Abstract
Of all the gynecologic tumors, ovarian cancer (OC) is known to be the deadliest. Advanced‐stages of OC are linked with high morbidity and low survival rates despite the immense amount of research in the field. Shortage of promising screening tools for early‐stage detection is one of the major challenges linked with the poor survival rate for patients with OC. In OC, therapeutic management is used with multidisciplinary approaches that includes debulking surgery, chemotherapy, and (rarely) radiotherapy. Recently, there is an increasing interest in using immunomodulation for treating OC. Relapse rates are high in this malignancy and averages around every 2‐years. Further treatments after the relapse are more intense, increasing the toxicity, resistance to chemotherapy drugs, and financial burden to patients with poor quality‐of‐life. A procedure that has been studied to help reduce the morbidity rate involves pre‐sensitizing cancer cells with standard therapy in order to produce optimal results with minimum dosage. Utilizing such an approach, platinum‐based agents are effective due to their increased response to platinum‐based chemotherapy in relapsed cases. These chemo‐drugs also help address the issue of drug resistance. After conducting an extensive search with available literature and the resources for clinical trials, information is precisely documented on current research, biomarkers, options for treatment and clinical trials. Several schemes for enhancing the therapeutic responses for OC are discussed systematically in this review with an attempt in summarizing the recent developments in this exciting field of translational/clinical research.
Keywords: biomarkers, clinical trials, ovarian cancer, ovarian cancer screening
This review article provides an in‐depth information on current treatment strategies and available clinical trials for ovarian cancer. Systematic review of strategies for enhancing the therapeutic response in ovarian carcinoma summarizing the recent developments in translational and clinical research.
1. INTRODUCTION
Ovarian cancer (OC) is the deadliest cancer among women placing it with 4th place for all the fatal disease among women. Cancer statistics from 2019 show that the estimated number of new cases is 22 240 with deaths around 14 170 cases.1 There are three histological types associated with the disease. The most common is epithelial OC (EOC). Patients with this fatal disease have only 45.6% 5‐year survival rate.2 The survival rate in general increases up to 70% if effective early stage detection is possible. Early‐stage detection rate for this disease is as low as 20%. For most of the patients the late stage detection with advanced stage of cancer leads to low survival rate of 35%. In the case of recurrent EOC there is no satisfactory cure till to date.
Several aspects influence the progression of the disease. Genetic and epigenetic factors are the most important ones among them. Nearly 10%‐15% of familial OCs result from breast cancer gene mutations BRCA1 and BRCA2.3 The characteristic feature of these cancers is that they are multifocal and progress rather quickly. Mutations and the loss of the TP53 function are found in 60%‐80% of the familial and sporadic cases of the disease.3 These oncogenes will turn on different signaling pathways that leads to pathogenicity. Higher rate of thrombosis associated with OC is due to such activation of coagulation pathways by OC.4, 5
2. OVARIAN CARCINOMA‐PATHOBIOLOGY
Ovarian carcinoma is heterogeneous in nature. The disease progresses through several molecular level changes. Mainly there are three areas in the ovary where the tumor is developed. Surface epithelium is where majority of the malignancy is developed from. It is presented in different type of histology. Serous ovarian carcinoma (SOC) is the most common one and is presented at old age. Endometrioid carcinoma is presented at young age and associated with endometriosis. Mucinous carcinoma and clear cell carcinoma are also presented at young age. The other areas where the OC is developed are the germ cells and stroma.
The complexity in these malignancies arises from the microenvironment affected by changes in genetic factors. The degree of complexity varies according to the changes in epigenetic factors too. Understanding the tumor microenvironment is key for its diagnosis, treatment options, and survival. The microenvironment varies for different type of ovarian carcinomas with changes in gene expression leads to different tumor markers. The tumor markers play crucial role for the development of targeted therapies.3 Abnormal expression of homeobox (HOX) has been shown in histologic types developed at the embryonic stage.6 HOXA9 is absent in normal ovarian cells. High‐level expression of HOXA9 in SOC is found at the embryonic stage during fallopian tube formation. Abnormal level of HOXA10 is linked to endometroid carcinoma and HOXA11is linked to mucinous carcinoma.6 As far as the treatment goes, platinum‐ and taxane‐based chemotherapy have been shown to be successful for serous and endometrioid cancers, compared with clear‐cell cancers and mucinous histology type cancers.3
3. OC SCREENING TESTS
Low endurance rate for OC patients is because of the late‐stage detection and diagnosis of the disease. Early stage detection and diagnostic tools for screening OC are not efficient. Research is in progress for developing efficient diagnostic processes for OC. Transvaginal ultrasound (TVUS) is one of the current screening process for OC. Blood test for CA125, a tumor marker for OC is another common screening test for OC.7 Transvaginal ultrasound will identify the growth and masses in the scanned area. It will not differentiate between the malignant and benign masses. CA125 is elevated in ovarian carcinoma. But it is not specific to OC, hence a combination of TVUS in patients with high levels of CA125 can be a better screening tool for OC diagnosis.8
4. BIOMARKERS FOR OC
As the disease progresses, it gets even harder to treat and manage the patients. Only 20% of those affected cases have an early detection of the ailment. Many healthcare professionals confused OC with other urologic, abdominal, and gynecologic diseases because of the overlap in signs and symptoms, resulting in late detections. Ovaries do not have a peritoneal covering; therefore, the cancer spreads locally to the peritoneal cavity, resulting in symptoms. Absence of effective testing tools and equipments further delay the detection process for OC.9 As noted earlier, the early detection is crucial in increasing survival rates for advanced‐stage OC patients. Biomarkers are divided into diagnostic, prognostic, predictive, and response categories. Poor sensitivity and lack of specificity are the challenges for majority of biomarkes that have been studied. Although, the common biomarkers currently used are CA125, Human Epididymis Protein 4 (HE4), and mesothelin,9, 10 and their use in combination is often feasible.
4.1. CA125
Currently, the disease progression and treatment efficacy in OC patients is monitored using TVUS and elevated CA125 expression.11, 12 Elevated CA125 levels are present in about 80% of advance stage OC patients. For early stage OC patients elevated CA125 level is present in 50% only.13 New studies from Jennings group demonstrated the association of Neu5Gc‐glycans and SubB2M for detecting CA125 and using as an effective tool for the diagnosis and outcomes in stage II and IV patients.14
4.2. HE4
This is another maker for OC. Elevated HE4 expression is present in OC patients compared with normal and other nonmalignant diseases for women.15, 16 HE4 and CA125 are the biomarkers used in a study of women (n = 531) who has pelvic masses. 93.8% of these women were predicted for high‐risk ovarian carcinoma.15 In the US, HE4 is only approved as maker for OC for disease recurrence or progression.
4.3. Mesothelin
A 40‐kD protein associated with cell survival, tumor progression, and adherence. It is present in normal mesothelial cells. Increased levels of mesothelin is presented in blood samples of 40%‐67% of patients with OC.16, 17, 18, 19 The high expression level of mesothelin in OC identifies it as strong candidate for targeted therapy.
4.4. OVA1
A multiple biomarker‐based test OVA1 (Ovarian Malignancy Algorithm) is currently used for the evaluation of risk level of OC patients.20 Microglobulin Beta2, CA125, transthyretin (pre‐albumin), ApoA1, and transferrin are the biomarkers in OVA1. OVA1 analyze serum levels of these biomarkers. The OVA1 algorithm combines the results of these levels with information on the menopausal status of the patient for OC risk group classification.
