Abstract
Iron is an essential nutrient for bacterial growth, replication, and metabolism. Humans store iron bound to various proteins such as hemoglobin, haptoglobin, transferrin, ferritin, and lactoferrin, limiting the availability of free iron for pathogenic bacteria. However, bacteria have developed various mechanisms to sequester or scavenge iron from the host environment. Iron can be taken up by means of active transport systems that consist of bacterial small molecule siderophores, outer membrane siderophore receptors, the TonB-ExbBD energy-transducing proteins coupling the outer and the inner membranes, and inner membrane transporters. Some bacteria also express outer membrane receptors for iron-binding proteins of the host and extract iron directly from these for uptake. Ultimately, iron is acquired and transported into the bacterial cytoplasm. The siderophores are small molecules produced and released by nearly all bacterial species and are classified according to the chemical nature of their iron-chelating group (ie, catechol, hydroxamate, α-hydroxyl-carboxylate, or mixed types). Siderophore-conjugated antibiotics that exploit such iron-transport systems are under development for the treatment of infections caused by gram-negative bacteria. Despite demonstrating high in vitro potency against pathogenic multidrug-resistant bacteria, further development of several candidates had stopped due to apparent adaptive resistance during exposure, lack of consistent in vivo efficacy, or emergence of side effects in the host. However, cefiderocol, with an optimized structure, has advanced and has been investigated in phase 1 to 3 clinical trials. This article discusses the mechanisms implicated in iron uptake and the challenges associated with the design and utilization of siderophore-mimicking antibiotics.
Keywords: β-lactams, iron transport, monobactams, siderophore, siderophore-antibiotic conjugate
IRON HOMEOSTASIS IN HUMANS
Iron plays pivotal roles in metabolic pathways, oxygen transport, and immune function in humans, and maintaining balanced iron availability (homeostasis) is important for a healthy body. Iron deficiency leads to poor prognoses in long-term diseases and increased susceptibility to infection [1], as does iron overload [2, 3]. Iron homeostasis occurs through regulation of duodenal absorption and recycling of iron stores. Under normal physiological conditions, nearly three-quarters of body iron is found as hemoglobin, with the remainder stored intracellularly as ferritin or bound to extracellular proteins [4]. The normal serum level of iron is 10–30 µM, giving between 12% and 50% saturation of the iron-binding capacity (60–75 µM); higher levels are symptomatic of iron overload [5, 6].
Iron is also an essential nutrient for bacterial growth, replication, and metabolism, and the human body has numerous defense mechanisms that reduce the availability of iron to invading pathogens [7]. Tissue damage resulting from infection can alter local iron homeostasis by enhancing iron-scavenging and macrophage sequestration of iron, heme, and hemoglobin [8, 9]. The serum protein transferrin (also called serotransferrin) creates a bacteriostatic environment by sequestering free iron [10]. The affinity of transferrin for ferric iron is high at physiological pH but decreases at lower pH [11]. This facilitates the release and internalization of complexed iron following interaction with specific receptors on erythroid cells, lymphocytes, and macrophages [12–14]. The analogous lactoferrin is widely expressed in secretory fluids (milk, saliva, and tears), in secondary granules of polymorphonuclear cells, and in some pancreatic duct cells. Its affinity for iron is 300-times higher than that of transferrin and increases further in acidic conditions. This promotes transfer of iron from transferrin to lactoferrin during inflammation, when the local pH is decreased by accumulation of organic acids [15]. Lactoferrin possesses intrinsic antimicrobial activity owing to both its binding to lipopolysaccharide and its catalyzing formation of peroxides with concomitant reduction of ferric iron, which together increase membrane permeability and trigger lysis [16–18]. Iron may also be transported around the body by mammalian siderophores, such as catechols or citrate, bound to siderocalin [19]. Siderocalin is found in neutrophil granules, uterine secretions, and, at particularly high levels, in serum during bacterial infection, where it contributes to the host defense [19–22]. Iron uptake in the small intestine is regulated by hepcidin, an oligopeptide hormone synthesized in the liver [23, 24]. Hepcidin production is greatly increased during infection and inflammation, and it has been reported to have direct antimicrobial activity [25, 26]. Iron bound to hepcidin is transported into cells by ferroportin, enabling macrophages, hepatocytes, and enterocytes to retain iron that would otherwise be released into the bloodstream.
BACTERIAL IRON ACQUISITION
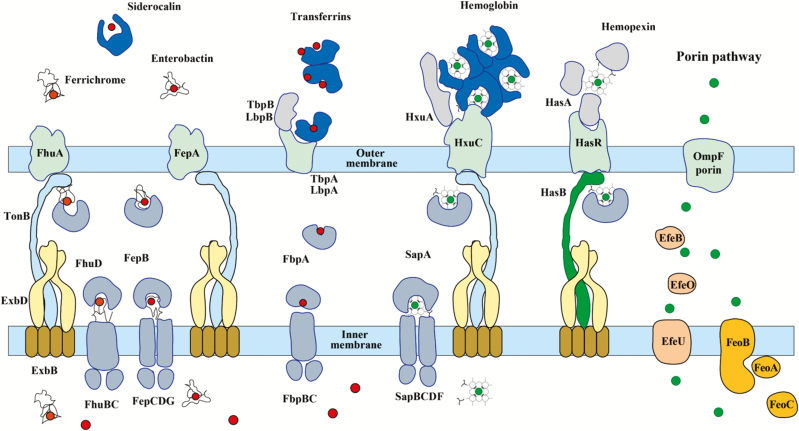
Bacteria use a number of strategies to acquire the iron essential for growth [27]. The most important mechanisms (Figure 1) mobilize ferric iron (Fe3+), the dominant iron form in oxygen-rich environments, but bacteria also take up aqueous ferrous iron (Fe2+) [28] or readily utilize ferrous iron bound in heme [29–31]. Most bacteria secrete powerful ferric iron–chelating molecules called siderophores to scavenge iron from their environment [32]. Siderophores have very high ferric-ion association constants (1020 to 1030 M− 1), and they effectively remove iron from the host’s iron–protein complexes [33]. The iron–siderophore complexes are recognized by uptake systems in bacteria [34]. In gram-negative bacteria, the first step is binding to specific outer membrane receptors (Figure 1), which facilitate their passage across the outer membrane. The translocation is driven by proteins of the TonB family and the energy-transducing complex ExbB/ExbD in the cytoplasmic membrane [35–39]. The iron–siderophore complexes are released into the periplasm, where they may be bound by further components of the transport systems for onward translocation to the cytoplasm or be catabolized to release the iron for uptake by alternative transport mechanisms.
Figure 1.
