Abstract
Database linkage is a common strategy to expand analytic possibilities. Our group recently completed a linkage between the SRTR and PHIS databases for pediatric heart transplant recipients. The aim of this project was to expand the linkage between SRTR and PHIS to include liver, kidney, lung, heart-lung, and small bowel transplants. All patients (<21 years) who underwent liver, kidney, lung, heart-lung, or small bowel transplant were identified from the PHIS database using APR-DRG codes (2002–2018). Linkage was performed in a stepwise approach using indirect identifiers. Hospital costs were estimated based on hospital charges and cost-to-charge ratios, inflated to 2018 dollars and described by transplant type. A total of 14,061 patients overlapped between databases. Of these, 13,388 (95.2%) were uniquely linked. Linkage success ranged from 92.6% to 97.8% by organ system. A total of 12,940 (92%) patients had complete cost data. Hospitalization costs were greatest for patients undergoing small bowel transplantation with a median cost of $734,454 (IQR $336,174 – $1,504,167), followed by heart $565,386 (IQR $352,813 – $999,216), heart-lung $471,573 (IQR $328,523 – 992,438), lung $303,536 (IQR $215,383 – $612,749), liver $200,448 (IQR $130,880 – $357,897), and kidney transplant $94,796 (IQR $73,157 -$131,040). We report a robust linkage between the SRTR and PHIS databases, providing an invaluable tool to assess resource utilization in solid organ transplant recipients. Our analysis provides contemporary cost data for pediatric solid organ transplantation from the largest U.S. sample reported to date. It also provides a platform for expanded analyses in the pediatric transplant population.
Introduction:
Large databases are increasingly utilized for pediatric solid organ transplantation research. Given relatively small patient numbers at each pediatric transplant center, this has enabled studies with increased statistical power through combining the collective experience across multiple centers. Many large databases are readily available that can facilitate research in solid organ transplantation [1–3]. These include clinical registries, administrative databases, and research datasets [2]. While analytic power is increased through large patient numbers, each database has inherent limitations and lack of data granularity can pose a challenge [1, 4]. Database linkage is increasingly common and allows researchers to leverage the strengths of each database, creating new opportunities and avenues for clinical research.
Database linkage has been effectively utilized across multiple disciplines including congenital heart disease and congenital heart surgery [4, 5], pediatric oncology [6, 7], and organ transplantation [8–11]. Our group recently reported the successful linkage of pediatric heart transplant recipients between the Scientific Registry of Transplant Recipients (SRTR) database and the Pediatric Health Information Systems (PHIS) administrative database [10]. This effort has provided valuable insight into hospital resource utilization in pediatric heart transplant recipients [12, 13] and also facilitated novel analyses that were not previously possible using existing datasets [14]. The aim of this project was to expand the linkage between SRTR and PHIS to include pediatric liver, kidney, lung, heart-lung, and small bowel transplants.
Methods:
This study used data from the SRTR. The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere. The Health Resources and Services Administration, U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The SRTR collects and maintains data regarding organ transplantation in the United States. Data are derived from multiple sources including the OPTN, transplant programs, organ procurement organizations, histocompatibility laboratories, the Centers for Medicare and Medicaid Services, and the National Technical Information Service’s Death Master File. The SRTR database includes data from every organ transplant and waitlist addition within the U.S. since October 1987.
The PHIS administrative database collects clinical and daily resource utilization data for hospital encounters from >50 tertiary children’s hospitals. This includes data from inpatient hospitalizations, observation, ambulatory surgery, and emergency department visits. This database stores diagnosis and procedural ICD-9 and ICD-10 codes, payer information, along with encounter-level hospital charge and cost data [10].
