Single Dose | |
| Parameter | (n = 6) | (n = 8) | (n = 43) |
| Cmax (μg/mL) | 156 (7.9) | 153 (12.9) | 89.7 (20.5) |
| AUC0–∞ (μg × h/mL) | 389.7 (9.0) | 366.5 (14.0) | 386.1 (17.2) |
| t1/2,z (h) | 2.74 (10.2) | 2.72 (21.6) | 2.41 (14.0) |
Source: Adapted from [27, 31].
Abbreviations: AUC0–∞, area under the concentration–time curve extrapolated from time zero to infinity; Cmax, maximum plasma concentration; t1/2, terminal elimination half-life.
aData are expressed as geometric mean (coefficient of variance % of geometric mean).
bEvery 8 h, for 10 d.
Figure 1.
Mean (standard deviation) plasma concentrations of cefiderocol following single-dose administration of cefiderocol 2000 mg infused over 1 hour and 3 hours . Adapted from [27] and [31]. This article was published in Clinical Therapeutics, Sanabria C, et al. Effect of Cefiderocol, a Siderophore Cephalosporin, on QT/QTc Interval in Healthy Adult Subjects, 2019; 41(9):1724–36.e4, Copyright Elsevier (2019).
In a mass-balance study using [14C]-labeled cefiderocol, renal excretion was found to be the major route of elimination of cefiderocol, with 98.59% of total radioactivity detected in urine and only a minor proportion (2.79%) detected in feces [28, 29]. Cefiderocol was the major radioactive component in urine and accounted for 90.57% of the administered dose. Evaluation of the plasma AUC for total radioactivity showed that cefiderocol accounted for 92.3% and pyrrolidine chlorobenzamide, a degradation product, accounted for 4.70%; other metabolites of cefiderocol were present at lower levels (ie, <2% of the plasma AUC for total radioactivity). Total radioactivity was predominantly associated with plasma, with little partitioning into red blood cells [28, 29].
In a thorough QT/QTc study, all point estimates for the time-matched placebo- and baseline-adjusted QT interval corrected using the Fridericia formula (ddQTcF interval), with moxifloxacin being positive control, were <5 msec, and the upper bound of the 90% confidence interval (CI) was well <10 msec at each time point after initiation of the infusion. Thus, single 2 g and 4 g doses of cefiderocol did not prolong the ddQTcF interval, which would have been considered as clinically relevant, and met the criteria associated with a negative thorough QT/QTc assessment [31].
The phase 1 studies indicated that the single cefiderocol dose of up to 4000 mg and the multiple dose of up to 2000 mg were well tolerated in healthy subjects [27, 31]. In the single-dose part of the study, 9 adverse events that might have been related to study drug (ie, diarrhea, abdominal pain, nausea, rash, blood present in urine, increased white blood cells) were reported in the cefiderocol group [27]. In the multiple-dose part of the study, the adverse events that might have been related to cefiderocol were rash, pyrexia, abdominal pain, oropharyngeal pain, headache, increased or decreased blood thyroid-stimulating hormone, elevated liver enzymes, increased blood urea level, blood present in urine, increased white blood cell count, and increased blood creatine phosphokinase [27]. However, similar adverse events were also reported in the respective placebo groups [27]. Cefiderocol is an iron-chelating agent, so blood iron levels were also investigated; however, there was no correlation between the dose of cefiderocol and change in iron levels at any time point [27].
PHARMACOKINETICS AND TOLERABILITY IN SUBJECTS WITH IMPAIRED RENAL FUNCTION
In the phase 1 renal impairment study [30], total clearance (CL) and elimination half-life correlated with indices of renal function. Ratios (90% CIs) of AUC in subjects with renal impairment compared with ratios in those with normal renal function were 1.0 (0.8–1.3), 1.5 (1.2–1.9), 2.5 (2.0–3.3), and 4.1 (3.3–5.2) for mild (estimated glomerular filtration rate [eGFR] of 60 to <90 mL/minute/1.73 m2), moderate (eGFR 30 to <60 mL/minute/1.73 m2), severe (eGFR <30 mL/minute/1.73 m2), and end-stage renal disease (ESRD) requiring hemodialysis, respectively, indicating that AUC increases with worsening severity of renal impairment. The Cmax and fraction of unbound drug in plasma were similar between renal impairment groups and those with normal renal function (ie, protein binding ranged between 53% and 65% at 1 hour and 8 hours across various renal function status). The volume of distribution of cefiderocol was not significantly altered in subjects with renal impairment [30]. Approximately 60% of cefiderocol was removed by hemodialysis of 3–4 hours.