Iron transporters in gram-negative bacteria and metal availability in the host during infection. In a healthy individual, ferric iron (Fe3+; red circles) circulates bound by transferrin in the blood, and ferrous iron (Fe2+; green circles) is complexed in heme, which is bound by hemoglobin within red blood cells but can be released by hemolysis during infection. Free Fe2+ is uncommon; however, when available, it enters through the general porin pathway. Free heme is scavenged by hemopexin. Secreted bacterial siderophores remove iron from transferrins and ferritin, and the siderophore–iron complexes are bound by cognate receptors at the bacterial surface. Similarly, secreted hemophores such as HasA and HxuC can remove heme from hemoglobin and hemopexin. Enterobacteria also possess outer membrane receptors for heme. In Neisseria, iron transferrins are bound by outer membrane receptors comprising 2 subunits (eg, LbpA and LbpB for lactoferrin) and forced to release 1 of the bound iron ions. Catecholate-mediated iron acquisition (eg, by enterobactin) can be inhibited by the innate immune protein lipocalin-2 (siderocalin or neutrophil gelatinase-associated lipocalin), which binds and sequesters catechols.
The siderophore strategy is disrupted by the human protein siderocalin, which can also sequester bacterial siderophores and prevent their uptake by bacteria [40–43]. However, bacteria often display redundancy in their deployment of siderophores and can utilize alternatives, such as the fungal ferrichrome, in order to escape siderocalins [44, 45]. Some gram-negative bacteria, especially Neisseria spp., can acquire ferric iron directly from lactoferrin and serum transferrin [46], and many bacteria can exploit the ferrous iron bound in heme as a nutritional source. The heme or heme proteins are bound by cell surface receptors and transported into the cytoplasm, where the tetrapyrrole ring is cleaved in order to release the iron [47]. Gram-negative bacteria also secrete extracellular heme-binding proteins (hemophores), such as hemopexin, which sequester heme and deliver it to active uptake systems [48–50].
Bacteria use a wide variety of ligands in their siderophores [32], the principal ligands being α-hydroxy acids (eg, citrate, vibrioferrin, staphyloferrin A), catechols (eg, enterobactin, bacillibactin), and hydroxamates (eg, ferrichrome, deferoxamine, ornibactin). Some species produce siderophores that combine different ligands (eg, azotobactin, pyoverdine) [41]. The ligands are typically bidentate and, together with other ligands, form pseudo-octahedral, hexadentate coordination complexes with ferric iron [51]. Siderophores such as enterobactin or ferrichrome are optimized for binding, as each molecule carries 3 bidentate ligands.
ILLICIT TRANSPORT BY IRON UPTAKE SYSTEMS
The iron–siderophore uptake systems provide access to the periplasm across the otherwise poorly permeable outer membrane. It is therefore not surprising that bacteria have evolved ways to exploit these systems to deliver toxic compounds that hinder the growth of competing species. The natural antibiotics, known as sideromycins, mimic hydroxamate siderophores [52]. This small group of antibiotics includes albomycin, produced by Actinomyces subtropicus; ferrimycin A1, produced by Streptomyces griseoflavus; and salmycins A–D, produced by Streptomyces violaceus [53–55]. No new members of this structurally diverse group have been identified, although a genome-mining approach has paved the way for structural variation around albomycin [56]. Many Enterobacteriaceae secrete microcins that are conjugated post-translationally with endogenous catecholate siderophores [57–59]. Genome mining, using the readily identified genes responsible for conjugating the siderophore and peptide [60], has yielded many new variants [61].
SYNTHETIC SIDEROPHORE–DRUG CONJUGATES
Research efforts exploiting a “Trojan horse” strategy started in the 1980s, with the aim of developing agents that would facilitate the uptake of antibiotics into gram-negative bacteria in a way similar to albomycin [32, 62, 63]. For the most part, the activity of these model compounds was not greater than that of the parent antibiotic [32], and additional challenges emerged including solubility issues, inadequate passage across the cytoplasmic membrane, and a lack of release of the active antibiotic [64]. The β-lactam antibiotics, whose targets (the penicillin-binding proteins [PBPs] essential for cell wall biosynthesis) are located in the periplasmic space, have been the antibiotic class where this strategy has been most successfully used, with 4 compounds, cefetecol, BAL30072, GSK3342830, and cefiderocol, reaching clinical trials [32, 65].
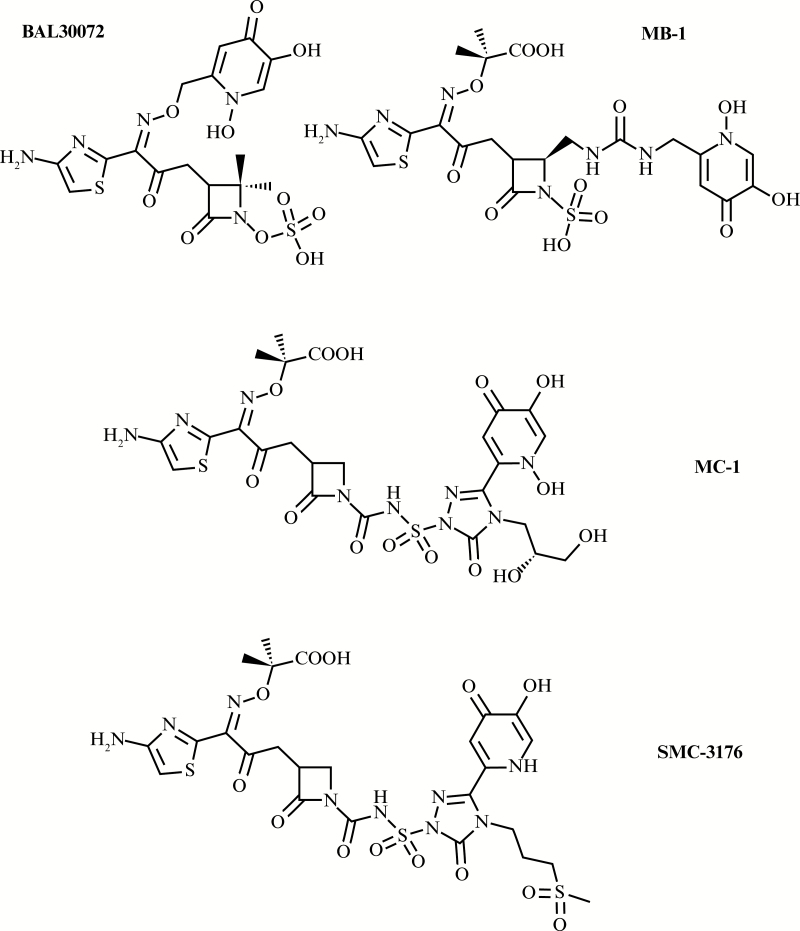
BAL30072 (Figure 2) is a monocyclic β-lactam, an analog of the monosulfactam tigemonam, conjugated with the catechol isostere, hydroxypyridone [66]. Other hydroxypyridone-conjugated monocyclic β-lactams that have recently received experimental investigation include a monobactam MB-1 [67] and 2 monocarbams MC-1 and SMC-3176 [68, 69]. These 4 compounds (Figure 2) exhibited potent activity against β-lactamase–producing Enterobacteriaceae, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia, but the activities of MB-1, MC-1, and SMC-3176 against Acinetobacter spp. were limited. Monocyclic β-lactams are not readily hydrolyzed by the class B metallo-β-lactamases and class C serine-β-lactamases [68, 69]. MB-1, MC-1, and SMC-3176 carry bulky substituents that improve stability toward class A, C, and D extended-spectrum β-lactamases and carbapenemases, but the bulk of these substituents prevents binding to the active site of the target PBP3 in Acinetobacter spp. [70, 71]. Of these 4 molecules, only BAL30072 entered into clinical trials, but its development by Basilea Pharmaceutica was suspended in phase 1.
