Abstract
The vascular system of the spinal cord is particularly complex and vulnerable. Damage to the main vessels or alterations to the regulation of blood flow will result in a reduction or temporary cessation of blood supply. The resulting tissue hypoxia may be brief: acute, or long lasting: chronic. Damage to the vascular system of the spinal cord will develop after a traumatic event or as a result of pathology. Traumatic events such as road traffic accidents, serious falls and surgical procedures, including aortic cross-clamping, will lead to an immediate cessation of perfusion, the result of which may not be evident for several days, but may have long-term consequences including neurodegeneration. Pathological events such as arterial sclerosis, venous occlusion and spinal cord compression will result in a progressive reduction of blood flow, leading to chronic hypoxia. While in some situations the initial pathology is exclusively vascular, recent research in neurodegenerative disease has drawn attention to concomitant vascular anomalies in disorders, including amyotrophic lateral sclerosis, spinal muscular atrophy and muscular sclerosis. Understanding the role of, and tissue response to, chronic hypoxia is particularly important in these cases, where inherent neural damage exacerbates the vulnerability of the nervous system to stressors including hypoxia.
Subject terms: Neurodegeneration, Spinal cord diseases
Facts
Damage to the spinal cord vascular system results in acute or chronic hypoxia.
Hypoxia causes progressive and irreversible damage, which can be initially difficult to detect.
Hypoxia is damaging to neurones, especially those already affected by intrinsic, neurological disease.
Open questions
What is the effect of chronic hypoxia on the nervous system?
Is undiagnosed chronic hypoxia a causative or confounding factor in neurodegenerative disease?
Should the functionality of the spinal cord vascular system be taken into account in the treatment of neurodegenerative disease?
Background
Neurodegenerative diseases encompass a wide range of disorders that have the deterioration and death of neurones in the central nervous system (CNS) as a common link. While often grouped together, the origin of the pathology is highly variable, and may be intrinsic, due to genetic factors, extrinsic, caused by external damage or a combination of risk factors. Even within the same disease, in cases like amyotrophic lateral sclerosis (ALS), there is a broad array of mutated genes, such as SOD-1 or SEXT, which result in the same symptomatology by which they are then grouped1,2. While neurone-specific alterations tend to be very disease specific, and research has shown that frequently there is some sort of peripheral pathology that exacerbates neural damage. For example, inflammation in ALS and multiple sclerosis, or microglial damage in brain pathologies such as Alzheimer’s, aggravates the underlying disease3,4. Recently, there has been significant research into vascular damage and the role of hypoxia in brain neurodegeneration in diseases such as Alzheimer’s and other forms of dementia. This line of research has not been explored in depth in relation to spinal cord neurodegeneration. However, the existing studies show great potential to provide further understanding of disease aetiology and risk factors, especially considering that they are relevant to inherited disease and traumatic injury of the spinal cord.
Spinal cord vascular system
Any research into the role of hypoxia in neurodegeneration in the spinal cord needs to consider whether the hypoxia is acute or chronic (Table 1). Acute hypoxia is short term, caused by a transient decrease in blood flow to an area, followed by a critical decrease in oxygen level. In contrast, chronic hypoxia results from a long-term reduction of normal oxygen levels5. Hypoxia studies also need to take into account the complexity of the vascular supply, and most importantly its particularly low efficiency6. The main vessel network develops during the first 6 months of embryonic development, and then its layout remains relatively unchanged to adulthood7. Extraspinal vessels (chiefly branches of the aorta8) are responsible for the majority of the blood flow that arrives into the system9. They connect with the intrinsic arterial system, which can be divided into a central and a peripheral system. The central system supplies mainly the grey matter through the sulcal arteries, which are longitudinally connected by the anterior spinal artery10. Depending on the type of radicular artery, they can also feed the dura mater or the nerve roots close to them, and sometimes they can be feeders of the anterior or posterior spinal arteries11. The white matter is mostly supplied by the peripheral system or pial network, which covers the exterior of the spinal cord, from where they branch perpendicularly into the cord. Similarly to the sulcal arteries, the pial network is connected longitudinally by the two posterior spinal arteries10. The posterior and anterior arteries are highly interconnected along the spinal cord at a capillary level12, with the main point of contact being at the termination of the cauda equina12,13 (Fig. 1).
Table 1.
Summary of the major causes of vascular damage to the spinal cord; the sites of initial damage and the long-term consequences of that damage
| Initial damage | Consequences | Key references | ||
|---|---|---|---|---|
| Hypoxia caused by traumatic events | Acute spinal cord hypoxia | Traumatic accidents (e.g. car accidents, falls), surgery can cut temporarily blood flow to the spinal cord | Neural necrosis within 6 h and up to 34–48 after hypoxic episode. Long-lasting damage, normally irreversible | Richards et al.23, Gravereaux et al.24, Ahuja et al.25, Kato et al.26, Long et al.38 |
| Long-term spinal cord compression | Damage to the spinal cord can result in chronic compression of the spinal cord paired with a prolonged decrease of the blood supply | Decrease of vascular microvasculature. Slow neural damage, eventually irreversible (after 9 weeks) | Cheng et al.37, Long et al.38, Kurokawa et al.39, Kasahara et al.40 | |
| Hypoxia due to chronic disease | Vascular alterations | Vascular pathology (e.g. arteriovenous fistulas) can result in a prolonged decrease of the blood supply | Similar to spinal cord compression. Shown to be damaging to oligodendrocytes (demyelination) | Hurst et al.49, Larsson et al.50, Jellemaet al.51, Duncombe et al.54, Shibata et al.57 |
| Motor neurone disease and muscular sclerosis | Vascular anomalies have been detected in some neurodegenerative diseases (e.g. ALS, SMA), resulting in alterations of the normal blood supply | Neural damage and demyelination likely to be increased. Potential negative effect in neurone-focused treatments | Somers et al.61, Zhong et al.62, Nobutoki and Ihara63, Miyazaki et al.64, Davies et al.72, Desai et al. n.d., Hua et al.81 |
Fig. 1. The blood supply of the spinal cord originates mainly from the aorta.