4.5. DOvEEgene
It is an ongoing clinical trial (NCT02288676) study sponsored by McGill University, Canada. In advanced OC, treatment efficiency was studied using computed tomography (CT) perfusion.21
Numerous studies, and trials involving OC biomarkers that are being conducted across the globe. A summary of the ongoing biomarker studies in OC detection is given in Table S1 (Source: http://Clinicaltrial.gov).
5. OC TREATMENT STRATEGIES
The treatment strategies for different type of cancer depends on its pathological stages. Early detection will help to have the treatment options that are promising and effective. Current treatment options are combining debulking surgery and drug treatment and radiation therapy. Some of the advanced level treatment options include targeted therapy, immunotherapy, and hormone therapy. Chemotherapy is the most vital part of OC treatment. Chemotherapeutic agents can be administered via intravenously (IV), intraperitonially (IP), or by IV/IP combination. In neo adjuvant treatment plan chemotherapy was done before the surgery. IP/IV combination delivery of chemotherapeutic agents is the preferred mode drug administration for OC patients with cytoreduced disease.22, 23, 24 Treatment of peritoneal area is most effective when the chemotherapeutic agents are administered via IP route.25 Compared with the IV carboplatin chemotherapy, the IP carboplatin chemotherapy is well tolerated in advance stage OC patients undergoing surgery followed by neoadjuvant.26
Chemotherapeutic agents will be selected for treatment based on the stage of OC. Platinum containing drugs (cisplatin and carboplatin) and taxane family (paclitaxel and docetaxel) are frequently used chemotherapeutic agents for treatment of OC.27 Carboplatin is the preferred choice over cisplatin due to its reduced toxicity, and side effects with equivalent response rate and survival outcomes.28, 29, 30 Sensitivity of the chemotherapeutic agent is important during the drug selection process of OC. Gemcitabine, doxorubicin, and bevacizumab are the drugs used for treatment for cisplatin and carboplatin‐resistant ovarian carcinoma.31, 32, 33 Usage of high‐dose chemotherapeutic agent will lead to complications due to side effects and can result in termination of treatment plan. Since the OC cells undergo molecular level changes over the time and may lead to resistance to chemotherapy. A list of currently approved chemotherapeutic agents for OC therapy and their mechanism(s) for anti‐cancer activity is summarized in Table S2.
6. ROLE OF APOPTOTIC GENES AND TARGETED THERAPY
The biological phenomena by which the body gets rid of unnecessary cells in order to maintain homeostasis is known as apoptosis. OC, among others, has several genes working against apoptosis, which allows cancerous cells to flourish instead of being killed off. Candidates involved in both intrinsic and extrinsic pathways were studied. Bcl‐2 family proteins and Tyrosine‐protein kinases, respectively, facilitates intrinsic and extrinsic apoptosis, while inhibitor of apoptosis (IAP) proteins are associated with both intrinsic and extrinsic pathways. Bcl2 is anti‐apoptotic 34 and is expressed in high concentration in OC. 35, 36 Additionally, Bcl2 modulates resistance to chemotherapy and decreases survival, along with Bcl‐X and Mcl‐1 in OC patients.36, 37, 38 Conversely, Bid, Bad, Bax, and Kak all respond to the treatment by inducing apoptosis 39 and improve the survival. Clinical trials for treatment with Bcl‐2 inhibitors improved the response to cisplatin, and this has also been seen in preclinical models of OC studies.39, 40
Another anti‐apoptotic gene family is the IAP proteins. Survivin is a well characterized inhibitor for apoptotic proteins present in ovarian and other type of cancer cells.41 Survivin plays a significant role in cell division and thus control apoptosis. Animal studies have shown that targeting survivin with suppressor drugs resulted in tumor growth suppression and enhanced sensitivity to chemotherapeutic agents.41
Tyrosine‐protein kinase Met (c‐Met) linked to poor treatment outcomes for cancer chemotherapy is upregulated in OC.42, 43, 44, 45 Increased levels of c‐Met impacts cell proliferation, infiltration, angiogenesis, and endurance.46, 47, 48 Antiapoptotic activity of c‐Met linked to chemo resistance for therapies.46 Radiotherapy induces c‐Met expression and triggers the series of signals that increases the pro‐survival process and spreads the response of treatments.49 An in vitro study has shown that by treating OC cells with c‐Met inhibitors, cell proliferation has been significantly reduced and increased apoptosis of cancer cells was observed.50
A class of transcription factors known as specific proteins (Sp) regulates VEGF (vascular endothelial growth factor) expression with functional variation. Thus, Sp transcription factors have crucial impact on tumor expansion and metastasis.51 Association of Sp transcription factors in anti‐cancer activity is illustrated in Figure 1.
Figure 1.
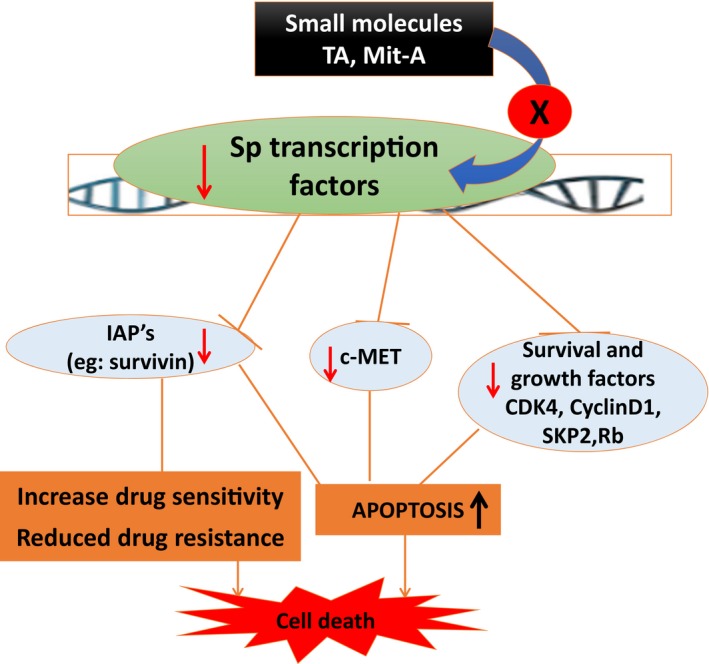
Association of Sp transcription factors in anti‐cancer activity. Small molecules like tolfenamic acid (TA), Mithramycin A are shown to inhibit specificity protein (Sp) family of transcription factors and will result in increased apoptosis of cancer cells
7. MULTIPLE DRUG RESISTANCE
Research is in progress for understanding the mechanism of drug resistance in OC. Some of these mechanisms include increased DNA repair, overexpression of surface p 170‐glycoprotein, increased cellular levels of glutathione (GSH) and glutathione S‐transferases causing de‐toxification of platinum agents and taxol.52 Cancer cells develop certain transport proteins that help them to eliminate the effective dosages of drugs from the cells causing multiple drug resistance (MDR).53, 54
The “Classical” MDR is resulted from higher level of MDR‐1 gene that code for 170‐kD ATP‐dependent glycoprotein Pgp. Pgp causes reduction of cellular levels of cytotoxic drugs within the cells by transporting the drug outside the cells against the concentration gradient. Chemotherapy often upregulates the expression of P‐gp on cancer cells resulting in MDR. Resistance to multiple drugs is associated with P‐gp overexpression and includes paclitaxel, vincristine, and doxorubicin.55, 56, 57, 58 Cells that do not express P‐gp acquire other methods for drug resistance. Amplification of MDR‐associated protein gene (MRP) has been found in such cells that encodes a protein MRP, which expelling the drug out of the cells.58, 59 The MRP1 coded by ABCC1 and MRP2 coded by ABCC2 genes. They induce resistance to many cancer drugs, especially with the widely used cisplatin in OC.55, 56, 57, 58 A schematic representation of the proteins involved in drug resistance mechanism of commonly used chemotherapeutic agents for OC is given in Figure 2.
