Abstract
Endometriosis is a common gynaecological condition characterized by severe pelvic pain and/or infertility. The combination of nonspecific symptoms and invasive laparoscopic diagnostics have prompted researchers to evaluate potential biomarkers that would enable a non-invasive diagnosis of endometriosis. Endometriosis is an inflammatory disease thus different cytokines represent potential diagnostic biomarkers. As panels of biomarkers are expected to enable better separation between patients and controls we evaluated 40 different cytokines in plasma samples of 210 patients (116 patients with endometriosis; 94 controls) from two medical centres (Slovenian, Austrian). Results of the univariate statistical analysis showed no differences in concentrations of the measured cytokines between patients and controls, confirmed by principal component analysis showing no clear separation amongst these two groups. In order to validate the hypothesis of a more profound (non-linear) differentiating dependency between features, machine learning methods were used. We trained four common machine learning algorithms (decision tree, linear model, k-nearest neighbour, random forest) on data from plasma levels of proteins and patients’ clinical data. The constructed models, however, did not separate patients with endometriosis from the controls with sufficient sensitivity and specificity. This study thus indicates that plasma levels of the selected cytokines have limited potential for diagnosis of endometriosis.
Subject terms: Diagnostic markers, Diagnostic markers
Introduction
Endometriosis is a common benign gynaecological disease where endometrium like tissue is displaced and found outside the uterine cavity at ectopic locations. Endometriosis affects mainly women of reproductive age and is associated with pelvic pain and infertility1,2. Based on location of endometriotic lesions three types of endometriosis can be defined: ovarian, peritoneal and deep infiltrating endometriosis3,4. Laparoscopic visualization of the lesions followed by histological examination enables confirmation of endometriosis and according to the extent and location of lesions the disease is classified into four stages (minimal, mild, moderate and severe). The combination of non-specific symptoms and invasive laparoscopic procedure needed for the definitive diagnosis, results in up to 10 years of delay from the start of the symptoms to the definitive diagnosis of endometriosis1,5. Although several theories have been proposed that attempt to explain reasons for the clinical manifestation of endometriosis (metaplasia, transplantation), Sampson’s theory of retrograde menstruation still remains most widely accepted1,6–9. This theory states that the blood containing endometrial cells flows through the fallopian tubes into the pelvic cavity during menstruation, leading to ectopic endometrial lesions. Endometriosis is also an oestrogen-dependent and chronic inflammatory disease. Thus, several additional factors, such as impaired immune system, genetic and epigenetic predispositions as well as environmental factors were shown to play a role in determining whether an individual will develop the condition10. Endometrial tissue, which is displaced at different parts of the peritoneal cavity, induces inflammation. Inflammation is a complex process which is regulated by cytokines, a vast and diverse group of proteins that have a key role in the proliferation, activation of B cells, adhesion and cell chemotaxis. Cytokines via inflammation can therefore influence the onset and progression of endometriosis11. These proteins include growth factors, interferons, interleukins (IL) and chemokines12,13. Chemokines are a small (8–10 kDa) group of pro-inflammatory polypeptides and signal proteins as they induce chemotaxis and are involved in the inflammatory response. Based on the distance between the first two cysteine residues chemokines can be divided into four groups; namely C (γ chemokines), CC (β chemokines), CXC (α chemokines), and CX3C (δ chemokines). The CXC group of chemokines can be further subdivided according to the presence/absence of ELR (glutamic acid-leucine-arginine) motif14. Since cytokines and chemokines can be released into the bloodstream their plasma/serum concentrations can easily be determined and thus represent potential biomarkers for the non-invasive diagnosis of endometriosis. There have been several thorough review papers published by May et al., Rižner, Gupta et al. and Nisenblat et al. describing potential biomarkers for endometriosis, reported from 1984 to 201515–18. In addition, Borrelli et al. systematically reviewed published studies on chemokines as potential biomarkers of endometriosis where in total 27 different chemokines have been evaluated where the majority of the studies focused on the diagnostic potential of CXCL8, CCL2 and CCL519. The authors of these systematic reviews emphasized the importance of employing high quality standardized procedures when evaluating biomarkers for the diagnosis of endometriosis. Starting from sample collection and storage to collecting more detailed clinical data. These reviews emphasized also a need for multicentre validation studies performed on an independent set of patients from different populations.
In our previous study we evaluated the concentrations of 16 cytokines and other secretory proteins in peritoneal fluid and serum samples from patients with ovarian endometriosis, benign ovarian cysts and healthy women20. In peritoneal fluid the models with the highest diagnostic accuracies included: (i) IL-8 and the ratio of ficolin2 to glycodelin (ii) the ratio of biglycan to leptin and also the ratio of RANTES to IL-6; both in combination with age; the model with the highest diagnostic accuracy had an area under the curve (AUC) of 0.9. In serum the best characteristics were shown for models including: (i) the ratio between leptin and glycodelin and (ii) the ratio between ficolin2 and glycodelin; again both in combination with age; where the models with the highest diagnostic accuracies had a slightly lower AUC of 0.86 and 0.85, respectively20. The present study was performed on a different set of patient samples that were collected from two medical centres (Slovenian, Austrian) and included evaluation of 40 different cytokines - mainly chemokines in plasma samples. We decided to evaluate a different set of proteins from aforementioned studies in order to broaden the set of potential biomarkers for further validation studies that could include previous, as well as potential novel biomarkers. To the best of our knowledge this is the first study that evaluated such a broad spectrum of inflammatory proteins in plasma samples from a large, well-defined group of patients with different types of endometriosis. Aims of the present study were therefore to evaluate whether a single cytokine or combination of cytokines in a large, well-defined patient population can differentiate patients with endometriosis from control patients. If we identified cytokines with diagnostic potential we planned to design a diagnostic model with sufficient sensitivity and specificity, based on the plasma concentrations of cytokines and gathered patients’ clinical data, and with the use of appropriate statistical and bioinformatics analysis.
Materials and Methods
Study design and sample source
The prospective case-control study was approved by both (i.e. Slovenian and Austrian) National Medical Ethics Committees (0120-127/2016-2 and EMMA 545/2010, respectively) and all the participants signed their written informed consent before being included in the study. Inclusion criteria comprised endometriosis-like symptoms (i.e. infertility and/or pain) as well as benign gynaecological conditions (i.e. different types of cysts and/or myomas). Exclusion criteria included pregnancy, age below 18 or above 50 years, menopausal status, gynaecological malignancies, other types of cancer, cancelled operation, HIV infection and the presence of haemolysis in plasma samples. The aim was to collect approximately 200 samples, with approximately one-to-one ratio of patients and controls to achieve more than 80% statistical power (probability to reject null hypothesis if it is false) and less than 5% Type I error rate under assumption that mean concentrations of cytokines noticeably differ between conditions.