The PHIS and SRTR databases were linked at the patient level using indirect identifiers in a stepwise approach. All available patients ≤21 years of age were included. The SRTR database records were limited to transplants that overlapped with data available in the PHIS database (2002–2018). These dates were chosen based on our prior experience that data was generally insufficient for successful linkage prior to 2002 [10]. PHIS transplant encounters were identified using the All-Patient Refined Diagnosis Related Group (3M Health Information Systems; APR-DRG) code for heart and/or lung transplantation (APR-DRG 002), liver and/or small intestine transplant (APR-DRG 001), and renal transplant (APR-DRG 440). Linkage of PHIS encounters with SRTR data was performed in a three-step process after selecting patients by the type of transplant; 1) Uniquely linked patients were identified by matching hospital, date of birth, sex, and date of transplant, 2) unlinked records were then matched by hospital, date of birth, and sex, and 3) all remaining unlinked records were matched using hospital and date of birth. The strategy using sequential removal of identifiers was intended to account for the possibility of mismatches between datasets. However, removal of identifiers limits the ability to uniquely identify records. Patients with records that were unable to be uniquely linked between datasets were excluded from the linked cohort but included in a cohort to compare characteristics between linked and unlinked patients. Characteristics of linked and unlinked patients were compared using the chi-square test or Wilcoxon rank sum test, as appropriate.
A validation cohort was constructed using patients from Vanderbilt University Medical Center for kidney and liver transplant recipients (pediatric small bowel and lung transplants are not routinely performed at our center). Encrypted medical record numbers were extracted from the PHIS database and cross-validated against center data to assess accuracy of the data linkage. Institutional data were also reviewed to assess the reasons why unlinked patients were unable to be uniquely identified.
From the linked database, all patients with complete cost and resource utilization data were identified. PHIS includes hospital charges and these were converted to costs using hospital- and year-specific cost-to-charge ratios. All costs were adjusted for inflation to 2018 dollars using the medical component of the Consumer Price Index. Costs were assessed for the entire transplant hospitalization and then divided into pre- and post-transplant periods. Component costs were calculated including pharmacy, laboratory, imaging, supply, clinical, and other (primarily room and nursing charges) costs. Costs were compared by patient diagnosis and age at transplant using the Kruskal-Wallis test. All statistical analyses were performed in SAS version 9.4 (SAS Institute; Cary, NC) or STATA version 15 (StataCorp LLC; College Station, TX) with p<0.05 considered statistically significant.
This project was approved by SRTR, PHIS, as well as the Vanderbilt University Institutional Review Board.
Results:
A total of 14,061 patients were available for linkage with overlapping data between the SRTR and PHIS databases. Of these, 13,388 (95.2%) were successfully linked using the 3-step linkage algorithm. The linkage success ranged from 92.6% for kidney transplants to 97.8% for heart-lung transplants (Table 1). Greater than 99% of linked patients were uniquely matched on the first step of the linkage algorithm. Cost data were available for 96.7% of linked patients, representing 92% of the entire cohort available for linkage.
Table 1.
Linkage success by organ transplant type
| Transplant type |
Total available for linkage |
Linked | Cost data available |
|---|---|---|---|
| Heart* | 3,178 | 3,046 (95.9%) | 2,896 (91.1%) |
| Small bowel | 316 | 305 (96.5%) | 304 (96.2%) |
| Heart-Lung | 46 | 45 (97.8%) | 42 (91.3%) |
| Lung | 597 | 577 (96.7%) | 562 (94.1%) |
| Liver | 4,849 | 4,714 (97.2%) | 4,595 (94.8%) |
| Kidney | 5,075 | 4,701 (92.6%) | 4,541 (89.5%) |
| Total | 14,061 | 13,388 (95.2%) | 12,940 (92%) |
Linkage from 2002–2018 for all organs except heart transplant (2002–2016)
Data include multi-organ transplants (i.e. liver-small bowel, heart-kidney)
Characteristics of the linked patients by type of transplant are shown in Table 2 and the comparisons of linked and unlinked patients are provided in supplemental tables 1–6. There were no significant differences noted between linked and unlinked patients receiving small bowel or heart-lung transplants. There were significant differences in disease etiology between linked and unlinked patients for heart, liver, and kidney transplants. There were also statistically significant differences in age distribution and transplant type (cadaveric vs. living donor) for liver and kidney transplant recipients between linked and unlinked groups.