Figure 2.
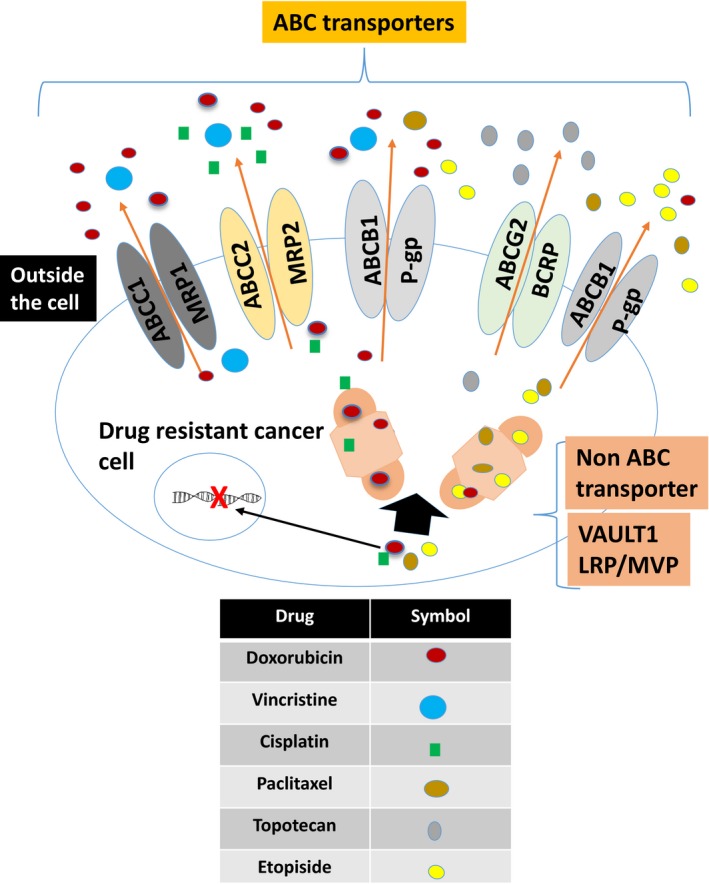
Dug resistance mechanisms: A schematic representation of proteins involved in drug resistance mechanism of commonly used chemotherapeutic agents for ovarian cancer chemotherapy. The classical multiple drug resistance produced by ABC transporters and non‐ABC transporters are illustrated
In yeast and mammalian system, copper transporter CTR1 is one of the transporters mediating uptake of platinum compounds.60 Cisplatin reduces its cellular influx by rapid degradation of CTR1 resulting in drug resistance.61, 62 Increased CTR1 expression by the cells results in an increased platinum concentration and decreased resistance to cisplatin.62 Combination of platinum drugs with bortezomib, a modulator for copper transporter expression, is a current option for platinum‐resistant solid tumors.63
Several epigenetic changes have been observed in cisplatin‐resistant human cells that open new avenues to study drug resistance among the OC cells. It has been observed that certain individual cells among the cancer cell population attain the reversible state of drug tolerance to prevent the eradication of the population by potential lethal exposure.64 Altering the chromatin state and engaging IGF‐1, insulin‐like growth factor will increase drug resistance. Treating with Inhibitors of IGF‐1 receptor will reverse this process and can be an important therapeutic strategy. In addition, by inactivating the cytotoxic genes like folate binding gene (FBP) in cancer cells, DNA hypermethylation plays critical role(s) in generating the multiple drug‐resistant phenotypes.65
Another well‐known carrier, breast cancer resistant protein coded by ABCG2 is found to be overexpressed in ovarian 66 and breast cancers.67 Upregulated BRCP is known to protect cancer cells from topotecan 66, 68 and mitoxantrone.67, 68 The mechanism of normal epithelial cells changes to drug resistant cancerous cells is by activating epithelial‐mesenchymal transition (EMT).34 Beginning of their transformation malignant epithelial cells go through angiogenesis and massive propagation.69 Cellular level changes occurring during the transition process transform epithelial cells to mesenchymal cells. These mesenchymal cells have anti apoptotic with increased migratory capacity and invasiveness.70 E‐cadherin a suppressor of insensitivity and motility is downregulated by transcription factors like Twist, Snail and Slug which are key coordinators for EMT.71 Snail and Twist are overexpressed in paclitaxel‐resistant EOC cells which is predicted by the molecular level modifications during EMT.72, 73
Since the EMT is mediated by several signaling pathways,74 it has become clearer that by halting these pathways, EMT can be reverted as well as some biological effects like drug sensitivity.75 Overexpression of endothelin‐1 and endothelin A receptor has been shown to enable EOC cells with increased resistance to chemo‐drugs, and thus, increase their relative survival capacity.76 In advanced‐stage EOC, ET (Endothelin) 1 and ET A receptor, ETAR pathways are overexpressed.77 The ET‐1 and ETAR are overexpressed with increased MAP kinase (MAPK) and protein kinase B phosphorylation, c‐1ell proliferation, in drug resistant EOC cells.76 In a study, treatment of cancer cells with the drugs that can block ETAR‐driven EMT, inhibition of tumor progression was seen, and chemo resistance has been overcome. EMT markers are used as a tool in several randomized clinical trials to develop personalized therapies. Clinical trial based on the aspirin treatment (NCT02602938) for metastatic breast and colorectal is an example of circulating tumor cells (CTC)s with EMT features.78
8. IMMUNOTHERAPIES
Immunotherapy involves various methods enhancing immune system. Exploiting the immune system for tumor recession is an ancient procedure as in 2600 BC The Pharaoh Imhotep self‐infected to enable tumor recession.79 Native and adaptive immunity of the patient are activated in the beginning of the tumor formation.80, 81 In the later stages of tumor growth, the tumor microenvironment inhibits the immune system in targeting cancer.
Anti‐tumor lymphocytes from healthy adults and patients are used in treatment using adoptive cell transfer to stimulate cancer decline.82 In this approach, the autologous T cells are collected from patient's peripheral blood or resected tumor tissue or tumor‐infiltrating lymphocytes (TIL) and those cells are expanded or manipulated ex vivo, an environment different from the patients tumor microenvironment (TME). These T cells cultured ex vivo and recombinant interleukin 2 are given back to patients.83
Use of cancer vaccines is another approach to bring about immune activation. T‐cell responses were stimulated by activating the antigen‐presenting cells.84 More recently, 11 heavily treated patients with platinum‐resistant OC (PROC) have been treated with GL‐ONC1 on a phase Ib protocol [NCT02759588]. GL‐ONC1 is a modified vaccinia virus developed by Genelux Corporation (San Diego, CA) that causes tumor cell oncolysis, immune activation through release of oncoproteins, presentation of both foreign and tumor antigens by dendritic cells, and durable anti‐cancer T cell tumor‐specific memory. In this phase Ib trial [NCT02759588], patients received a minimum of three prior lines of therapy, with five patients having had at least five prior lines. Nine patients had PROC, one was platinum refractory, and one was intermediate platinum sensitive (7 months prior PFS). Ten patients progressed on their prior line of therapy and nine had ascites or pleural effusions. The trial involved intraperitoneal (IP) infusion of GL‐ONC1 monotherapy that was given at higher dosages and contained provisions for dose‐escalation every three patients. Two separate IP instillations were performed 24 hours apart through a tunneled catheter system. The primary objectives were measurement of toxicity and secondary endpoints were anti‐tumor response. Encouraged by these preliminary outcomes, a clinical trial for first dose cohort is currently underway.