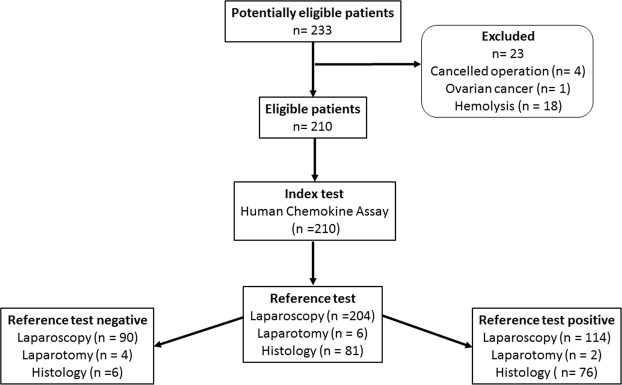
Patient enrolment took place from March 2013 to September 2016 at the Departments of Obstetrics and Gynaecology, University Medical Centre Ljubljana, Slovenia and the Medical University Vienna, Austria. At both Departments of Gynecology patients were recruited by senior gynecologists with the help of study nurses. Blood samples were analyzed in 2016. The time interval between recruitment/surgery and blood analysis (index test) was few weeks to 3 years. On the day of the surgery (Vienna) or one day to one week before surgery (Ljubljana) blood samples were collected according to a strict standard operating procedure. Blood samples of 4 ml were taken into BD Vacutainer tubes, (#368861, Becton Dickinson and Company, NJ, USA). Within one hour after collection the samples were centrifuged at 1400 g for 10 min at 4 °C. The plasma was aspirated and samples were aliquoted into 100 μL volumes and stored at −80 °C until analysis. Participants were interviewed regarding their ethnic origin, life style (i.e. diet, smoking status, sport and recreation, stress level), medical history especially with regards to different types of pain that are associated with endometriosis (pelvic pain, dysmenorrhea, dyschezia and dyspareunia) as well as medication intake a week prior to surgery, the use of oral contraceptives and hormonal therapy, current or in the three months prior to surgery. The intensity of dysmenorrhea and dyspareunia were evaluated using a validated visual analogue scale of 10 points. The reference test was laparoscopy (in exceptional cases laparotomy) with visualization of typical lesions and histological evaluation. Laparoscopy and laparotomy were performed by expert surgeons with at least ten years of experience. In total out of 233 patients 210 met inclusion criteria of whom 116 were laparoscopically (or by laparotomy) and histologically characterized by the presence of endometriosis and 94 by the absence of it (Table 1, Fig. 1).
Table 1.
Clinical characteristics of the study participants (§Mann-Whitney test for continuous variables and Fisher’s or Chi-square test for categorical variables; P values calculated by comparing controls to patients with endometriosis); ns, not significant.
| Characteristic | Subgroup | Controls n = 94 |
Patients with endometriosis n = 116 |
P-value§ | ||
|---|---|---|---|---|---|---|
| Frequency | [%] | Frequency | [%] | |||
| Age (years) | <26 | 17 | 18.1 | 17 | 14.7 | ns |
| 26–29.9 | 19 | 20.2 | 22 | 19.0 | ||
| 30–35.9 | 26 | 27.7 | 49 | 42.2 | ||
| 36–40.9 | 21 | 22.3 | 22 | 19.0 | ||
| >41 | 11 | 11.7 | 6 | 5.2 | ||
| BMI (kg/m2) | <18.5 | 3 | 3.2 | 11 | 9.5 | <0.05 |
| 18.6–24.9 | 59 | 62.8 | 78 | 67.2 | ||
| 25–29.9 | 25 | 26.6 | 16 | 13.8 | ||
| >30 | 7 | 7.4 | 11 | 9.5 | ||
| Smoking status | Nonsmoker | 45 | 47.9 | 68 | 58.6 | ns |
| Smoker | 31 | 33.0 | 29 | 25.0 | ||
| Occasional smoker | 5 | 5.3 | 6 | 5.2 | ||
| Former smoker | 12 | 12.8 | 12 | 10.3 | ||
| Missing data | 1 | 1.1 | 1 | 0.9 | ||
| Menstrual phase | Proliferative | 41 | 43.6 | 49 | 42.2 | ns |
| Secretory | 41 | 43.6 | 59 | 50.9 | ||
| Anovulatory | 2 | 2.1 | 0 | 0 | ||
| Oral contraceptives | 4 | 4.3 | 6 | 5.2 | ||
| Missing data | 6 | 6.4 | 2 | 1.7 | ||
| Hormonal therapy three months prior to surgery | No | 86 | 91.5 | 104 | 89.7 | ns |
| Yes | 8 | 8.5 | 12 | 10.3 | ||
| Missing data | 0 | 0 | 0 | 0 | ||
| Oral contraceptives three months prior to surgery | No | 83 | 88.3 | 101 | 87.1 | ns |
| Yes | 11 | 11.7 | 15 | 12.9 | ||
| Missing data | 0 | 0 | 0 | 0 | ||
| Medication intake a week prior to surgery | No | 55 | 58.5 | 62 | 53.4 | ns |
| Yes | 39 | 41.5 | 54 | 46.6 | ||
| Missing data | 0 | 0 | 0 | 0 | ||
|
Additional pathologies/conditions Cysts |
No | 63 | 67.0 | 102 | 87.9 | |
| <0.01 | ||||||
| Yes | 31 | 33.0 | 14 | 12.1 | ||
| Fallopian tube related | No | 79 | 84.0 | 113 | 97.4 | <0.01 |
| Yes | 15 | 16.0 | 3 | 2.6 | ||
| Uterus related | No | 86 | 91.5 | 107 | 92.2 | ns |
| Yes | 8 | 8.5 | 9 | 7.8 | ||
| Adenomyosis | No | 92 | 97.9 | 113 | 97.4 | ns |
| Yes | 2 | 2.1 | 3 | 2.6 | ||
| Adhesions | No | 80 | 85.1 | 89 | 76.7 | ns |
| Yes | 14 | 14.9 | 27 | 23.3 | ||
| Inflammation related conditions | No | 83 | 88.3 | 113 | 97.4 | <0.05 |
| Yes | 11 | 11.7 | 3 | 2.6 | ||
| Borderline ovarian tumour | No | 92 | 97.9 | 116 | 100 | ns |
| Yes | 2 | 2.1 | 0 | 0 | ||
Figure 1.
Flowchart of patient recruitment.
Additional pathologies/conditions were identified after the surgical procedure. The phase of the menstrual cycle was estimated based on the date of the last menstruation and the thickness, as well as appearance, of the endometrium determined by ultrasound. The study was designed to meet the principles of the Declaration of Helsinki (Ethical Principles for Medical Research Involving Human Subjects), Oviedo Convention (Protecting Human Rights in the Biomedical Field) and the Code of Medical Ethics.
