Abstract
Several ongoing clinical trials are investigating the use of immuno-targeting therapy with programmed cell death protein-1 and programmed death-ligand 1 (PD-L1) inhibitors for triple-negative breast cancer. However, the role of PD-L1 expression in HER2-positive breast cancer remains unclear. We investigated the clinicopathological utility of PD-L1 expression in HER2-positive breast cancer. Cohort A included 248 patients with invasive breast cancer (all subtypes). Cohort B included 126 HER2-positive patients who received neoadjuvant chemotherapy (NAC) concomitant with trastuzumab. The relationship of PD-L1 expression on the cancer cells with clinicopathological factors including pathological complete response (pCR) and prognosis was investigated. In cohort A, 8.1% patients were PD-L1-positive; PD-L1 positivity showed a correlation with high degree of tumor-infiltrating lymphocytes (TILs), estrogen receptor negativity, progesterone receptor negativity, and high histological grade. In cohort B, 17.5% patients were PD-L1-positive; PD-L1 positivity showed a significant correlation with high degree of TILs and high abundance of CD8-positive TILs. The pCR rates were related to TILs and PD-L1 expression. Among PD-L1-negative patients, high CD8-positive TILs were associated with significantly better prognosis. In conclusion, 17.5% of HER2-positive type patients were PD-L1-positive. PD-L1 expression was associated with response to NAC with trastuzumab in patients with HER2-positive breast cancer.
Subject terms: Predictive markers, Breast cancer, Tumour immunology
Introduction
Mononuclear immune cells located in tumor tissues [also referred to as tumor-infiltrating lymphocytes (TILs)] play an important role in tumor immunity1,2. The degree of TIL is considered an important prognostic factor and a predictive factor for the treatment of breast cancer patients, especially those with estrogen receptor (ER)-negative type3–6. In 2014, the International Working Group established guidelines pertaining to the evaluation of the degree of TIL in invasive breast cancer7. On the basis of these guidelines, we previously found that TIL-expression was a potent predictor of the response to neoadjuvant chemotherapy (NAC) with trastuzumab in patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer4.
Programmed cell death protein-1 (PD-1) and programmed death-ligand 1 (PD-L1) are considered as immune checkpoint factors that inhibit the immune reaction to cancer cells8,9. Thus, these factors have attracted attention as novel therapeutic targets in the context of many cancer types10–12. In lung cancer, immunohistochemical (IHC) assay of PD-L1 expression is the companion and/or complementary diagnostic tool for the PD-1/PD-L1 immune checkpoint inhibitors13. Several clinical trials have investigated the use of PD-1/PD-L1 immune checkpoint inhibitors in patients with invasive breast cancer14–16. In addition, the PD-L1 antibody SP142 was used as the companion diagnosis in a clinical trial that investigated the use of atezolizumab (a PD-L1 inhibitor) for treatment of triple-negative breast cancer14. However, the clinical utility of PD-L1 evaluation in the context of HER2-positive breast cancer is not clear.
The present study investigated the relationship of PD-L1 expression with several clinicopathological factors, including the outcome and pathological response to NAC with trastuzumab in patients with HER2-positive breast cancer.
Results
Clinicopathological and prognostic utility of PD-L1 expression
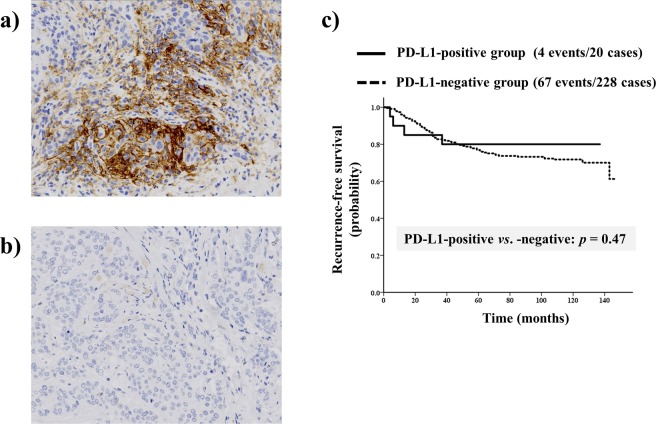
In cohort A (n = 248), 20 patients (8.1%) were PD-L1-positive (Fig. 1a) while 228 (91.9%) patients were PD-L1-negative (Fig. 1b). PD-L1 positivity showed a significant association with ER negative status (p < 0.0001), PgR negative status (p < 0.0001), high TIL expression (p < 0.0001) and histological grade 3 (p < 0.0001) (Table 1). In addition, 1.3% of the hormone receptor (HR)-positive/HER2-negative type, 11.6% of the HER2-positive type and 27.7% of the triple-negative type were classified as PD-L1-positive breast cancer in cohort A. The median recurrence-free survival (RFS) in cohort A was 128 (range, 1–147) months. PD-L1 expression was not a significant prognostic factor in any of the subtypes of breast cancer (hazard ratio = 0.69, 95% confidence interval (CI) 0.25–1.88, p = 0.47; Fig. 1c). On multivariate analyses, PD-L1 expression was not an independent prognostic factor (hazard ratio = 0.51, 95% confidence interval (CI) 0.17–1.56, p = 0.24; Table S1).