Manipulation of immune checkpoints has become the modern revolution in cancer immunotherapy. Cytotoxic T‐Lymphocyte‐Associated Protein 4 (CTLA‐4) and Programmed Cell Death Protein 1 (PD‐1) are Immune checkpoint linked to T cells. These proteins control the equilibrium of immune response and tolerance upon influencing the T lymphocyte activity. Activated T lymphocyte functions as an inhibitor via “negative feedback loop” mechanism and protect normal tissues from tumor‐derived immune response.85 These proteins are over expressed in OC patients and their natural anti‐cancer immunity is at disadvantage. Currently, several monoclonal antibodies (mAb) against CTLA‐4 and other proteins and its ligand are used in clinics.86, 87, 88 FDA approved mAb's targeted against immune checkpoints for various cancers are given in Table 1.
Table 1.
Currently approved monoclonal antibodies targeted against Immune checkpoint proteins
| Drug name | Immune checkpoint target | Current approval as of June, 2019 |
|---|---|---|
| Ipilimumab | CTLA‐4 | Melanoma, renal cell carcinoma (combined with nivolumab), colorectal cancer |
| Pembrolizumab | PD‐1 | Nonsmall cell lung cancer (NSCLC), squamous cell carcinoma of head and neck (SCCHN), classic Hodgkin's lymphoma, large B‐cell lymphoma, urothelial cancer, micro‐satellite instability‐ high (MSI_H)or mismatch repair deficient (dMMR) cancers, gastric or GEJ adenocarcinoma and cervical ca |
| Nivolumab | PD‐1 | NSCLC, melanoma, RCC, classic HL, squamous cell carcinoma of head and neck (SCCHN), urothelial cancer (UC), MSI‐H or dMMR colorectal cancer, hepatocellular cancer |
| Avelumab | PD‐L1 | Merkel cell carcinoma (MCC), UC |
| Durvalumab | PD‐L1 | UC, NSCLC |
| Atezolizumab | PD‐L1 | UC, NSCLC |
| Tremelimumab | CTLA‐4 | Awaiting approval |
Abbreviations: CTLA‐4, cytotoxic T lymphocyte‐associated antigen 4; PD‐1: programmed cell death 1; PD‐L1: programmed cell death ligand 1.
Source: FDA Approved drugs: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
9. IMMUNOTHERAPY FOR OC
The success rate for immunotherapy for OC treatment is very low and there is not yet any FDA approval for immune therapies for OC. Motivated by some of the recent encouraging results in other closely‐related tumor types, the scientific community has also started adapting immunotherapy to treat gynecologic cancers. Antibodies and T cells responsive for cancer are detected from ascites, blood, and tumor of advanced‐stage OC patients.89 Since it is known that the TILs expression level is linked to increased survival rate in OC patients, immunotherapies are highly potential for effective treatment outcomes, similar to other cancers.90 Table S1 includes current clinical trials listed on http://www.ClinicalTrial.gov that are recruiting patients. Schematic representation for the emerging immunotherapies for OC is given in Figure 3.
Figure 3.
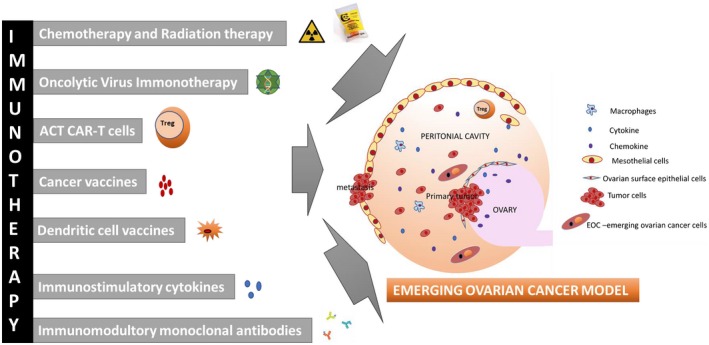
Emerging immunotherapies for ovarian cancer. A schematic representation with the details on cancer vaccines, dendritic cell vaccines, adoptive T‐cell transfer, immunostimulatory cytokines are some of the techniques explained in this review
10. INHIBITORS AND MODULATOR
Important factors that help predict the tumor responses to inhibitors for immune checkpoint are the effector immune cell availability, approachability, and the tumor dependence on immune checkpoint pathways. TILs and PD‐L1 are identified as the markers to predict immune response to inhibitors. Using these markers, higher than 50% of advanced SOC estimated to act in response to immune checkpoint inhibitors via adaptive immune resistance. It is nearly absent low‐grade SOC, about 25% in other pathological types cancers.91 Currently, several immune checkpoint inhibitors are tested in animal studies and some of them are in clinical studies for OC treatment.82, 92
Preliminary data from clinical studies with all the current inhibitors reveals limited efficacy in OC with 15% rates of objective response, ORR. Hence it is very important future studies are required to identify more biomarkers for immune checkpoint inhibitors. 17% of ORR with a rate of 83% disease control is reported for a phase I study of olaparib (Poly ADP ribose polymerase [PARP] inhibitor) and durvalumab (anti‐PD‐L1).93 Table S3 shows the currently recruiting clinical trials (as of August, 2019) that utilizes immunotherapy for patients with OC.
11. VACCINES USED IN OC THERAPY
Vaccines used in cancer therapy will activate the immune cells for the elimination of cancerous cells. Specific tumor‐associate antigens (TAAs) are administered using various methods. Some of the common vaccines tested as cancer vaccines are developed using various methods, epigenetic, and genetic. Vaccines are administered as it or along with cytokines or other accelerating factors.94
CA125, p53 protein, folate receptor‐alpha (FRα), human epidermal growth factor receptor‐2 (HER2), and cancer‐testis antigens, like melanoma‐associated antigen A4 (MAGE‐A4) and New York‐esophageal squamous cell carcinoma11 (NY‐ESO‐1) are potential TAA molecules found in OC..95 Cancer Vaccine therapeutic investigation is an actively growing area in OC research. Currently, there are mainly pilot and phase I or II trials on the use of therapeutic vaccines in OC.85, 96 A list of ongoing vaccine studies in OC (Source: http://www.ClinicalTrial.gov) is given in Table S4.
NY‐ESO‐1, a potential molecule for targeted vaccine due to its over expression in OC exhibited stimulated immune response specific to T cells.97 Combination therapy of NY‐ESO‐1 with DNA methylation inhibitors and chemotherapy was administered in patients with recurrent disease for therapeutic efficacy enhancement.83 This increases the NY‐ESO‐1 antibody availability, T‐cell responses that lead to clinical response in OC patients.
As noted earlier, oncolytic virus (OV) has shown synergy when combined with checkpoint inhibitor antibodies.98 Oncolytic virus immunotherapy or combination of OV with other molecules that are immune‐stimulatory or induce immunogenic responses are encouraging avenues to explore as novel therapeutic options for OC.82
Her2/Neu, tumor antigen presents in almost 90% of the recurrent OC cases. Clinical study of 11 patients were given Her2/Neu packed antigen autologous dendritic cells combined with antigens of telomerase reverse transcriptase (human) and pan‐DR peptides.99 90% 3‐year overall survival was reported as the outcome for this study of patients in remission with advanced OC.
Whole tumor cell vaccines will induce immunologic reaction for a larger range of antigens compared to specific TAA.100 Broader reaction with T cells can also be induced using whole vaccine.100 Another important avenue in vaccine therapy is personalized peptide vaccines developed from individual tumor depending on the human leukocyte antigen and IgG expressions.101 Clinical research showed that patient with Pt‐ sensitive cancer have 39.3 months overall survival rate. For patients with Pt resistant cancer overall survival rate is 16.2 months.