Table 2.
Characteristics of linked patients by organ transplanted
| Heart N=3,046 |
Small Bowel N=305 |
Heart Lung N=45 |
Lung N=577 |
Liver N=4,714 |
Kidney N=4,701 |
|
|---|---|---|---|---|---|---|
| Age | ||||||
| <1 year | 933 (30.6%) | 52 (17.1%) | 5 (10.9%) | 50 (8.7%) | 1294 (27.5%) | 2 (<0.1%) |
| 1–5 years | 729 (23.9%) | 159 (52.1%) | 7 (15.2%) | 81 (14%) | 1796 (38.1%) | 888 (18.9%) |
| 6–10 years | 431 (14.2%) | 46 (15.1%) | 4 (8.7%) | 103 (17.9%) | 656 (13.9%) | 827 (17.6%) |
| 11–17 years | 876 (28.8%) | 40 (13.1%) | 29 (63%) | 317 (54.9%) | 920 (19.5%) | 2548 (54.2%) |
| 18–21 years | 77 (2.5%) | 8 (2.6%) | 1 (2.2%) | 26 (4.5%) | 48 (1%) | 436 (9.3%) |
| Male sex | 1657 (54.4%) | 177 (58%) | 22 (48.9%) | 247 (42.8%) | 2334 (49.5%) | 2749 (58.5%) |
| Race | ||||||
| Caucasian | 1798 (59%) | 207 (67.9%) | 34 (75.6%) | 409 (70.9%) | 2663 (56.5%) | 2131 (45.3%) |
| African American | 561 (18.4%) | 48 (15.7%) | 2 (4.4%) | 40 (6.9%) | 636 (13.5%) | 852 (18.1%) |
| Hispanic | 513 (16.8%) | 41 (13.4%) | 8 (17.8%) | 101 (17.5%) | 990 (21%) | 1421 (30.2%) |
| Other | 174 (5.7%) | 9 (3%) | 1 (2.2%) | 27 (4.7%) | 425 (9%) | 297 (6.3%) |
| Blood type | ||||||
| O | 1377 (45.2%) | 142 (46.6%) | 17 (37.8%) | 250 (43.3%) | 2283 (48.4%) | 2386 (51.3%) |
| A | 1148 (37.7%) | 108 (35.4%) | 22 (48.9%) | 220 (38.1%) | 1612 (34.2%) | 1529 (32.9%) |
| B | 393 (12.9%) | 38 (12.5%) | 3 (6.7%) | 79 (13.7%) | 622 (13.2%) | 593 (12.7%) |
| AB | 128 (4.2%) | 17 (5.6%) | 3 (6.7%) | 28 (4.9%) | 197 (4.2%) | 143 (3.1%) |
| Era | ||||||
| 2002–2009 | 1366 (44.8%) | 212 (69.5%) | 25 (55.6%) | 289 (50.1%) | 1939 (41.1%) | 1789 (38.1%) |
| 2010–2018* | 1680 (55.2%) | 93 (30.5%) | 20 (44.4%) | 288 (49.9%) | 2775 (58.9%) | 2912 (61.9%) |
| Diagnosis | ||||||
| CM 1404 (46.6%) | Short gut 203 (66.6%) | PHN 28 (62.2%) | CF 286 (49.6%) | Biliary atresia 1796 (38.1%) | Congenital 1870 (39.8%) | |
| CHD 1439 (47.8%) | FBD 68 (22.3%) | CHD 10 (22.2%) | PHN 109 (18.9%) | Metabolic 723 (15.3%) | Glomerular 1198 (25.5%) | |