The incidence of adverse events did not appear to have any correlation with the degree of renal impairment. Single 1000-mg intravenous doses of cefiderocol were generally well tolerated in subjects with impaired renal function except for 1 subject whose infusion was discontinued due to urticaria [30]. The most frequently reported adverse event was contact dermatitis (unrelated to cefiderocol), reported for 3 subjects [30]. Furthermore, no clinically significant changes in physical examination, 12-lead electrocardiogram, QTcF/QTcB (QT interval corrected using the Bazett’s formula) parameters, or clinical laboratory investigations were observed [30].
DRUG INTERACTIONS
Drug–drug interactions (DDIs) via transporters are well known in clinical practice [38], which may require dose modification or close monitoring of adverse events when agents are coadministered. Based on 50% inhibitory concentration values and clinically relevant concentrations of cefiderocol, in vitro studies suggested low or no potential for DDI of cefiderocol for the organic anion transporting polypeptide (OATP) 1B1, multidrug and toxin extrusion (MATE) 1, P glycoprotein, breast cancer resistance protein (BCRP), and bile salt export pump (BSEP); however, inhibitory potential was demonstrated via OAT1, OAT3, organic cation transporter (OCT) 1, OCT2, OATP1B3, and MATE2-K [unpublished data]. These findings indicated the need for clinical DDI studies according to the regulatory guidances [39-41]. A phase 1 clinical DDI study was conducted to investigate the inhibitory potential of cefiderocol on the pharmacokinetics of substrates of the transporters: (1) furosemide for OAT1 and OAT3; (2) metformin for OCT1, OCT2, and MATE2-K; and (3) rosuvastatin for OATP1B3 [32]. In this study, geometric mean ratios (coadministration [substrate + cefiderocol] / substrate alone) of Cmax and AUC, and their 90% CIs, respectively, were 1.00 (0.71–1.42) and 0.92 (0.73–1.16) for furosemide (for OAT1 and OAT3), 1.09 (0.92–1.28) and 1.03 (0.93–1.15) for metformin (for OCT1, OCT2, and MATE2-K), and 1.28 (1.12–1.46) and 1.21 (1.08–1.35) for rosuvastatin (for OATP1B3). These results demonstrate that there are no clinically significant effects of cefiderocol on the pharmacokinetics of these substrates. The in vitro and in vivo findings indicate that cefiderocol is unlikely to affect the pharmacokinetics of coadministered drugs that are substrates of gut, hepatic, and renal transporters [32].
MONTE CARLO SIMULATIONS AND DOSING RATIONALE FOR PATIENTS WITH VARYING RENAL FUNCTION
The population pharmacokinetic models were developed based on 1348 plasma and 276 urine concentration data collected from 54 healthy subjects, and 633 plasma and 30 dialysate concentration data collected from 37 subjects with varying renal function [35]. The 3-compartment model was selected as a structural model because this described the pharmacokinetic data of cefiderocol with varying renal function and posthemodialysis session better than the 2-compartment model [35]. The pharmacodynamic target implicated in the modeling was 75% ƒT > MIC, which is required to achieve a bactericidal effect based on the previously discussed animal infection models [14–16]. The Monte Carlo simulations using the population pharmacokinetic model from healthy subjects confirmed that the PTA at the dose of 2 g every 8 hours with either 1- or 3-hour infusion was achieved in >90% of patients for 75% fT > MIC with MICs ≤4 μg/mL. However, based on evidence in our preclinical investigation [25] and in clinical trials in terms of clinical cure rates with β-lactam antibiotics [8], the prolonged infusion (ie, 3-hour infusion) was selected as the standard dose regimen for cefiderocol.