Biomarker measurements
All methods were performed in accordance with the relevant guidelines and regulations. The Luminex xMAP multiplexing and the Bio-Plex Pro™ Human Chemokine Assay platforms (#171ak99mr2, lots: #64025638, #64040537 Bio-Rad Laboratories, CA, USA) were used according to the manufacturer’s protocol. Briefly, the method is based on 5.5 μm polystyrene beads that are labelled with two different fluorescent dyes in different ratios assigned for each individual antibody, thus enabling quantification of 40 different cytokines, mainly chemokines in each sample (Table 2). The intra assay and inter assay variability of the Human Chemokine Assay, as specified by the producer, was 2–6% CV and 2–8% CV, respectively. The samples were anonymized and the person performing the assays was blind to identity of the samples and the result of the surgery. According to the producers’ instruction manual plasma was diluted fourfold prior to analysis. Bio-Plex™ Manager Software with a 5-parameter logistic regression modelling was used to calculate final concentrations. Calibrations and verifications were performed prior to every analysis with the use of commercially available and recommended kits (MPX-PVER-K25, MPX-CAL-K25; Luminex, Austin, Texas, USA). Clinical data that were obtained from the patients (i.e. metadata) was included in the statistical modelling. The data were processed using Microsoft Excel 2003, and for statistical analysis we used GraphPad Prism Software version 5.00 for Windows (San Diego, CA, USA), R programming language21 version 3.4.3 (2017-11-30) – “Kite-Eating-Tree” and R Studio version 1.1.383 with packages such as mice, caret and ggplot2. Corrected P value of <0.05 was considered significant.
Table 2.
Plasma concentrations of the measured cytokines in pg/mL (with no significant differences between patients with endometriosis and controls).
| Controls; n = 94 | Patients with endometriosis; n = 116 | Controls; n = 94 | Patients with endometriosis; n = 116 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean ± SD | Range | Median | Mean ± SD | Range | Median | Mean ± SD | Range | Median | Mean ± SD | Range | ||
| CCL21 | 4812.6 | 5198.8 ± 1769.4 | 2491.1–11051.8 | 4966.4 | 5695.7 ± 2031.5 | 1870.2–14517.3 | IL-16 | 339.3 | 347.6 ± 111.7 | 97.9–609.3 | 341.7 | 351.4 ± 128.7 | 82.4–967.4 |
| CXCL13 | 17.4 | 26.1 ± 30.8 | 8.1–205.4 | 17.9 | 21.6 ± 18.4 | 3.6–157.5 | CXCL10 | 26.6 | 32.7 ± 24.7 | 13.3–226.2 | 26.5 | 31.3 ± 20.7 | 7.8–170.7 |
| CCL27 | 584.0 | 607.3 ± 239.3 | 82.8–1255.1 | 637.9 | 658.9 ± 265.2 | 172.7–1675.3 | CXCL11 | 109.7 | 138.0 ± 122.5 | 12.1–1068.74 | 122.9 | 141.1 ± 79.8 | 44.2–657.8 |
| CXCL5 | 165.1 | 168.4 ± 112.8 | 11.1–494.2 | 141.7 | 159.2 ± 108.8 | 6.83–440.6 | CCL22 | 586.5 | 626.9 ± 258.6 | 203.3–2024.0 | 578.6 | 598.9 ± 205.6 | 149.5–1236.6 |
| CCL11 | 76.0 | 78.2 ± 13.8 | 50.1–116.1 | 73.4 | 74.6 ± 13.0 | 46.5–104.6 | MIF | 955.4 | 1398.0 ± 1550.4 | 167.9–10307.6 | 830.0 | 1338.4 ± 1425.3 | 192.9–8217.7 |
| CCL24 | 98.4 | 122.2 ± 99.8 | 3.4–595. 3 | 89.9 | 117.7 ± 95.8 | 8.3–447.8 | CCL2 | 19.0 | 19.8 ± 7.4 | 3.9–47.0 | 17.5 | 18.6 ± 6.9 | 8.7–65.2 |
| CCL26 | 10.5 | 9.1 ± 5.2 | 0.1–20.9 | 8.9 | 8.3 ± 4.6 | 0.4–18.2 | CCL8 | 15.4 | 16.6 ± 8.2 | 3.9–72.4 | 15.7 | 15.9 ± 6.1 | 2.8–37.9 |
| CX3CL1 | 102.2 | 120.2 ± 83.2 | 56.7–695.2 | 99.6 | 108.1 ± 47.1 | 50.9–519.3 | CCL7 | 41.3 | 43.8 ± 11.6 | 29.2–103.5 | 39.2 | 41.1 ± 7.9 | 29.2–74.0 |
| CXCL6 | 20.0 | 21.3 ± 8.4 | 10.1–47.9 | 21.0 | 22.5 ± 9.3 | 8.5–59.3 | CCL13 | 17.7 | 23.4 ± 15.5 | 5.0–103.6 | 18.3 | 21.6 ± 11.9 | 6.9–66.0 |
| GMCSF | 5.7 | 5.9 ± 3.7 | 1.0–14.5 | 4.3 | 4.9 ± 3.6 | 1.0–10.6 | TNF-α | 14.2 | 15.1 ± 5.4 | 9.2–50.6 | 13.4 | 13.9 ± 2.8 | 6.4–23.6 |
| CXCL1 | 149.6 | 155.4 ± 33.0 | 92.5–322.7 | 145.2 | 148.5 ± 30.6 | 92.5–276.6 | CCL17 | 19.4 | 28.3 ± 26.5 | 6.4–168.6 | 22.5 | 27.9 ± 19.9 | 3.8–115.2 |
| CXCL2 | 56.3 | 86.0 ± 74.1 | 23.5–367.9 | 65.4 | 102.1 ± 90.8 | 16.9–426.9 | CCL25 | 259.0 | 256.2 ± 87.0 | 112.9–648.2 | 241.5 | 240.6 ± 69.7 | 112.9–545.1 |
| CCL1 | 44.2 | 44.7 ± 12.0 | 26.8–83.0 | 42.7 | 42.6 ± 10.4 | 24.6–71.0 | CXCL9 | 119.7 | 185.3 ± 422.9 | 50.6–4189.5 | 121.3 | 170.5 ± 359.2 | 68.9–3925.5 |
| IFN-γ | 1.8 | 1.9 ± 0.6 | 1.1–5.9 | 1.8 | 1.8 ± 0.4 | 1.1–3.6 | CCL3 | 5.2 | 5.6 ± 2.6 | 3.8–29.2 | 4.9 | 5.1 ± 0.8 | 3.6–7.6 |
| IL-1β | 10.3 | 13.4 ± 17.5 | 2.5–129.0 | 11.6 | 12.9 ± 13.2 | 1.5–143.4 | CCL15 | 4540.9 | 5893.6 ± 4562.9 | 1259.4–26163.1 | 4113.2 | 5262.9 ± 3497.7 | 893.2–21597.0 |
| IL-2 | 3.5 | 4.1 ± 5.0 | 1.3–50.1 | 3.5 | 3.4 ± 1.0 | 1.3–5.8 | CCL20 | 7.6 | 16.4 ± 56.0 | 3.6–512.1 | 7.4 | 8.9 ± 5.6 | 4.0–54.1 |
| IL-4 | 9.0 | 9.4 ± 3.2 | 2.7–19.4 | 10.0 | 9.7 ± 3.3 | 0.9–16.5 | CCL19 | 51.7 | 59.3 ± 37.3 | 24.9–284.8 | 48.3 | 54.6 ± 24.1 | 20.0–167.3 |
| IL-6 | 8.1 | 10.1 ± 8.4 | 3.4–76.8 | 8.1 | 8.2 ± 2.4 | 3.9–15.5 | CCL23 | 316.9 | 324.8 ± 155.1 | 19.2–681.2 | 339.0 | 332.3 ± 150.4 | 9.8–827.4 |
| IL-8 | 8.9 | 10.3 ± 7.6 | 4.1–76.8 | 8.9 | 9.0 ± 2.5 | 3.9–19.3 | CXCL16 | 372.4 | 384.8 ± 122.3 | 111.6–715.4 | 361.4 | 380.2 ± 130.7 | 130.1–799.8 |
| IL-10 | 18.9 | 20.7 ± 10.4 | 10.1–82.0 | 19.9 | 20.4 ± 8.6 | 7.0–73.3 | CXCL12 | 1164.7 | 1130.0 ± 305.8 | 423.8–2075.7 | 1189.1 | 1174.4 ± 308.5 | 552.3–2882.1 |
Statistics
For univariate statistical analysis two sided Wilcoxon rank-sum test (Mann-Whitney U test) was used to assess statistical significance of the difference in plasma concentrations of 40 different cytokines and chemokines between endometriosis patients (i.e. also according to the different types of endometriosis) and control group of women. Results of the univariate analysis were then also corrected according to Bonferroni’s correction for multiple testing. To assess the normality of the distributions Shapiro-Wilk test was used. Fisher’s exact and Chi-square tests were used for comparison of categorical variables. Results of the descriptive analysis (i.e. patient’s clinical data) were presented as mean ± standard deviation (SD) while the concentrations of the measured proteins were presented as median and also as mean ± SD (Tables 1 and 2, respectively). Before further analysis we excluded proteins with reported out of range concentrations (i.e. GM-CSF, CXCL5). Apart from the remaining single proteins additional variables were constructed which represented ratios of the protein’s concentrations. Batch effect between samples collected in different centres was identified with principle component analysis (PCA) and removed using mean-centring and normalisation of standard deviations of all protein features across samples from each batch.