| ReTx 168 (5.5%) | Other 34 (11.1%) | Other 7 (15.6%) | Other 182 (31.5%) | Hepatic failure 530 (11.2%) | Tubular/interstitial 365 (7.8%) | |
| Other 35 (1.1%) | Malignancy 439 (9.3%) | Familial/genetic 362 (7.7%) | ||||
| Cirrhosis 285 (6%) | Other 906 (19.3%) | |||||
| Other 941 (20%) | ||||||
Abbreviations: CF – Cystic fibrosis; CHD – Congenital heart disease; CM – Cardiomyopathy; FBD – Functional bowel disorder; PHN – Pulmonary hypertension; ReTx – Retransplant
Era 2 includes 2010 – 2018 for all groups except heart transplant (2010 – 2016)
Linkage validation was performed using kidney and liver transplants at our institution. A total of 31 liver and 67 kidney transplant recipients from our institution were successfully linked between databases. Of these, 100% were accurately identified for both transplant types. There was a total of 6 unlinked patients from our institution (1 liver and 5 kidney transplants). The reasons for linkage failure varied in these cases. A total of 3 patients had date mismatches (date of transplant N=1 or date of birth N=2). The remaining unlinked patients failed due to issues with APR-DRG coding. A total of 2 patients did not have a APR-DRG code for transplantation, and 1 patient was a multi-organ transplant and the documented APR-DRG represented the concurrently transplanted organ.
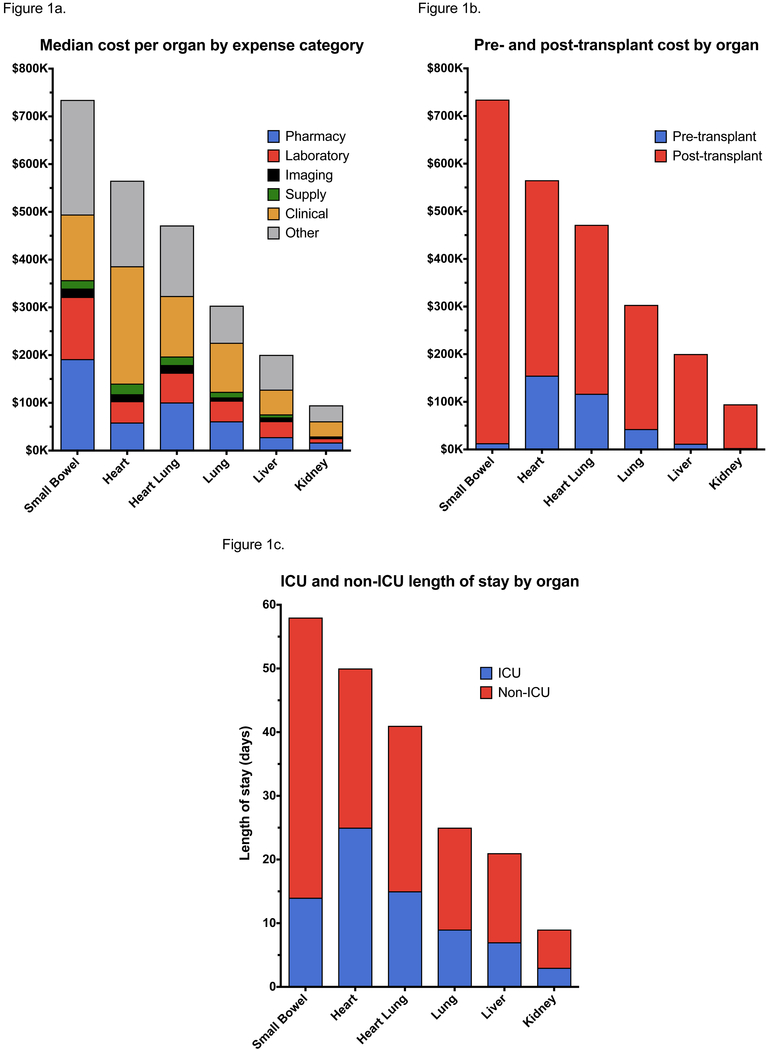
Total costs by organ transplant, including the distribution by expense category, are shown in Figure 1a. Hospitalization costs were greatest for patients undergoing small bowel transplantation with a median cost of $734,454 (IQR $336,174 – $1,504,167), followed by heart $565,386 (IQR $352,813 – $999,216), heart-lung $471,573 (IQR $328,523 – 992,438), lung $303,536 (IQR $215,383 – $612,749), liver $200,448 (IQR $130,880 – $357,897), and kidney transplant $94,796 (IQR $73,157 -$131,040). Room and nursing charges (other category) as well as charges for clinical services contributed most significantly to overall costs across organ systems with pharmacy costs contributing significantly to the cost of small bowel transplants.
