Abstract
Extragonadal germ cell tumors (EGGCTs) are uncommon neoplasms, which arise in anatomical locations other than gonads. The pathogenesis of these neoplasms is still poorly understood and it is a matter of debate if they really represent extragondal primary neoplasms or rather extragondal metastasis from occult gonadal neoplasms. The actual observations suggest that EGGCTs represent a unique entity, so their biology and behavior are substantially different from gonadal counterparts. The diagnosis of EGGCTs is often challenging, and differential diagnosis is particularly wide. Nevertheless, a correct diagnosis is essential for the correct management of the patient. We summarize the state of art about EGGCTs, with particular emphasis on diagnosis and prognosis.
Keywords: choriocarcinoma, chromosome 12p, embryonal carcinoma, extragonadal germ cell tumors, SALL4, seminoma, SOX2, teratoma, yolk sac tumor
Extragonadal germ cell tumors (EGGCTs) are uncommon neoplasms with peculiar molecular and pathological features; the diagnosis of these neoplasms may be challenging, due to wide differential diagnosis depending to the variable anatomical location. The authors summarize the state of art about EGGCTs, with particular emphasis on diagnosis and prognosis.
1. INTRODUCTION
Extragonadal germ cell tumors (EGGCTs) are a heterogeneous group of tumors of neoplastic germ cells arising from extragonadal anatomical locations, without evidence of gonadal primary tumors. EGGCTs include seminomatous tumors, including only classical seminoma, and nonseminomatous tumors (NST), including embryonal carcinoma (EC), teratoma (mature or immature), yolk sac carcinoma (YST) and choriocarcinoma. EGGCTs constituted by two or more histotypes are referred to as Mixed Germ Cell Tumors. Although EGGCTs are morphologically identical to their gonadal counterparts, current knowledge about these neoplasms shows that they represent a unique entity and their biology is substantially different from their gonadal counterparts.
2. EPIDEMIOLOGY
EGGCTs are uncommon neoplasms. Stang et al. have recently reported epidemiologic data related to the incidence and survival of EGGCTs in the US from 1973 to 2007.1 According to this series, the overall incidence ranges from 1.8 to 3.4/1 million; females are less commonly affected than males and the highest incidence has been observed in white males (56.3/1 million).1 EGGCTs may localize in almost every structure along the midline of the body, from the brain to the coccyx. However, the most common anatomical locations are represented by the mediastinum, retroperitoneum, and brain,1 while the pineal gland and sacrococcygeal area are fewer common locations.2, 3 Isolated cases have been reported in bladder,4 prostate,5 paratesticular adnexa,6 vulva,7 placenta, pelvis, uterus,8 kidney,9 nasal sinuses10 and other sites. The anatomical distribution of EGGCT is summarized in Figure 1. Epidemiology of EGGCTs varies widely in age and gender groups of patients. Particularly, Teratoma is the most common EGGCT in prepuberal age regardless of the gender. It could be observed as pure or mixed tumor mainly associated to YST or, more rarely, to EC. In prepuberal patients, the most common anatomical sites include the sacrococcygeal area, intracranial, mediastinum, head and neck and peritoneum.11, 12 Sacrococcygeal teratoma is one of the most common congenital tumors, with a prevalence of 1/27 000 live births and a male‐to‐female ratio of 1:4.1 Mediastinal masses are rarely EGGCTs, representing up to 16% of all mediastinal neoplasms in adults and up to 19%‐25% in population younger than 18 years.13 It is well recognized that recurrences or metastasis from neonatal pure teratomas can acquire a different phenotype including mixed teratoma‐YST or even pure YST.14 These two different possibilities could be related to an inadequate sampling of the surgical sample, but also to a real “progression” of the neoplasm. It seems that in older patients, sacrococcygeal location and presence of histological immaturity are frequently associated with a higher incidence of mixed teratoma‐YST.11 In addition, metastases from a mixed EGGCT are frequently constituted by teratomatous component. The teratomatous metastasis is also observed sometimes even in case of pure EGGCT other than teratoma; in these clinical settings, an unrecognized mixed component or a posttherapy differentiation is supposed.15 In adults, the epidemiology of EGGCTs depends on sex. Teratoma is by far the most common histotype in females, representing up to 90% of all EGGCTs. Teratoma, seminoma, YST and mixed tumors are quite equally represented in males.16 Seminoma is extremely rare before puberty and particularly in children less than 10 years of age.17
Figure 1.
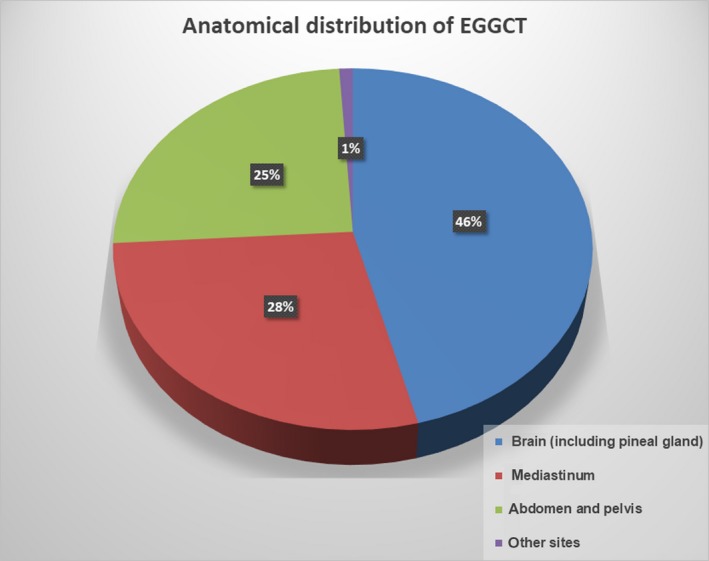
Anatomical distribution of extragonadal germ cell tumors (EGGCTs)
3. ETIOPATHOGENESIS
The exact etiopathogenesis of EGGCTs is not clearly demonstrated. Indeed, we still refer to the classical hypothesis of the “embryonic” origin of such neoplasms from germ cell precursors erroneously arrested in midline migration during the embryogenesis.18 Primordial GC originating from the proximal epiblast normally migrate to the genital ridge following the body midline. Particularly the thymus could be a preferential site for primordial GC arrest, because of high expression of KIT ligands, notoriously involved in primordial GC proliferation.19 Although this theory is widely accepted, some Authors proposed alternative etiopathogenetic explanations. McKenney et al. argued that EGGCTs could represent metastases developed from an undiagnosed or regressed (“burned out”) primary GC tumors in the gonads.20 This theory seems to be corroborated by some observations. Indeed, the possibility of various degrees of regression in cases of gonadal GCTs has been well documented. Although this usually presents as focal areas occupied by fibrous scars at the periphery of the neoplasm, it can be diffuse and affect the whole neoplasm.21 Furthermore, survival rates are lower in EGGCTs than in primary gonadal GCTs, as expected by metastatic disease.