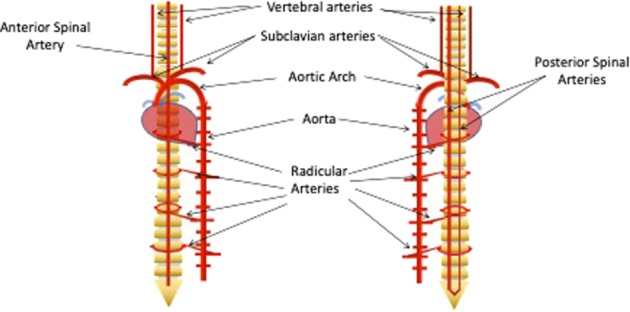
The network of vessels that surround the cord is connected by three main vessels: the anterior spinal artery and the two posterior spinal arteries
The spinal cord is particularly vulnerable to damage within the vascular network. While tissue oxygen levels at the spinal cord are the same as the oxygen levels in the brain (35–39 mmHg), the blood flow is between 40 and 60% lower11. The level of vulnerability may also vary depending on the spinal cord section. The cervical area is well supplied, and receives its main blood flow coming from radicular arteries branching from the vertebral and subclavian arteries. The superior extent of the upper anterior spinal artery is formed at this point by the union of the vessels arising from each vertebral artery, while the superior extent of the posterior spinal arteries are formed by anastomoses between branches of the vertebral arteries and posterior inferior cerebral arteries. The lower cervical spinal cord is supplied by a vessel originating from the deep cervical artery, which in turn originated from the costocervical trunk that branches from the subclavian artery14. The sacral sections have numerous connections to the lateral sacral arteries15. Both the thoracic and the lumbar areas feed mainly from segmental arteries branching directly from the aorta. However, the distance between the main points of entry into the spinal cord blood network is a lot greater in the thoracic area11.
The lower number of segmental arteries means that occlusion of any artery feeding into the thoracic spinal cord can potentially be significantly more damaging than in any other region, and that both the thoracic and lumbar areas are far more at risk in the event of aortic occlusion or damage. This is especially true of the artery of Adamkiewicz, the main radicular artery of the spinal cord. This artery branches from the aorta at a variable point in the lumbar or thoracic region, but most frequently between T9 and T12, and rather less frequently between T5 and T8 or L1 and L2. It passes though the intervertebral foramen, and makes a hairpin turn in the anterior spinal cord, where it anastomoses with the anterior spinal artery16. The occlusion of the artery of Adamkiewicz has been frequently shown to result in paraplegia17. Within the cord (Fig. 2), the capillary network density is always much higher in grey matter compared with white matter18. Grey matter artery density can also vary, as it is correlated to the metabolic demand of each region of the spinal cord19. Within grey matter, there is a decrease in vascularity at the ends of the posterior horns, their irrigation being decreased in comparison with the rest of the grey matter11. As motor neurones are situated in this area of lower vessel density, they are at higher risk than other cells of being affected by any alterations to the blood supply or any damage to the microvasculature. A low vessel density increases the likelihood of the collateral circulation being insufficient to protect the surrounding tissue from damage in the case of loss of perfusion, and therby contributes to the higher vulnerability of the tissue.
Fig. 2.
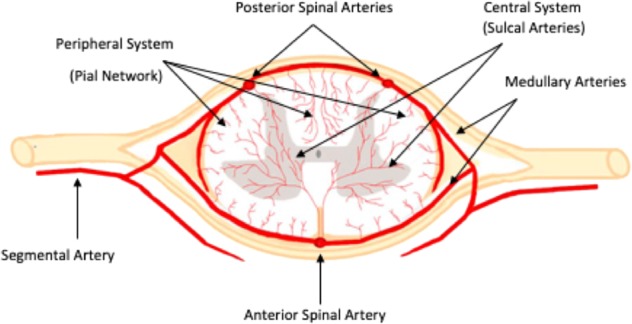
The three major blood vessels feed the medulla through the peripheral and central systems
Haemodynamics are also highly complex, and can make the consequences of vessel damage difficult to predict. Blood flow can be ascending or descending within the cord, which creates numerous watershed areas where opposing currents meet20. Watershed areas have a higher likelihood of becoming hypoxic during any event that results in nearby areas being subjected to abnormal pressure21. Again, this can be particularly damaging at the thoracic region, where the larger distance between radicular arteries results in larger watershed areas11. Blood flow can be regulated rapidly by mechanisms that regulate blood and tissue pressure, even changing its direction, but this regulation may not work correctly in an unhealthy spinal cord. The fragility of this system needs to be taken into consideration in cases of non-traumatic hypoxia. Chronic near- or actual hypoxic conditions may mean that any disease-related (acute) hypoxia may not induce a normal protective response, and could potentially aggravate degeneration due to improper regulation of cellular processes.