Figure 1.
Median total a) cost with breakdown by expense category, b) pre- and post-transplant costs, and c) total and ICU length of stay by type of organ transplant
Costs divided by the pre- and post-transplant periods are shown in Table 3 and Figure 1b. The majority of costs are incurred in the post-transplant period across all organs. However, thoracic organ transplants incurred the greatest costs in the pre-transplant period. Total hospital length of stay, divided by ICU and non-ICU care, are shown in Figure 1c. Length of stay was greatest for patients undergoing small bowel transplants with a median total length of stay of 58 days, followed by heart (50 days), heart-lung (41 days), lung (25 days), liver (21 days), and kidney transplants (9 days). Length of stay directly correlated with total transplant hospitalization costs.
Table 3.
Total, pre-, and post-transplant hospitalization costs by organ
| Pre-transplant | Post-transplant | Total | |
|---|---|---|---|
| Heart | $138,173 ($0 – $405,002) | $365,750 ($257,767 – $554,252) | $565,386 ($352,813 – $999,216) |
| Small bowel | $12,119 ($0 – $109,013) | $668,774 ($303,075 – $1,256,947) | $734,454 ($336,174 – $1,504,167) |
| Heart Lung | $115,417 ($0 – $422,182) | $350,380 ($224,420 – $568,236) | $471,573 ($328,523 – 992,438) |
| Lung | $40,335 ($1,192 – $182,185) | $245,216 ($179,380 – $378,903) | $303,536 ($215,383 – $612,749) |
| Liver | $10,716 ($2,142 – $73,756) | $166,847 ($110,966 – $286,089) | $200,448 ($130,880 – $357,897) |
| Kidney | $2,891 ($0 – $6,354) | $89,048 ($67,806 – $123,303) | $94,796 ($73,157 – $131,040) |
. Costs inflated to 2018 U.S. dollars and expressed as median (25% – 75%)
To assess the ability of the linked database to enable a more detailed assessment of resource utilization across the spectrum of pediatric solid organ transplants, transplant hospitalization costs were assessed based on patient age and disease etiology for all transplant types (Table 4). Patients <1 year of age represent the group with the highest costs across all transplant types. This difference reached statistical significance for all groups, except heart-lung transplants, which had the fewest number of linked patients. There are also notable differences in cost based on disease etiology across the different types of solid organ transplants.
Table 4.