Genetic studies recently allowed a better understanding of the etiopathogenesis of EGGCTs. Aneuplody and Chromosome 12 abnormalities are the most frequent genetic aberrations observed in postpuberal GCTs. In particular, Chromosome 12 abnormalities, most often resulting in 12p overexpression, have been demonstrated in 96% of mediastinal seminoma.22 Limited data are actually known about the genetic alteration of non‐seminomatous GCTs. An increased number of chromosome 12p copies, mainly as isochromosome i(12p), have been most commonly described.23 i(12p) is considered highly specific for the germ cell origin of postpuberal neoplasm, but not in prepuberal gonadal neoplasm.24, 25 While the genetic alterations of gonadal GCTs have been better described, 12p status of EGGCTs are yet to be adequately studied. Recently Gurda et al. described the absence of chromosome 12p alterations, including i(12p), in 11 prepubertal and five postpubertal mature sacrococcygeal teratomas and immature prepubertal sacrococcygeal teratomas.26 These data suggest that i(12p) could be less important in the pathogenesis of EGGCTs when compared with postpuberal gonadal counterparts, underlining a higher proximity to prepuberal gonadal neoplasms. Some genetic syndromes present increased incidence of EGGCTs, including Klinefelter, Down and Li‐Fraumeni syndromes.27 The recurrent amplification of specific chromosome arms, reciprocal deletions and K‐RAS mutations seem to be the most common primary somatic features of GCTs.28, 29 A recent study demonstrated genomic amplification of SOX2, supporting the theory that SOX2 has a relevant oncogenic role in the etiopathogenesis of EC.30
4. HISTOPATHOLOGY
Morphological and immunohistochemical features of EGGCTs are the same as observed in the gonadal counterparts. Solid somatic malignancies can develop in the context of an EGGCT, mainly in pure teratoma or mixed EGGCT in adult males.31 Sarcomas (embryonal rhabdomyosarcoma, leiomyosarcoma, angiosarcoma and neuroblastoma) and adenocarcinoma are the most commonly reported neoplasms in this clinic‐pathological setting.31 The histological features of EGGCTs are shown in Figures 2 and 3.
Figure 2.
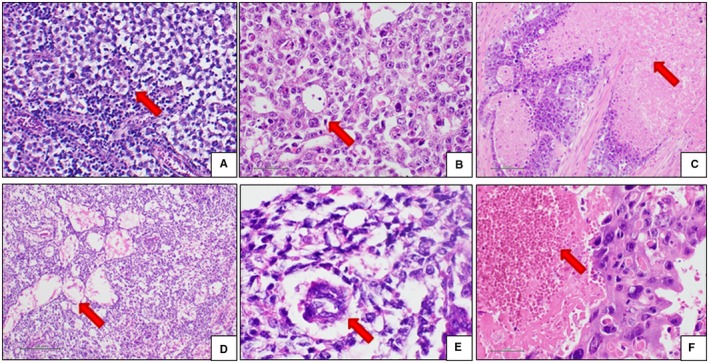
Histological features of EGGCTs. A, Seminoma. The neoplastic cells are arranged in solid lobules (red arrow) separated by thin fibrous septa containing lymphocytes. The cells are large‐sized, with abundant clear cytoplasm, roundish and relatively regular nucleus and prominent nucleolus. B, EC. The neoplastic cells are quite similar to seminoma cells, but the microscopic appearance is more variable. In this case, the architectural pattern is glandular (red arrow) and solid, and the neoplastic cells are more pleomorphic and atypical. C, EC. Coagulative necrosis (red arrow) is a diagnostic clue of EC. Attention must be paid to differentiating real coagulative necrosis from ischemic necrosis, a possible event in large or traumatized seminomas. D, YST. Relatively bland neoplastic cells arranged in the typical cystic architectural pattern (red arrow). E, YST. Schiller‐Duval bodies (red arrow) are a diagnostic clue, but they are present in about 50% of cases. F, Choriocarcinoma. The neoplastic cells are very large in size, with abundant slightly eosinophilic cytoplasm and atypical nuclei. Large hemorrhages are typically seen (red arrow). EC, embryonal carcinoma; EGGCTs, extragonadal germ cell tumors; YST, yolk sac tumor
Figure 3.