Acute hypoxia in spinal cord traumatic injuries
Traumatic injuries are one of the main potential causes of damage to the spinal cord. Spinal cord injuries affect thousands of people every year, and their effect can last a lifetime22. These injures are mainly caused by car accidents or falls, as well as violence and sport-related accidents, so they can affect a wide range of people23. Similarly, surgery involving vessels that supply the spinal cord, such as those clamped during surgery for thoracoabdominal aneurysms, generates a considerable risk of damage due to ischaemia resulting from the operation, with very similar consequences24. There are several factors in a traumatic injury that can lead to ischaemia in the spinal cord. Both the decrease/loss of perfusion and also autonomic nerve dysfunction can lead to loss of blood pressure regulation and excessive relaxation/dilation of blood vessels, generally resulting in acute hypotension. If the damage is significant and affects pulmonary function, oxygen levels in blood may also be reduced25. Even after a short (15–20 min) episode of hypoxia, neural necrosis will rapidly ensue within 6 h, and damaged cells will continue to die from apoptosis for 24–48 h26. This last apoptotic stage can be greatly aggravated by the cytokine cascades and microglia that were activated by the initial necrotic cell damage27. Although this damage can be long lasting, and normally irreversible, these changes due to acute hypoxia can be triggered by a very short period of initial hypoxic damage.
Chronic hypoxia due to vessel compression
While acute compression, which significantly reduces blood flow, can have a very similar outcome to a spinal cord injury28, chronic compression can be more difficult to detect but may still have long-term consequences. Chronic compression has been hypothesised as the origin of several myelopathies. Cervical myelopathies appear in elderly individuals and are one of the most common spinal cord disorders at late ages29. They are caused by compression of the spinal cord canal, generally caused by degeneration or abnormal ossification of the ligaments and vertebrae30. Unlike spinal cord injury, where the main damage is related to the main vessels, the vascular hypothesis of this disease considers damage to the microvasculature a more likely cause of the ischaemia31. Other myelopathies in this group include Surfer’s myelopathy, caused by hyperextension of the back due to the inadequate practice of the sport32, or Hirayama disease, a disorder that appears as progressive weakness and dysfunction of the upper body and limbs in young people33,34. It is also possible for spinal cord vascular compression to appear as a secondary effect of metastatic tumours35. Taken together, the incidence of these diseases is low; a study of Hirayama disease in Japan found only 333 cases between 1996 and 199836, and the prevalence of degenerative cervical myelopathy in the United States has been estimated at 4.04/100,000 people per year30. Consequently, most of the research on the effects of long-term spinal cord compression has been done in mouse models. Chronic compression has been shown to result in a decrease of vascular density37, especially of the microvasculature38, and to a significant decrease in spinal cord blood flow39. Damage of this kind can be very slow to develop and will eventually cause irreversible damage, but might be reversible if detected in the early stages. Studies on rats showed that neural damage could be recovered from when chronic compression was present for up to 6 weeks, but this capacity for recovery disappeared if the compression lasted longer than 9 weeks40. While early diagnosis for early relief of the compression would probably be the best option, for example with surgery41, diagnosis tends to happen after neurological symptoms have already appeared, which is by definition too late. This applies both to arterial and venous compression; while most research is focussed on the former, there is also related compression of the venous plexus at the cauda equina, which could be correlated with neurodegeneration, particularly with lumbar stenosis42–44.
Chronic hypoxia due to vascular alterations
Blood flow to the spinal cord can also be altered by vascular damage. These cases, while scarce, are particularly interesting due to the damage being caused exclusively by alterations of the blood flow. Both traumatic injuries and compression damage have a physical trauma factor that can affect neuronal well-being and alter the normal state of the surrounding tissue. The disarray of the normal blood distribution can have several causes. An unusual example would be a case where a myelopathy developed due to cholesterol-related arteriosclerosis45. Most cases are related to arteriovenous malformations, the most common being spinal–dural arteriovenous fistulas, with a prevalence of 5–10 cases per million46. This disorder appears in the form of vascular lesions at the spinal cord, sometimes due to genetic diseases, and generally in the thoracic area47,48. The lesions tend to result in alterations of normal blood flow, arterialisation of intramedullary veins and eventual ischaemia and necrosis49–51. This type of disease is frequently misdiagnosed, due to the symptoms being nerve-related (extremity weakness, pain and sensory malfunction48,52), and therefore confused with other forms of degenerative spinal disease48. While there is little research specific to spinal cord in this area, lately it has become more relevant in brain disease, particularly in small-vessel disease. Small-vessel disease effects tend to be long term. It is considered one of the main causes for vascular cognitive impairment, and is related to about 45% of new dementia cases were diagnosed globally every year53. Models for this disease focusing on hypoperfusion of the nervous tissue have shown that a decrease in the blood flow results in chronic hypoxia, particularly in the white matter54. It has also been shown to cause alterations in the blood-brain barrier and inflammation54–56. Particularly relevant for potential spinal cord research is that chronic hypoxia has been shown to be particularly damaging for oligodendrocytes and oligodendrocyte precursors, resulting in axon demyelination57.