Total hospitalization costs based on age group and patient diagnosis
| Median total cost (25% – 75%)a | p valueb | Median total cost (25% – 75%)a | p valueb | ||
|---|---|---|---|---|---|
| Heart | Lung | ||||
| All patients | $565,386 ($352,813 – $999,216) | All patients | $303,536 ($215,383 – $612,749) | ||
| Age group | Age group | ||||
| <1 year | $773,672 ($481,651 – $1,328,535) | <0.001 | <1 year | $894,072 ($626,844 – $1,205,729) | <0.001 |
| 1–5 years | $558,965 ($370,562 – $1,032,231) | 1–5 years | $744,555 ($326,089 – $1,259,554) | ||
| 6–10 years | $509,097 ($300,410 – $883,710) | 6–10 years | $286,506 ($214,892 – $513,860) | ||
| 11–17 years | $442,081 ($302,528 – $768,100) | 11–17 years | $260,678 ($196,443 – $365,899) | ||
| 18–21 years | $426,773 ($281,276 – $575,252) | 18–21 years | $417,033 ($232,810 – $496,122) | ||
| Diagnosis | Diagnosis | ||||
| CM | $529,434 ($328,834 – $913,094) | <0.001 | CF | $263,722 ($201,560 – $375,930) | <0.001 |
| CHD | $642,271 ($395,260 – $1,115,489) | PHN | $525,742 ($282,361 – $1,042,119) | ||
| ReTx | $431,414 ($294,189 – $752,525) | Other | $377,697 ($221,120 – $862,374) | ||
| Small Intestine | Heart-Lung | ||||
| All patients | $734,454 ($336,174 – $1,504,167) | All patients | $471,573 ($328,523 – 992,438) | ||
| Age group | Age group | ||||
| <1 year | $1,345,228 ($680,705 – $2,093,655) | 0.001 | <1 year | $755,853 ($481,473 – $1,004,960) | 0.323 |
| 1–5 years | $729,215 ($316,200 – $1,389,008) | 1–5 years | $681,394 ($471,573 – $1,045,348) | ||
| 6–10 years | $387,959 ($233,241 – $1,162,244) | 6–10 years | $277,638 ($206,764 – $2,059,976) | ||
| 11–17 years | $632,135 ($335,001 – $1,298,084) | 11–18 years | $410,401 ($315,850 – $947,663) | ||
| 18–21 years | $911,134 ($335,972 – $1,193,398) | Diagnosis | |||
| Diagnosis | PHN | $520,462 ($346,002 – $779,802) | 0.352 | ||
| Short gut | $823,202 ($323,469 – $1,824,330) | 0.077 | CHD | $829,878 ($297,621 – $1,162,582) | |
| FBD | $586,934 ($346,991 – $1,254,395) | Other | $377,946 ($211,102 – $1,061,056) | ||
| Other | $749,033 ($277,840 – $1,091,067) | ||||
| Kidney | Liver | ||||
| All patients | $94,796 ($73,157 -$131,040) | All patients | $200,448 ($130,880 – $357,897) | ||
| Age group | Age group | ||||
| <1 year | $151,552 ($106,868 – $196,236) | <0.001 | <1 year | $251,740 ($157,040 – $480,304) | |
| 1–5 years | $107,458 ($79,650 – $151,027) | 1–5 years | $191,682 ($126,509 – $330,490) | ||
| 6–10 years | $95,725 ($74,335 – $131,460) | 6–10 years | $173,742 ($123,300 – $279,067) | <0.001 | |
| 11–17 years | $90,410 ($70,097 – $121,878) | 11–17 years | $174,305 ($118,044 – $294,040) | ||
| 18–21 years | $99,731 ($77,744 – $139,856) | 18–21 years | $214,019 ($112,632 – $400,989) | ||
| Diagnosis | Diagnosis | ||||
| Congenital | $95,169 ($73,282 – $128,556) | 0.002 | Biliary atresia/hypoplasia | $192,426 ($129,059 – $325,567) | <0.001 |
| Glomerular | $98,903 ($74,760 – $140,371) | Metabolic disease | $189,855 ($117,044 – $321,241) | ||
| Tubular/interstitial | $93,775 ($71,482 – $131,303) | Acute hepatic failure | $185,289 ($130,172 – $289,713) | ||
| Familial/genetic | $91,877 ($72,867 – $124,881) | Malignancy | $172,335 ($117,337 – $266,757) | ||
| Other | $92,299 ($71,919 – $124,556) | Cirrhosis | $242,370 ($160,553 – $395,327) | ||
| Other | $240,205 ($135,816 – $680,646) | ||||
Costs inflated to 2018 U.S. dollars
p values from the Kruskal Wallis test
Abbreviations: CF – Cystic fibrosis; CHD – Congenital heart disease; CM – Cardiomyopathy; FBD – Functional bowel disorder; PHN – Pulmonary hypertension; ReTx – Retransplant
Discussion:
We report successful extension of the linkage between SRTR and PHIS databases to include pediatric liver, kidney, lung, heart-lung, and small bowel transplants. Prior studies utilizing indirect identifiers for data linkage have demonstrated that this technique results in a robust and accurate merger of datasets [4–10]. Similarly, our linkage algorithm was highly successful in uniquely identifying patients between databases. Additionally, our internal validation suggests that none of the linked patients were incorrectly matched.