Figure 1.
Immunohistochemical staining of programmed death-ligand 1 (PD-L1) to assay protein expression in breast cancer tissue showing (a) positive-staining and (b) negative-staining in the cytoplasm. (c) Cumulative survival of all subtypes of breast cancer patients stratified according to PD-L1 expression. PD-L1 was not a significant prognostic factor in any of the subtypes of breast cancer.
Table 1.
Correlation of PD-L1 expression with clinicopathological factors in invasive breast cancer.
| PD-L1 expression | N | p | |||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| TILs | High | 18 (58.1%) | 13 (41.9%) | 31 | <0.0001 |
| Intermediate | 39 (95.1%) | 2 (4.9%) | 41 | ||
| Low | 171 (97.2%) | 5 (2.8%) | 176 | ||
| ER | Negative | 63 (77.8%) | 18 (22.2%) | 81 | <0.0001 |
| Positive | 165 (99.8%) | 2 (1.2%) | 167 | ||
| PgR | Negative | 87 (83.7%) | 17 (16.3%) | 104 | <0.0001 |
| Positive | 141 (97.9%) | 3 (2.1%) | 144 | ||
| HER2 | Positive | 38 (88.4%) | 5 (11.6%) | 43 | 0.35 |
| Negative | 190 (92.7%) | 15 (7.3%) | 205 | ||
| Subtypes | HR-positive and HER2-negative | 156 (98.7%) | 2 (1.3%) | 158 | <0.0001 |
| HER2-positive | 38 (88.4%) | 5 (11.6%) | 43 | ||
| Triple negative | 34 (72.3%) | 13 (27.7%) | 47 | ||
| Histological grade | Grade 3 | 125 (86.2%) | 20 (13.8%) | 145 | <0.0001 |
| Grade 1/2 | 103 (100.0%) | 0 (0.0%) | 103 | ||
| Pathological tumor size | pT 3/4 | 110 (90.9%) | 11 (9.1%) | 121 | 0.56 |
| pT 1/2 | 118 (92.9%) | 9 (7.1%) | 127 | ||
| Pathological nodal status | Positive | 101 (91.0%) | 10 (9.0%) | 111 | 0.62 |
| Negative | 127 (92.7%) | 10 (7.3%) | 137 | ||
Abbreviations: PD-L1, programmed death-ligand 1; TILs, tumor-infiltrating lymphocytes; ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor.
Correlation of PD-L1 expression with clinicopathological factors including pathological response to NAC with trastuzumab
In cohort B (n = 126), 22 patients (17.5%) were PD-L1-positive, while 104 patients (82.5%) were PD-L1-negative (Table 2). PD-L1 positivity showed a significant association with high expression of TIL (p < 0.0001), high abundance of CD8-positive TIL (p = 0.00087) and histological grade 3 (p = 0.043) (Table 1). The distribution of clinicopathological factors including PD-L1 expression in the pCR and non-pCR groups is presented in Table 3. The pCR rate showed a significant correlation with PD-L1 expression (p = 0.027). The pCR rate in PD-L1-positive patients (86.4%) was significantly greater than that in PD-L1-negative patients (61.5%) (Table 3). As shown in Table 3, pCR also showed a significant association with high (40–90%) TIL expression (p = 0.027), ER negativity (p < 0.0001), PgR negativity (p = 0.00015), high (≥30%) Ki67 labelling index (p = 0.0052) and histological grade 3 (p = 0.0038). However, on multivariate analysis, none of these factors was an independent predictor of pCR (Table 3).