Figure 2.
Structures of the monocyclic β-lactam-siderophore conjugates that have been evaluated for clinical development—BAL30072 (terminated in phase 1), MB-1 (preclinical investigation only), MC-1 (preclinical investigation only), and SMC-3176 (preclinical investigation only).
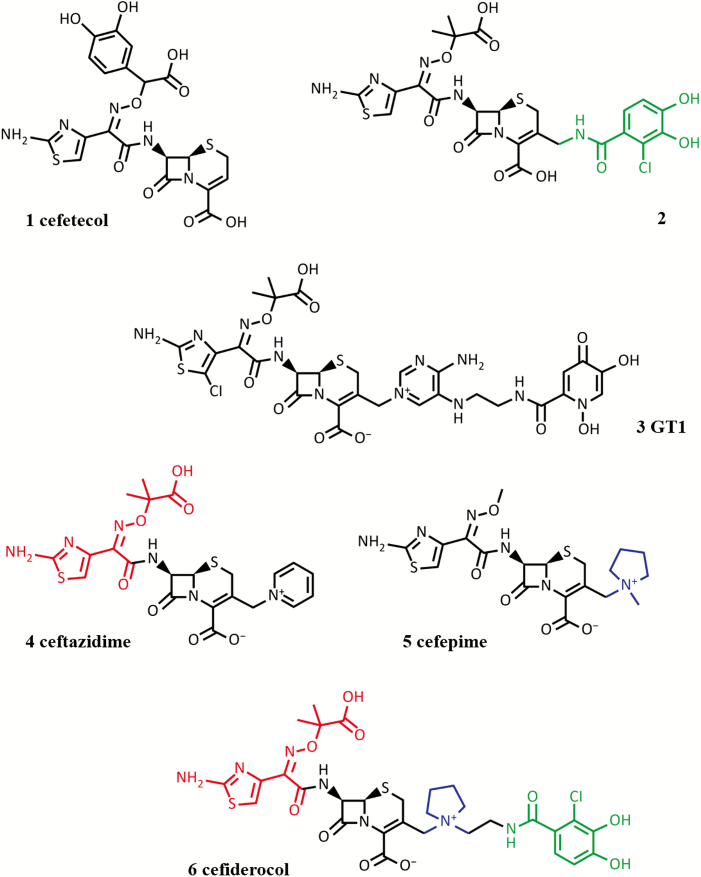
Catechol-conjugated cephalosporins have been investigated experimentally by many research groups [32, 72]. Of the early conjugates, cefetecol (Figure 3, compound 1) entered into human clinical trials, but its development was terminated in phase 1. The potent in vitro activity of these early compounds did not translate into good in vivo efficacy, largely because mammalian catechol O-methyltransferase (COMT) methylates 1 phenol group of the catechol [72], leading to loss of activity as the metabolized compound is no longer a substrate for the uptake systems. Decreasing the pKa of the catechol moiety by the introduction of an electron-withdrawing halogen atom (eg, Figure 3, compound 2) led to compounds that retained excellent potency, were more stable toward COMT, and had extended pharmacokinetic half-lives [73]. However, none of these compounds reached the market owing to a lack of stability against β-lactamase–mediated hydrolysis, unwanted side effects, and, in some cases, simple economics. More recently, Shionogi and GlaxoSmithKline (GSK) ran a joint discovery program around such conjugated cephalosporins. GSK registered a phase 1 trial to evaluate safety, tolerability, and pharmacokinetics of an ascending intravenous single-dose and repeat dose of GSK3342830 (NCT02751424) [74]; however, the trial was suspended, and no details of activity or structure of the compound are available. Shionogi pursued S-649266 (previously also known as GSK2696266), now called cefiderocol (Figure 3, compound 6), which has progressed satisfactorily through clinical trials (see below).
Figure 3.
Structures of the siderophore-conjugated cephalosporins that have been evaluated for clinical development. Compound 1: cefetecol (terminated in phase 1). Compound 2 (preclinical investigation only [73]): the halogen-substituted catechol moiety (highlighted in green), optimized for in vivo stability. Compound 3: GT-1 (currently in preclinical investigation). Compound 6: cefiderocol (phase 1 to phase 3 clinical development), which shares the halogen-substituted catechol moiety shown in compound 2. For comparison, structures are also shown for ceftazidime (compound 4), with which cefiderocol shares the bulky 7-acylamino side chain (highlighted in red) that confers β-lactamase stability, and cefepime (compound 5), with which cefiderocol shares the 3’ side chain with a quaternary ammonium function (highlighted in blue) that confers β-lactamase stability and good penetration into gram-negative bacteria.
Hydroxypyridone, the catechol isostere, has also been investigated with cephalosporins [32], and GT-1 (LCB10-0200) is a new hydroxypyridone-conjugated cephalosporin in development by Geom Therapeutics and LegoChem Biosciences, alone and in combination with a β-lactamase inhibitor (GT055, LCB18-055). GT-1 (Figure 3, compound 3) is active against many gram-negative pathogens, including P. aeruginosa and Acinetobacter baumannii. Addition of the new β-lactamase inhibitor improves activity against Enterobacteriaceae [75].
RESISTANCE TO SIDEROPHORE-CONJUGATED Β-LACTAMS
Resistance to any of the conjugates with a catechol ligand, or catechol isosteres, can be acquired in Escherichia coli (and other Enterobacteriaceae) through loss of the TonB energy-transducing protein or the catecholate receptors Cir and Fiu, which preferentially transport monomeric catecholate siderophores [76–78]. Similarly, loss of the putative catecholate receptors PiuA, PiuD, and PirA or TonB in P. aeruginosa and A. baumannii results in elevated minimum inhibitory concentrations (MICs) for the conjugated monocyclic β-lactams and cefiderocol [77, 79–83].