Role of chronic hypoxia in motor neurone disease and muscular sclerosis
Alterations of the vascular system have proven to be sufficient to trigger neural pathology, suggesting that vascular alterations in diseases where neurones are already affected by other factors could be exacerbating the pathology. Two families of disease where this may be a factor are amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA). Both of these diseases commonly have their origin in genetic defects, whether there are several genes involved, as in ALS2, or just one, as in SMA58. They overlap in several aspects of the pathologies59,60, and vascular defects could also be a common trait61–64. Alterations in the superoxide dismutase-1 (SOD-1) gene are correlated with 20% of familial and 2% of sporadic cases of ALS65, which are known to affect the vascular system, particularly the microvasculature62. Histological analysis has shown alterations in the structure of the neurovascular unit64, which importantly, occur prior to neurone damage62,64. This in turn suggests that they may influence the onset of neurodegeneration in ALS, especially since cell death has been linked to an increase in hypoxia biomarkers66,67, and exposure to intermittent hypoxia advances disease progression in mouse models68. Similarly, histology of SMA spinal cords has shown a significant reduction in vascular density, along with hypoxia in motor neurone cell bodies61. It has also been speculated that astrocytic malfunction could affect capillary blood flow regulations, which along with high levels of vasoconstrictor hormones could increase the chances of chronic hypoperfusion63.
Besides ALS and SMA, there are other motor neurone diseases where chronic hypoxia could be a factor. The role of chronic hypoxia in axonal demyelination is of particular interest in demyelinating disease like multiple sclerosis (MS)69. Unlike ALS and SMA, the causes of MS are far from clear, but are thought to be a mix of complex genetic factors and external factors like Epstein–Barr virus infecton70,71. Research done in MS shows that not only can hypoxia result in damage following a similar pattern to the lesions in early disease, but that an animal model would show less damage under the same conditions if supplied with highly oxygenated breathing air (80–95% oxygen)72,73. This shows that chronic hypoxia likely plays a role in the disease, which fits with the findings of decreased vascularity in the brain74, even if there is no information about spinal cord vascularity. It also opens the possibility of highly oxygenated air used as a treatment to slow down the damage caused by MS.
These findings are likely also relevant in SMA, where a novel treatment has been recently approved for patients. SMA is the result of the homozygous mutation of the SMN1 gene, resulting in a failure to produce the survival motor neurone protein (SMN)58, which is necessary for survival beyond the embryonic stage75. SMA only occurs as a disease because of the existence of the survival motor neurone 2 (SMN2) gene, which is identical to SMN1 save for a single mutation at exon 7 that results in its exclusion during splicing. Due to this variation, only a small amount of functional SMN is produced by this SMN2 gene76. Multiple copies of the SMN2 gene allow for a sufficient amount of SMN protein to be produced to ensure survival, but not to ensure full health. The treatment consists of intrathecal injections of an antisense oligonucleotide that promotes inclusion of exon 7 in SMN2, resulting in higher levels of full-length SMN protein being produced in patient cells. While promising, the delivery by intrathecal injections means that the vascular system remains untreated77. SMA has always been considered a neurodegenerative disease, but SMN is a ubiquitous protein that is expressed in every cell and tissue78. More recently, considerable evidence has shown a significant systemic pathology61,79,80 that includes organ, blood cell and vascular alterations. Given the findings of vascular dysfunction at a pre-symptomatic stage, and considering that this treatment is delivered to very young children, future damage caused by chronic dysfunction of perfusion cannot be ignored. This is supported by mouse models of acute SMA, where peripheral delivery of therapeutics has been shown to be required for long-term effectiveness81.
Chronic and acute hypoxia response
In order to better predict the possible consequences of spinal cord hypoxia on neurones, it is necessary to consider the natural response of the organism to hypoxia. The basis of the hypoxia response is relatively well known. It is based on the promotor protein HIF1, whose activation depends on the α-subunit of the protein that accumulates when cellular oxygen levels fall82. HIF1 (Fig. 3) will trigger the production of a wide range of proteins among whose functions are regulation of apoptosis, metabolism and angiogenesis83, in a response time that can be as short as 30 min84. However, the HIF1 response tends to have a more significant role in the response to acute hypoxia. Its activation requires very low oxygen levels (around 1% O2), and within a relatively short period of time, <72 h, it is downregulated85. In chronic hypoxia, the main response appears to come from a different HIF form: HIF-2α. This protein appears during milder hypoxia (around 5% O2)85 and remains active for a much longer period of time86 during which its major function seems to be in promoting vascular development87 and increasing erythropoiesis88. Understanding the nature of this response is especially important in cases where the vascular system has already been shown to malfunctioning51,62,64. In such cases, the main response of the organism to ameliorate damage may be completely ineffective, especially in diseases like ALS, where the HIF1 response has been shown to be defective in monocytes, suggesting that the hypoxia molecular response could be affected89. It has been shown that dysregulation of HIF1 can result in a decrease of vascular endothelial growth factor (VEGF) expression, with a consequent decrease in angiogenesis90,91. Outside of disease, the main situation where an organism needs to adapt to lower-than-normal oxygen conditions is related to changes in altitude92. Research in this area has shown that the first response in the brain is to increase blood flow93, followed by an increase of red cell volume92 and an increase in angiogenesis94. Assuming that the same pattern occurs in the spinal cord, it is hard to say what could happen in diseases where vascular development and blood flood regulation are already affected.