Table 2.
Correlation of PD-L1 expression with clinicopathological factors in HER2-positive breast cancer.
| PD-L1 expression | N | p | |||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| TILs | High | 6 (26.1%) | 17 (73.9%) | 23 | <0.0001 |
| Intermediate | 34 (91.9%) | 3 (8.1%) | 37 | ||
| Low | 64 (97.0%) | 2 (3.0%) | 66 | ||
| CD8-positive TILs | High | 65 (74.7%) | 22 (25.3%) | 87 | 0.00087 |
| Low | 30 (100.0%) | 0 (0.0%) | 30 | ||
| ER | Negative | 62 (80.5%) | 15 (19.5%) | 77 | 0.45 |
| Positive | 42 (85.7%) | 7 (14.3%) | 49 | ||
| PgR | Negative | 74 (81.3%) | 17 (18.7%) | 91 | 0.56 |
| Positive | 30 (85.7%) | 5 (14.3%) | 35 | ||
| Ki67 | High (≥30%) | 70 (77.8%) | 20 (22.2%) | 90 | 0.36 |
| Low (<30%) | 34 (94.4%) | 2 (5.6%) | 36 | ||
| Histological grade | Grade 3 | 85 (79.4%) | 22 (20.6%) | 107 | 0.043 |
| Grade 1/2 | 19 (100.0%) | 0 (0.0%) | 19 | ||
| Clinical tumor size | cT 3/4 | 38 (86.4%) | 6 (13.6%) | 44 | 0.41 |
| cT 1/2 | 66 (80.5%) | 16 (19.5%) | 82 | ||
| Clinical nodal status | Positive | 68 (80.0%) | 17 (20.0%) | 85 | 0.28 |
| Negative | 36 (87.8%) | 5 (12.2%) | 41 | ||
Abbreviations: PD-L1, programmed death-ligand 1; TILs, tumor-infiltrating lymphocytes; ER, estrogen receptor; PgR, progesterone receptor HER2, human epidermal growth factor receptor 2.
Table 3.
Relationship between pathological complete response and clinicopathological factors including PD-L1 expression.
| Non-pCR | pCR | pCR ratio (%) | p | |||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| PD-L1 | Positive | 3 | 19 | 86.4 | 0.027 | 0.34 |
| Negative | 40 | 64 | 61.5 | |||
| TILs | 40–90% | 3 | 20 | 87.0 | 0.018 | 0.40 |
| 0–40% | 40 | 63 | 61.2 | |||
| CD8-positive TILs | High | 26 | 61 | 70.1 | 0.18 | — |
| Low | 13 | 17 | 56.7 | |||
| ER | Negative | 15 | 62 | 80.5 | <0.0001 | 0.058 |
| Positive | 28 | 21 | 42.9 | |||
| PgR | Negative | 22 | 69 | 75.8 | 0.00015 | 0.25 |
| Positive | 21 | 14 | 40.0 | |||
| Ki67 | High (≥30%) | 24 | 66 | 73.3 | 0.0052 | 0.15 |
| Low (<30%) | 19 | 17 | 47.2 | |||
| Histological grade | Grade 3 | 31 | 76 | 71.0 | 0.0038 | 0.28 |
| Grade 1/2 | 12 | 7 | 36.8 | |||
| Clinical tumor size | cT 3/4 | 17 | 27 | 61.4 | 0.43 | — |
| cT 1/2 | 26 | 56 | 68.3 | |||
| Clinical nodal status | Positive | 29 | 56 | 65.9 | 1.00 | — |
| Negative | 14 | 27 | 65.9 | |||
Abbreviations: PD-L1, programmed death-ligand 1; TILs, tumor-infiltrating infiltrating lymphocytes; ER, estrogen receptor; PgR, progesterone receptor; pCR, pathological complete response.
We assessed the relationship of pCR rate with the combination of the following 6 biological markers: PD-L1, TILs, ER, PgR, histological grade 3, and Ki67. All 126 patients were classified into 7 groups (score 0–6). The pCR rates of patients with score 5–6, score 4, score 3, score 2, and score 0–1 were 94.1%, 80.0%, 64.0%, 52.0%, and 14.3%, respectively. This scoring was a significant predictor of pCR (p < 0.0001) (Fig. 2).