Another novel therapeutic method is to explore the use cancer cells from the patient to deliver viruses to the tumor.102 Tumor microenvironment can be manipulated using cancer cells as virus vehicles. Changing a “cold” cancer to a “hot” cancer potentiates anti‐cancer reaction. This a promising strategy for OC patients who do not get benefitted from current therapies due to suboptimal immune infiltration. Schematic representation for the emerging immunotherapies for OC is given in Figure 3.
12. ADOPTIVE CELLULAR THERAPY‐ADOPTIVE T‐CELL TRANSFER
Peripheral blood lymphocytes (PBLs) are separated from patients’ blood and will be used for the isolation of tumor‐specific lymphocytes. Tumor‐specific PBLs will be grown to supply back to the patient. Anti‐cancer action of PBLs can also be enhanced by genetic modification.103 Clinical trials of ACT for OC are ongoing. ACT has shown around 72% reaction rates that last for more than three years in metastatic melanoma occurring at.83 This is a very promising outcome that can be translated into OC therapy by optimizing the conditions for Adoptive cellular therapy (ACT) in OC therapy.
13. CAR‐T‐CHIMERIC ANTIGEN RECEPTOR T
The major limitation to the ACT trials in the beginning stages were the need for isolation and culturing of functional cancer responsive T cells. The emergence of engineered T cells has become a promising tool to enhance the cancer immune therapy.82, 104, 105 Tumor‐specific targeting can be gained using patients receptors of T cell and CARs. The tumor recognition in a major histocompatibility complex (MHC) can be achieved by CAR‐T cells. T‐cell stimulation and selectivity of antigen features are combined in one combination molecule.105 The initial group of CARs was examined in OC and other tumors inducing modest responses.105, 106 At the very first CAR T‐cells trial in OC, cancer load was not reduced in patients.106 FRα,107 HER‐2,108 CA125 (MUC16),109, 110 and mesothelin 111 are ensuring antigens for CARS. CAR‐T therapeutic efficacy still need to be improved in OC. Combination treatment modalities to overcome these problems may be a novel approach. Combining inhibitors for immune checkpoint with CAR‐T cells is a better therapeutic option for OC.112 A schematic representation of emerging immunotherapies for OC is given in Figure 3.
14. FUTURE PERSPECTIVES
Regardless of the extensive developments in OC therapy, it is still the deadliest malignancy in women. The biggest hurdle is the shortage of efficient screening procedure that helps to detect the tumor at an early stage. Even though the curing rate for beginning stage OC patient is 90%, about 20% of OC is detected as early as stage1. This reveals the need for future research for finding biomarkers that are more responsive and specific for detection of OC at an early stage.
Surgery and chemotherapy are conventional therapy for ovarian carcinoma. Poor prognosis with a recurring progressive cancer is the major challenge to the treatment. The prognosis varies for each patient and it depends on the level of response to preliminary therapy. Drug administration by IP is the efficient way to target OC cells situated the peritoneal area. Removal of all the residual tissues by surgery followed by chemotherapy, is the most ideal cure for OC.
Sensitivity to chemotherapeutic agents is a crucial parameter in therapeutic efficacy. Research to identify biomarkers for apoptosis and chemo resistance in OC therapy should be one of the prime goals in cancer research (eg, Caris® Assays). Newly identified microRNA biomarkers linked to platinum drug resistance are let‐7109 and ATP11B.108 Another upcoming are of research in OC is combination therapy of drugs and other small molecules that can enhance therapeutic efficacy by increasing drug sensitivity and reducing drug resistance. Currently, several studies are in progress with the compounds that modify Bcl2 proteins family,82, 85 and agents for targeting DNA repair and inhibit PARP.113, 114, 115 To improve drug sensitivity to cisplatin, small molecules like triethylenetetramine, genistein, butathione sulfoximine, and rapamycin are under the stage of preclinical testing.116 Triethylenetetramine inhibits telomerase, induces anti‐antiogenesis and acts as anti‐cancer agent. Triethylenetetramine reversed cisplatin resistance in OC cells. Genistein showed anti‐cancer activity in both pediatric and adult cancer models. Triethylenetetramine sensitized OC cells against cisplatin. Butathione sulfoximine also showed sensitizing effects in gastric and OC cells against cisplatin. Laboratory studies also demonstrated the effect of rapamycin for inducing the effect of cisplatin in OC breast cancer and lung cancer cells.
Another promising technique for OC therapy is silencing gene expression. By this technique, specific sets of genes can be targeted and altered, and it requires less dosage. This technique is still the subject of ongoing research to overcome its challenges like stability and compound delivery to a target site.110, 111 MicroRNAs are used as targeted molecules for diagnosis is another growing field. Developing clinical trials with more molecules similar to what we explained above will help to overcome the challenges in OC therapy.
Diab et al have done a review of targeted therapy for OC for 2010‐2017.117 Targeted therapies are evolving in three main fields, angiogenesis, signaling, and apoptosis. The VEGF pathway is focused for angiogenesis. PI3K/Akt and the MAP kinase pathways critically involved signaling cascades. McCabe et al118 have conducted examination of trials in EOC to investigate the association between platinum‐resistance and response to anti‐angiogenic agents. The analysis revealed that novel anti‐angiogenic therapies would be beneficiary for the patients with platinum‐resistant EOC.
Dose‐dense chemotherapy is the promising option currently available for the patients with poor responses to chemotherapy. PARP inhibitors are the most emerging class of new drugs in combination therapy with the traditional chemotherapy drugs listed in Figure 3. Bevacizumab is recently approved for EOC treatment. The common challenge with anti‐angiogenic agents is the non‐availability of efficient biomarkers. Folate receptor targeting required further research to consider as one of the treatment options. Despite cost issues, regular Breast cancer susceptibility gene (BRCA) screening should be done for all OC patients for a better selection of targeted therapy. Knowledge about tumor microenvironment and immune suppressive pathways are crucial for newer immunotherapeutic approaches toward OC. For a personalized medical treatment, systematic data analysis of molecular and genetical categorization of various types of OC with precision is required.119 A schematic representation of some of the most common strategies for improving therapeutic responses is given in Figure 4.
Figure 4.
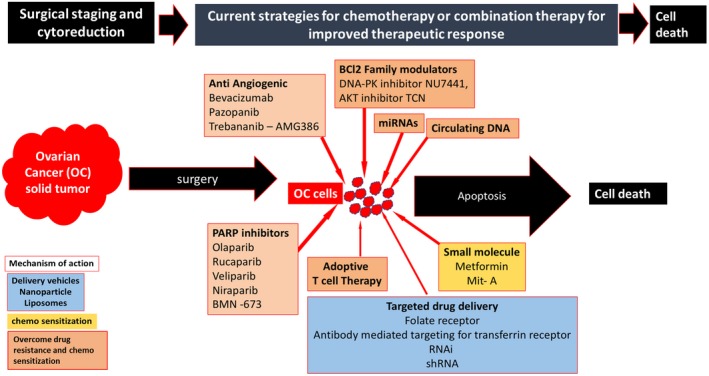
Current strategies for improving therapeutic response. Effective Targeted therapy, Usage of PARP inhibitors, combination therapy, immunotherapy, and usage of chemosensitizers are some of the future strategies for improved therapeutic responses for ovarian cancer treatment
15. CONCLUSIONS
Ovarian cancer affects the lives of many women around us. Despite continued efforts and steady improvements in treatment over the past few decades, OC still remains the deadliest malignancy in women. The poor clinical outcome is due to the deficiency of effective tools for detecting the disease at an early stage, chemotherapy resistance and increased heterogeneity of the disease. The vast majority of cases have high‐grade papillary serous histology marked by p53 mutations and 25% of cases have either inherited or acquired mutations in BRCA. Primary therapy is initiated with cytoreductive surgery and chemotherapy. Even with optimization of treatment protocols that have improved PFS, only limited gains in OS. Ultimately, approximately 80% of patients develop PROC. Once this occurs, further chemotherapy response rates are about 10%‐15% and survival averages 9‐12 months. Therefore, we are critically in need of developing novel therapies to improve cure rates and provide effective long‐term disease stability for PROC.