The merger of these databases allows for expanded analyses in the pediatric solid organ transplant population and leverages the strengths of each database. The PHIS administrative database provides an invaluable tool to assess costs and resource utilization among tertiary children’s hospitals. In addition to this, data granularity is increased through the documentation of diagnosis and procedure ICD codes and APR-DRG coding. Given that PHIS only captures data from hospital encounters, long-term outcome data are lacking. The SRTR database provides additional transplant-specific data that are not available in PHIS. More importantly, SRTR is better suited to assess patient outcomes with respect to graft/patient survival and post-transplant complications, regardless of whether these events were associated with a hospital encounter.
Our group previously reported the linkage of pediatric heart transplant recipients between the PHIS and SRTR databases. This effort has allowed for novel analyses that would not be possible with either database in isolation. This includes an in-depth assessment of costs and resource utilization [12, 13, 15], assessment of practice variation, and the ability to identify and describe outcomes for subpopulations of interest [14, 16]. We anticipate that the extension of this linkage to include other pediatric solid organ transplants will similarly expand analytic possibilities.
This analysis demonstrates the advantages and potential utility of using this unique linked database to assess resource utilization. Solid organ transplantation represents a considerable expenditure, which varies based on the type of transplant. Data from Medicare demonstrate that kidney transplantation has the lowest cost compared to other organ transplants, with an average per person per year reimbursement of $75K for those with a functioning graft at 1-year [17]. This is followed by liver transplant ($154K per person per year), heart transplant ($272K per person per year), and small bowel transplant ($301K per person per year) [17]. However, these data are not readily generalizable to the pediatric population, which has greater heterogeneity and significant differences in pre- and post-operative care compared to the adult population. The linkage between PHIS and SRTR will allow for the most in-depth assessment of solid organ transplant costs from the largest pediatric cohort to date. The linked PHIS-SRTR database may also enable improved cost-effectiveness analyses by linking resource utilization with patient outcomes.
This project has inherent limitations. Patients who were listed but not transplanted are not included in the linkage, limiting the ability to perform analyses on this group. The date of transplant provides a discrete identifier that was utilized in our linkage algorithm. Patients who were listed but not transplanted may be able to be linked in the future, but a different linkage strategy will be necessary. There are some notable differences between patients who were successfully linked and those who were not. The etiology for and the impact of these differences remains unclear. However, the number of unlinked patients remains small and therefore are unlikely to significantly impact future analyses. While daily post-transplant cost has been assessed in previous studies and may be of interest in the current study, it is impractical to compare daily costs across transplant types as the daily cost is skewed by the cost of the transplant procedure so that those with shorter lengths of stay demonstrate the highest daily cost. Additionally, due to differences in billing practices it is difficult to reliably exclude the cost of transplant procedures from this calculation. Lastly, there may be missing and/or erroneous data in either database. While this represents a limitation of any large database, the PHIS-SRTR merger may help to minimize the amount of missing data and allow cross-verification across databases.
This project demonstrates the successful merger of the PHIS administrative database and SRTR database using indirect identifiers. This linkage will allow an in-depth assessment of resource utilization in pediatric solid organ transplantation from the largest reported U.S. cohort to date. This linkage also provides a platform for expanded analyses that would not be possible with either database in isolation.