The dose regimens for patients with impaired renal function were adjusted for renal function groups (mild [eGFR of 60 to <90 mL/minute/1.73 m2], moderate [eGFR 30 to <60 mL/minute/1.73 m2], severe [eGFR <30 mL/minute/1.73 m2], and ESRD [patients requiring hemodialysis]) based on the results in the renal impairment study [30]. The objective was to attain a daily AUC for patients with renal impairment that was comparable to that for subjects with normal renal function achieved with cefiderocol 2 g every 8 hours. Based on the modeling, the adjusted dose regimens would provide >90% PTA for 75% fT > MIC for strains with an MIC of ≤4 µg/mL in any impaired renal function group. The dose regimens based on renal function, including patients undergoing continuous or intermittent renal replacement therapy, are presented in Table 2. A dose of 0.75 g every 12 hours with 3-hour infusion plus a supplemental dose of 0.75 g administered after a standard 4-hour hemodialysis session will provide >90% PTA for 75% fT > MIC of ≤4 μg/mL in patients requiring intermittent hemodialysis [35].
Table 2.
Proposed Dose Regimens Based on Renal Function
| Augmented renal function (CrCL ≥120 mL/min) | 2 g every 6 h, 3-h infusion |
| Normal renal function (CrCL 90 to <120 mL/min) | 2 g every 8 h, 3-h infusion |
| Mild renal impairment (CrCL 60 to <90 mL/min) | 2 g every 8 h, 3-h infusion |
| Moderate renal impairment (CrCL 30 to <60 mL/min) | 1.5 g every 8 h, 3-h infusion |
| Severe renal impairment (CrCL 15 to <30 mL/min) | 1 g every 8 h, 3-h infusion |
| ESRD (CrCL <15 mL/min) | 0.75 g every 12 h, 3-h infusion |
| Patient requiring intermittent hemodialysis | 0.75 g every 12 h, 3-h infusiona |
| Patient with CVVH | 1 g every 12 h, 3-h infusion |
| Patient with CVVHD or CVVHDF | 1.5 g every 12 h, 3-h infusion |
Source: Adapted from [35, 44].
Abbreviations: CrCL, creatinine clearance estimated by Cockcroft-Gault equation; CVVH, continuous venovenous hemofiltration; CVVHD, continuous venovenous hemodialysis; CVVHDF, continuous venovenous hemodiafiltration; ESRD, end-stage renal disease.
aThe supplemental (third) dose of 0.75 g with 3-h infusion will be administered after the completion of intermittent hemodialysis on dialysis days.
One of the target patient populations expected to receive cefiderocol will be seriously ill and/or ventilated patients, some of whom will have ARC [42, 43]. The Monte Carlo simulations of patients, with creatinine clearance (CrCL) up to 185 mL/minute calculated by the Cockcroft-Gault equation [44], demonstrated that a more frequent administration of cefiderocol (ie, 2 g every 6 hours, infused over 3 hours) would provide adequate drug exposure for >90% of patients with ARC (ie, >120 mL/ minute of CrCL) infected with strains with an MIC of ≤4 μg/mL [35].
As actual data for subjects receiving continuous renal replacement therapy (CRRT), including continuous venovenous hemofiltration (CVVH), continuous venovenous hemodialysis (CVVHD), and continuous venovenous hemodiafiltration (CVVHDF), are not available for cefiderocol, clearance with hemodialysis (CLHD) of cefiderocol for CRRT was predicted based on the reported CLHD of cefepime for CRRT [45, 46]. The pharmacokinetic characteristics of cefiderocol [27, 30] are similar to those of cefepime [47-49]. The renal excretion in unchanged form (cefiderocol: 61.5–68.4%; cefepime: 80%), half-life (2–3 hours for both), and volume of distribution (cefiderocol: 13.5–26.6 L; cefepime: 16.9–19.3 L) are comparable between the 2 agents. The molecular weights of cefiderocol and cefepime are <1000 Da, which are in the dialyzable range. The unbound fraction (fu) of cefiderocol is half of that of cefepime (cefiderocol fu: 0.422; cefepime fu: 0.8; respectively) [50]. Therefore, by adjusting the difference in fu, CLHD of cefiderocol by CRRT was considered predictable based on that of cefepime. Based on the predicted CLHD of cefiderocol, the plasma concentration profiles of cefiderocol in patients receiving CRRT [45] were simulated with the selected dose regimens of 1 g every 12 hours, 1.5 g every 12 hours, and 1.5 g every 12 hours for patients receiving CVVH, CVVHD, and CVVHDF, respectively. The PTA was >90% against bacterial strains with MICs ≤4 μg/mL at the selected dose regimens with 3-hour infusions for patients receiving CRRT.