Targeted therapy is the fast growing modalities for cancer treatment. For targeted therapy drugs or small molecules will be used to block tumor growth. More studies should be done on combination therapies involving one or more of these small molecules as modulators for OC treatment. Such research should be augmented to fight chemo resistance better treatment outcomes. Inhibitors for various genes involved in the signal pathway in tumor growth should be another area of focus for future studies for OC treatment. Study of the various molecules involved in tumor micro environment at various stages of tumor metastasis is crucial for the development of better immunotherapies for OC.120 How each one of these molecules control various treatment strategies and the immune system of the patient. Immune and cellular therapies coupled with genetic testing and precision assays (biomarkers) are promising strategies for better clinical outcomes. Novel strategies and rapid growth of research in medical field will lead to better therapeutic schemes to minimize ill health and improved life expectancy for patients with OC.
CONFLICT OF INTEREST
None declared.
Supporting information
Chandra A, Pius C, Nabeel M, et al. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019;8:7018–7031. 10.1002/cam4.2560
Funding information
Supported by NIMHD (grant #: 2U54 MD006882‐06), NCI (grant #: 1P20CA233355‐01) and NHLBI (grant #: R25HL125447).
REFERENCES
- 1. DeSantis CE, Miller KD, Dale W, et al. Cancer statistics for adults aged 85 years and older. CA Cancer J Clin. 2019. 10.3322/caac.21577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oronsky B, Ray CM, Spira AI, Trepel JB, Carter CA, Cottrill HM. A brief review of the management of platinum‐resistant–platinum‐refractory ovarian cancer. Med Oncol. 2017;34(6):103. [DOI] [PubMed] [Google Scholar]
- 3. Bast RC Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9(6):415‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang X, Wang E, Kavanagh JJ, Freedman RS. Ovarian cancer, the coagulation pathway, and inflammation. J Transl Med. 2005;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holmes CE, Levis JE, Ornstein DL. Activated platelets enhance ovarian cancer cell invasion in a cellular model of metastasis. Clin Exp Metas. 2009;26(7):653‐661. [DOI] [PubMed] [Google Scholar]
- 6. Cheng W, Liu J, Yoshida H, Rosen D, Naora H. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005;11(5):531‐537. [DOI] [PubMed] [Google Scholar]
- 7. Bosse K, Rhiem K, Wappenschmidt B, et al. Screening for ovarian cancer by transvaginal ultrasound and serum CA125 measurement in women with a familial predisposition: a prospective cohort study. Gynecol Oncol. 2006;103(3):1077‐1082. [DOI] [PubMed] [Google Scholar]
- 8. Olivier RI, Lubsen‐Brandsma MA, Verhoef S, van Beurden M. CA125 and transvaginal ultrasound monitoring in high‐risk women cannot prevent the diagnosis of advanced ovarian cancer. Gynecol Oncol. 2006;100(1):20‐26. [DOI] [PubMed] [Google Scholar]
- 9. Yurkovetsky ZR, Linkov FY, E Malehorn D, Lokshin AE. Multiple biomarker panels for early detection of ovarian cancer. Future Oncol. 2006;2(6):733‐741. [DOI] [PubMed] [Google Scholar]
- 10. Bandiera E, Zanotti L, Fabricio AS, et al. Cancer antigen 125, human epididymis 4, kallikrein 6, osteopontin and soluble mesothelin‐related peptide immunocomplexed with immunoglobulin M in epithelial ovarian cancer diagnosis. Clin Chem Lab Med. 2013;51(9):1815‐1824. [DOI] [PubMed] [Google Scholar]
- 11. Moro F, Pasciuto T, Djokovic D, et al. Role of CA125/CEA ratio and ultrasound parameters in identifying metastases to the ovaries in patients with multilocular and multilocular‐solid ovarian masses. Ultrasound Obstet Gynecol. 2019;53:116‐123. [DOI] [PubMed] [Google Scholar]
- 12. van Nagell JR Jr., DePriest PD, Ueland FR, et al. Ovarian cancer screening with annual transvaginal sonography: findings of 25,000 women screened. Cancer. 2007;109(9):1887‐1896. [DOI] [PubMed] [Google Scholar]
- 13. Prorok PC, Andriole GL, Bresalier RS, et al. Design of the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(6 Suppl):273s‐309s. [DOI] [PubMed] [Google Scholar]
- 14. Shewell LK, Wang JJ, Paton JC, Paton AW, Day CJ, Jennings MP. Detection of N‐glycolylneuraminic acid biomarkers in sera from patients with ovarian cancer using an engineered N‐glycolylneuraminic acid‐specific lectin SubB2M. Biochem Biophys Res Commun. 2018;507(1–4):173‐177. [DOI] [PubMed] [Google Scholar]
- 15. Clarke‐Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med. 2009;361(2):170‐177. [DOI] [PubMed] [Google Scholar]
- 16. Shah CA, Lowe KA, Paley P, et al. Influence of ovarian cancer risk status on the diagnostic performance of the serum biomarkers mesothelin, HE4, and CA125, Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2009;18(5):1365‐1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Creaney J, van Bruggen I, Hof M, et al. Combined CA125 and mesothelin levels for the diagnosis of malignant mesothelioma. Chest. 2007;132(4):1239‐1246. [DOI] [PubMed] [Google Scholar]
- 18. Hassan R, Remaley AT, Sampson ML, et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res. 2006;12(2):447‐453. [DOI] [PubMed] [Google Scholar]
- 19. Yen MJ, Hsu CY, Mao TL, et al. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin Cancer Res. 2006;12(3 Pt 1):827‐831. [DOI] [PubMed] [Google Scholar]
- 20. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(6):588‐594. [DOI] [PubMed] [Google Scholar]
- 21. Ng CS, Zhang Z, Lee SI, et al. CT perfusion as an early biomarker of treatment efficacy in advanced ovarian cancer: an ACRIN and GOG study. Clin Cancer Res. 2017;23(14):3684‐3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tewari D, Java JJ, Salani R, et al. Long‐term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2015;33(13):1460‐1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2016;(1):Cd005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wright AA, Cronin A, Milne DE, et al. Use and effectiveness of intraperitoneal chemotherapy for treatment of ovarian cancer. J Clin Oncol. 2015;33(26):2841‐2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanner EJ, Black DR, Zivanovic O, et al. Patterns of first recurrence following adjuvant intraperitoneal chemotherapy for stage IIIC ovarian cancer. Gynecol Oncol. 2012;124(1):59‐62. [DOI] [PubMed] [Google Scholar]
- 26. Provencher DM, Gallagher CJ, Parulekar WR, et al. OV21/PETROC: a randomized Gynecologic Cancer Intergroup phase II study of intraperitoneal versus intravenous chemotherapy following neoadjuvant chemotherapy and optimal debulking surgery in epithelial ovarian cancer. Ann Oncol. 2018;29(2):431‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum‐based chemotherapy versus conventional platinum‐based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO‐OVAR‐2.2 trial. Lancet. 2003;361(9375):2099‐2106. [DOI] [PubMed] [Google Scholar]
- 28. Hogberg T, Glimelius B, Nygren P. A systematic overview of chemotherapy effects in ovarian cancer. Acta Oncol. 2001;40(2–3):340‐360. [DOI] [PubMed] [Google Scholar]
- 29. Aabo K, Adams M, Adnitt P, et al. Chemotherapy in advanced ovarian cancer: four systematic meta‐analyses of individual patient data from 37 randomized trials. Advanced Ovarian Cancer Trialists' Group. Br J Cancer. 1998;78(11):1479‐1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neijt JP, Engelholm SA, Tuxen MK, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol. 2000;18(17):3084‐3092. [DOI] [PubMed] [Google Scholar]
