Abstract
BACKGROUND: One way to overcome the genetic and molecular variations within glioblastoma is to treat each tumour on an individual basis. To facilitate this, we have developed a microfluidic culture paradigm that maintains human glioblastoma tissue ex vivo. METHODS: The assembled device, fabricated using a photolithographic process, is composed of two layers of glass bonded together to contain a tissue chamber and a network of microchannels that allow continued tissue perfusion. RESULTS: A total of 128 tissue biopsies (from 33 patients) were maintained in microfluidic devices for an average of 72 hours. Tissue viability (measured with Annexin V and propidium iodide) was 61.1% in tissue maintained on chip compared with 68.9% for fresh tissue analysed at commencement of the experiments. Other biomarkers, including lactate dehydrogenase absorbance and trypan blue exclusion, supported the viability of the tissue maintained on chip. Histological appearances remained unchanged during the tissue maintenance period, and immunohistochemical analysis of Ki67 and caspase 3 showed no significant differences when compared with fresh tissues. A trend showed that tumours associated with poorer outcomes (recurrent tumours and Isocitrate Dehydrogenase - IDH wildtype) displayed higher viability on chip than tumours linked with improved outcomes (low-grade gliomas, IDH mutants and primary tumours). conclusions: This work has demonstrated for the first time that human glioblastoma tissue can be successfully maintained within a microfluidic device and has the potential to be developed as a new platform for studying the biology of brain tumours, with the long-term aim of replacing current preclinical GBM models and facilitating personalised treatments.
Introduction
Gliomas make up 81% [1] of brain tumours, and of all gliomas, glioblastoma (GBM) represents nearly half (47%) [2] of all cases. The World Health Organization (WHO) classification of tumors of the central nervous system uses histological and genetic markers to identify 5 forms of GBM [3]. The Cancer Genome Atlas group used genetic fingerprinting studies, patient demographics and survival data to identify 4 clinically relevant subtypes [4]. These classification methods are not mutually exclusive and are increasingly combined to characterise an array of genetically and phenotypically different types of GBM. Multiple forms exist, but regardless of subtype, the diagnosis of a GBM is a devastating one with a poor prognosis, which has remained essentially unchanged in over 90 years. Stupp et al. [5] showed the addition of temozolomide (TMZ) to radiotherapy and surgery increased survival by 2.5 months compared with surgery and radiotherapy alone. Despite this survival benefit, the nature of the disease, which sees widespread permeation of tumour cells along white matter tracts, ensures that therapies such as even TMZ eventually fail. Median survival ranges from 9 to 15 months [6,7]. Undeterred by decades of research and treatments, GBM kills 97.3% of patients within 5 years of diagnosis [8]. While addressing tumour stem cells and treatment resistance in GBM, potential cures must resolve the complex issue of tumour heterogeneity, optimising treatment based on the individual tumour type.
Current strategies aimed at developing new treatments for GBM are encumbered with an imperfect process of drug screening as well as the absence of a good model of the disease. Cell cultures, currently the mainstay of preclinical assays, do not recapitulate the infiltrative nature of the tumour and possess significant molecular and pathological differences from human gliomas [9]. Mouse models [10] and patient-derived xenografts (PDXs) [11] provide a solution to this particular issue but are costly and time-consuming, making these models only suitable in drug-development testing, with no scope to apply them to a personalised approach.
A more ideal treatment paradigm involves being able to study patient tumours in the laboratory—freshly biopsied at the time of surgical debulking and kept in a viable state long enough to analyse response to various treatment modalities—and offering treatment to patients based on specific results. Just as microbiologists rationalise treatments based on culture and sensitivity, an approach that allows analysis of human GBM tissue ex vivo could personalise treatment and improve patient outcomes.
Microfluidics (MF) is a fast-growing area of research that allows experimentation with mimicry of natural conditions. Fluid flow through microdevices takes place at a submillilitre scale, with Reynold numbers less than 100 [12], where viscous as opposed to inertial forces dictate flow. The resultant laminar flow means diffusion becomes the predominant form of molecular interactions, as is seen in cells and tissues. MF devices have been used to explore a wide range of biological processes, with devices created to mimic complex organs [13] or facilitate molecular tests [14]. More recently, devices that maintain whole tissues ex vivo for 3–7 days have been created, allowing chemotherapy and radiotherapy testing and correlating response to in vivo tumour behaviour [[15], [16], [17], [18], [19]]. As a preclinical model, such a system would effectively mimic the native tumour microenvironment while avoiding prolonged incubation periods required with PDX. As a clinical model, it would allow testing of individual tumour samples within a short time frame, potentially allowing key analyses of the tumour to asses for subtype and response to specific treatment and allowing stratification of the patients to particular drug sensitivities. The issue of intratumour heterogeneity can be overcome by real-time measurements of the net response of the tumour to a single agent or combination therapy. Such a system introduces the desired degree of flexibility to deal with an adaptive tumour-like GBM and would significantly enhance clinical research, aiding scientists and clinicians in preclinical drug development, patient stratification for drug trials and predicting responses to treatment in the clinic.
Currently, no such model exists. The development of such a platform would be instrumental in reducing the mortality associated with the disease and bring the elusive cure closer to our reach. The aim of the current research project is to establish a robust MF tissue culture paradigm for the maintenance of human GBM tissue. Using an MF device similar to that previously described [20], GBM tissue biopsies from surgery were successfully maintained within the MF device, with continued perfusion and withdrawal of medium, over a 3-day period.