POPULATION PHARMACOKINETICS IN PATIENTS WITH CUTI OR AUP
In the APEKS-cUTI study [33, 34], the pharmacokinetics were evaluated based on plasma and urine concentrations of cefiderocol following a sparse sampling design, collecting blood samples for pharmacokinetic analysis (3× blood and 2× urine samples per patient) on day 3 during treatment. The population pharmacokinetic models were refined using additional data from 710 plasma concentrations from 238 patients treated in the study [36]. The population pharmacokinetic models were developed with each of 3 renal function markers: body surface area–adjusted eGFR, absolute eGFR, and CrCL. A clear relationship of CL of cefiderocol with each renal function parameter was found. Although CrCL was the best predictor of cefiderocol clearance, the final population pharmacokinetic models with all 3 renal function markers adequately described plasma cefiderocol concentrations. Body weight and the disease status (with or without infection) were significant covariates on the pharmacokinetics of cefiderocol. The CL and volume of distribution of cefiderocol in infected patients were 26% and 36% higher, respectively, than in subjects without infection, suggesting modestly lower exposure in patients with infection [36].
The mean of urine cefiderocol concentrations for 8 patients in the cUTI study were 2710 μg/mL (range, 953–5520) at 2 hours after the start of infusion and 1520 μg/mL (range, 336–4220) at 6 hours after the start of infusion. These concentrations were much higher than the MIC values (median, 0.06 µg/mL [range, ≤0.004–8 µg/mL]; lowest concentration of the antibiotic at which 90% of the isolates were inhibited [MIC90] 1 µg/mL) of the pathogens isolated in patients included in the analysis population [36]. Based on phase 1 clinical data [30], in all renal impairment groups the urine concentration of cefiderocol was much higher than the MIC of a susceptible organism [unpublished data].
Our results in the APEKS-cUTI study [34] suggested that patients who are not critically ill may have augmented renal clearance (ie, 23 of 238 patients had CrCL ≥120 mL/minute), and potentially could be underdosed with β-lactams [37, 42, 51]. A more frequent dose (every 6 hours) was proposed for patients with ARC [35].
CONCLUSIONS
Cefiderocol, a novel parenteral siderophore cephalosporin, exhibits potent efficacy in vitro and in vivo against most gram-negative bacteria, including carbapenem-resistant strains of Enterobacteriaceae, P. aeruginosa, A. baumannii, and S. maltophilia. Cefiderocol pharmacokinetics are linear. Cefiderocol is primarily excreted unchanged via the kidneys with elimination half-life of 2–3 hours. No accumulation of cefiderocol was observed following multiple dosing every 8 hours. The developed population pharmacokinetic models described the pharmacokinetics of cefiderocol well in healthy subjects, in subjects with varying renal function, and in patients with cUTI or AUP. Renal function markers were the most influential covariates on the pharmacokinetic profile, as expected. A 2-g dose every 8 hours, infused over 3 hours was selected as a standard dose regimen based on the PTA for target fT > MIC. Dose adjustment based on renal function is proposed to ensure that a similar exposure to cefiderocol can be achieved in patients with normal or impaired renal function as well as in patients with ARC. Of note, even if patients with an infection are not critically ill, they may have ARC, as observed in the APEKS-cUTI study. The target exposures with the proposed dose regimens are conservatively estimated to provide a bactericidal activity in plasma for gram-negative pathogens with MICs of ≤4 µg/mL. The comprehensive PK/PD profiling of cefiderocol, a novel siderophore cephalosporin, is promising in terms of dosing recommendations provided for a range of patient populations with varying renal function or disease status. Based on available evidence, cefiderocol was well tolerated in phase 1 studies as described above, and in a phase 2 study in patients with cUTI [34, 52].
Notes
Acknowledgments. Editorial support was provided by Highfield (Oxford, United Kingdom), sponsored by Shionogi Inc (Florham Park, New Jersey).
Financial support. This review article was sponsored by Shionogi & Co, Ltd (Osaka, Japan), but R. E. did not receive any fee for his authorship.