In addition, MB-1 and SMC-3176 may be vulnerable to adaptive resistance (ie, reversible resistance observed only in the presence of the antibiotic) in P. aeruginosa [81, 84], a phenomenon that has previously been observed for this organism exposed to aminoglycosides and polymyxins [85–89]. The mechanisms underlying adaptive resistance are unclear and the net effect probably depends on specific combinations of environmental conditions, strains, and antibiotics. Adaptive resistance has been attributed to decreased outer membrane permeability and action of efflux pumps [85, 90, 91]. Indeed, MB-1 was potentiated by combination with an efflux pump inhibitor [92]. It was also suggested that, for MB-1, competition between the natural siderophores and the synthetic conjugate may contribute to the effect [92]. However, activity of BAL30072 against Burkholderia pseudomallei is independent of the ability of the bacteria to take up malleobactin and pyochelin, the siderophores utilized by this organism [93], and activity against P. aeruginosa was not affected by competition with endogenous siderophores at physiological expression levels [94]. Furthermore, extensive in vivo testing did not suggest a propensity for adaptive resistance during exposure to BAL30072 [95–100]. Similarly, cefiderocol has demonstrated good in vivo efficacy in rat models of respiratory infection [101] and the neutropenic mouse thigh infection model [102–104]. In a recent study comparing the in vivo efficacies of cefiderocol, MB-1, and SMC-3167 against P. aeruginosa strains that exhibited variable susceptibility toward MB-1 and SMC-3167 in previous investigations [81, 84], the attenuated efficacies of MB-1 and SMC-3167 were confirmed, whereas cefiderocol showed sustained inhibitory effects consistent with expectation from the MICs determined in iron-depleted medium [103]. It remains to be proven that the discrepancy between observed activity of some of the siderophore conjugates in vitro and their efficacy in animal models of infection in vivo is caused by a resistance phenomenon. It is possible that the inconsistency is simply attributable to differences in expression of the multiple iron uptake pathways between the in vitro test medium and the infection site in vivo.
Normal growth media recommended for susceptibility testing contain iron at concentrations that are many times higher than the normal free iron concentration in blood. These concentrations are sufficient to repress the expression of siderophores and most iron-uptake pathways. These media are clearly inappropriate for determination of the activity of the siderophore compounds and it has been customary to add a chelating agent, such as ovotransferrin (conalbumin) [105] or 2,2’ bipyridyl [66] or to remove iron using ion-exchange resins [81]. This results in increased siderophore production, induction of the iron-uptake systems, and, consequently, increased susceptibility toward the siderophore-conjugated antibiotics. However, P. aeruginosa is well known to adapt its iron homeostasis to local conditions of infection [106, 107]. Without information about the actual expression levels under the various in vitro susceptibility testing conditions, it is unclear whether any of the proposed in vitro tests is appropriate for prediction of in vivo efficacy and, therefore, for properly identifying adaptive resistance for this species.
SIDEROPHORE CONJUGATES CURRENTLY IN DEVELOPMENT
Cefiderocol is an advanced-generation cephalosporin that combines the optimized chloro-catechol iron-binding moiety, similar to earlier siderophore cephalosporins (Figure 3, compounds 1, 2), with features conferring β-lactamase stability, such as the quaternary ammonium function in the 3’ side chain, similar to cefepime (Figure 3, compound 5), and a bulky 7-acylamino side chain, similar to ceftazidime (Figure 3, compound 4). Cefiderocol therefore shows potent activity against a wide range of gram-negative bacteria that produce serine-β-lactamases [108–115] because it is poorly hydrolyzed by these enzymes, including the Klebsiella pneumoniae carbapenemases [116]. It is not clear which structural features lead to the remarkable stability of cefiderocol toward metallo-β-lactamases, where unexpectedly low catalytic efficiencies (kcat/KM) were reported for imipenemase metallo-β-lactamase-1 (IMP-1), Verona integron-encoded metallo-β-lactamase (VIM-2), L1, and New Delhi metallo-β-lactamase-1 (NDM-1) [116].
The molar ratio of cefiderocol to ferric iron in the equilibrium complex was found to be 1:1 [108], lower than the expected 3:1 reported for monocarbams [117]. It seems likely that, as observed with BAL30072 [118], other parts of the molecule provide secondary ligands to fulfill the coordination requirements of the ferric ion. The example of BAL30072 strongly suggests that a lower stoichiometry might be the one recognized by the receptor, so that to focus only on the highest stoichiometry might be misleading for siderophores with fewer than 6 donors. The possibly unique iron-chelating modality of cefiderocol may allow it to escape the mechanisms that underlie the putative adaptive resistance observed with MB-1, MC-1, and SMC-3167. The clinical development of cefiderocol is continuing, with the recent completion of a phase 2 trial to study efficacy and safety of intravenous cefiderocol vs imipenem/cilastatin in complicated urinary tract infections (APEKS-cUTI) [119] and an ongoing phase 3 trial studying the efficacy of cefiderocol in the treatment of adult patients with serious infections caused by carbapenem-resistant gram-negative pathogens (CREDIBLE-CR) [120].
CONCLUSIONS
Cefiderocol is the first siderophore-conjugated antibiotic to progress beyond phase 1 human safety trials. Its unique combination of structural features has helped to avoid problems earlier conjugated cephalosporins encountered. Developing a standardized in vitro testing method should be feasible based on the apparent robust correlation between in vitro and in vivo models. The further development and use of cefiderocol in clinical practice for the treatment of infection will be watched with interest.
Notes
Acknowledgments. Editorial support was provided by Highfield, Oxford, United Kingdom, sponsored by Shionogi Inc, Florham Park, New Jersey.
Financial support. This article was sponsored by Shionogi & Co, Ltd, Osaka, Japan. The author did not receive any fee for his authorship.
Supplement sponsorship. This supplement is sponsored by Shionogi & Co., Ltd.