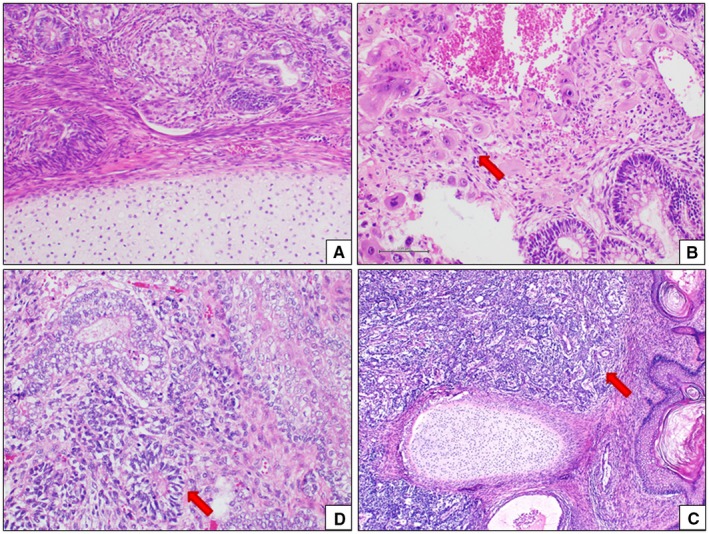
Histological features of mixed EGGCTs. Teratoma is characterizedby the coexistence in the same neoplasm of different mature or immature tissues (A). Mixed EGGCT often include a teratoma component (B: teratoma and chociocarcinoma (red arrow); (C) teratoma and EC (red arrow); (D) teratoma and YST (red arrow). EC, embryonal carcinoma; EGGCTs, extragonadal germ cell tumors; YST, yolk sac tumor
4.1. Seminoma
Macroscopically, seminoma is an expansive, lobulated mass with a tan‐gray cut surface. The mass ranges from 1 to 20 centimeters.32 Histologically, seminoma shows a lobular architecture with neoplastic nodules delimitated by incomplete thin fibrous septa. The neoplastic cells are large, with clear to lightly eosinophilic cytoplasm and regular nucleus with a prominent nucleolus. A lymphoid infiltrate constituted by small mature lymphocytes is commonly present, mostly in the thickness of the fibrous septa. A granulomatous infiltrate with multinucleated giant cells and syncytiotrophoblastic cells can be present.
4.2. EC
Macroscopically, EC is often a large mass when diagnosed, with infiltration features of the surrounding tissues. The cut surface generally shows hemorrhagic areas and necrosis. The histological architecture is variable and more often solid, but “epithelial” arrangements are possible, including glandular and papillary structures. The cells are large‐sized, with large atypical roundish nucleolated nuclei. Cytoplasmic features are quite variable including amphophilic, basophilic, eosinophilic or clear appearances. Coagulative necrosis, “epithelial” arrangements and nuclear atypia are the most important morphological features useful for differential diagnosis with seminoma.
4.3. YST
Macroscopically, YST is a large mass with whitish cut surface, frequently characterized by hemorrhagic, mixoid and necrotic areas. Histologically, several possible architectural patterns are possible, including reticular/microcystic, psudopapillary/endodermal sinus, myxomatous, hepatoid, and glandular and solid patterns. The reticular or microcystic pattern is more frequently observed and is characterized by irregular channels circumscribing small cystic spaces organized in a loose network. In about 50% of YSTs, Schiller‐Duval bodies are found. The neoplastic cells are medium to large‐sized, with evident cytoplasm, roundish nucleus and prominent nucleolus. Also in YST, some scattered syncytiotrophoblastic cells could be observed.
4.4. Teratoma
Macroscopically, a teratoma usually appears as a well‐demarcated and encapsulated mass with a variegated cut surface, which comprises soft or fleshy areas, cystic areas containing keratinaceous debris or hair shaft, mucoid or serous material. Teeth or bone can be present. Histologically, a teratoma is composed of several somatic tissues, with various degrees of maturation, arranged in a disorganized distribution. A teratoma is distinct in mature and immature forms, depending on the resemblance to adult or fetal tissues. In immature teratoma, neuroectodermal tissue constituting tubules and rosettes is the most common fetal‐appearing tissue observed. Rarely other fetal tissues including primitive mesenchymal tissue, cartilage, bone, rhabdomyoblasts are observed.
4.5. Choriocarcinoma
Macroscopically, choriocarcinoma is an extensively hemorrhagic mass, infiltrating the adjacent structures at the time of the diagnosis. Histologically, choriocarcinoma is composed by syncytiotrophoblastic and cytotrophoblastic cells. The former are giant multinucleated cells. The latter are mononuclear cells with eosinophilic cytoplasm, roundish nuclei and prominent nucleoli. Large hemorrhagic areas, dense vascularity and prominent cellular atypia are distinctive features.
5. CYTOPATHOLOGY
The diagnosis is often performed on an incisional bioptic or Fine Needle Aspiration Cytology (FNAC) sample. US‐ or CT‐guided FNAC may be a valid diagnostic alternative to biopsy for an accurate, rapid and reliable diagnosis of EGGCT. In particular, differentiation of EGGCTs in seminoma and nonseminoma tumors is possible also on cytological samples. FNAC smears with a homomorphous and dissociated cell population and few loose small clusters characterize seminoma. The cells are mononucleated and show large vesicular nuclei with frequent membrane irregularities. The nuclei show irregularly distributed chromatin. Chromatin clumping, parachromatin clearing and prominent, sometimes multiple nucleoli are present. The cytoplasm is often abundant, but may be scant or moderate and poorly defined, unless stripped. A characteristic tigroid (stripped) background is typically present on May‐Grümwald‐Giemsa stained smears. Mitotic figures may be observed. Lymphocytes, plasma cells and giant cells are observed in the background. Cytological features of seminoma are shown in Figure 4. Thymoma and large cell lymphoma represent the principal diagnosis differential in mediastinal EGGCT. FNAC smears of nonseminoma tumors show heterogeneous morphological aspects. Cohesive clusters or acinar structures constituted of cells with vacuolated cytoplasm and large nuclei are observed in YST. Extracellular matrix and hyaline globules may be also observed. Choriocarcinoma is characterized by giant multinucleated tumor cells. Numerous anaplastic isolated cells or hyperchromatic nuclei with nucleoli and scant, poorly defined basophilic cytoplasm arranged in glandular or papillary structures are observed in EC. Mitotic figures are frequent. Necrosis and hemorrhage may be observed on cytological smears. The diagnosis of teratoma could be suspected when squamous cells, columnar cells and mesenchymal portions are observed. Immature forms may be more difficult to diagnose on a cytological sample as well as mixed forms. Metastatic neoplasms of the lung or gastroenteric tract and melanoma represent their differential diagnosis.33, 34, 35
Figure 4.