Fig. 3.
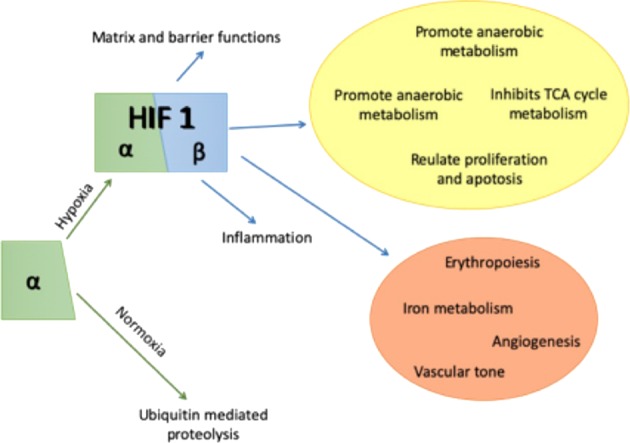
Hypoxia induces a HIF-1 mediated cellular response that involves a large range of cellular and systemic functions related to neuronal survival
Conclusion
Research on neurodegeneration is frequently centred on neurone-specific responses and processes. However, it is necessary to remember that regulation of the environment where the neurones are located depends significantly on the tissues surrounding them. Vascular tissue has been a significant focus for research in brain neurodegeneration95,96, yet there is still little research in this area with respect to spinal cord neurodegeneration. Considering the well-established fact that poor blood distribution and ischaemia alone can cause neural damage51, it is likely that the vascular system in its entirety (heart, vessels and circulating cells) is a factor that must be carefully considered in any neurodegenerative disease. This is especially relevant in diseases like ALS or SMA, where neurones have already been debilitated by other factors that increase their vulnerability to hypoxic stress.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by A. Yaron
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kenna KP, et al. Delineating the genetic heterogeneity of ALS using targeted high-throughput sequencing. J. Med. Genet. 2013;50:776–783. doi: 10.1136/jmedgenet-2013-101795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat. Rev. Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 3.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streit WJ, Braak H, Xue Q-S, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 2009;118:475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayer C, Shi K, Astner ST, Maftei C-A, Vaupel P. Acute versus chronic hypoxia: why a simplified classification is simply not enough. Int. J. Radiat. Oncol. Biol. Phys. 2011;80:965–968. doi: 10.1016/j.ijrobp.2011.02.049. [DOI] [PubMed] [Google Scholar]
- 6.Bosmia AN, Hogan E, Loukas M, Tubbs RS, Cohen-Gadol AA. Blood supply to the human spinal cord: Part I. Anatomy and hemodynamics. Clin. Anat. 2015;28:52–64. doi: 10.1002/ca.22281. [DOI] [PubMed] [Google Scholar]
- 7.Gillilan LA. The arterial blood supply of the human spinal cord. J. Comp. Neurol. 1958;110:75–103. doi: 10.1002/cne.901100104. [DOI] [PubMed] [Google Scholar]
- 8.Hoehmann, C. L., Hitscherich, K. & Cuoco, J. The artery of Adamkiewicz: vascular anatomy, clinical significance and surgical considerations. Artic. Int. J. Cardiovasc. Res. 10.4172/2324-8602.1000284 (2016).
- 9.Kawaharada N, et al. Magnetic resonance angiographic localization of the artery of Adamkiewicz for spinal cord blood supply. Ann. Thorac. Surg. 2004;78:846–851. doi: 10.1016/j.athoracsur.2004.02.085. [DOI] [PubMed] [Google Scholar]
- 10.Melissano G, et al. Angio-CT imaging of the spinal cord vascularisation: a pictorial essay. Eur. J. Vasc. Endovasc. Surg. 2010;39:436–440. doi: 10.1016/j.ejvs.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Martirosyan NL, et al. Blood supply and vascular reactivity of the spinal cord under normal and pathological conditions. J. Neurosurg. Spine. 2011;15:238–251. doi: 10.3171/2011.4.SPINE10543. [DOI] [PubMed] [Google Scholar]
- 12.Romanes GJ. The arterial blood supply of the human spinal cord. Paraplegia. 1965;2:199–207. doi: 10.1038/sc.1964.37. [DOI] [PubMed] [Google Scholar]
- 13.Singh, U., Siver, J. R., Welply, J. C., Silver, J. R. & Welply, N. C. Hypotensive infarction of the spinal cord. Spinal Cord32, 314–322 (1994). [DOI] [PubMed]
- 14.Turnbull IM. Blood supply of the spinal cord: normal and pathological considerations. Neurosurgery. 1973;20:56–84. doi: 10.1093/neurosurgery/20.CN_suppl_1.56. [DOI] [PubMed] [Google Scholar]
- 15.Dommisse GF. The blood supply of the spinal cord: a critical vascular zone in spinal surgery. J. Bone Jt. Surg. Br. 1974;56-B:225–235. doi: 10.1302/0301-620X.56B2.225. [DOI] [PubMed] [Google Scholar]
- 16.Charles YP, Barbe B, Beaujeux R, Boujan F, Steib JP. Relevance of the anatomical location of the Adamkiewicz artery in spine surgery. Surg. Radiol. Anat. 2011;33:3–9. doi: 10.1007/s00276-010-0654-0. [DOI] [PubMed] [Google Scholar]
- 17.Fried LC, Aparicio O. Experimental ischemia of the spinal cord: Histologic studies after anterior spinal artery occlusion. Neurology. 2012;23:289–289. doi: 10.1212/WNL.23.3.289. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z-A, Nonaka H, Hatori T. The microvasculature of the spinal cord in the human adult. Neuropathology. 1997;17:32–42. doi: 10.1111/j.1440-1789.1997.tb00008.x. [DOI] [Google Scholar]
- 19.Shamji MF, Maziak DE, Shamji FM, Ginsberg RJ, Pon R. Circulation of the spinal cord: an important consideration for thoracic surgeons. Ann. Thorac. Surg. 2003;76:315–321. doi: 10.1016/S0003-4975(03)00139-5. [DOI] [PubMed] [Google Scholar]