Supplement sponsorship. This supplement is sponsored by Shionogi & Co., Ltd.
Potential conflicts of interest. T. K. and T. W. are employees of Shionogi & Co, Ltd. R. E. is a consultant for Shionogi Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Neuner EA, Gallagher JC. Pharmacodynamic and pharmacokinetic considerations in the treatment of critically ill patients infected with carbapenem-resistant Enterobacteriaceae. Virulence 2017; 8:440–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bassetti M, Vena A, Croxatto A, Righi E, Guery B. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018; 7:212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics 2017. Available at: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf. Accessed 1 February 2019.
- 4. Tacconelli E, Carrara E, Savoldi A, et al. . Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18:318–27. [DOI] [PubMed] [Google Scholar]
- 5. Wright H, Bonomo RA, Paterson DL. New agents for the treatment of infections with gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect 2017; 23:704–12. [DOI] [PubMed] [Google Scholar]
- 6. Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998; 26:1–12. [DOI] [PubMed] [Google Scholar]
- 7. Osthoff M, Siegemund M, Balestra G, Abdul-Aziz MH, Roberts JA. Prolonged administration of β-lactam antibiotics—a comprehensive review and critical appraisal. Swiss Med Wkly 2016; 146:w14368. [DOI] [PubMed] [Google Scholar]
- 8. Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis 2018; 18:108–20. [DOI] [PubMed] [Google Scholar]
- 9. Ito A, Kohira N, Bouchillon SK, et al. . In vitro antimicrobial activity of S-649266, a catechol substituted siderophore cephalosporin, when tested against non-fermenting gram-negative bacteria. J Antimicrob Chemother 2016; 71:670–7. [DOI] [PubMed] [Google Scholar]
- 10. Kohira N, West J, Ito A, et al. . In vitro antimicrobial activity of a siderophore cephalosporin, S-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother 2015; 60:729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ito A, Toba S, Nishikawa T, et al. . S-649266, a novel siderophore cephalosporin: binding affinity to PBP and in vitro bactericidal activity. Poster presented at: 25th European Congress of Clinical Microbiology and Infectious Diseases, Copenhagen, Denmark, 25–28 April 2015. Poster 0250. [Google Scholar]
- 12. Nakamura R, Toba S, Tsuji M, Yamano Y, Shimada J. A novel siderophore cephalosporin: IV. In vivo efficacy in various murine infection models. Poster presented at: 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5–9 September 2014. Poster F-1558. [Google Scholar]
- 13. Yamano Y. In vitro activity of cefiderocol against a broad range of clinically important gram-negative bacteria. Clin Infect Dis 2019; 69(Suppl 7):544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakamura R, Toba S, Ito A, Tsuji M, Yamano Y, Shimada J. A novel siderophore cephalosporin: V. Pharmacodynamic assessment in murine thigh infection models. Poster presented at: 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5–9 September 2014. Poster F-1559. [Google Scholar]
- 15. Horiyama T, Toba S, Nakamura R, Tsuji M, Yamano Y, Shimada J. A novel siderophore cephalosporin: VII. Magnitude of PK/PD parameter required for efficacy in murine thigh infection model. Poster presented at: 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5–9 September 2014. Poster F-1561. [Google Scholar]
- 16. Horiyama T, Toba S, Nakamura R, Tsuji M, Yamano Y, Shimada J. A novel siderophore cephalosporin: VI. Magnitude of PK/PD parameter required for efficacy in murine lung infection model. Poster presented at: 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5–9 September 2014. Poster F-1560. [Google Scholar]
- 17. Yamano Y, Nakamura R, Sato T, Tsuji M, Echols R. Correlation of cefiderocol between in vivo efficacy murine thigh/lung infection models and MIC determined in iron-depleted conditions. Poster presented at: IDWeek,San Diego, CA, 4–8 October 2017. Poster 1524. [Google Scholar]
- 18. Takemura M, Nakamura R, Sato T, Tsuji M, Yamano Y. In vivo pharmacokinetic/pharmacodynamic (PK/PD) assessment of cefiderocol against Stenotrophomonas maltophilia in a neutropenic murine lung infection model. Poster presented at: 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain, 21–24 April 2018. Poster P0189. [Google Scholar]