Figure 2.
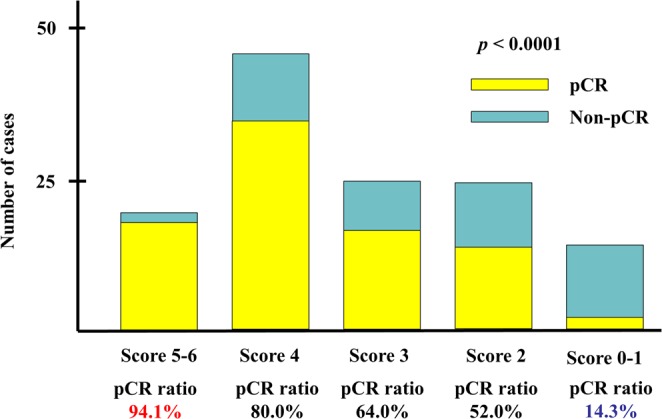
The pathological complete response (pCR) prediction scores and their relationship with pCR. The pCR score, consisting of programmed death-ligand 1 (PD-L1), tumor-infiltrating lymphocytes (TILs), estrogen receptor (ER), progesterone receptor (PgR), Ki67, and histological grade 3 significantly predicted pCR.
Prognostic utility of PD-L1 expression in HER2-positive type
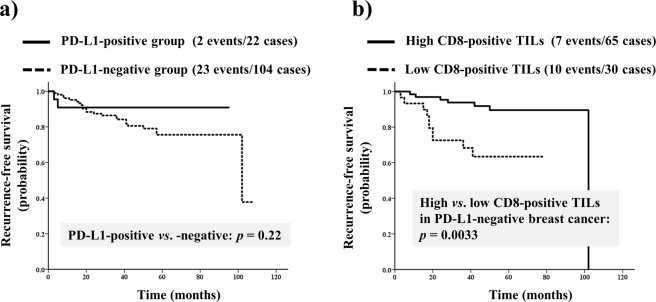
The median survival in cohort B (n = 126) was 52.5 (range, 3–108) months. In this cohort of HER2-positive patients, PD-L1 expression was not a significant prognostic factor (hazard ratio = 0.40, 95% CI 0.09–1.71, p = 0.22; Fig. 3a). The other 5 biological markers associated with pCR (i.e. PD-L1, TILs, ER, PgR, histological grade 3, and Ki67) showed no significant association with prognosis (Table S2). However, among patients with PD-L1-negative breast cancer, survival in the high CD8-positive TIL expression group was significantly longer than that in the low CD8-positive TIL expression group (hazard ratio = 4.58, 95% CI 1.66–12.67, p = 0.0033; Fig. 3b).
Figure 3.
(a) Cumulative survival of patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer stratified according to programmed death-ligand 1 (PD-L1) expression. PD-L1 was not a significant prognostic factor in HER2-positive breast cancer. (b) Survival curves stratified according to the degrees of CD8-positive tumor-infiltrating lymphocytes (TILs) in PD-L1-negative/HER2-positive patients. Among PD-L1-negative/HER2-positive patients, recurrence-free survival was significantly better in the high CD8-positive TILs group than in the low CD8-positive TILs group.
Discussion
PD-1 is expressed on the surface of lymphocytes, whereas its ligand PD-L1 is expressed on the surface of cancer cells as well as lymphocytes8. PD-1 and PD-L1 belong to the immune checkpoint family of proteins, which inactivate T-lymphocytes. Within the tumor microenvironment, PD-1/PD-L1 expression suppresses the immune response by killer/cytotoxic T cells against cancer cells8. This type of mechanism, mediated via modulation of PD-1 and PD-L1 binding, represents the resistance of tumor cells to anti-tumor immunity. Discovery of methods to overcome these mechanisms of tumor resistance while maintaining the initial anti-tumor immune response is a key area of immune oncology research9. Over the previous three decades, various PD-1/PD-L1 inhibitors have been developed for the treatment of several types of cancer17. Clinical trials have suggested the efficacy of PD-L1 inhibitors against solid tumors, including skin cancer18, lung cancer19, and bladder cancer20. In a worldwide clinical study, use of a PD-L1 inhibitor in combination with nab-paclitaxel was shown to significantly prolong the progression-free survival of patients with metastatic triple-negative breast cancer compared to nab-paclitaxel monotherapy14.