- 20.Jellinger K. Circulation disorders of the spinal cord. Acta Neurochir. 1972;26:327–337. doi: 10.1007/BF01407076. [DOI] [PubMed] [Google Scholar]
- 21.Bolton B. The blood supply of the human spinal cord. J. Neurol. Psychiatry. 1939;2:137. doi: 10.1136/jnnp.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerhart KA, Bergstrom E, Charlifue SW, Menter RR, Whiteneck GG. Long-term spinal cord injury: functional changes over time. Arch. Phys. Med. Rehabil. 1993;74:1030–1034. doi: 10.1016/0003-9993(93)90057-H. [DOI] [PubMed] [Google Scholar]
- 23.Richards C, MacKenzie N, Roberts S, Escorpizo R. People with spinal cord injury in the United States. Am. J. Phys. Med. Rehabil. 2017;96:S124–S126. doi: 10.1097/PHM.0000000000000620. [DOI] [PubMed] [Google Scholar]
- 24.Gravereaux EC, et al. Risk of spinal cord ischemia after endograft repair of thoracic aortic aneurysms. J. Vasc. Surg. 2001;34:997–1003. doi: 10.1067/mva.2001.119890. [DOI] [PubMed] [Google Scholar]
- 25.Ahuja CS, Martin AR, Fehlings M. Recent advances in managing a spinal cord injury secondary to trauma. F1000Research. 2016;5:F1000Research. doi: 10.12688/f1000research.7586.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato H, et al. Neuronal apoptosis and necrosis following spinal cord ischemia in the rat. Exp. Neurol. 1997;148:464–474. doi: 10.1006/exnr.1997.6707. [DOI] [PubMed] [Google Scholar]
- 27.Gong G, et al. Glycyrrhizin attenuates rat ischemic spinal cord injury by suppressing inflammatory cytokines and HMGB1. Acta Pharmacol. Sin. 2012;33:11–18. doi: 10.1038/aps.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivlin AS, Tator CH. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg. Neurol. 1978;10:38–43. [PubMed] [Google Scholar]
- 29.Young WF. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. Am. Fam. Physician. 2000;62:1064–1070. [PubMed] [Google Scholar]
- 30.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy. Spine. 2015;40:E675–E693. doi: 10.1097/BRS.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 31.Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60:S1-35–S1-41. doi: 10.1227/01.NEU.0000215383.64386.82. [DOI] [PubMed] [Google Scholar]
- 32.Shuster A, Franchetto A. Surfer’s myelopathy—an unusual cause of acute spinal cord ischemia: a case report and review of the literature. Emerg. Radiol. 2011;18:57–60. doi: 10.1007/s10140-010-0913-8. [DOI] [PubMed] [Google Scholar]
- 33.Hirayama K, et al. Focal cervical poliopathy causing juvenile muscular atrophy of distal upper extremity: a pathological study. J. Neurol. Neurosurg. Psychiatry. 1987;50:285–290. doi: 10.1136/jnnp.50.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirayama K. Juvenile muscular atrophy of distal upper extremity (Hirayama disease) Intern. Med. 2008;39:283–290. doi: 10.2169/internalmedicine.39.283. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert RW, Kim J-H, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann. Neurol. 1978;3:40–51. doi: 10.1002/ana.410030107. [DOI] [PubMed] [Google Scholar]
- 36.Tashiro K, et al. Nationwide survey of juvenile muscular atrophy of distal upper extremity (Hirayama disease) in Japan. Amyotroph. Lateral Scler. 2006;7:38–45. doi: 10.1080/14660820500396877. [DOI] [PubMed] [Google Scholar]
- 37.Cheng X, et al. Three-dimensional alteration of cervical anterior spinal artery and anterior radicular artery in rat model of chronic spinal cord compression by micro-CT. Neurosci. Lett. 2015;606:106–112. doi: 10.1016/j.neulet.2015.08.050. [DOI] [PubMed] [Google Scholar]
- 38.Long H-Q, et al. Value of micro-CT for monitoring spinal microvascular changes after chronic spinal cord compression. Int. J. Mol. Sci. 2014;15:12061–12073. doi: 10.3390/ijms150712061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurokawa R, Murata H, Ogino M, Ueki K, Kim P. Altered blood flow distribution in the rat spinal cord under chronic compression. Spine. 2011;36:1006–1009. doi: 10.1097/BRS.0b013e3181eaf33d. [DOI] [PubMed] [Google Scholar]
- 40.Kasahara K, Nakagawa T, Kubota T. Neuronal loss and expression of neurotrophic factors in a model of rat chronic compressive spinal cord injury. Spine. 2006;31:2059–2066. doi: 10.1097/01.brs.0000231893.21964.f2. [DOI] [PubMed] [Google Scholar]
- 41.Nemani VM, Kim HJ, Piyaskulkaew C, Nguyen JT, Riew KD. Correlation of cord signal change with physical examination findings in patients with cervical myelopathy. Spine (Phila. Pa. 1976). 2015;40:6–10. doi: 10.1097/BRS.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 42.Olmarker K, Rydevik B, Holm S, Bagge U. Effects of experimental graded compression on blood flow in spinal nerve roots. A vital microscopic study on the porcine cauda equina. J. Orthop. Res. 1989;7:817–823. doi: 10.1002/jor.1100070607. [DOI] [PubMed] [Google Scholar]