Potential conflicts of interest. M. G. P. P. holds shares in Basilea Pharmaceutica International Ltd. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tansarli GS, Karageorgopoulos DE, Kapaskelis A, Gkegkes I, Falagas ME. Iron deficiency and susceptibility to infections: evaluation of the clinical evidence. Eur J Clin Microbiol Infect Dis 2013; 32:1253–8. [DOI] [PubMed] [Google Scholar]
- 2. Kontoghiorghes GJ, Kolnagou A, Skiada A, Petrikkos G. The role of iron and chelators on infections in iron overload and non iron loaded conditions: prospects for the design of new antimicrobial therapies. Hemoglobin 2010; 34:227–39. [DOI] [PubMed] [Google Scholar]
- 3. Nairz M, Schroll A, Sonnweber T, Weiss G. The struggle for iron— a metal at the host-pathogen interface. Cell Microbiol 2010; 12:1691–702. [DOI] [PubMed] [Google Scholar]
- 4. Brock JH. Benefits and dangers of iron during infection. Curr Opin Clin Nutr Metab Care 1999; 2:507–10. [DOI] [PubMed] [Google Scholar]
- 5. Kasvosve I, Delanghe J. Total iron binding capacity and transferrin concentration in the assessment of iron status. Clin Chem Lab Med 2002; 40:1014–8. [DOI] [PubMed] [Google Scholar]
- 6. Yamanishi H, Iyama S, Yamaguchi Y, Kanakura Y, Iwatani Y. Total iron-binding capacity calculated from serum transferrin concentration or serum iron concentration and unsaturated iron-binding capacity. Clin Chem 2003; 49:175–8. [DOI] [PubMed] [Google Scholar]
- 7. Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol 2014; 2:946–53. [DOI] [PubMed] [Google Scholar]
- 8. Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr 2010; 30:105–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol 2015; 15:500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ong ST, Ho JZ, Ho B, Ding JL. Iron-withholding strategy in innate immunity. Immunobiology 2006; 211:295–314. [DOI] [PubMed] [Google Scholar]
- 11. Aisen P, Leibman A, Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem 1978; 253:1930–7. [PubMed] [Google Scholar]
- 12. Egan TJ, Zak O, Aisen P. The anion requirement for iron release from transferrin is preserved in the receptor-transferrin complex. Biochemistry 1993; 32:8162–7. [DOI] [PubMed] [Google Scholar]
- 13. Núñez MT, Gaete V, Watkins JA, Glass J. Mobilization of iron from endocytic vesicles. The effects of acidification and reduction. J Biol Chem 1990; 265:6688–92. [PubMed] [Google Scholar]
- 14. Ponka P, Lok CN. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol 1999; 31:1111–37. [DOI] [PubMed] [Google Scholar]
- 15. Mazurier J, Spik G. Comparative study of the iron-binding properties of human transferrins. I. Complete and sequential iron saturation and desaturation of the lactotransferrin. Biochim Biophys Acta 1980; 629:399–408. [DOI] [PubMed] [Google Scholar]
- 16. Brock JH, . De Sousa M. Iron in Immunity, Cancer, and Inflammation. New York, NY: Wiley, 1989. ISBN 0-471-92150–5. [Google Scholar]
- 17. Farnaud S, Evans RW. Lactoferrin–a multifunctional protein with antimicrobial properties. Mol Immunol 2003; 40:395–405. [DOI] [PubMed] [Google Scholar]
- 18. Levin RE, Kalidas S, Gopinadhan P, Pometto A.. Food Biotechnology. Boca Raton, FL: CRC/Taylor & Francis, 2006: ISBN-13 978-0-8247-5329-0. [Google Scholar]
- 19. Correnti C, Strong RK. Mammalian siderophores, siderophore-binding lipocalins, and the labile iron pool. J Biol Chem 2012; 287:13524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson GJ, McLaren GD. Iron Physiology and Pathophysiology in Humans. New York, NY: Springer; 2012. ISBN 9781603274845. [Google Scholar]
- 21. Paragas N, Qiu A, Hollmen M, Nickolas TL, Devarajan P, Barasch J. NGAL-siderocalin in kidney disease. Biochim Biophys Acta 2012; 1823:1451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sia AK, Allred BE, Raymond KN. Siderocalins: siderophore binding proteins evolved for primary pathogen host defense. Curr Opin Chem Biol 2013; 17:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ganz T. Hepcidin and its role in regulating systemic iron metabolism. Hematology Am Soc Hematol Educ Program 2006; 1:29–35. [DOI] [PubMed] [Google Scholar]
- 24. Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr 2006; 26:323–42. [DOI] [PubMed] [Google Scholar]
- 25. Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 2001; 276:7806–10. [DOI] [PubMed] [Google Scholar]
- 26. Jiang XF, Liu ZF, Lin AF, Xiang LX, Shao JZ. Coordination of bactericidal and iron regulatory functions of hepcidin in innate antimicrobial immunity in a zebrafish model. Sci Rep 2017; 7:4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wandersman C, Delepelaire P. Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol 2004; 58:611–47. [DOI] [PubMed] [Google Scholar]
- 28. Lau CK, Krewulak KD, Vogel HJ. Bacterial ferrous iron transport: the Feo system. FEMS Microbiol Rev 2016; 40:273–98. [DOI] [PubMed] [Google Scholar]
- 29. Smith AD, Wilks A. Extracellular heme uptake and the challenges of bacterial cell membranes. Curr Top Membr 2012; 69:359–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Contreras H, Chim N, Credali A, Goulding CW. Heme uptake in bacterial pathogens. Curr Opin Chem Biol 2014; 19:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hantke K. Ferrous iron transport. In: Crosa J, Mey A, Payne S, eds. Iron Transport in Bacteria. Washington, DC: ASM Press, 2004:178–84. [Google Scholar]
- 32. Page MG. Siderophore conjugates. Ann N Y Acad Sci 2013; 1277:115–26. [DOI] [PubMed] [Google Scholar]
- 33. Raymond KN, Müller G, Matzanke BF. Complexation of iron by siderophores a review of their solution and structural chemistry and biological function. Top Curr Chem 1984; 123:49–102. [Google Scholar]
- 34. Chu BC, Garcia-Herrero A, Johanson TH, et al. . Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals 2010; 23:601–11. [DOI] [PubMed] [Google Scholar]
- 35. Karlsson M, Hannavy K, Higgins CF. ExbB acts as a chaperone-like protein to stabilize TonB in the cytoplasm. Mol Microbiol 1993; 8:389–96. [DOI] [PubMed] [Google Scholar]
- 36. Larsen RA, Thomas MG, Postle K. Protonmotive force, ExbB and ligand-bound FepA drive conformational changes in TonB. Mol Microbiol 1999; 31:1809–24. [DOI] [PubMed] [Google Scholar]
- 37. Paquelin A, Ghigo JM, Bertin S, Wandersman C. Characterization of HasB, a Serratia marcescens TonB-like protein specifically involved in the haemophore-dependent haem acquisition system. Mol Microbiol 2001; 42:995–1005. [DOI] [PubMed] [Google Scholar]
- 38. Fardeau S, Mullié C, Dassonville-Klimpt A, Audic N, Sasaki A, Sonnet P. Bacterial iron uptake: a promising solution against multidrug resistant bacteria. Science against microbial pathogens: communicating current research and technological advances. In: Méndez-Vilas A, ed. FORMATEX, Microbiology book series number 3. Vol. 2. Badajoz, Spain: Formatex Research Center, 2011:695–704. [Google Scholar]