- 19. Tsuji M, Horiyama T, Toba S, Nakamura R, Yamano Y. S-649266, a novel siderophore cephalosporin: in vivo efficacy in murine infection model caused by multidrug-resistant gram-negative bacteria. Poster presented at: 25th European Congress of Clinical Microbiology and Infectious Diseases, Copenhagen, Denmark, 25–28 April 2015. Poster 0253. [Google Scholar]
- 20. Ito A, Ota M, Nakamura R, Tsuji M, Sato T, Yamano Y. In vitro and in vivo activity of cefiderocol against Stenotrophomonas maltophilia clinical isolates. Poster presented at: IDWeek, San Francisco, CA, 3–7 October 2018. Poster P1366. [Google Scholar]
- 21. Matsumoto S, Kanazawa S, Nakamura R, Tsuji M, Sato T, Yamano Y. In vivo efficacy of cefiderocol against carbapenem-resistant gram-negative bacilli in murine urinary tract infection models. Poster presented at: IDWeek, San Diego, CA, 4–8 October 2017. Poster P1512. [Google Scholar]
- 22. Horiyama T, Singley CM, Nakamura R, et al. . S-649266, a novel siderophore cephalosporin: VIII. Efficacy against Pseudomonas aeruginosa and Acinetobacter baumannii in rat lung infection model with humanized exposure profile of 2 g dose with 1 h infusion. Poster presented at: 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5–9 September 2014. Poster F-1556. [Google Scholar]
- 23. Tsuji M, Horiyama T, Nakamura R, et al. . S-649266, a novel siderophore cephalosporin: efficacy against Klebsiella pneumoniae, producing NDM-1 or KPC in rat lung infection model with recreated humanized exposure profile of 2 g dose with 1 or 3 h infusion. Poster presented at: IDWeek,Philadelphia, PA, 8–12 October 2014. Poster 248. [Google Scholar]
- 24. Takemura M, Matsumoto S, Miyagawa S, Sato T, Tsuji M, Yamano Y. Efficacy of humanized cefiderocol exposure against Stenotrophomonas maltophilia in a rat respiratory tract infection model. Poster presented at: 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain, 21–24 April 2018. Poster P0190. [Google Scholar]
- 25. Matsumoto S, Singley CM, Hoover J, et al. . Efficacy of cefiderocol against carbapenem-resistant gram-negative bacilli in immunocompetent-rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrob Agents Chemother 2017; 61:e00700–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of gram-negative bacteria in the murine thigh infection model. Antimicrob Agents Chemother 2017; 61:e01022–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saisho Y, Katsube T, White S, Fukase H, Shimada J. Pharmacokinetics, safety, and tolerability of cefiderocol, a novel siderophore cephalosporin for gram-negative bacteria, in healthy subjects. Antimicrob Agents Chemother 2018; 62:e02163–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miyazaki S, Katsube T, Narukawa Y, Migoya E. Metabolism and excretion of [14C]-cefiderocol, a siderophore cephalosporin, and drug-drug interaction potential via transporters of cefiderocol in healthy subjects. Poster presented at: 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain, 21–24 April 2018. Poster 0188. [Google Scholar]
- 29. Miyazaki S, Katsube T, Shen H, Tomek C, Narukawa Y. Metabolism, excretion, and pharmacokinetics of [(14) C]-cefiderocol (S-649266), a siderophore cephalosporin, in healthy subjects following intravenous administration. J Clin Pharmacol 2019. doi: 10.1002/jcph.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katsube T, Echols R, Arjona Ferreira JC, Krenz HK, Berg JK, Galloway C. Cefiderocol, a siderophore cephalosporin for gram-negative bacterial infections: pharmacokinetics and safety in subjects with renal impairment. J Clin Pharmacol 2017; 57:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanabria C, Migoya E, Mason JW, et al. . Effect of cefiderocol, a siderophore cephalosporin, on QT/QTc interval in healthy adult subjects. Clin Ther 2019. doi: 10.1016/j.clinthera.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 32. Katsube T, Miyazaki S, Narukawa Y, Hernandez-Illas M, Wajima T. Drug-drug interaction of cefiderocol, a siderophore cephalosporin, via human drug transporters. Eur J Clin Pharmacol 2018; 74:931–8. [DOI] [PubMed] [Google Scholar]
- 33. Portsmouth S, Veenhuyzen D, Echols R, et al. . Cefiderocol compared with imipenem/cilastatin in the treatment of adults with complicated urinary tract infections with or without pyelonephritis or acute uncomplicated pyelonephritis: results from a multicenter, double-blind, randomized study (APEKS-cUTI). Oral presentation at: 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 22–25 April 2017. Oral presentation 0250D. [Google Scholar]