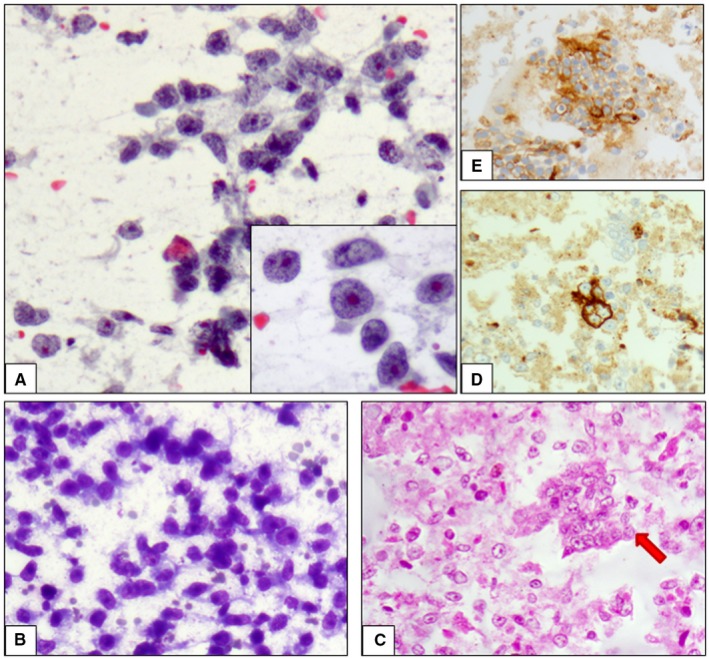
Cytological features of seminoma. A, Papanicolaou‐stained smears of seminoma are characterized by a dissociated cell population and few loose small clusters. The cells show large vesicular nuclei and prominent nucleoli and chromatin clumping (Inset). B, May‐Grümwald‐Giemsa stained smears show the characteristic tigroid (stripped) background. C, The realization of a cell block is needful and allows the evaluation of architectural disposition of neoplastic cells and the realization of immunohistochemical tests. In this case, neoplastic cells are organized in single elements and small solid fragments (red arrow), and show positivity for CD117 (D) and PLAP (E)
6. IMMUNOHISTOCHEMISTRY
Immunohistochemistry (IHC) is very helpful in order to establish the diagnosis of different components occurring in EGGCT. The most useful IHC markers are listed in Table 1. Seminoma expresses several factors of pluripotency regulation also expressed by normal pluripotent germ cell tumors and normal gonocytes, such as PLAP, POU5F1(OCT4), NANOG, SOX2, REX1, UTF1, KIT (CD117) or LIN28.36, 37, 38, 39, 40, 41, 42, 43, 44, 45 Some of these embryonic factors, like NANOG and oct4, are also expressed by EC; however, CD30 and SOX2 are more specific markers for diagnosis of EC, allowing differential diagnosis with seminoma.46YST reproduces the immunophenotype of human yolk sac and early endoderm, and consequently expresses alpha fetoprotein (αFP), Glypican‐3, Villin, SALL4 and LIN28.47 However, the most informative markers for YST commonly used in clinical practice are αFP, Glypican‐3 and SALL4.48 ZBTB16 (Zinc finger and BTB domain‐containing protein 16) is a factor involved in cell cycle progression, recently proposed as possible diagnostic marker for YST. Indeed ZBTB16 is highly sensitive and specific for YST, as it is expressed in 91.6% of extragonadal and metastatic YST.49 Cytokeratins are expressed by nonseminomatous GCTs and may have a diagnostic role in differentiating seminoma from nonseminomatous mimickers like EC and YSTs. Nevertheless, a dot‐like positivity of low molecular weight cytokeratin has been demonstrated in almost 80% of mediastinal seminomas.50 beta human chorionic gonadotropin (β‐hCG) is the most sensitive marker for choriocarcinoma, which also expresses Glypican‐3 and SALL4.51 The diagnosis of teratoma generally does not require IHC, but it could be needed in the case of suspect immature component. Indeed S100 and synaptophysin are observed in neural components, whilst desmin and myogenin in muscle components and S100 and in cartilagineous components.
Table 1.
Immunohistochemical features of EGGCTs
| Histotype | CK | αFP | βhCG | CD117 | PLAP | CD30 | OCT4 | SALL4 | Glypican 3 | NANOG | LIN28 | SOX2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SE | Nega | Neg | Negb | Pos | Pos | Neg | Pos | Pos | Neg | Pos | Pos | Neg |
| EC | Pos | Neg/Pos | Negb | Neg | Neg | Pos | Pos | Pos | Neg/Pos | Pos | Pos/Neg | Pos |
| YSC | Pos | Pos | Negb | Neg | Neg/Pos | Neg/Pos | Neg | Pos | Pos | Neg | Neg | Neg |
| PT | Pos | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| CHC | Pos | Neg | Pos | Neg | Neg | Neg | Neg | Posc | Pos | Neg | Neg | Neg/Pos |
Abbreviations: αFP, alpha fetoprotein; β‐hCG, beta human chorionic gonadotropin; CHC, choriocarcinoma; EC, embryonal carcinoma; PT, pure teratoma; SE, seminoma; YSC, yolk sac tumor.
Dot‐like positivity can be observed.
Syncytiotrophoblastic cells are positive, if present.
Positive in mononuclear trophoblastic cells.