- 39. Sverzhinsky A, Fabre L, Cottreau AL, et al. . Coordinated rearrangements between cytoplasmic and periplasmic domains of the membrane protein complex ExbB-ExbD of Escherichia coli. Structure 2014; 22:791–7. [DOI] [PubMed] [Google Scholar]
- 40. Abergel RJ, Clifton MC, Pizarro JC, et al. . The siderocalin/enterobactin interaction: a link between mammalian immunity and bacterial iron transport. J Am Chem Soc 2008; 130:11524–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hider RC, Kong X. Chemistry and biology of siderophores. Nat Prod Rep 2010; 27:637–57. [DOI] [PubMed] [Google Scholar]
- 42. Hoette TM, Clifton MC, Zawadzka AM, Holmes MA, Strong RK, Raymond KN. Immune interference in Mycobacterium tuberculosis intracellular iron acquisition through siderocalin recognition of carboxymycobactins. ACS Chem Biol 2011; 6:1327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Allred BE, Correnti C, Clifton MC, Strong RK, Raymond KN. Siderocalin outwits the coordination chemistry of vibriobactin, a siderophore of Vibrio cholerae. ACS Chem Biol 2013; 8:1882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fischbach MA, Lin H, Liu DR, Walsh CT. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat Chem Biol 2006; 2:132–8. [DOI] [PubMed] [Google Scholar]
- 45. Valdebenito M, Müller SI, Hantke K. Special conditions allow binding of the siderophore salmochelin to siderocalin (NGAL-lipocalin). FEMS Microbiol Lett 2007; 277:182–7. [DOI] [PubMed] [Google Scholar]
- 46. Ekins A, Khan AG, Shouldice SR, Schryvers AB. Lactoferrin receptors in gram-negative bacteria: insights into the iron acquisition process. Biometals 2004; 17:235–43. [DOI] [PubMed] [Google Scholar]
- 47. Tong Y, Guo M. Bacterial heme-transport proteins and their heme-coordination modes. Arch Biochem Biophys 2009; 481:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krieg S, Huché F, Diederichs K, et al. . Heme uptake across the outer membrane as revealed by crystal structures of the receptor-hemophore complex. Proc Natl Acad Sci U S A 2009; 106:1045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zambolin S, Clantin B, Chami M, et al. . Structural basis for haem piracy from host haemopexin by Haemophilus influenzae. Nat Commun 2016; 7:11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Porcheron G, Garénaux A, Proulx J, Sabri M, Dozois CM. Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front Cell Infect Microbiol 2013; 3:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Raymond KN, Allred BE, Sia AK. Coordination chemistry of microbial iron transport. Acc Chem Res 2015; 48:2496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Braun V, Pramanik A, Gwinner T, Köberle M, Bohn E. Sideromycins: tools and antibiotics. Biometals 2009; 22:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gause GF. Recent studies on albomycin, a new antibiotic. Br Med J 1955; 2:1177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bickel H, Mertens P, Prelog V, Seibl J, Walser A. Constitution of ferrimycin A1. Antimicrob Agents Chemother 1965; 5:951–7. [PubMed] [Google Scholar]
- 55. Vertesy L, Aretz W, Fehlhaber HW, Kogler H. Salmycin A-D. Antibiotika aus Streptomyces violaceus DSM 8286, mit siderophor-aminoglycosid-struktur. Helv Chim Acta 1995; 78:46–60. [Google Scholar]
- 56. Zeng Y, Kulkarni A, Yang Z, et al. . Biosynthesis of albomycin δ(2) provides a template for assembling siderophore and aminoacyl-tRNA synthetase inhibitor conjugates. ACS Chem Biol 2012; 7:1565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hantke K, Nicholson G, Rabsch W, Winkelmann G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc Natl Acad Sci U S A 2003; 100:3677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Destoumieux-Garzón D, Peduzzi J, Thomas X, Djediat C, Rebuffat S. Parasitism of iron-siderophore receptors of Escherichia coli by the siderophore-peptide microcin E492m and its unmodified counterpart. Biometals 2006; 19:181–91. [DOI] [PubMed] [Google Scholar]
- 59. Morin N, Lannelue I, Connil N, Cottenceau M, Pons AM, Sablé S. Mechanism and bactericidal activity of microcin L in Escherichia coli and Salmonella enterica. Antimicrob Agents Chemother 2011; 55:899–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nolan EM, Walsh CT. Investigations of the MceIJ-catalyzed posttranslational modification of the microcin E492 C-terminus: linkage of ribosomal and nonribosomal peptides to form “Trojan horse” antibiotics. Biochemistry 2008; 47:9289–99. [DOI] [PubMed] [Google Scholar]
- 61. Vassiliadis G, Destoumieux-Garzón D, Lombard C, Rebuffat S, Peduzzi J. Isolation and characterization of two members of the siderophore-microcin family, microcins M and H47. Antimicrob Agents Chemother 2010; 54:288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Möllmann U, Heinisch L, Bauernfeind A, Köhler T, Ankel-Fuchs D. Siderophores as drug delivery agents: application of the “Trojan horse” strategy. Biometals 2009; 22:615–24. [DOI] [PubMed] [Google Scholar]
- 63. Bilitewski U, Blodgett JA, Duhme-Klair A-K, et al. . Chemical and biological aspects of nutritional immunity— perspectives for new anti-infectives targeting iron uptake systems. Angew Chem 2017; doi: 10.1002/ange.201701586. [DOI] [PubMed] [Google Scholar]
- 64. Ji C, Miller MJ. Chemical syntheses and in vitro antibacterial activity of two desferrioxamine B-ciprofloxacin conjugates with potential esterase and phosphatase triggered drug release linkers. Bioorg Med Chem 2012; 20:3828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bush K, Page MGP. What we may expect from novel antibacterial agents in the pipeline with respect to resistance and pharmacodynamic principles. J Pharmacokinet Pharmacodyn 2017; 44:113–32. [DOI] [PubMed] [Google Scholar]
- 66. Page MG, Dantier C, Desarbre E. In vitro properties of BAL30072, a novel siderophore sulfactam with activity against multiresistant gram-negative bacilli. Antimicrob Agents Chemother 2010; 54:2291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brown MF, Mitton-Fry MJ, Arcari JT, et al. . Pyridone-conjugated monobactam antibiotics with gram-negative activity. J Med Chem 2013; 56:5541–52. [DOI] [PubMed] [Google Scholar]
- 68. Murphy-Benenato KE, Dangel B, Davis HE, et al. . SAR and structural analysis of siderophore-conjugated monocarbam inhibitors of Pseudomonas aeruginosa PBP3. ACS Med Chem Lett 2015; 6:537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Murphy-Benenato KE, Bhagunde PR, Chen A, et al. . Discovery of efficacious Pseudomonas aeruginosa-targeted siderophore-conjugated monocarbams by application of a semi-mechanistic pharmacokinetic/pharmacodynamic model. J Med Chem 2015; 58:2195–205. [DOI] [PubMed] [Google Scholar]