- 34. Portsmouth S, van Veenhuyzen D, Echols R, et al. . Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2018; 18:1319–28. [DOI] [PubMed] [Google Scholar]
- 35. Katsube T, Wajima T, Ishibashi T, Arjona Ferreira JC, Echols R. Pharmacokinetic/pharmacodynamic modeling and simulation of cefiderocol, a parenteral siderophore cephalosporin, for dose adjustment based on renal function. Antimicrob Agents Chemother 2017; 61:e01381–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawaguchi N, Katsube T, Echols R, Wajima T. Population pharmacokinetic analysis of cefiderocol, a parenteral siderophore cephalosporin, in healthy subjects, subjects with various degrees of renal function, and patients with complicated urinary tract infection or acute uncomplicated pyelonephritis. Antimicrob Agents Chemother 2018; 62:e01391–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roberts JA, Abdul-Aziz MH, Lipman J, et al. . Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 2014; 14:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. International Transporter Consortium, Giacomini KM, Huang SM, et al. Membrane transporters in drug development. Nat Rev Drug Discov 2010; 9:215–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. US Food and Drug Administration. In vitro metabolism- and transporter-mediated drug–drug interaction studies 2017. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/vitro-metabolism-and-transporter-mediated-drug-drug-interaction-studies-guidance-industry. Accessed 4 August 2019.
- 40. US Food and Drug Administration. Clinical drug interaction studies—study design, data analysis, and clinical implications 2017. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-study-design-data-analysis-and-clinical-implications-guidance. Accessed 4 August 2019.
- 41. European Medicines Agency. Guideline on the investigation of drug interactions 2012. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-drug-interactions_en.pdf. Accessed 4 August 2019.
- 42. Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet 2010; 49:1–16. [DOI] [PubMed] [Google Scholar]
- 43. Hobbs AL, Shea KM, Roberts KM, Daley MJ. Implications of augmented renal clearance on drug dosing in critically ill patients: a focus on antibiotics. Pharmacotherapy 2015; 35:1063–75. [DOI] [PubMed] [Google Scholar]
- 44. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. [DOI] [PubMed] [Google Scholar]
- 45. Katsube T, Wajima T, Ishibashi T, Arjona Ferreira JC, Echols R. Dose adjustment of S-649266, a siderophore cephalosporin, for patients requiring haemodialysis. Poster presented at: 26th European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, Netherlands, 9–12 April 2016. Poster 1311. [Google Scholar]
- 46. Malone RS, Fish DN, Abraham E, Teitelbaum I. Pharmacokinetics of cefepime during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother 2001;45:3148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barbhaiya RH, Forgue ST, Gleason CR, et al. . Safety, tolerance, and pharmacokinetic evaluation of cefepime after administration of single intravenous doses. Antimicrob Agents Chemother 1990; 34:1118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barbhaiya RH, Forgue ST, Gleason CR, et al. . Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob Agents Chemother 1992; 36:552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barbhaiya RH, Knupp CA, Forgue ST, Matzke GR, Guay DR, Pittman KA. Pharmacokinetics of cefepime in subjects with renal insufficiency. Clin Pharmacol Ther 1990; 48:268–76. [DOI] [PubMed] [Google Scholar]
- 50.Hospira Inc. Maxipime (cefepime hydrochloride) for injection. Prescribing information. Lake Forest, IL: Hospira Inc, 2018. [Google Scholar]
- 51. Hites M, Taccone FS, Wolff F, et al. . Broad-spectrum β-lactams in obese non-critically ill patients. Nutr Diabetes 2014; 4:e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Echols R, Ariyasu M, Nagata TD. Pathogen-focused clinical development to address unmet medical need: cefiderocol targeting carbapenem resistance. Clin Infect Dis 2019; 69(Suppl 7):559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]