- 31. Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum‐sensitive recurrent ovarian cancer: an intergroup trial of the AGO‐OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24(29):4699‐4707. [DOI] [PubMed] [Google Scholar]
- 32. Sehouli J, Camara O, Schmidt M, et al. Pegylated liposomal doxorubicin (CAELYX) in patients with advanced ovarian cancer: results of a German multicenter observational study. Cancer Chemother Pharmacol. 2009;64(3):585‐591. [DOI] [PubMed] [Google Scholar]
- 33. Ferrandina G, Ludovisi M, Lorusso D, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol. 2008;26(6):890‐896. [DOI] [PubMed] [Google Scholar]
- 34. Thiery JP. Epithelial‐mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442‐454. [DOI] [PubMed] [Google Scholar]
- 35. Witham J, Valenti MR, De‐Haven‐Brandon AK, et al. The Bcl‐2/Bcl‐XL family inhibitor ABT‐737 sensitizes ovarian cancer cells to carboplatin. Clin Cancer Res. 2007;13(23):7191‐7198. [DOI] [PubMed] [Google Scholar]
- 36. Eliopoulos AG, Kerr DJ, Herod J, et al. The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and Bcl‐2. Oncogene. 1995;11(7):1217‐1228. [PubMed] [Google Scholar]
- 37. Adams JM, Cory S. The Bcl‐2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26(9):1324‐1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vogler M. BCL2A1: the underdog in the BCL2 family. Cell Death Differ. 2012;19(1):67‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang H, Zhang Z, Wei X, Dai R. Small‐molecule inhibitor of Bcl‐2 (TW‐37) suppresses growth and enhances cisplatin‐induced apoptosis in ovarian cancer cells. J Ovarian Res. 2015;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zeitlin BD, Zeitlin IJ, Nor JE. Expanding circle of inhibition: small‐molecule inhibitors of Bcl‐2 as anticancer cell and antiangiogenic agents. J Clin Oncol. 2008;26(25):4180‐4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamamoto T, Tanigawa N. The role of survivin as a new target of diagnosis and treatment in human cancer. Med Electron Microsc. 2001;34(4):207‐212. [DOI] [PubMed] [Google Scholar]
- 42. Lengyel E, Prechtel D, Resau JH, et al. C‐Met overexpression in node‐positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int J Cancer. 2005;113(4):678‐682. [DOI] [PubMed] [Google Scholar]
- 43. Jagadeeswaran R, Ma PC, Seiwert TY, et al. Functional analysis of c‐Met/hepatocyte growth factor pathway in malignant pleural mesothelioma. Can Res. 2006;66(1):352‐361. [DOI] [PubMed] [Google Scholar]
- 44. Kong DS, Song SY, Kim DH, et al. Prognostic significance of c‐Met expression in glioblastomas. Cancer. 2009;115(1):140‐148. [DOI] [PubMed] [Google Scholar]
- 45. Di Renzo MF, Olivero M, Katsaros D, et al. Overexpression of the Met/HGF receptor in ovarian cancer. Int J Cancer. 1994;58(5):658‐662. [DOI] [PubMed] [Google Scholar]
- 46. Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12(2):89‐103. [DOI] [PubMed] [Google Scholar]
- 47. Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915‐925. [DOI] [PubMed] [Google Scholar]
- 48. Appleman LJ. MET signaling pathway: a rational target for cancer therapy. J Clin Oncol. 2011;29(36):4837‐4838. [DOI] [PubMed] [Google Scholar]
- 49. De Bacco F, Luraghi P, Medico E, et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J Natl Cancer Inst. 2011;103(8):645‐661. [DOI] [PubMed] [Google Scholar]
- 50. Kim HJ, Yoon A, Ryu JY, et al. c‐MET as a potential therapeutic target in ovarian clear cell carcinoma. Sci Rep. 2016;6:38502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Safe S, Abdelrahim M. Sp transcription factor family and its role in cancer. Eur J Cancer. 2005;41(16):2438–2448. [DOI] [PubMed] [Google Scholar]
- 52. Johnson SW, Laub PB, Beesley JS, Ozols RF, Hamilton TC. Increased platinum‐DNA damage tolerance is associated with cisplatin resistance and cross‐resistance to various chemotherapeutic agents in unrelated human ovarian cancer cell lines. Can Res. 1997;57(5):850‐856. [PubMed] [Google Scholar]
- 53. Stavrovskaya AA. Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry. 2000;65(1):95‐106. [PubMed] [Google Scholar]
- 54. Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22(47):7537‐7552. [DOI] [PubMed] [Google Scholar]
- 55. Surowiak P, Materna V, Denkert C, et al. Significance of cyclooxygenase 2 and MDR1/P‐glycoprotein coexpression in ovarian cancers. Cancer Lett. 2006;235(2):272‐280. [DOI] [PubMed] [Google Scholar]
- 56. Januchowski R, Sterzynska K, Zaorska K, et al. Analysis of MDR genes expression and cross‐resistance in eight drug resistant ovarian cancer cell lines. J Ovarian Res. 2016;9(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8(5):411‐424. [DOI] [PubMed] [Google Scholar]
- 58. Cole SP, Bhardwaj G, Gerlach JH, et al. Overexpression of a transporter gene in a multidrug‐resistant human lung cancer cell line. Science. 1992;258(5088):1650‐1654. [DOI] [PubMed] [Google Scholar]
- 59. Sodani K, Patel A, Kathawala RJ, Chen ZS. Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer. 2012;31(2):58‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA. 2002;99(22):14298‐14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol. 2006;70(4):1390‐1394. [DOI] [PubMed] [Google Scholar]
- 62. Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum‐containing cancer drugs. Mol Pharmacol. 2010;77(6):887‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kilari D, Guancial E, Kim ES. Role of copper transporters in platinum resistance. World J Clin Oncol. 2016;7(1):106‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sharma SV, Lee DY, Li B, et al. A chromatin‐mediated reversible drug‐tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chang X, Monitto CL, Demokan S, et al. Identification of hypermethylated genes associated with cisplatin resistance in human cancers. Can Res. 2010;70(7):2870‐2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maliepaard M, van Gastelen MA, de Jong LA, et al. Overexpression of the BCRP/MXR/ABCP gene in a topotecan‐selected ovarian tumor cell line. Can Res. 1999;59(18):4559‐4563. [PubMed] [Google Scholar]
- 67. Zhang F, Throm SL, Murley LL, et al. MDM2 antagonist nutlin‐3a reverses mitoxantrone resistance by inhibiting breast cancer resistance protein mediated drug transport. Biochem Pharmacol. 2011;82(1):24‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26(1):39‐57. [DOI] [PubMed] [Google Scholar]
- 69. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57‐70. [DOI] [PubMed] [Google Scholar]
- 70. Kalluri R, Neilson EG. Epithelial‐mesenchymal transition and its implications for fibrosis. J Clin Investig. 2003;112(12):1776‐1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 72. Wang X, Ling MT, Guan XY, et al. Identification of a novel function of TWIST, a bHLH protein, in the development of acquired taxol resistance in human cancer cells. Oncogene. 2004;23(2):474‐482. [DOI] [PubMed] [Google Scholar]
- 73. Kajiyama H, Shibata K, Terauchi M, et al. Chemoresistance to paclitaxel induces epithelial‐mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31(2):277‐283. [PubMed] [Google Scholar]
- 74. Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265‐273. [DOI] [PubMed] [Google Scholar]
- 75. Bagnato A, Rosano L. Understanding and overcoming chemoresistance in ovarian cancer: emerging role of the endothelin axis. Current Oncol. 2012;19(1):36‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rosano L, Cianfrocca R, Spinella F, et al. Acquisition of chemoresistance and EMT phenotype is linked with activation of the endothelin A receptor pathway in ovarian carcinoma cells. Clin Cancer Res. 2011;17(8):2350‐2360. [DOI] [PubMed] [Google Scholar]