- 70. Han S, Zaniewski RP, Marr ES, et al. . Structural basis for effectiveness of siderophore-conjugated monocarbams against clinically relevant strains of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 2010; 107:22002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Han S, Caspers N, Zaniewski RP, et al. . Distinctive attributes of β-lactam target proteins in Acinetobacter baumannii relevant to development of new antibiotics. J Am Chem Soc 2011; 133:20536–45. [DOI] [PubMed] [Google Scholar]
- 72. Hubschwerlen C. b-Lactam antibiotics. In: Taylor JB, Triggle DJ (Eds). Comprehensive Medicinal Chemistry II. Vol. 7: Therapeutic Areas II: Cancer, Infectious Diseases, Inflammation & Immunology and Dermatology; Antibacterials; chapter 7.17. Amsterdam; London: Elsevier; 2007: 479–518. ISBN: 978-0-08-045044-5. [Google Scholar]
- 73. Bird TG, Arnould JC, Bertrandie A, Jung FH. Pharmacokinetics of catechol cephalosporins. The effect of incorporating substituents into the catechol moiety on pharmacokinetics in a marmoset model. J Med Chem 1992; 35:2643–51. [DOI] [PubMed] [Google Scholar]
- 74. Tenero D, Farinola N, Berkowitz EM, et al. . Pharmacokinetics, safety, and tolerability evaluation of single and multiple doses of GSK3342830 in healthy volunteers. Clin Pharmacol Drug Dev 2018; doi: 10.1002/cpdd.637. [DOI] [PubMed] [Google Scholar]
- 75. Hackel M, Biek D, Cho YL, Sahm D. In vitro activity of GT-1 and GT-1/GT-055 against recent gram-negative clinical isolates. Poster presented at: American Society of Microbiology Annual Meeting (ASM-Microbe) 2018, June 7–11, 2018. Atlanta, GA; Poster 575. Available at: https://www.ihma.com/app/uploads/Geom_P40_GT-1_ASM-2018_FINAL.pdf [Google Scholar]
- 76. Nikaido H, Rosenberg EY. Cir and Fiu proteins in the outer membrane of Escherichia coli catalyze transport of monomeric catechols: study with beta-lactam antibiotics containing catechol and analogous groups. J Bacteriol 1990; 172:1361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ito A, Sato T, Ota M, et al. . In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against gram-negative bacteria. Antimicrob Agents Chemother 2017; 62:e01454–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kohira N, Nakamura R, Ito A, et al. . Resistance acquisition studies of cefiderocol by serial passage and in vitro pharmacodynamic model under human simulated exposure. Poster presented at: American Society of Microbiology Annual Meeting (ASM-Microbe) 2018, June 7–11, 2018. Atlanta, GA; Poster 623. [Google Scholar]
- 79. McPherson CJ, Aschenbrenner LM, Lacey BM, et al. . Clinically relevant gram-negative resistance mechanisms have no effect on the efficacy of MC-1, a novel siderophore-conjugated monocarbam. Antimicrob Agents Chemother 2012; 56:6334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. van Delden C, Page MG, Köhler T. Involvement of Fe uptake systems and AmpC β-lactamase in susceptibility to the siderophore monosulfactam BAL30072 in Pseudomonas aeruginosa. Antimicrob Agents Chemother 2013; 57:2095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kim A, Kutschke A, Ehmann DE, et al. . Pharmacodynamic profiling of a siderophore-conjugated monocarbam in Pseudomonas aeruginosa: assessing the risk for resistance and attenuated efficacy. Antimicrob Agents Chemother 2015; 59:7743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ito A, Nishikawa T, Matsumoto S, et al. . Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2016; 60:7396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Moynié L, Luscher A, Rolo D, et al. . Structure and function of the PiuA and PirA siderophore-drug receptors from Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob Agents Chemother 2017; 61:pii:e02531-16. doi: 10.1128/AAC.02531-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tomaras AP, Crandon JL, McPherson CJ, et al. . Adaptation-based resistance to siderophore-conjugated antibacterial agents by Pseudomonas aeruginosa. Antimicrob Agents Chemother 2013; 57:4197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gilleland HE Jr, Farley LB. Adaptive resistance to polymyxin in Pseudomonas aeruginosa due to an outer membrane impermeability mechanism. Can J Microbiol 1982; 28:830–40. [DOI] [PubMed] [Google Scholar]
- 86. Daikos GL, Jackson GG, Lolans VT, Livermore DM. Adaptive resistance to aminoglycoside antibiotics from first-exposure down-regulation. J Infect Dis 1990; 162:414–20. [DOI] [PubMed] [Google Scholar]
- 87. Barclay ML, Begg EJ, Chambers ST, Thornley PE, Pattemore PK, Grimwood K. Adaptive resistance to tobramycin in Pseudomonas aeruginosa lung infection in cystic fibrosis. J Antimicrob Chemother 1996; 37:1155–64. [DOI] [PubMed] [Google Scholar]
- 88. Fernández L, Gooderham WJ, Bains M, McPhee JB, Wiegand I, Hancock RE. Adaptive resistance to the “last hope” antibiotics polymyxin B and colistin in Pseudomonas aeruginosa is mediated by the novel two-component regulatory system ParR-ParS. Antimicrob Agents Chemother 2010; 54:3372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Skiada A, Markogiannakis A, Plachouras D, Daikos GL. Adaptive resistance to cationic compounds in Pseudomonas aeruginosa. Int J Antimicrob Agents 2011; 37:187–93. [DOI] [PubMed] [Google Scholar]
- 90. Motta SS, Cluzel P, Aldana M. Adaptive resistance in bacteria requires epigenetic inheritance, genetic noise, and cost of efflux pumps. PLoS One 2015; 10:e0118464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sandoval-Motta S, Aldana M. Adaptive resistance to antibiotics in bacteria: a systems biology perspective. Wiley Interdiscip Rev Syst Biol Med 2016; 8:253–67. [DOI] [PubMed] [Google Scholar]
- 92. Tomaras AP, Crandon JL, McPherson CJ, Nicolau DP. Potentiation of antibacterial activity of the MB-1 siderophore-monobactam conjugate using an efflux pump inhibitor. Antimicrob Agents Chemother 2015; 59:2439–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mima T, Kvitko BH, Rholl DA, Page MG, Desarbre E, Schweizer HP. In vitro activity of BAL30072 against Burkholderia pseudomallei. Int J Antimicrob Agents 2011; 38:157–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Page MGP, Müller C, Hofer B, Desarbre E, Dreier J, Vidal F. The role of iron transport in the activity of the siderophore sulfactam BAL30072 against Pseudomonas aeruginosa. Poster presented at: 20th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2010, April 10–13, 2010. Vienna, Austria; Poster P-1241. [Google Scholar]
- 95. Miriagou V, Papagiannitsis C, Kotsakis S, et al. . Efficacy of BAL30072, alone and combined with meropenem, against VIM-producing Enterobacteria in a murine thigh infection model. Poster presented at: 20th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2010, April 10–13, 2010. Vienna, Austria; Poster P-1240. [Google Scholar]