Machine learning
Machine learning algorithms such as decision tree22, generalised linear model23, weighted k-nearest neighbour24 and random forest25 were applied to identify proteins or panels of proteins that would discriminate patients with endometriosis from the controls. R packages rpart, GLMNET, KKNN and RandomForest were used to implement aforementioned models. Selected machine learning methods represent very popular, however, intrinsically different classes of classification algorithms. Each employed method is sufficiently simple to produce interpretable results, but at the same time powerful enough to model complex and often non-linear interactions between input features. In order to ensure robustness of the reported results, 4-fold repeated cross-validation (4-fold repeated CV) technique has been used. For each classifier average accuracy across all the folds and repetitions were reported. Reported accuracy has been compared to the accuracy of the hypothetical random classifier trained on the same data to assess the diagnostic potential of the trained models. At times when number of samples was not equal in modelled groups, balanced accuracy which takes into account imbalanced representation of samples was applied instead of regular accuracy. We have included additional clinical data into our analysis such as the use of hormonal therapy and/or oral contraception three months prior to surgery, medication intake a week prior to surgery as potential important confounders or effect modifiers. The obtained metadata are included in the Table 1 and in the Supplementary Table S1.
Results
Characteristics of the patient’s cohorts
Our case group comprised 116 patients with different types of endometriosis (Tables 1 and S1). Staging of endometriosis was done according to the revised American Society for Reproductive Medicine classification3. Minimal to mild endometriosis was present in 72 patients (62%) and moderate to severe in 40 patients (35%) and for four (3%) patients the information regarding the extent of endometriosis was not known. Patients with endometriosis were 32 ± 6 years of age (range between 19 and 50 years) and with a body mass index (BMI) of 23 ± 5 kg/m2 (range between 16 and 50 kg/m2). According to the menstrual phase 59 patients (51%) were in their secretory and 49 (42%) in their proliferative phase (Table 1), six (5%) patients were on oral contraceptives at the time of the hospitalization, and for two (2%) patients this information was missing.
Patients with benign gynaecological conditions (i.e. different types of cysts and/or myoma), unexplained infertility and/or severe pain where laparoscopy excluded the presence of endometriosis totalled 94 controls. Controls were 32 ± 8 years of age (range between 18 and 50 years) and with a BMI of 24 ± 4 kg/m2 (range between 18 and 42 kg/m2). In total 41 (44%) controls were in secretory and the same number of controls were in proliferative phase of their menstrual cycle (Table 1) while four (4%) controls were taking oral contraceptives at the time of the surgery and for eight (8%) controls the information was missing or the phase of the menstrual cycle could not be determined.
Three months prior to surgery the vast majority of our study participants was not on hormonal therapy, only 8.5% controls and 10.3% of endometriosis patients used hormonal therapy (mainly progesterone and progestins), and additional 11.7% controls and 12.9% patients with endometriosis was on oral contraception (Table 1). A week before surgery 54 patients with endometriosis (47%) and 39 controls (42%) were taking medications, mostly analgesics, anti-inflammatory and anti-rheumatic products and psychoanaleptics. More than half of the patients with endometriosis (59%) and less than a half of controls (48%) were non-smokers (Table 1). Sport or recreation two days before surgery was reported for 39 patients with endometriosis (34%) and 19 (20%) controls.
The two study groups did not differ in age, menstrual phase, use of hormonal therapy and oral contraceptives three months prior to surgery, use of other medications a week before surgery, and smoking status. However, they differed in BMI distribution (P < 0.05), frequency of dysmenorrhea (P < 0.01), intensity of dysmenorrhea (P < 0.05) and in presence of additional pathologies/conditions such as fallopian tube related pathologies (P < 0.01), cysts (P < 0.01) and inflammation related conditions (P < 0.05). Most of the study participants (59%) were of Slovene or Austrian origin and all of the participants were of European descent. This clinical information is summarized in Tables 1 and S1.
Levels of cytokines in patients with endometriosis and in control
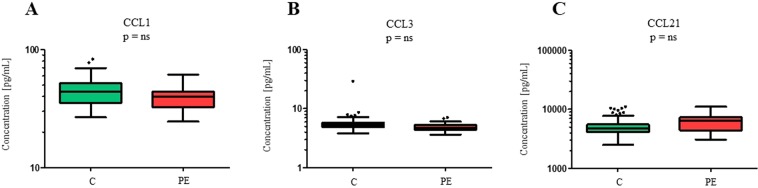
In all 210 plasma samples concentrations of all 40 different chemokines were measured (Table 2). Univariate statistical analysis revealed that there are no statistically significant differences in cytokine levels between all patients and controls. We also compared plasma concentrations of cytokines from patients with different types of endometriosis with controls where we identified eleven potential biomarkers for a specific type of endometriosis. In total we have identified seven potential biomarkers for peritoneal endometriosis (i.e. CCL21, CCL11, CCL26, CX3CL1, CCL1, IL-6, and CCL3), two for the presence of peritoneal and ovarian endometriosis (i.e. CXCL11, CXCL12), one for peritoneal and deep infiltrating endometriosis (i.e. IFN-γ) and two for all three types of endometriosis (i.e. CCL15, CXCL12). The most differential proteins for peritoneal endometriosis CCL1, CCL3 and CCL21 (Fig. 2) but after correction for multiple testing a boundary of the statistical significance was set at P < 0.001 and the differences in the concentrations of these proteins were not statistically significant.