- 77. Rosano L, Spinella F, Di Castro V, et al. Endothelin‐1 promotes epithelial‐to‐mesenchymal transition in human ovarian cancer cells. Can Res. 2005;65(24):11649‐11657. [DOI] [PubMed] [Google Scholar]
- 78. Santamaria PG, Moreno‐Bueno G, Portillo F, Cano A. EMT: present and future in clinical oncology. Mol Oncol. 2017;11(7):718‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jessy T. Immunity over inability: the spontaneous regression of cancer. J Nat Sci Biol Med. 2011;2(1):43‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329‐360. [DOI] [PubMed] [Google Scholar]
- 81. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235‐271. [DOI] [PubMed] [Google Scholar]
- 82. McCloskey CW, Rodriguez GM, Galpin K, Vanderhyden BC. Ovarian cancer immunotherapy: preclinical models and emerging therapeutics. Cancers. 2018;10(8):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21(2):233‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lohmueller J, Finn OJ. Current modalities in cancer immunotherapy: immunomodulatory antibodies, CARs and vaccines. Pharmacol Ther. 2017;178:31‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pakish JB, Jazaeri AA. Immunotherapy in gynecologic cancers: are we there yet? Curr Treat Options Oncol. 2017;18(10):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte‐associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100(14):8372‐8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med. 2012;366(26):2443‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti‐PD‐L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455‐2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor‐infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538‐18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Barnett JC, Bean SM, Whitaker RS, et al. Ovarian cancer tumor infiltrating T‐regulatory (T(reg)) cells are associated with a metastatic phenotype. Gynecol Oncol. 2010;116(3):556‐562. [DOI] [PubMed] [Google Scholar]
- 91. Gaillard SL, Secord AA, Monk B. The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gynecol Oncol Res Pract. 2016;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hartkopf AD, Fehm T, Wallwiener M, Lauer U. Oncolytic viruses to treat ovarian cancer patients—a review of results from clinical trials. Geburtshilfe Frauenheilkd. 2012;72(2):132‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yigit R, Massuger LF, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol. 2010;117(2):366‐372. [DOI] [PubMed] [Google Scholar]
- 94. Kuhn I, Bauzon M, Green N, Seymour L, Fisher K, Hermiston T. OvAd1, a novel, potent, and selective chimeric oncolytic virus developed for ovarian cancer by 3D‐directed evolution. Mol Ther Oncolytics. 2017;4:55‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Uusi‐Kerttula H, Davies JA, Thompson JM, et al. Ad5NULL‐A20: a tropism‐modified, alphavbeta6 Integrin‐selective oncolytic adenovirus for epithelial ovarian cancer therapies. Clin Cancer Res. 2018;24(17):4215‐4224. [DOI] [PubMed] [Google Scholar]
- 96. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti‐PD‐1 immunotherapy. Cell. 2017;170(6):1109‐1119.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Besser MJ, Shapira‐Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short‐term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16(9):2646‐2655. [DOI] [PubMed] [Google Scholar]
- 98. Dummer R, Hoeller C, Gruter IP, Michielin O. Combining talimogene laherparepvec with immunotherapies in melanoma and other solid tumors. Cancer Immunol Immunother. 2017;66(6):683‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Freedman RS, Edwards CL, Kavanagh JJ, et al. Intraperitoneal adoptive immunotherapy of ovarian carcinoma with tumor‐infiltrating lymphocytes and low‐dose recombinant interleukin‐2: a pilot trial. J Immunother Emphasis Tumor Immunol. 1994;16(3):198‐210. [DOI] [PubMed] [Google Scholar]
- 101. Santoiemma PP, Powell DJ Jr. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. 2015;16(6):807‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Power AT, Wang J, Falls TJ, et al. Carrier cell‐based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15(1):123‐130. [DOI] [PubMed] [Google Scholar]
- 103. Arulanandam R, Batenchuk C, Angarita FA, et al. VEGF‐mediated induction of PRD1‐BF1/Blimp1 expression sensitizes tumor vasculature to oncolytic virus infection. Cancer Cell. 2015;28(2):210‐224. [DOI] [PubMed] [Google Scholar]
- 104. Yang JC, Rosenberg SA. Adoptive T‐Cell therapy for cancer. Adv Immunol. 2016;130:279‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21(2):215‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene‐modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106‐6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kandalaft LE, Powell DJ Jr, Coukos G. A phase I clinical trial of adoptive transfer of folate receptor‐alpha redirected autologous T cells for recurrent ovarian cancer. J Transl Med. 2012;10:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lanitis E, Dangaj D, Hagemann IS, et al. Primary human ovarian epithelial cancer cells broadly express HER2 at immunologically‐detectable levels. PLoS ONE. 2012;7(11):e49829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chekmasova AA, Rao TD, Nikhamin Y, et al. Successful eradication of established peritoneal ovarian tumors in SCID‐Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res. 2010;16(14):3594‐3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Felder M, Kapur A, Gonzalez‐Bosquet J, et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014;13:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93(1):136‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yoon DH, Osborn MJ, Tolar J, Kim CJ. Incorporation of immune checkpoint blockade into chimeric antigen receptor T cells (CAR‐Ts): combination or built‐in CAR‐T. Int J Mol Sci. 2018;19(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pujade‐Lauraine E. New treatments in ovarian cancer. Ann Oncol. 2017;28(suppl_8):viii57‐viii60. [DOI] [PubMed] [Google Scholar]
- 114. Wiggans AJ, Cass GK, Bryant A, Lawrie TA, Morrison J. Poly(ADP‐ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer. Cochrane Database Syst Rev. 2015;(5):Cd007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ratner ES, Sartorelli AC, Lin ZP. Poly (ADP‐ribose) polymerase inhibitors: on the horizon of tailored and personalized therapies for epithelial ovarian cancer. Curr Opin Oncol. 2012;24(5):564‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yellepeddi VK, Vangara KK, Kumar A, Palakurthi S. Comparative evaluation of small‐molecule chemosensitizers in reversal of cisplatin resistance in ovarian cancer cells. Anticancer Res. 2012;32(9):3651‐3658. [PubMed] [Google Scholar]
- 117. Diab Y, Muallem MZ. Targeted therapy in ovarian cancer. A comprehensive systematic review of literature. Anticancer Res. 2017;37(6):2809‐2815. [DOI] [PubMed] [Google Scholar]
- 118. McCabe N, El-Helali A, Steele C, et al. Platinum based chemotherapy selects for PDGFRα dependent angiogenesis. J Clin Oncol. 2018;36(15_suppl):5578–5578. [Google Scholar]
- 119. Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018;81(1):17‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Rodriguez GM, Galpin K, McCloskey CW, Vanderhyden BC. The tumor microenvironment of epithelial ovarian cancer and its influence on response to immunotherapy. Cancers. 2018;10(8):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