Several studies have shown the clinicopathological significance of PD-L1 expression and the usefulness of PD-1/PD-L1 inhibitors in the treatment of triple-negative breast cancer16,21,22. However, only a few clinical trials have investigated the role of PD-1/PD-L1 inhibitors in patients with HER2-positive breast cancer. Results of the PANACEA clinical trial pertaining to patients with metastatic HER2-positive breast cancer, showed a 15.2% objective response rate to pembrolizumab-trastuzumab combination therapy in PD-L1-positive patients as against 0% in PD-L1-negative patients23. In HER2-positive breast cancer, the anti-tumor immune response is also an important predictor of therapeutic response and prognosis. In our previous study, high expression of TILs in the primary tumor was associated with significant improvement in pCR rate after NAC with trastuzumab4. Trastuzumab is an anti-HER2-targeting drug, which binds and inhibits HER2 dimerization, resulting in inhibition of the downstream phosphatidylinositol 3-kinase cascade24,25. In addition, trastuzumab induces anti-tumor effects by promoting the activation of natural killer cells through antibody-dependent-cellular cytotoxicity (ADCC)26,27. In our previous study, NAC with trastuzumab was found to induce an increase in the number of TILs in 20% of non-pCR cases, compared with pre-treatment measurements. Furthermore, the group with high TILs in the residual tumor showed a significantly better prognosis than the group with low TILs4. Trastuzumab induces infiltration of lymphocytes in the tumor independent of the ADCC activity. Perez et al.28 conducted a study using large-scale transcriptomic data; they found a strong association between the gene sets related to immune function and the effect of trastuzumab therapy in patients with HER2-positive breast cancer. In the present study, all PD-L1-positive cases showed a high density of CD8-positive TILs. In contrast, in the PD-L1-negative cases, the positivity of CD8-positive TILs was a significant prognostic factor. Collectively, these findings suggest that trastuzumab is strongly associated with killer/cytotoxic T cell activity, in addition to ADCC activity4.
The effectiveness of combination therapy with immune checkpoint inhibitors and anti-HER2 agents against early-stage HER2-positive breast cancer is an important topic in medical oncology. However, further investigation is necessary to determine the most suitable patients for such combination therapy29. PD-L1 expression in breast cancer cells may be a predictive biomarker of response to PD-1/PD-L1 immune checkpoint inhibitors. However, there is no clear consensus on the evaluation method for PD-L1 expression with respect to the selection of the PD-L1 antibody clone and the appropriate percentage cutoff level to determine PD-L1 positivity and negativity30. Representative PD-L1 antibodies include 22C3, 28-8, SP263, and SP142. SP142 binds to the cytoplasmic domain of PD-L1, whereas 22C3 and 28-8 bind to its extracellular domain31,32. Of note, 22C3 was used in a companion diagnostic assay for non-small-cell lung cancer in a clinical trial of pembrolizumab therapy. In that study, the cutoff values were set at ≥50% and ≥1% for first- and second-line treatment, respectively33,34. Furthermore, 28-8 was used in a complementary diagnostic assay in a clinical trial of nivolumab therapy for non-small-cell lung cancer; in this study, the cutoff value was set at ≥1% for second-line treatment8,35. In the IMpassion130 trial14 – assessing the efficacy of atezolizumab against triple-negative breast cancer – SP142 was used with a cutoff value of ≥1%. However, in the Blueprint Working Group study, the positivity rate obtained with the use of SP142 was lower than that obtained with 22C3, 28-8, and SP26336. Furthermore, in a study by Sun et al.37, the PD-L1 expression patterns were found to differ depending on the antibody clone used; they reported a positivity rate of 19.3% with use of SP142. In the present study, we used the PD-L1 antibody SP142 with a cutoff value of ≥1%. On this basis, 1.3% of HR-positive/HER2-negative cases, 11.6% of HER2-positive cases, and 27.7% of triple-negative cases were classified as PD-L1-positive. Further prospective studies are warranted to identify appropriate antibodies for PD-L1 companion and complementary diagnoses in breast cancer.