Figure 2.
Box plots comparing plasma levels of the three cytokines that differ between the control group of patients and patients with peritoneal endometriosis in the univariate analysis. Plasma levels of cytokines are presented as Tukey box-and-whiskers plots with median, the box from the 25th to 75th percentiles, and whiskers correspond to the 25th percentile minus 1.5 times IQR (interquartile range) and to the 75th percentile plus 1.5 IQR. After correction for multiple testing no statistical difference (ns) was observed. Plasma concentrations of the cytokines are represented on a logarithmic scale. C, controls; PE, peritoneal endometriosis.
Analysis of cytokines by different machine learning approaches did not allow separation between cases and controls
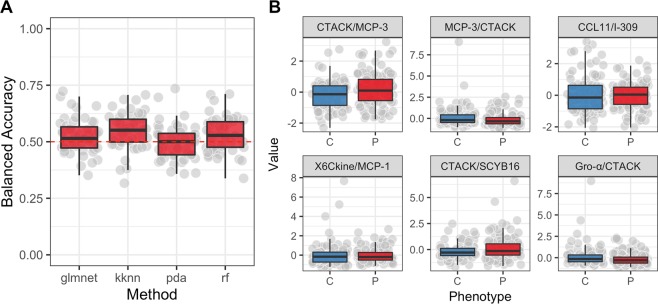
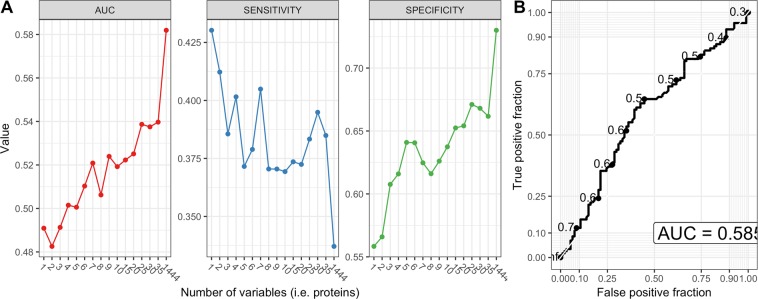
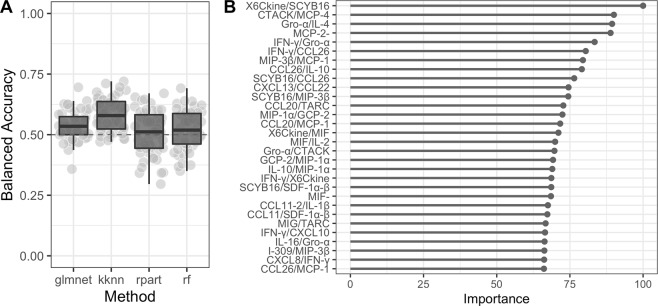
As more biomarkers potentially increase the reliability of a diagnostic test we decided to use machine learning to evaluate whether a panel of proteins with or without incorporation of metadata can differentiate among our two phenotypes. Results of the PCA showed there was no meaningful separation between patients with endometriosis and the controls based on the measured plasma levels of cytokines (Fig. 3). The highest average classification performance was achieved by the random forest algorithm (balanced accuracy = 59%, see Fig. 4a) with signal of six most influential features (i.e. proteins) illustrated as boxplots in Fig. 4. Obtained accuracy was not sufficiently different from random chance. Next, we trained the random forest model on different numbers of protein features to test a hypothesis that model trained on fewer proteins would generate better diagnostic characteristics (i.e. higher sensitivity, specificity and AUC) rather than using the whole panel of proteins and protein ratios at once. Results showed that a combination of three proteins would generate the highest combination of the selected diagnostic characteristics with a sensitivity of 40%, specificity of 65% and an AUC of 0.61 (Fig. 5), which, however is still far from being acceptable for diagnostics.
Figure 3.
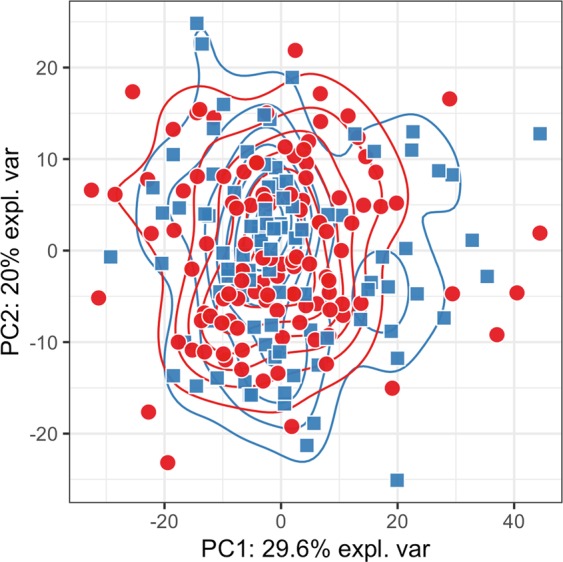
Principal component analysis plot. Data from the protein concentrations were scaled and normalized. The PCA plot is based on the whole protein set and coloured according to the disease status (red circles - patients with endometriosis; blue squares - controls). Transformed data show no meaningful grouping between patients with endometriosis and controls.
Figure 4.
Averaged classification performance of four classifiers and box plots for the selected features that were used for training a random forest model. (A) Four different classifiers were used based on the data from the training set with the highest average classification performance (i.e. accuracy) achieved with random forest (balanced accuracy of ~59%). (B) Box plots of the six most important features that were used for training a RandomForest model based on the training set and were the most differential between patients with endometriosis and controls. Red color designate patients with endometriosis and blue color controls. Machine Learning models used: glmnet, elastic-net regularized generalized models; kknn, Weighted k-Nearest Neighbors; rpart, Recursive Partitioning and Regression Trees; rf, RandomForest. Dashed red line indicates expected balanced accuracy of a random chance.
Figure 5.
Modelling results from the recursive feature elimination method. (A) Each dot that forms curves was chosen automatically by the random forest algorithm trained on the number of protein features specified by x-axis. The best performance was achieved by random forest that was trained on all 1444 protein concentrations or ratios of protein concentrations remaining after pre-processing which achieved AUC of 0.585 for all samples with a sensitivity of 34% and specificity of over 70%. (B) ROC curve based on the highest values of sensitivity, specificity and AUC. ROC, receiver operating characteristic; AUC, area under the curve.