- 43.Manaka M, Komagata M, Endo K, Imakiire A. Assessment of lumbar spinal canal stenosis by magnetic resonance phlebography. J. Orthop. Sci. 2003;8:1–7. doi: 10.1007/s007760300000. [DOI] [PubMed] [Google Scholar]
- 44.Ju J-H, et al. Patterns of epidural venous varicosity in lumbar stenosis. Korean J. Spine. 2012;9:244. doi: 10.14245/kjs.2012.9.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heigl F, Hettich R, Mauch E, Klingel R, Fassbender C. Lipoprotein(a)-hyperlipoproteinemia as cause of chronic spinal cord ischemia resulting in progressive myelopathy - successful treatment with lipoprotein apheresis. Clin. Res. Cardiol. Suppl. 2017;12:50–54. doi: 10.1007/s11789-017-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koch C. Spinal dural arteriovenous fistula. Curr. Opin. Neurol. 2006;19:69–75. doi: 10.1097/01.wco.0000200547.22292.11. [DOI] [PubMed] [Google Scholar]
- 47.Krings T, Geibprasert S. Spinal dural arteriovenous fistulas. AJNR Am. J. Neuroradiol. 2009;30:639–687. doi: 10.3174/ajnr.A1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maimon, S., Luckman, Y. & Strauss, I. in Advances and tEchnical Standards in Neurosurgery 111–137 (Springer, Cham, 2016). [DOI] [PubMed]
- 49.Hurst RW, Kenyon LC, Lavi E, Raps EC, Marcotte P. Spinal dural arteriovenous fistula: the pathology of venous hypertensive myelopathy. Neurology. 1995;45:1309–1313. doi: 10.1212/WNL.45.7.1309. [DOI] [PubMed] [Google Scholar]
- 50.Larsson, E. M., Desai, P., Hardin, C. W., Story, J. & Jinkins, J. R. Venous infarction of the spinal cord resulting from dural arteriovenous fistula: MR imaging findings. Am. J. Neuroradiol.12, 739–743 (1991). [PMC free article] [PubMed]
- 51.Jellema K, Tijssen CC, Gijn JVan. Spinal dural arteriovenous fistulas: a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain. 2006;129:3150–3164. doi: 10.1093/brain/awl220. [DOI] [PubMed] [Google Scholar]
- 52.Donghai W, et al. The diagnosis of spinal dural arteriovenous fistulas. Spine. 2013;38:E546–E553. doi: 10.1097/BRS.0b013e31828a38c4. [DOI] [PubMed] [Google Scholar]
- 53.Horsburgh K, et al. Small vessels, dementia and chronic diseases—molecular mechanisms and pathophysiology. Clin. Sci. 2018;132:851–868. doi: 10.1042/CS20171620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duncombe J, et al. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin. Sci. 2017;131:2451–2468. doi: 10.1042/CS20160727. [DOI] [PubMed] [Google Scholar]
- 55.Seo JH, et al. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J. Clin. Invest. 2013;123:782–786. doi: 10.1172/JCI65863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holland PR, et al. Gliovascular disruption and cognitive deficits in a mouse model with features of small vessel disease. J. Cereb. Blood Flow. Metab. 2015;35:1005–1014. doi: 10.1038/jcbfm.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- 58.Lefebvre S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 59.Yamazaki T, et al. FUS-SMN protein interactions link the motor neuron diseases ALS and SMA. Cell Rep. 2012;2:799–806. doi: 10.1016/j.celrep.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsuiji H, et al. Spliceosome integrity is defective in the motor neuron diseases ALS and SMA. EMBO Mol. Med. 2013;5:221–234. doi: 10.1002/emmm.201202303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Somers E, et al. Vascular defects and spinal cord hypoxia in spinal muscular atrophy. Ann. Neurol. 2016;79:217–230. doi: 10.1002/ana.24549. [DOI] [PubMed] [Google Scholar]
- 62.Zhong Z, et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nobutoki T, Ihara T. Early disruption of neurovascular units and microcirculatory dysfunction in the spinal cord in spinal muscular atrophy type I. Med. Hypotheses. 2015;85:842–845. doi: 10.1016/j.mehy.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 64.Miyazaki K, et al. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J. Neurosci. Res. 2011;89:718–728. doi: 10.1002/jnr.22594. [DOI] [PubMed] [Google Scholar]
- 65.Taylor JP, Brown RH, Cleveland DW. Decoding ALS: from genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu R, et al. Linking hypoxic and oxidative insults to cell death mechanisms in models of ALS. Brain Res. 2011;1372:133–144. doi: 10.1016/j.brainres.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 67.Nomura E, et al. Imaging hypoxic stress and the treatment of amyotrophic lateral sclerosis with dimethyloxalylglycine in a mice model. Neuroscience. 2019;415:31–43. doi: 10.1016/j.neuroscience.2019.06.025. [DOI] [PubMed] [Google Scholar]
- 68.Kim S-M, et al. Intermittent hypoxia can aggravate motor neuronal loss and cognitive dysfunction in ALS mice. PLoS ONE. 2013;8:e81808. doi: 10.1371/journal.pone.0081808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu. Rev. Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 70.Margarit BP, Monteiro GC, Herán IS, Delgado FR, Izquierdo AY. Esclerosis múltiple. Medicine-Programa de Formación Médica Continuada Acreditado. 2019;12:4587–4597. doi: 10.1016/j.med.2019.05.010. [DOI] [Google Scholar]