7. SERUM MARKERS
The diagnostic process of GCTs requires the measure of specific biochemical serum tumor markers (STMs). STMs results frequently elevated in EGGCTs and are helpful for both diagnosis and follow‐up. The most commonly increased STMs in EGGCT patients include αFP, β‐hCG and lactate dehydrogenase (LDH). Particularly, αFP is frequently increased in nonseminomatous EGGCTs (proportionally higher according to the disease stage), while it is never increased in pure seminoma patients.52 Serum αFP is almost always increased in pure YST or containing YST‐mixed GCT patients. Serum β‐hCG may be increased in pure seminoma,and nonseminomatous GCTs, in both cases more frequently in advanced disease.52 The presence of multinuclear trophoblastic‐like giant cells in a seminoma is related to β‐hCG production.53 Serum LDH, the less specific STM, increases in 40%‐60% of GCTs patients regardless of the histological type. Serum LDH may reach higher levels when a relevant gain of chromosome 12p is present, being LDHB gene located on 12p.54 STMs are recommended by the International Germ Cell Cancer Collaborative Group system to monitor the treatment and stratify the risk of nonseminomatous GSTs patients but not for seminoma patients.55 The interpretation of these “classical” serum markers is often difficult, because several different clinical conditions could affect their serum increase. Most recently, some micro‐RNA profiles (miRNAs) as in the embryonic stem cells have been linked to GCTs. These miRNAs types seem to be highly upregulated and detectable in the serum of GCT patients, regardless of age and sex of patients.56 Preliminary studies concerning the detection of miR‐371‐3 cluster, miR‐302 and miR‐367, showed great specificity (99%‐100%) and sensitivity in GCTs other than pure teratoma.57, 58, 59, 60 Furthermore, miRNAs have also been detected in pleural effusions and cerebrospinal fluid, in patients with GCTs.60, 61 Although the diagnostic utility of miRNAs looks very promising, these preliminary studies considered only testicular GCTs and further exploration is mandatory.
8. PROGNOSIS
Prognosis is strictly related to the patient age, histological type, and anatomic location. The latter affects the prognosis for the direct effects of the tumor on vital organs in such site.11 Although most neonatal and congenital extragonadal teratomas are benign, about 10% of congenital sacrococcygeal teratomas have a malignant behavior.62, 63 Congenital and neonatal extragonadal teratomas are usually immature and a YST component is often associated. Although the presence of admixed YST has unclear clinical significance, neonatal mixed teratomas seem to have a poor prognosis.64 In congenital and neonatal extragonadal teratomas the presence of immature neuroepithelium is not predictive per se of malignant behavior, but immature teratomas are more likely associated to admixed YST component.20 Other factors associated to the presence of admixed YST include relatively older patients and sacrococcygeal location.11 Hemorrhage is the most common lethal complication in neonatal patients with sacrococcygeal teratomas.65 Increasing age negatively affects the clinical behavior and the prognosis of completely resected EGGCTs, worsening at approximately 7 months.20 Histotype is the most important prognostic factor in adult EGGCTs, being seminomas long‐time survival rate of about 90% and nonseminomatous of about 45%.31 The presence of immature neuroepithelium in adult extragonadal teratomas has an important prognostic role.66, 67, 68, 69 Histologically, mature teratomas have a benign behavior regardless of patient's age; immature teratomas behave as aggressive tumors in adults. In EGGCTs adult patients, clinical and pathological staging, primary location in the mediastinum, increased serum β‐hCG and nonpulmonary visceral metastases are further independent prognostic factors related to shorter survival.31
9. CLINICAL MANAGEMENT
In the past, complete surgical resection of the tumoral masses was the standard therapy for EGGCTs patients. But EGGCTs, particularly mediastinal EGGCTs and EGGCTs including NST components, related with a poorer prognosis when treated with surgical resection alone. Recently, the introduction of cisplatin‐based chemotherapy in these clinical settings as in gonadal counterpart has dramatically improved the prognosis.70, 71, 72
Nonetheless, in several EGGCT patients a persistent residual mass after cisplatin‐based chemotherapy and conventional selvage chemotherapy could be observed. Thus, the residual mass resection after chemotherapy is necessary in order to obtain radical control of EGGCt morbidity. The presence of residual tumor is often due to the unresponsiveness of teratoma components in EGGCTs mass.15 Therefore, this multidimensional approach is needed to assess response, to remove residual disease and eventually to turn to additional chemotherapy.73
In the case of localized EGGCTs, patients can really benefit from radical surgery and subsequent chemiotherapy. Indeed, surgical excision demonstrated advantages in order of disease free‐survival, when complete excision can be performed.74
10. CONCLUSION