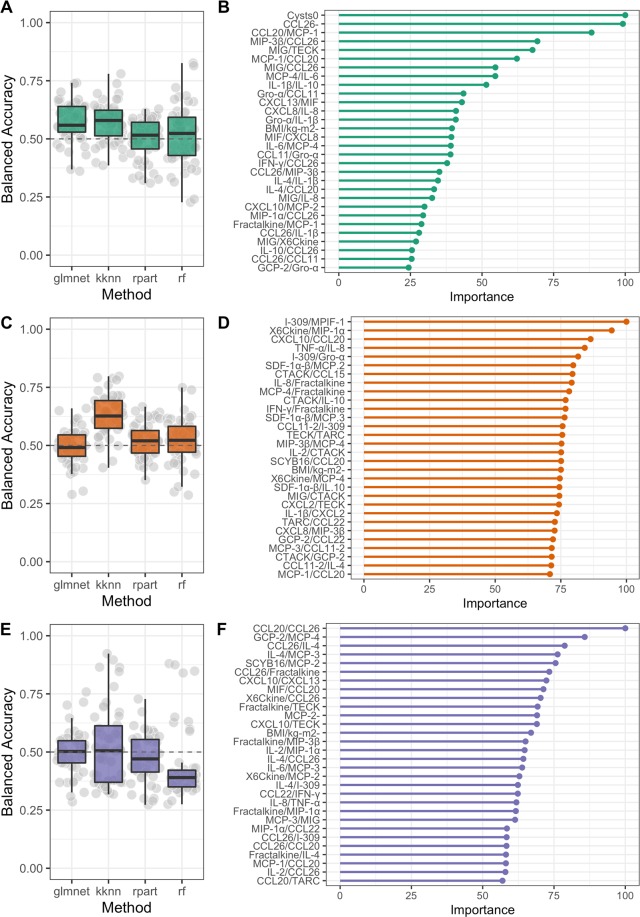
We then added metadata features (included in Tables 1 and S1) along with protein levels to the training data, but it did not improve the overall fit of the models and we could still not see a clear separation between patients with endometriosis and controls (Fig. 6). Performing separate analysis on individual types of endometriosis with the inclusion of metadata revealed no significant differences (Fig. 7). Comparing all four stages (minimal, mild, moderate and severe) of endometriosis as well as comparing minimal/mild with moderate/severe endometriosis with controls did not end up in significantly different features. Except for TNFα/CCL27 protein ratio that has been consistently reported by the random forest algorithm as the most valuable feature for separating patients with minimal/mild endometriosis from controls. However, despite high importance of TNFα/CCL27, the accuracy achieved by the algorithm remained modest (57.4%). We also did not observe any significant differences between patients and controls when divided with respect to the medication intake (use of any kind of medication or the use of nonsteroidal anti-inflammatory drugs or the use of any type of hormonal medication). Other personal and clinical data also showed no differences in the plasma profiles of the patients and controls.
Figure 6.
Modelling results after inclusion of metadata. (A) Random forest achieved the highest balanced accuracy on average (~0.55). (B) Both protein and metadata features ranked by their relative importance for RandomForest predictive performance. The last step was to evaluate if there is any clear separation between patients within individual types of endometriosis and controls with the inclusion of metadata. Results also showed that there is no improvement of discriminating performance of the classifiers if we look into individual type of endometriosis.
Figure 7.
Modelling results after inclusion of metadata for individual types of endometriosis and controls. Nested Cross-validation results for three machine learning methods on patients with ovarian (A,B) peritoneal (C,D) and deep infiltrating endometriosis (E,F) and control samples. 5-fold CV was repeated 10 times without any parameter learning or sharing allowed between the folds to ensure generalisation and robustness of the obtained models. Results suggest that machine learning models cannot differentiate between different types of endometriosis and controls with an accuracy that exceeds the one of random chance.
Discussion
Endometriosis is a common benign gynaecological condition that is characterized by the presence of endometrial lesions in the peritoneal cavity and is thus also described as a chronic inflammatory disease where diagnostic biomarkers that would be applicable for clinical use have not yet been identified16. Cytokines have already been investigated as potential biomarkers of endometriosis in the blood and/or peritoneal fluid. In addition to individual inflammatory proteins, also panels of cytokines in conjunction with other proteins have been studied, although the results of these studies varied16,19,26. The majority of these studies investigated IL-6 and INF-γ which are included in the activation and differentiation of inflammatory cells and are also involved in the pathogenesis of endometriosis27–30.
In the current study there were no statistically significant differences in cytokine plasma levels between patient with different types of endometriosis and controls. Although we found potential importance of the ratio TNFα/CCL27 for separating patients with minimal/mild endometriosis from the control group of patients, the accuracy achieved by the algorithm was insufficient. Univariate analysis revealed the lowest P values when we compared concentrations of cytokines/chemokines CCL1, CCL3, CCL21 in patients with peritoneal endometriosis and controls. We found no published studies evaluating blood levels of these cytokines/chemokines in patients with endometriosis Borrelli et al., evaluated the levels of CCL21 in the peritoneal fluid of 36 patients with endometriosis and 27 controls and reported no significant differences31. Other studies focused on the expression of the corresponding genes. Unchanged or changed expression (i.e. increased/decreased) in the endometrium from patients with endometriosis was reported for CCL21 with no explanation on how these changes might contribute to the aetiology or pathogenesis of endometriosis32–34. Expression and/or role of CCL1 and its receptor (i.e. CCR8) in endometrial tissue was studied by Shi et al.35,36 and revealed higher expression and their potential role in the pathogenesis of endometriosis. Although these three cytokines/chemokines identified in our study have so far not been sufficiently investigated in endometriosis our experimental data link changes in concentrations of these proteins to peritoneal endometriosis, which implies that CCL1, CCL3 and CCL21 might have a role in the aetiology and pathogenesis of this type of endometriosis.
The studies that evaluated blood concentrations of cytokines and chemokines as potential biomarkers of endometriosis are scarce, as the most studies so far evaluated the diagnostic potential of inflammatory proteins in peritoneal fluid and/or tissue samples (i.e. eutopic/ectopic endometrium) of patients with endometriosis. Kalu et al., evaluated a panel of 10 cytokines in peritoneal fluid and serum of women undergoing laparoscopy for unexplained infertility. Their study group comprised of women with minimal or mild endometriosis that were compared to the control group of women with unexplained infertility and absence of endometriosis. Elevated levels of CCL2, IL-8 and IL-6 were found in peritoneal fluid while the equivalent increase in serum samples was not found37. Similar study was later on conducted by Hassa et al. that evaluated the diagnostic potential of four cytokines (IL-2, IL-4, IL-10, IFN-γ) and immune cells in serum and peritoneal fluid of patients with endometriosis comparing to healthy group of patients38. No significant differences were observed when comparing control group with early and late stageendometriosis patients. Recently, Fan et al. evaluated seven cytokines including IL-10, IL-6, IL-4 and IL-2 in serum and peritoneal fluid from endometriosis patients and control patients and found significantly higher levels of IL-10 and lower levels of IL-2 in serum, but significantly higher levels of IL-2 in peritoneal fluid39. Amongstudies evaluating cytokines as blood biomarkers Rocha et al.40 followed a criteria for case-control studies where case and control groups originate from the same cohort41. In their study all of the patients presented with at least one endometriosis-like symptom (i.e. chronic pelvic pain and/or infertility and/or potential presence of endometrioma based on the ultrasound). After the laparoscopic operation and histological evaluation patients were divided into two groups; patients with endometriosis (n = 44) and control group of patients (n = 31). Concentrations of seven different cytokines (i.e. IL-2, IL-4, IL-6, IL-10, CCL2, CXCL10, and CCL11) were simultaneously determined using cytometric bead array and results showed that based on the panel of these cytokines and clinical data it was not possible to predict the presence of endometriosis in a group of symptomatic patients40.