Supplementary Material
Acknowledgments
Funding sources: This project was supported through internal funding from the Vanderbilt University Transplant Center.
Abbreviations:
- APR-DRG
All-Patient Refined Diagnosis Related Group
- CF
Cystic fibrosis
- CHD
Congenital heart disease
- CM
Cardiomyopathy
- FBD
Functional bowel disorder
- OPTN
Organ Procurement and Transplantation Network
- PHIS
Pediatric Health Information System
- PHN
Pulmonary hypertension
- ReTx
Retransplant
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Disclosures:
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
References:
- 1.Pasquali SK, Schumacher KR, and Davies RR, Can linking databases answer questions about paediatric heart failure? Cardiol Young, 2015. 25 Suppl 2: p. 160–6. [DOI] [PubMed] [Google Scholar]
- 2.Vener DF, et al. , Clinical Databases and Registries in Congenital and Pediatric Cardiac Surgery, Cardiology, Critical Care, and Anesthesiology Worldwide. World J Pediatr Congenit Heart Surg, 2017. 8(1): p. 77–87. [DOI] [PubMed] [Google Scholar]
- 3.Bennett TD, et al. , Existing data analysis in pediatric critical care research. Front Pediatr, 2014. 2: p. 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasquali SK, et al. , Linking clinical registry data with administrative data using indirect identifiers: implementation and validation in the congenital heart surgery population. Am Heart J, 2010. 160(6): p. 1099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs JP, et al. , Linking the congenital heart surgery databases of the Society of Thoracic Surgeons and the Congenital Heart Surgeons’ Society: part 1--rationale and methodology. World J Pediatr Congenit Heart Surg, 2014. 5(2): p. 256–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aplenc R, et al. , Merging of the National Cancer Institute-funded cooperative oncology group data with an administrative data source to develop a more effective platform for clinical trial analysis and comparative effectiveness research: a report from the Children’s Oncology Group. Pharmacoepidemiol Drug Saf, 2012. 21 Suppl 2: p. 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, et al. , Merging Children’s Oncology Group Data with an External Administrative Database Using Indirect Patient Identifiers: A Report from the Children’s Oncology Group. PLoS One, 2015. 10(11): p. e0143480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilmore AS, et al. , Linking the US transplant registry to administrative claims data: expanding the potential of transplant research. Med Care, 2007. 45(6): p. 529–36. [DOI] [PubMed] [Google Scholar]
- 9.Tovikkai C, et al. , Linkage of a national clinical liver transplant database with administrative hospital data: methods and validation. Transplantation, 2014. 98(3): p. 341–7. [DOI] [PubMed] [Google Scholar]
- 10.Godown J, et al. , A Unique Linkage of Administrative and Clinical Registry Databases to Expand Analytic Possibilities in Pediatric Heart Transplantation Research. Am Heart J, 2017. 194: p. 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Getz KD, et al. , Successful merging of data from the United Network for Organ Sharing and the Pediatric Health Information System databases. Pediatr Transplant, 2018. 22(5): p. e13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godown J, et al. , Mechanical circulatory support costs in children bridged to heart transplantation – analysis of a linked database. Am Heart J, 2018. 201: p. 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godown J, et al. , Changes in pediatric heart transplant hospitalization costs over time. Transplantation, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chew JD, et al. , Heart Transplantation in Children with Turner Syndrome: Analysis of a Linked Dataset. Pediatr Cardiol, 2018. 39(3): p. 610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godown J, et al. , Center Variation in Hospital Costs for Pediatric Heart Transplantation: The Relationship Between Cost and Outcomes. Pediatr Cardiol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duong SQ, et al. , Increased mortality, morbidities, and costs after heart transplantation in heterotaxy syndrome and other complex situs arrangements. J Thorac Cardiovasc Surg, 2019. 157(2): p. 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnitzler MA, et al. , OPTN/SRTR 2016 Annual Data Report: Economics. Am J Transplant, 2018. 18 Suppl 1: p. 464–503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.