IHC assessment revealed increased PD-L1 expression in cancer cells and low expression in normal epithelial cells8. However; PD-L1 expression on the tumor cells is heterogeneous, and PD-L1 inhibitors may bind to PD-L1-positive cancer cells as well as PD-L1-positive lymphocytes38. The difference between the effects of PD-L1 inhibitors on PD-L1-positive cancer cells and lymphocytes is not clear. In the current study, PD-L1 expression on tumor cells was not a prognostic biomarker in patients with HER2-positive breast cancer. In a recent study, PD-L1 expression on tumor cells was associated with high-risk clinicopathological parameters and poor prognosis, while PD-L1 expression on the TILs was associated with favourable survival outcomes. In the present study, we did not evaluate PD-L1 expression on TILs. Further studies are warranted to delineate the mechanisms that underlie PD-L1 expression in tumor cells and lymphocytes.
In conclusion, approximately 15% of HER2-positive breast cancers were found PD-L1-positive using the PD-L1 antibody SP142. PD-L1 expression on cancer cells was found to be a predictive biomarker for response to NAC with trastuzumab. A limitation of the present study is that the clinical utility of PD-L1 expression was not clear for HER2-positive breast cancer treated with immune check point inhibitors. Several studies are currently underway to identify biomarkers that may predict the response to these inhibitors; these broadly focus on the character of TILs, PD-L1 expression, the mutational landscape, and the gene signature. In this context, it is important to determine the differences in the characteristics of immune cells (e.g. CD8-positive TILs) prior to and after NAC with trastuzumab and to advance the evaluation of PD-L1 expression, with the objective to optimise the use of immune checkpoint inhibitors in the treatment of HER2-positive breast cancer.
Methods
Patient characteristics
Cohort A comprised of 248 consecutive female patients with invasive breast cancer who received breast cancer surgery without NAC at the Saitama Cancer Center, Saitama, Japan between 2000 and 2001 (Table S3).
Among patients with invasive breast cancer who underwent surgery at the Division of Breast Surgery in Saitama Cancer Center between 2005 and 2011, 126 consecutive patients with HER2-positive type who received NAC with trastuzumab were included in cohort B. All patients received 24 cycles of trastuzumab with NAC consisting of paclitaxel or docetaxel followed by anthracycline (Table S4). The details of the treatment administered in cohort B are described elsewhere39.
Ethical approval for this study was provided by the Institutional Review Board of the Saitama Cancer Center (Reference numbers: 533 and 534). Informed consent was obtained from all patients included in the study.
Histopathological evaluation
IHC and in-situ hybridisation were performed as described elsewhere39,40. Briefly, the following antibodies were used for IHC staining to determine the subtype: ER (1D5; DAKO, Copenhagen, Denmark), progesterone receptor (PgR; PgR636; DAKO), and HER2 (HercepTest; DAKO). Specimens with a nuclear staining rate ≥1% were considered HR-positive (ER and PgR). HER2 amplification was automatically stained (BenchMark® XT; Ventana Medical Systems, Tucson, Arizona, USA) with dual in-situ hybridisation (DISH; INFORM HER2 Dual ISH DNA Probe Cocktail Assay; Roche, Basel, Switzerland). Patients with ‘HER2 IHC-score 3+’ or ‘HER2 IHC-score 2+ and those positive for HER2 amplification by DISH’ were defined as HER2-positive type. IHC staining for Ki67 (MIB-1, DAKO) was performed automatically using an IHC machine (BenchMark® XT, Ventana Medical Systems, Inc.). The Ki67 labelling index (percentage of positivity) was calculated for approximately 500 cancer cells in hot to warm areas.
Pathological complete response (pCR) to NAC was evaluated in accordance with the guidelines of the Japanese Breast Cancer Society. The details of this evaluation are described elsewhere39. Residual noninvasive cancers or axillary lymph node metastases were not considered while determining the pCR.
Evaluation of biomarkers associated with tumor immunity
The surgical samples were used in cohort A, and the core needle biopsy specimens were used in cohort B.