In conclusion, EGGCTs represent a rare group of neoplasms, occurring more commonly in the mediastinum and retroperitoneum. The EGGCTs are histologically constituted by the same components observed in the gonadal counterparts, but they are characterized by different biologic behaviors, clinical features and poorer prognoses, mainly when constituted by nonsemonomatous components. The efficacy of multidimensional therapy, frequently practised in mediastinal EGGCTs, depends on both successful chemotherapy and surgery. Currently new therapeutic strategies are being studied to improve the prognosis, in order to obtain a longer overall survival, similar to that observed in the gonadal germ cell tumors.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by grant “Progetto ONCONET 2.0—Linea progettuale 14 per l'implementazione della prevenzione e diagnosi precoce del tumore alla prostata e testicolo—Regione Campania, Italy”
Ronchi A, Cozzolino I, Montella M, et al. Extragonadal germ cell tumors: Not just a matter of location. A review about clinical, molecular and pathological features. Cancer Med. 2019;8:6832–6840. 10.1002/cam4.2195
REFERENCES
- 1. Stang A, Trabert B, Wentzensen N, et al. Gonadal and extragonadal germ cell tumours in the United States, 1973‐2007. Int J Androl. 2012;35:616‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paradies G, Zullino F, Orofino A, Leggio S. Unusual presentation of sacrococcygeal teratomas and associated malformations in children: clinical experience and review of the literature. Ann Ital Chir. 2013;84:333‐346. [PubMed] [Google Scholar]
- 3. Arora RS, Alston RD, Eden TO, Geraci M, Birch JM. Comparative incidence patterns and trends of gonadal and extragonadal germ cell tumors in England, 1979 to 2003. Cancer. 2012;118:4290‐4297. [DOI] [PubMed] [Google Scholar]
- 4. Hanna NH, Ulbright TM, Einhorn LH. Primary choriocarcinoma of the bladder with the detection of isochromosome 12p. J Urol. 2002;167:1781. [PubMed] [Google Scholar]
- 5. Kleinhans B, Kalem T, Hendricks D, Arps H, Kaelble T. Extragonadal germ cell tumor of the prostate. J Urol. 2001;166:611‐612. [PubMed] [Google Scholar]
- 6. Yao XD, Hong YP, Ye DW, Wang CF. Primary yolk sac tumor of seminal vesicle: a case report and literature review. World J Surg Oncol. 2012;10:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Euscher ED. Unusual presentations of gynecologic tumors: extragonadal yolk sac tumor of the vulva. Arch Pathol Lab Med. 2017;141:293‐297. [DOI] [PubMed] [Google Scholar]
- 8. Stolnicu S, Szekely E, Molnar C, et al. Mature and immature solid teratomas involving uterine corpus, cervix, and ovary. Int J Gynecol Pathol. 2017;36:222‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar Y, Bhatia A, Kumar V, Vaiphei K. Intrarenal pure yolk sac tumor: an extremely rare entity. Int J Surg Pathol. 2007;15:204‐206. [DOI] [PubMed] [Google Scholar]
- 10. Chuang HC, Kang CJ, Lee LY. Sinonasal pure yolk sac tumor: a case report and literature review. Fetal Pediatr Pathol. 2014;33:127‐134. [DOI] [PubMed] [Google Scholar]
- 11. Heerema‐McKenney A, Harrison MR, Bratton B, Farrell J, Zaloudek C. Congenital teratoma: a clinicopathologic study of 22 fetal and neonatal tumors. Am J Surg Pathol. 2005;29:29‐38. [DOI] [PubMed] [Google Scholar]
- 12. Harms D, Schmidt D, Leuschner I. Abdominal, retroperitoneal and sacrococcygeal tumours of the newborn and the very young infant. Report from the Kiel PaediatricTumour Registry. Eur J Pediatr. 1989;148:720‐728. [DOI] [PubMed] [Google Scholar]
- 13. Takeda S, Miyoshi S, Ohta M, Minami M, Masaoka A, Matsuda H. Primary germ cell tumors in the mediastinum: a 50‐year experience at a single Japanese institution. Cancer. 2003;97:367‐376. [DOI] [PubMed] [Google Scholar]
- 14. Hawkins E, Issacs H, Cushing B, Rogers P. Occult malignancy in neonatal sacrococcygeal teratomas. A report from a Combined Pediatric Oncology Group and Children's Cancer Group study. Am J Pediatr Hematol Oncol. 1993;15:406‐409. [PubMed] [Google Scholar]
- 15. Reuter VE. The pre and post chemotherapy pathologic spectrum of germ cell tumors. Chest Surg Clin N Am. 2002;12:673‐694. [DOI] [PubMed] [Google Scholar]
- 16. Marchevsky AM, Wick MR. Pathology of the Mediastinum. Cambridge: Cambridge University Press; 2014. [Google Scholar]
- 17. Schneider DT, Calaminus G, Koch S, et al. Epidemiologic analysis of 1,442 children and adolescents registered in the German germ cell tumor protocols. Pediatr Blood Cancer. 2004;42:169‐175. [DOI] [PubMed] [Google Scholar]
- 18. Willis RA. The borderland of embryology and pathology. Bull N Y Acad Med. 1950;26:440‐460. [PMC free article] [PubMed] [Google Scholar]
- 19. Oosterhuis JW, Stoop H, Honecker F, Looijenga LH. Why human extragonadal germ cell tumours occur in the midline of the body: old concepts, new perspectives. Int J Androl. 2007;30:256‐263. [DOI] [PubMed] [Google Scholar]
- 20. McKenney JK, Heerema‐McKenney A, Rouse RV. Extragonadal germ cell tumors: a review with emphasis on pathologic features, clinical prognostic variables, and differential diagnostic considerations. Adv Anat Pathol. 2007;14:69‐92. [DOI] [PubMed] [Google Scholar]
- 21. Azzopardi JG, Mostofi FK, Theiss EA. Lesions of testes observed in certain patients with widespread choriocarcinoma and related tumors. The significance and genesis of hematoxylin‐staining bodies in the human testis. Am J Pathol. 1961;38:207‐225. [PMC free article] [PubMed] [Google Scholar]
- 22. Sung MT, Maclennan GT, Lopez‐Beltran A, Zhang S, Montironi R, Cheng L. Primary mediastinal seminoma: a comprehensive assessment integrated with histology, immunohistochemistry, and fluorescence in situ hybridization for chromosome 12p abnormalities in 23 cases. Am J Surg Pathol. 2008;32:146‐155. [DOI] [PubMed] [Google Scholar]