- 96. Gould JK, Sattar A, Daws GM, et al. . Comparative efficacy of BAL30072, aztreonam, meropenem and ceftazidime and effects of dose fractionation in a murine thigh infection model. Poster presented at: 52nd Interscience Congress on Antimicrobial Agents and Chemotherapy (ICAAC), 2012, September 9–12, 2012. San Francisco, CA; Poster F-842. [Google Scholar]
- 97. Gould JK, Sattar A, Thommes P, et al. . Efficacy of BAL30072 in murine thigh infection models of multi-resistant gram-negative bacteria. Poster presented at: 23rd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2013, April 27–30, 2013. Berlin, Germany; Poster P-908. [Google Scholar]
- 98. Weiss W, Pulse M, Nguyen P, et al. . In vivo efficacy of the novel monosulfactam BAL30072 alone and in combination with meropenem against clinically important gram-negative pathogens. Oral presentation at: 23rd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2013, April 27–30, 2013; Berlin, Germany; Oral presentation O182. [Google Scholar]
- 99. Sattar A, Vaddi S, Thommes P, et al. . Efficacy of BAL30072 in murine lung infection models of multi-resistant gram-negative bacteria. ePoster presented at: 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2015, April 25–28, 2015. Copenhagen, Denmark; ePoster EP153. [Google Scholar]
- 100. Thommes P, Sattar A, Burgess E, et al. . Efficacy of BAL30072 in combination with meropenem in murine thigh infection models of multi-resistant gram-negative bacteria. Poster presented at: 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2015, April 25–28, 2015. Copenhagen, Denmark; Poster P1380. [Google Scholar]
- 101. Matsumoto S, Singley CM, Hoover J, et al. . Efficacy of cefiderocol against carbapenem-resistant gram-negative bacilli in immunocompetent rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrob Agents Chemother 2017; 61:pii: e00700-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of gram-negative bacteria in a murine thigh infection model. Antimicrob Agents Chemother 2017; 61:pii:e01022-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ghazi IM, Monogue ML, Tsuji M, Nicolau DP. Humanized exposures of cefiderocol, a siderophore cephalosporin, display sustained in vivo activity against siderophore-resistant Pseudomonas aeruginosa. Pharmacology 2018; 101:278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ghazi IM, Monogue ML, Tsuji M, Nicolau DP. Pharmacodynamics of cefiderocol, a novel siderophore cephalosporin, in a Pseudomonas aeruginosa neutropenic murine thigh model. Int J Antimicrob Agents 2018; 51:206–12. [DOI] [PubMed] [Google Scholar]
- 105. Babini GS, Livermore DM. Effect of conalbumin on the activity of Syn 2190, a 1,5 dihydroxy-4-pyridon monobactam inhibitor of AmpC beta-lactamases. J Antimicrob Chemother 2000; 45:105–9. [DOI] [PubMed] [Google Scholar]
- 106. Cornelis P, Dingemans J. Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front Cell Infect Microbiol 2013; 3:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nguyen AT, O’Neill MJ, Watts AM, et al. . Adaptation of iron homeostasis pathways by a Pseudomonas aeruginosa pyoverdine mutant in the cystic fibrosis lung. J Bacteriol 2014; 196:2265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yamano Y, Tsuji M, Hackel M, Sahm D, Echols R. Mode of action of cefiderocol, a novel siderophore cephalosporin, active against highly resistant gram-negative bacteria including carbapenem-resistant strains of Enterobacteriaceae and non-fermenting bacteria. Oral presentation at: 27th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2017, April 22–25, 2017 Vienna, Austria; Oral presentation OS0750. [Google Scholar]
- 109. Kohira N, West J, Ito A, et al. . In vitro antimicrobial activity of a siderophore cephalosporin, s-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother 2016; 60:729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Falagas ME, Skalidis T, Vardakas KZ, Legakis NJ; Hellenic Cefiderocol Study Group Activity of cefiderocol (S-649266) against carbapenem-resistant gram-negative bacteria collected from inpatients in Greek hospitals. J Antimicrob Chemother 2017; 72:1704–8. [DOI] [PubMed] [Google Scholar]
- 111. Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant gram-negative bacilli from North America and Europe, including carbapenem non-susceptible isolates: the SIDERO-WT-2014 Study. Antimicrob Agents Chemother 2017; 61:pii: e00093–17. doi: 10.1128/AAC.00093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dobias J, Dénervaud-Tendon V, Poirel L, Nordmann P. Activity of the novel siderophore cephalosporin cefiderocol against multidrug-resistant gram-negative pathogens. Eur J Clin Microbiol Infect Dis 2017; 36:2319–27. [DOI] [PubMed] [Google Scholar]
- 113. Jacobs MR, Abdelhamed AM, Good CE, et al. . 1351. In vitro activity of cefiderocol (S-649266), a siderophore cephalosporin, against Enterobacteriaceae with defined extended-spectrum b-lactamases and carbapenemases. Open Forum Infect Dis 2018; 5:S413–4. [Google Scholar]
- 114. Jacobs MR, Abdelhamed AM, Good CE, et al. . ARGONAUT-I: activity of cefiderocol (S-649266), a siderophore cephalosporin, against gram-negative bacteria, including carbapenem-resistant nonfermenters and Enterobacteriaceae with defined extended-spectrum β-lactamases and carbapenemases. Antimicrob Agents Chemother 2018; 63:pii:e01801–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tsuji M, Hackel M, Yamanao Y, et al. . Cefiderocol in vitro activity against gram-negative clinical isolates collected in Europe: results from 3 SIDERO-WT surveillance studies during 2014–2017. Poster presented at: 29th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2019, April 13–16, 2019. Amsterdam, Netherlands; Poster P1852. [Google Scholar]
- 116. Ito-Horiyama T, Ishii Y, Ito A, et al. . Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother 2016; 60:4384–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Harrington JM, Gootz T, Flanagan M, et al. . Characterization of the aqueous iron(III) chelation chemistry of a potential Trojan horse antimicrobial agent: chelate structure, stability and pH dependent speciation. Biometals 2012; 25:1023–36. [DOI] [PubMed] [Google Scholar]
- 118. Scorciapino MA, Malloci G, Serra I, et al. . Complexes formed by the siderophore-based monosulfactam antibiotic BAL30072 and their interaction with the outer membrane receptor PiuA of P. aeruginosa. Biometals 2019; 32:155–70. [DOI] [PubMed] [Google Scholar]
- 119. Portsmouth S, van Veenhuyzen D, Echols R, et al. . Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2018; 18:1319–28. [DOI] [PubMed] [Google Scholar]
- 120. Study of S-649266 or best available therapy for the treatment of severe infections caused by carbapenem-resistant Gram-negative pathogens (CREDIBLE - CR) Available at: https://clinicaltrials.gov/ct2/show/NCT02714595. Accessed 4 March 2019.