PD-L1 expression was assessed by IHC using SP142 (dilution 1:50; Spring Biosience, USA). Breast cancer cells with a cytoplasmic and/or membrane staining rate ≥1% were classified as PD-L1-positive (Fig. 1a). Staining was assessed by an investigator specialising in breast pathology (MK) according to the evaluation method initially established for urothelial cancer41.
Haematoxylin and eosin-stained sections were used for the evaluation of TILs. For this purpose, the expression of mononuclear immune cells interposing between tumor nests (stromal TILs) was evaluated using an optical microscope (magnification: 200–400x); the evaluation was performed by an investigator specialised in breast pathology (MK). The expression of TIL, as previously reported3,4, was categorised into three groups by modifying the International Working Group criteria7: low (TILs: 0–10%); moderate (TILs: 10–40%); high (TILs: 40–90%). In cohort B, the expression of CD8 (DAKO, Denmark) in TILs was evaluated by IHC staining of core needle biopsy specimens of primary tumours. Presence of >25 CD8-positive TILs in one high-power field was defined as ‘high CD8-positive TIL expression’4.
For the assessment of the relationship between pCR rate and the combination of 6 biological markers (i.e. PD-L1, TILs, ER, PgR, histological grade 3, and Ki67), one point each was assigned for PD-L1-positivity, high TIL expression, ER-negativity, PgR-negativity, high Ki67 labelling index and histological grade 3.
Statistical analysis
The association of PD-L1 expression with pCR rate and clinicopathological factors was assessed using the Chi-squared test. Multivariate logistic regression analysis was performed to identify factors significantly associated with pCR. In addition, the association of PD-L1 and CD8 expressions with prognosis [RFS] was determined using the Cox proportional hazards model. Survival curves were drawn using the Kaplan–Meier method. All statistical analyses were performed using the SPSS statistical software version 24.0 (IBM, Armonk, New York, USA); p values ≤ 0.05 were considered indicative of statistical significance.
Ethics approval and consent to participate
This study was conducted according to the tenets of the Declaration of Helsinki. Ethic approval was granted by the Saitama Cancer Center Institutional Review Board (Reference numbers: 533 and 534). Informed consent was obtained from all patients included in the study.
Supplementary information
Author contributions
S.K. and K.I. collected the clinical data. M.K. assisted S.K. in the histopathological examinations. S.K. mainly performed statistical analyses. H.M., K.I. and M.K. assisted in designing the study and evaluating the results. T.F., J.H., T.O. and K.S. contributed to the theoretical organisation of the study.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
K.I. received remuneration from CHUGAI Pharmaceutical Co., Ltd. K.I. received research funding from PAREXEL International Corp., Puma Biotechnology, Inc., Merck Sharp & Dohme Ltd., Novartis Pharma K.K., GlaxoSmithKline K.K., Pfizer Inc., CHUGAI Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., and Daiichi Sankyo Co., Ltd. MK received honoraria from Taiho Pharmaceutical Co., Ltd. and CHUGAI Pharmaceutical Co., Ltd. F.T. received research funding from Eisai Co., Ltd. There were no conflicts of interest for any of the other authors.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-52944-6.
References
- 1.Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 3.Kurozumi S, et al. Prognostic significance of tumour-infiltrating lymphocytes for oestrogen receptor-negative breast cancer without lymph node metastasis. Oncol. Lett. 2019;17:2647–2656. doi: 10.3892/ol.2019.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurozumi S, et al. Prognostic utility of tumor-infiltrating lymphocytes in residual tumor after neoadjuvant chemotherapy with trastuzumab for HER2-positive breast cancer. Sci. Rep. 2019;9:1583. doi: 10.1038/s41598-018-38272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denkert C, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 6.Denkert C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 7.Salgado R, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurozumi S, et al. Significance of evaluating tumor-infiltrating lymphocytes (TILs) and programmed cell death-ligand 1 (PD-L1) expression in breast cancer. Med. Mol. Morphol. 2017;50:185–194. doi: 10.1007/s00795-017-0170-y. [DOI] [PubMed] [Google Scholar]