- 23. Taylor MD, Jones DR. Genetic markers of mediastinal tumors. Thorac Surg Clin. 2009;19:17‐27. [DOI] [PubMed] [Google Scholar]
- 24. Looijenga LH, Zafarana G, Grygalewicz B, et al. Role of gain of 12p in germ cell tumour development. APMIS. 2003;111:161‐171. [DOI] [PubMed] [Google Scholar]
- 25. Cheng L, Zhang S, MacLennan GT, et al. Interphase fluorescence in situ hybridization analysis of chromosome 12p abnormalities is useful for distinguishing epidermoid cysts of the testis from pure mature teratoma. Clin Cancer Res. 2006;12:5668‐5672. [DOI] [PubMed] [Google Scholar]
- 26. Gurda GT, VandenBussche CJ, Yonescu R, et al. Sacrococcygeal teratomas: clinico‐pathological characteristics and isochromosome 12p status. Mod Pathol. 2014;27:562‐568. [DOI] [PubMed] [Google Scholar]
- 27. Hasle H, Mellemgaard A, Nielsen J, Hansen J. Cancer incidence in men with Klinefelter syndrome. Br J Cancer. 1995;71:416‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taylor‐Weiner A, Zack T, O'Donnell E, et al. Genomic evolution and chemoresistance in germ‐cell tumours. Nature. 2016;540:114‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hartley AL, Birch JM, Kelsey AM, Marsden HB, Harris M, Teare MD. Are germ cell tumors part of the Li‐Fraumeni cancer family syndrome? Cancer Genet Cytogenet. 1989;42:221‐226. [DOI] [PubMed] [Google Scholar]
- 30. Eini R, Stoop H, Gillis AJ, Biermann K, Dorssers LC, Looijenga LH. Role of SOX2 in the etiology of embryonal carcinoma, based on analysis of the NCCIT and NT2 cell lines. PLoS ONE. 2014;9:e83585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bokemeyer C, Nichols CR, Droz JP, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J ClinOncol. 2002;20:1864‐1873. [DOI] [PubMed] [Google Scholar]
- 32. Bokemeyer C, Droz JP, Horwich A, et al. Extragonadal seminoma: an international multicenter analysis of prognostic factors and long term treatment outcome. Cancer. 2001;91:1394‐1401. [PubMed] [Google Scholar]
- 33. Gupta R, Mathur SR, Arora VK, Sharma SG. Cytologic features of extragonadal germ cell tumors: a study of 88 cases with aspiration cytology. Cancer. 2008;114:504‐511. [DOI] [PubMed] [Google Scholar]
- 34. Chao TY, Nieh S, Huang SH, Lee WH. Cytology of fine needle aspirates of primary extragonadal germ cell tumors. Acta Cytol. 1997;41:497‐503. [DOI] [PubMed] [Google Scholar]
- 35. Collins KA, Geisinger KR, Wakely PE Jr, Olympio G, Silverman JF. Extragonadal germ cell tumors: a fine‐needle aspiration biopsy study. Diagn Cytopathol. 1995;12:223‐229. [DOI] [PubMed] [Google Scholar]
- 36. Skotheim RI, Monni O, Mousses S, et al. New insights into testicular germ cell tumorigenesis from gene expression profiling. Cancer Res. 2002;62:2359‐2364. [PubMed] [Google Scholar]
- 37. Looijenga LH, Stoop H, de Leeuw HP, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63:2244‐2250. [PubMed] [Google Scholar]
- 38. Sperger JM, Chen X, Draper JS, et al. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci U S A. 2003;100:13350‐13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Almstrup K, Hoei‐Hansen CE, Wirkner U, et al. Embryonic stem cell‐like features of testicular carcinoma in situ revealed by genome‐wide gene expression profiling. Cancer Res. 2004;64:4736‐4743. [DOI] [PubMed] [Google Scholar]
- 40. Juric D, Sale S, Hromas RA, et al. Gene expression profiling differentiates germ cell tumors from other cancers and defines subtype‐specific signatures. Proc Natl Acad Sci U S A. 2005;102:17763‐17768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Korkola JE, Houldsworth J, Chadalavada RS, et al. Down‐regulation of stem cell genes, including those in a 200‐kb gene cluster at 12p13.31, is associated with in vivo differentiation of human male germ cell tumors. Cancer Res. 2006;66:820‐827. [DOI] [PubMed] [Google Scholar]
- 42. Biermann K, Heukamp LC, Steger K, et al. Genome‐wide expression profiling reveals new insights into pathogenesis and progression of testicular germ cell tumors. Cancer Genomics Proteomics. 2007;4:359‐367. [PubMed] [Google Scholar]
- 43. Gashaw I, Dushaj O, Behr R, et al. Novel germ cell markers characterize testicular seminoma and fetal testis. Mol Hum Reprod. 2007;13:721‐727. [DOI] [PubMed] [Google Scholar]
- 44. Kristensen DM, Nielsen JE, Skakkebæk NE, et al. Presumed pluripotency markers UTF‐1 and REX‐1 are expressed in human adult testes and germ cell neoplasms. Hum Reprod. 2008;23:775‐782. [DOI] [PubMed] [Google Scholar]
- 45. West JA, Viswanathan SR, Yabuuchi A, et al. A role for Lin28 in primordial germ‐cell development and germ‐cell malignancy. Nature. 2009;460:909‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leroy X, Augusto D, Leteurtre E, Gosselin B. CD30 and CD117 (c‐kit) used in combination are useful for distinguishing embryonal carcinoma from seminoma. J Histochem Cytochem. 2002;50:283‐285. [DOI] [PubMed] [Google Scholar]
- 47. Nogales FF, Quiñonez E, López‐Marín L, Dulcey I, Preda O. A diagnostic immunohistochemical panel for yolk sac (primitive endodermal) tumours based on an immunohistochemical comparison with the human yolk sac. Histopathology. 2014;65:51‐59. [DOI] [PubMed] [Google Scholar]
- 48. Wang F, Liu A, Peng Y, et al. Diagnostic utility of SALL4 in extragonadal yolk sac tumors: an immunohistochemical study of 59 cases with comparison to placental‐like alkaline phosphatase, alpha‐fetoprotein, and glypican‐3. Am J Surg Pathol. 2009;33:1529‐1539. [DOI] [PubMed] [Google Scholar]