Recently, Aalamat et al. published a systematic review on the use of multiplex technology for identification of potential novel biomarkers of endometriosis among inflammation associated proteins42. They reported that the majority of studies that adapted multiplex technology evaluated potential novel biomarkers of endometriosis in peritoneal fluid20,31,43–47. Although peritoneal fluid is collected by a semi-invasive method it is the most representative sample that closely reflects inflammatory changes that are associated with the pathogenesis of endometriosis48. Based on the literature and our published studies20, we conclude that cytokines and chemokines in peritoneal fluid have a far greater diagnostic potential for endometriosis than their plasma or serum concentrations. Results of our study are also in concordance with the study conducted by Lee et al. that evaluated the diagnostic potential of pro-inflammatory oxylipins and cytokines in serum samples of 103 women undergoing laparoscopy. Results of their study showed limited diagnostic potential of the measured circulating biomarkers for the diagnosis of endometriosis, warranting additional studies to evaluate the exact role of systemic inflammation in endometriosis49.
Although we evaluated a broad spectrum of inflammatory proteins in plasma samples of patients with different types of endometriosis and controls with several different multifactorial benign gynaecological conditions, both within a well-defined cohort, included detailed protocols, obtained a large set of clinical data, included different nationalities, combined with high throughput methodology and advanced statistical approaches, our results were consistent with several previous studies indicating limited diagnostic potential of circulating cytokines for the diagnosis of endometriosis. Having said this, presented results need to be considered carefully as they might be subject to various sources of bias and noise. Self-reporting of metadata by patients, undetected technical batch effects, unpredictable statistical fluctuations are all potential sources of bias and thus, limiting factors of the current study.
Conclusions
In this study we evaluated the diagnostic potential of 40 different cytokines in plasma samples from 210 patients with different types of endometriosis and control group of patients from two medical centres. Although several studies have associated inflammation with the development and progression of endometriosis, and inflammatory cytokines in endometrial tissue, peritoneal fluid and blood have been evaluated as potential biomarkers for endometriosis, the published results are inconsistent and identified no clinically useful biomarker to date. Based on the evaluated plasma concentrations of these 40 different cytokines, clinical data and appropriate statistical analysis we were unable to develop a diagnostic algorithm that would separate patients with endometriosis from the control group of patients with sufficient sensitivity and specificity. For development of a model with potential clinical applicability, which would enable diagnosis of patients with endometriosis with sufficient accuracy, further approaches of targeted and non-targeted “omics” technologies will be needed in conjunction with appropriate statistical/bioinformatics methods. These have to be followed by independent validation studies to confirm the results obtained in a research setting.
Supplementary information
Table S1: Detailed clinical characteristics of the study participants.
Acknowledgements
The authors thank their study participants, who kindly donated their samples and time. The authors thank the personnel of the Department of Obstetrics and Gynaecology, University Medical Centre Ljubljana, Ljubljana, Slovenia, and especially Tanja Lončar and Klara Primc, for their support in the enrolling of the study participants. The authors also thank Mrs. Vera Troha Poljančič and Prof. Dr. Joško Osredkar at the University Medical Centre Ljubljana, Clinical Institute of Clinical Chemistry and Biochemistry for processing the samples. The authors acknowledge Prof. Jaak Vilo at the University of Tartu for his guidance and support. D.F. and H.P. were supported by Estonian Research Council grants [PSG59, IUT34-4] and European Regional Development Fund through EXCITE Center of Excellence. The preparation of this manuscript was supported by grant J3-1755 from the Slovenian Research Agency to T.L.R.
Author contributions
T.K. carried out the experiments, helped with the clinical data and wrote the manuscript. A.V., M.G., R.W. enrolled patients into the study and gathered clinical data. D.F. carried out the statistical evaluation/analysis, prepared the figures and contributed to the manuscript. H.P. supervised statistical analysis and reviewed the manuscript. T.L.R. designed the study and contributed to writing the manuscript. All authors reviewed the final manuscript.
Data availability
All data are fully available without restriction.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tamara Knific and Dmytro Fishman.
Supplementary information
is available for this paper at 10.1038/s41598-019-52899-8.
References
- 1.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Burney Richard O. Biomarker development in endometriosis. Scandinavian Journal of Clinical and Laboratory Investigation. 2014;74(sup244):75–81. doi: 10.3109/00365513.2014.936692. [DOI] [PubMed] [Google Scholar]
- 3.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 67, 817–821 (1997). [DOI] [PubMed]
- 4.Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997;68:585–596. doi: 10.1016/S0015-0282(97)00191-X. [DOI] [PubMed] [Google Scholar]
- 5.Ahn SH, Singh V, Tayade C. Biomarkers in endometriosis: challenges and opportunities. Fertil. Steril. 2017;107:523–532. doi: 10.1016/j.fertnstert.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Sampson JA. The development of the implantation theory for the origin of peritoneal endometriosis. Am. J. Obstet. Gynecol. 1940;40:549–557. doi: 10.1016/S0002-9378(40)91238-8. [DOI] [Google Scholar]
- 7.Berkkanoglu M, Arici A. Immunology and endometriosis. Am. J. Reprod. Immunol. 2003;50:48–59. doi: 10.1034/j.1600-0897.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 8.Nap AW, Groothuis PG, Demir AY, Evers JL, Dunselman GA. Pathogenesis of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2004;18:233–244. doi: 10.1016/j.bpobgyn.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Olive DL, Schwartz LB. Endometriosis. N. Engl. J. Med. 1993;328:1759–1769. doi: 10.1056/NEJM199306173282407. [DOI] [PubMed] [Google Scholar]
- 10.Bulun SE. Endometriosis. N. Engl. J. Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 11.Hornung D, Bentzien F, Wallwiener D, Kiesel L, Taylor RN. Chemokine bioactivity of RANTES in endometriotic and normal endometrial stromal cells and peritoneal fluid. Mol. Hum. Reprod. 2001;7:163–168. doi: 10.1093/molehr/7.2.163. [DOI] [PubMed] [Google Scholar]
- 12.Riccio L, et al. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018 doi: 10.1016/j.bpobgyn.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Khan, M. M. Role of cytokines. In: Immunopharmacology. 33–59 (Springer, 2016).
- 14.Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 15.May KE, et al. Peripheral biomarkers of endometriosis: a systematic review. Hum. Reprod. Update. 2010;16:651–674. doi: 10.1093/humupd/dmq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rižner TL. Noninvasive biomarkers of endometriosis: myth or reality? Expert Rev. Mol. Diagn. 2014;14:365–385. doi: 10.1586/14737159.2014.899905. [DOI] [PubMed] [Google Scholar]
- 17.Nisenblat, V. et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev, CD012179, 10.1002/14651858.CD012179 (2016). [DOI] [PMC free article] [PubMed]
- 18.Gupta D, et al. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;4:CD012165. doi: 10.1002/14651858.CD012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borrelli GM, Abrao MS, Mechsner S. Can chemokines be used as biomarkers for endometriosis? A systematic review. Hum. Reprod. 2014;29:253–266. doi: 10.1093/humrep/det401. [DOI] [PubMed] [Google Scholar]
- 20.Kocbek V, Vouk K, Bersinger NA, Mueller MD, Rižner TL. Panels of cytokines and other secretory proteins as potential biomarkers of ovarian endometriosis. J. Mol. Diagn. 2015;17:325–334. doi: 10.1016/j.jmoldx.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Team, R. C. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2014).