- 9.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkin J, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer J, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darvin P, et al. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp. Mol. Med. 2018;50:165. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hersom M, Jørgensen JT. Companion and complementary diagnostics-focus on PD-L1 expression assays for PD-1/PD-L1 checkpoint inhibitors in non-small cell lung cancer. Ther. Drug Monit. 2018;40:9–16. doi: 10.1097/FTD.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 14.Schmid P, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 15.Nanda R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swoboda A, Nanda R. Immune checkpoint blockade for breast cancer. Cancer Treat. Res. 2018;173:155–165. doi: 10.1007/978-3-319-70197-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018;362:k3529. doi: 10.1136/bmj.k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Angelo SP, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: A preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4:e180077. doi: 10.1001/jamaoncol.2018.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Socinski MA, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 20.Powles T, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 21.Mittendorf EA, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang M, et al. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget. 2017;8:31347–31354. doi: 10.18632/oncotarget.15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loi S, et al. Abstract GS2-06: Phase Ib/II study evaluating safety and efficacy of pembrolizumab and trastuzumab in patients with trastuzumab-resistant HER2-positive metastatic breast cancer: Results from the PANACEA (IBCSG 45-13/BIG 4-13/KEYNOTE-014) study. Cancer Res. 2018;78(Supplement):GS2–06. [Google Scholar]
- 24.Lewis GD, et al. Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol. Immunother. 1993;37:255–263. doi: 10.1007/BF01518520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sliwkowski MX, et al. Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J. Biol. Chem. 1994;269:14661–14665. [PubMed] [Google Scholar]
- 26.Hurvitz SA, et al. Analysis of Fcgamma receptor IIIa and IIa polymorphisms: lack of correlation with outcome in trastuzumab-treated breast cancer patients. Clin. Cancer Res. 2012;18:3478–3486. doi: 10.1158/1078-0432.CCR-11-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341:1192–1198. doi: 10.1126/science.1241145. [DOI] [PubMed] [Google Scholar]
- 28.Perez EA, et al. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the North Central Cancer Treatment Group n9831 Adjuvant Trastuzumab Trial. J. Clin. Oncol. 2015;33:701–708. doi: 10.1200/JCO.2014.57.6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holgado E, Perez-Garcia J, Gion M, Cortes J. Is there a role for immunotherapy in HER2-positive breast cancer? NPJ Breast Cancer. 2018;4:21. doi: 10.1038/s41523-018-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandini S, Massi D, Mandalà M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016;100:88–98. doi: 10.1016/j.critrevonc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Wang S, Bindeman W. Clinical applications of PD-L1 bioassays for cancer immunotherapy. J. Hematol. Oncol. 2017;10:110. doi: 10.1186/s13045-017-0479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parra ER, Villalobos P, Mino B, Rodriguez-Canales J. Comparison of different antibody clones for immunohistochemistry detection of programmed cell death ligand 1 (PD-L1) on non-small cell lung carcinoma. Appl. Immunohistochem. Mol. Morphol. 2018;26:83–93. doi: 10.1097/PAI.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garon EB, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 34.Mino-Kenudson M. Programmed cell death ligand-1 (PD-L1) expression by immunohistochemistry: Could it be predictive and/or prognostic in non-small cell lung cancer? Cancer Biol. Med. 2016;13:157–170. doi: 10.20892/j.issn.2095-3941.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borghaei H, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsao MS, et al. PD-L1 Immunohistochemistry comparability study in real-life clinical samples: Results of Blueprint Phase 2 Project. J. Thorac. Oncol. 2018;13:1302–1311. doi: 10.1016/j.jtho.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun WY, Lee YK, Koo JS. Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. J. Transl. Med. 2016;14:173. doi: 10.1186/s12967-016-0925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang F, Zheng P. Tumor cells versus host immune cells: whose PD-L1 contributes to PD-1/PD-L1 blockade mediated cancer immunotherapy? Cell Biosci. 2018;8:34. doi: 10.1186/s13578-018-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurozumi S, et al. ER, PgR, Ki67, p27(Kip1), and histological grade as predictors of pathological complete response in patients with HER2-positive breast cancer receiving neoadjuvant chemotherapy using taxanes followed by fluorouracil, epirubicin, and cyclophosphamide concomitant with trastuzumab. BMC Cancer. 2015;7:622. doi: 10.1186/s12885-015-1641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurozumi S, et al. Power of PgR expression as a prognostic factor for ER-positive/HER2-negative breast cancer patients at intermediate risk classified by the Ki67 labeling index. BMC Cancer. 2017;17:354. doi: 10.1186/s12885-017-3331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenberg JE, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.