- 71.Veroni C, Serafini B, Rosicarelli B, Fagnani C, Aloisi F. Transcriptional profile and Epstein-Barr virus infection status of laser-cut immune infiltrates from the brain of patients with progressive multiple sclerosis. J. Neuroinflammation. 2018;15:18. doi: 10.1186/s12974-017-1049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davies AL, et al. Neurological deficits caused by tissue hypoxia in neuroinflammatory disease. Ann. Neurol. 2013;74:815–825. doi: 10.1002/ana.24006. [DOI] [PubMed] [Google Scholar]
- 73.Desai RA, et al. Cause and prevention of demyelination in a model multiple sclerosis lesion. Ann. Neurol. 2016;79:591–604. doi: 10.1002/ana.24607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zivadinov R, et al. Decreased brain venous vasculature visibility on susceptibility-weighted imaging venography in patients with multiple sclerosis is related to chronic cerebrospinal venous insufficiency. BMC Neurol. 2011;11:128. doi: 10.1186/1471-2377-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monani UR, et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn(-/-) mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 2000;9:333–339. doi: 10.1093/hmg/9.3.333. [DOI] [PubMed] [Google Scholar]
- 76.Monani UR, et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999;8:1177–1183. doi: 10.1093/hmg/8.7.1177. [DOI] [PubMed] [Google Scholar]
- 77.Kichula EA, Yum SW, Brandsema J. Spinal muscular atrophy: entering the treatment age. Curr. Pediatr. Rep. 2018;6:9–15. doi: 10.1007/s40124-018-0150-2. [DOI] [Google Scholar]
- 78.Groen EJN, et al. Temporal and tissue-specific variability of SMN protein levels in mouse models of spinal muscular atrophy. Hum. Mol. Genet. 2018;27:2851–2862. doi: 10.1093/hmg/ddy195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szunyogova E, et al. Survival motor neuron (SMN) protein is required for normal mouse liver development. Sci. Rep. 2016;6:34365. doi: 10.1038/srep34365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomson AK, et al. Survival of motor neurone protein is required for normal postnatal development of the spleen. J. Anat. 2017;230:337–346. doi: 10.1111/joa.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hua Y, et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature. 2011;478:123–126. doi: 10.1038/nature10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Déry M-AC, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int. J. Biochem. Cell Biol. 2005;37:535–540. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 83.Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 84.Ruscher K, et al. Induction of hypoxia inducible factor 1 by oxygen glucose deprivation is attenuated by hypoxic preconditioning in rat cultured neurons. Neurosci. Lett. 1998;254:117–120. doi: 10.1016/S0304-3940(98)00688-0. [DOI] [PubMed] [Google Scholar]
- 85.Holmquist-Mengelbier L, et al. Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 86.Henze AT, Acker T. Feedback regulators of hypoxia-inducible factors and their role in cancer biology. Cell Cycle. 2010;9:2749–2763. doi: 10.4161/cc.9.14.12249. [DOI] [PubMed] [Google Scholar]
- 87.Rankin EB, et al. Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene. 2008;27:5354–5358. doi: 10.1038/onc.2008.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J. Clin. Invest. 2007;117:862–865. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moreau C, et al. Deregulation of the hypoxia inducible factor-1α pathway in monocytes from sporadic amyotrophic lateral sclerosis patients. Neuroscience. 2011;172:110–117. doi: 10.1016/j.neuroscience.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 90.Rivard A, et al. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J. Biol. Chem. 2000;275:29643–29647. doi: 10.1074/jbc.M001029200. [DOI] [PubMed] [Google Scholar]
- 91.Oosthuyse B, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 92.Xu K, LaManna JC. Chronic hypoxia and the cerebral circulation. J. Appl. Physiol. 2006;100:725–730. doi: 10.1152/japplphysiol.00940.2005. [DOI] [PubMed] [Google Scholar]
- 93.Borgström L, Jóhannsson H, Siesjö BK. The relationship between arterial PO2 and cerebral blood flow in hypoxic hypoxia. Acta Physiol. Scand. 1975;93:423–432. doi: 10.1111/j.1748-1716.1975.tb05832.x. [DOI] [PubMed] [Google Scholar]
- 94.Boero JA, Ascher J, Arregui A, Rovainen C, Woolsey TA. Increased brain capillaries in chronic hypoxia. J. Appl. Physiol. 1999;86:1211–1219. doi: 10.1152/jappl.1999.86.4.1211. [DOI] [PubMed] [Google Scholar]
- 95.Román GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/S1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 96.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]