- 22.Therneau, T., Atkinson B., Ripley, B. rpart: Recursive Partitioning and Regression Trees. R package version 4.1–11, https://CRAN.R-project.org/package=rpart (2017).
- 23.Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010;33:1–22. doi: 10.18637/jss.v033.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schliep, K. & Hechenbichler, K. kknn: Weighted k-Nearest Neighbors. R package version 1.3.1. https://CRAN.R-project.org/package=kknn (2016).
- 25.Liaw, A., Wiener, M. Classification and regression by randomForest. R news 2.3. 18–22 (2002).
- 26.May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum. Reprod. Update. 2011;17:637–653. doi: 10.1093/humupd/dmr013. [DOI] [PubMed] [Google Scholar]
- 27.Li S, et al. Role of Interleukin-6 and Its Receptor in Endometriosis. Med Sci Monit. 2017;23:3801–3807. doi: 10.12659/MSM.905226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitawaki J, et al. Interferon-gamma gene dinucleotide (CA) repeat and interleukin-4 promoter region (-590C/T) polymorphisms in Japanese patients with endometriosis. Hum. Reprod. 2004;19:1765–1769. doi: 10.1093/humrep/deh337. [DOI] [PubMed] [Google Scholar]
- 30.Chiang CM, Hill JA. Localization of T cells, interferon-gamma and HLA-DR in eutopic and ectopic human endometrium. Gynecol. Obstet. Invest. 1997;43:245–250. doi: 10.1159/000291866. [DOI] [PubMed] [Google Scholar]
- 31.Borrelli GM, Kaufmann AM, Abrao MS, Mechsner S. Addition of MCP-1 and MIP-3beta to the IL-8 appraisal in peritoneal fluid enhances the probability of identifying women with endometriosis. J. Reprod. Immunol. 2015;109:66–73. doi: 10.1016/j.jri.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Kopelman A, et al. Analysis of Gene Expression in the Endocervical Epithelium of Women With Deep Endometriosis. Reprod. Sci. 2016;23:1269–1274. doi: 10.1177/1933719116638179. [DOI] [PubMed] [Google Scholar]
- 33.Bellelis P, et al. Transcriptional changes in the expression of chemokines related to natural killer and T-regulatory cells in patients with deep infiltrative endometriosis. Fertil. Steril. 2013;99:1987–1993. doi: 10.1016/j.fertnstert.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 34.Chand AL, et al. Laser capture microdissection and cDNA array analysis of endometrium identify CCL16 and CCL21 as epithelial-derived inflammatory mediators associated with endometriosis. Reprod. Biol. Endocrinol. 2007;5:18. doi: 10.1186/1477-7827-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi YL, Luo XZ, Zhu XY, Li DJ. Combination of 17beta-estradiol with the environmental pollutant TCDD is involved in pathogenesis of endometriosis via up-regulating the chemokine I-309-CCR8. Fertil. Steril. 2007;88:317–325. doi: 10.1016/j.fertnstert.2006.11.129. [DOI] [PubMed] [Google Scholar]
- 36.Shi YL, et al. Effects of combined 17beta-estradiol with TCDD on secretion of chemokine IL-8 and expression of its receptor CXCR1 in endometriotic focus-associated cells in co-culture. Hum. Reprod. 2006;21:870–879. doi: 10.1093/humrep/dei414. [DOI] [PubMed] [Google Scholar]
- 37.Kalu E, et al. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J. Obstet. Gynaecol. Res. 2007;33:490–495. doi: 10.1111/j.1447-0756.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 38.Hassa H, Tanir HM, Tekin B, Kirilmaz SD, Sahin Mutlu F. Cytokine and immune cell levels in peritoneal fluid and peripheral blood of women with early- and late-staged endometriosis. Arch. Gynecol. Obstet. 2009;279:891–895. doi: 10.1007/s00404-008-0844-8. [DOI] [PubMed] [Google Scholar]
- 39.Fan YY, et al. Expression of inflammatory cytokines in serum and peritoneal fluid from patients with different stages of endometriosis. Gynecol Endocrinol. 2018;34:507–512. doi: 10.1080/09513590.2017.1409717. [DOI] [PubMed] [Google Scholar]
- 40.Rocha AL, Vieira EL, Maia LM, Teixeira AL, Reis FM. Prospective Evaluation of a Panel of Plasma Cytokines and Chemokines as Potential Markers of Pelvic Endometriosis in Symptomatic Women. Gynecol. Obstet. Invest. 2016;81:512–517. doi: 10.1159/000443956. [DOI] [PubMed] [Google Scholar]
- 41.Zondervan KT, Cardon LR, Kennedy SH. What makes a good case-control study? Design issues for complex traits such as endometriosis. Hum. Reprod. 2002;17:1415–1423. doi: 10.1093/humrep/17.6.1415. [DOI] [PubMed] [Google Scholar]
- 42.O DF, et al. Multiplex immunoassays in endometriosis: An array of possibilities. Front Biosci (Landmark Ed) 2017;22:479–492. doi: 10.2741/4496. [DOI] [PubMed] [Google Scholar]
- 43.Wickiewicz D, et al. Diagnostic accuracy of interleukin-6 levels in peritoneal fluid for detection of endometriosis. Arch. Gynecol. Obstet. 2013;288:805–814. doi: 10.1007/s00404-013-2828-6. [DOI] [PubMed] [Google Scholar]
- 44.Bersinger NA, Dechaud H, McKinnon B, Mueller MD. Analysis of cytokines in the peritoneal fluid of endometriosis patients as a function of the menstrual cycle stage using the Bio-Plex(R) platform. Arch. Physiol. Biochem. 2012;118:210–218. doi: 10.3109/13813455.2012.687003. [DOI] [PubMed] [Google Scholar]
- 45.Mier-Cabrera J, Jimenez-Zamudio L, Garcia-Latorre E, Cruz-Orozco O, Hernandez-Guerrero C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG. 2011;118:6–16. doi: 10.1111/j.1471-0528.2010.02777.x. [DOI] [PubMed] [Google Scholar]
- 46.Podgaec S, et al. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum. Reprod. 2007;22:1373–1379. doi: 10.1093/humrep/del516. [DOI] [PubMed] [Google Scholar]
- 47.Jorgensen H, et al. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil. Steril. 2017;107:1191–1199 e1192. doi: 10.1016/j.fertnstert.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 48.Rižner TL. Diagnostic potential of peritoneal fluid biomarkers of endometriosis. Expert Rev. Mol. Diagn. 2015;15:557–580. doi: 10.1586/14737159.2015.1015994. [DOI] [PubMed] [Google Scholar]
- 49.Lee YH, et al. Limited value of pro-inflammatory oxylipins and cytokines as circulating biomarkers in endometriosis - a targeted ‘omics study. Sci. Rep. 2016;6:26117. doi: 10.1038/srep26117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Detailed clinical characteristics of the study participants.
Data Availability Statement
All data are fully available without restriction.