- 49. Xiao GQ, Priemer DS, Wei C, Aron M, Yang Q, Idrees MT. ZBTB16 is a sensitive and specific marker in detection of metastatic and extragonadal yolk sac tumour. Histopathology. 2017;71:562‐569. [DOI] [PubMed] [Google Scholar]
- 50. Moran CA, Suster S, Przygodzki RM, Koss MN. Primary germ cell tumors of the mediastinum: II. Mediastinal seminomas–a clinicopathologic and immunohistochemical study of 120 cases. Cancer. 1997;80:691‐698. [DOI] [PubMed] [Google Scholar]
- 51. Alvarado‐Cabrero I, Hernández‐Toriz N, Paner GP. Clinicopathologic analysis of choriocarcinoma as a pure or predominant component of germ cell tumor of the testis. Am J Surg Pathol. 2014;38:111‐118. [DOI] [PubMed] [Google Scholar]
- 52. Gilligan TD, Seidenfeld J, Basch EM, et al. American Society of Clinical Oncology clinical practice guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28:3388‐3404. [DOI] [PubMed] [Google Scholar]
- 53. Rajpert‐De Meyts E, Nielsen JE, Skakkebaek NE, Almstrup K. Diagnostic markers for germ cell neoplasms: from placental‐like alkaline phosphatase to micro‐RNAs. Folia Histochem Cytobiol. 2015;53:177‐188. [DOI] [PubMed] [Google Scholar]
- 54. von Eyben FE, de Graaff WE, Marrink J, et al. Serum lactate dehydrogenase isoenzyme 1 activity in patients with testicular germ cell tumors correlates with the total number of copies of the short arm of chromosome 12 in the tumor. Mol Gen Genet. 1992;235:140‐146. [DOI] [PubMed] [Google Scholar]
- 55. International Germ Cell Cancer Collaborative Group . International germ cell consensus classification: a prognostic factor‐based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;28:207‐214. [DOI] [PubMed] [Google Scholar]
- 56. Rijlaarsdam MA, van Agthoven T, Gillis AJ, et al. Identification of known and novel germ cell cancer‐specific (embryonic) miRs in serum by high‐throughput profiling. Andrology. 2015;3:85‐91. [DOI] [PubMed] [Google Scholar]
- 57. Dieckmann KP, Spiekermann M, Balks T, et al. MicroRNAs miR‐371‐3 in serum as diagnostic tools in the management of testicular germ cell tumours. Br J Cancer. 2012;107:1754‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gillis AJ, Rijlaarsdam MA, Eini R, et al. Targeted serum miRNA (TSmiR) test for diagnosis and follow‐up of (testicular) germ cell cancer patients: a proof of principle. Mol Oncol. 2013;7:108‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Syring I, Bartels J, Holdenrieder S, Kristiansen G, Müller SC, Ellinger J. Circulating serum miRNA (miR‐367‐3p, miR‐371a ‐3p, miR‐372‐3p and miR‐373‐3p) as biomarkers in patients with testicular germ cell cancer. J Urol. 2015;193:331‐337. [DOI] [PubMed] [Google Scholar]
- 60. Spiekermann M, Belge G, Winter N, et al. MicroRNA miR‐371a‐3p in serum of patients with germ cell tumours: evaluations for establishing a serum biomarker. Andrology. 2015;3:78‐84. [DOI] [PubMed] [Google Scholar]
- 61. Murray MJ, Bell E, Raby KL, et al. A pipeline to quantify serum and cerebrospinal fluid microRNAs for diagnosis and detection of relapse in paediatric malignant germ‐cell tumours. Br J Cancer. 2016;114:151‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lakhoo K. Neonatal teratomas. Early Hum Dev. 2010;86:643‐647. [DOI] [PubMed] [Google Scholar]
- 63. Swamy R, Embleton N, Hale J. Sacrococcygeal teratoma over two decades: birth prevalence, prenatal diagnosis and clinical outcomes. Prenat Diagn. 2008;28:1048‐1051. [DOI] [PubMed] [Google Scholar]
- 64. Gonzalez‐Crussi F, Winkler RF, Mirkin DL. Sacrococcygeal teratomas in infants and children: relationship of histology and prognosis in 40 cases. Arch Pathol Lab Med. 1978;102:420‐425. [PubMed] [Google Scholar]
- 65. Kremer ME, Wellens LM, Derikx JP, et al. Hemorrhage is the most common cause of neonatal mortality in patients with sacrococcygeal teratoma. J Pediatr Surg. 2016;51:1826‐1829. [DOI] [PubMed] [Google Scholar]
- 66. Moran CA, Suster S. Primary germ cell tumors of the mediastinum: I. Analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer. 1997;80:681‐690. [PubMed] [Google Scholar]
- 67. Chieffi P. Molecular targets for the treatment of testicular germ cell tumors. Mini‐Rev Med Chem. 2007;7:755‐759. [DOI] [PubMed] [Google Scholar]
- 68. Chieffi P, Franco R, Portella G. Molecular and cell biology of testicular germ cell tumors. Int Rev Cell Mol Biol. 2009;278:277‐308. [DOI] [PubMed] [Google Scholar]
- 69. Chieffi P, Chieffi S, Franco R, Sinisi A. Recent advances in the biology of germ cell tumors: implications for the diagnosis and treatment. J Endocrinol Inv. 2012;35:1015‐1020. [DOI] [PubMed] [Google Scholar]
- 70. Nichols CR. Mediastinal germ cell tumors. Clinical features and biologic correlates. Chest. 1991;99:472‐479. [DOI] [PubMed] [Google Scholar]
- 71. Nichols CR, Saxman S, Williams SD, et al. Primary mediastinal nonseminomatous germ cell tumors. A modern single institution experience. Cancer. 1990;65:1641‐1646. [DOI] [PubMed] [Google Scholar]
- 72. Dulmet EM, Macchiarini P, Suc B, Verley JM. Germ cell tumors of the mediastinum. A 30‐year experience. Cancer. 1993;72:1894‐1901. [DOI] [PubMed] [Google Scholar]
- 73. Kesler KA, Rieger KM, Hammoud ZT, et al. A 25‐year single institution experience with surgery for primary mediastinal nonseminomatous germ cell tumors. Ann Thorac Surg. 2008;85:371‐378. [DOI] [PubMed] [Google Scholar]
- 74. Liu Y, Wang Z, Peng ZM, Yu Y. Management of the primary malignant mediastinal germ cell tumors: experience with 54 patients. Diagn Pathol. 2014;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]