Material and Methods
Fabricating and Assembling the Chip
The MF device was fabricated using standard photolithography and wet etching techniques. A photomask containing the layout of the microchannel network was developed by photoreduction using a computer-assisted design package—AutoCAD LT software (Autodesk Ltd.). The photomask was aligned onto a 30 × 30-mm crown white glass, precoated with photoresist. The precoated glass, left in contact with the photomask, was exposed to ultraviolet radiation for just over a minute, creating the desired pattern and allowing further development and etching in 1% (v/v) hydrofluoric acid at 65 °C in a heated ultrasonic bath. The etched glass chip was then cleaned and thermally bonded with a top layer (with corresponding predrilled holes) at 600 °C for 3 hours. The completed device consisting of the two glass plates bonded together is represented in Figure 1. The thinner bottom (1 mm) layer has a network of microchannels of 190-μm width and 70-μm depth, which diverge into exit microchannels.
Figure 1.
Experimental setup. (A) Inlet and outlet (access) channels as well as a central channel were drilled into the top glass layer. Channel networks were etched into the bottom layer, producing channels 190 μm in width and 70 μm in depth. The tissue chamber was sealed with a microport and PDMS-filled adaptor. The tissue chamber has a volume of approximately 20 μL. Adapted from Hattersley et al [34]. (B) Pictures of the chip. Note: Two outlet holes glued with glass coverslip. (C) A picture of the assembled microfluidic chip. (D) Schematic of microfluidic setup (Adapted from Dawson et al.) [60]. (E) The device with GBM tissue, connected to syringes and syringe pump.
Further modifications of the basic unit were required: a semipermeable barrier (circular mesh) was secured onto the bottom of the tissue cavity, followed by sealing a PEEK microport (Anachem) to the surface of the top glass layer such that the circular tissue cavity could be enclosed using an English-threaded adapter (Anachem). The adapter contained a hollow central portion that was filled with Polydimethylsiloxane (PDMS - Dow Corning) to allow gaseous exchange into the tissue compartment. The fabricated device was finalised by connecting 0.8-mm (internal diameter) by 1.58-mm (external diameter) ethylene tetrafluoroethylene Teflon tubing (Anachem) to the inlet and outlet channels with epoxy adhesive and a graphite ferrule. For these experiments, only one inlet and one outlet channel were required. The completed device was sterilised with 70% (v/v) ethanol/distilled water before use.
Recruitment
The study received ethics approval from the Yorkshire and the Humber - Humber Bridge research ethics comittee (13/YH/0238) and Hull and East Yorkshire NHS Trust (R1584). Case notes, clinical imaging, and multidisciplinary team outcome plans for potential participants admitted to the recruiting hospital were reviewed, to identify cases where a GBM tumour was suspected.
Inclusion Criteria
Patients aged >18 years undergoing a diagnostic procedure or planned tumour resection where a sample of brain tumour tissue was expected to be taken, with a principal diagnosis of GBM, were included.
Exclusion Criteria
Participants lacking capacity to give informed consent were excluded.
Experimental SetUp
At operation, the most cellular portion (i.e., avoiding necrotic tissue) was acquired and immediately placed in a 50-ml polypropylene tube containing the maintenance media (Table 1). Samples were sectioned within 60 min of collection into 10- to 15-mg pieces, approximately 2 × 2 × 2 mm, and placed into the central chamber of the MF device, sealed with the microport adaptor. The tumour chip was connected to a 20-ml 3-Part Luer Slip Syringe (BD) containing the appropriate media and attached to a calibrated PhD 2000 Syringe Pump (Harvard) within a Perspex box with a thermostat regulated temperature of 37°C. Medium was infused into the chip and tissue at a rate of 4 μL min−1 (Figure 1E). In conjunction with tissue maintained within the MF device, a sample of the fresh GBM tissue was prepared for baseline histology and immunochemistry; additional tissue biopsies were enzymatically disaggregated, in preparation for flow cytometry studies.
Table 1.
Composition of the Culture Media Used in the Microfluidic Experiments
| Culture Medium |
|---|
| Dulbecco's Modified Eagle Medium, fortified with 4.5 g/L of glucose |
| Foetal bovine serum, 10% (v/v) heat inactivated |
| Penicillin (0.1 U/ml)/streptomycin (0.1 g/ml) mixture |
| Sodium pyruvate (5 mM) |
| HEPES (25 mM) |
The tissue on chip was perfused continuously for 72 hours, and the effluent samples were collected every 2 hours during the day and overnight samples were collected over a range of 12–18 hours. Infusions were stopped after 72 hours, and tissue samples were removed from the device for post-chip analyses.
Chip Analysis
Samples removed from the MF device underwent a number of different analyses.
Histological Preparations
Haematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) were performed for tissue processed after tissue maintenance in the MF device as well as those obtained fresh from the patient. Tissues were initially placed in 1.5 ml of 4% paraformaldehyde and processed for paraffin embedding. Wax-embedded tissues were cut (5 μm) and stained with H&E. IHC was performed using the Leica Bond III automated immunostaining platform (Leica) in conjunction with the Leica Bond Polymer Refine DAB detection kit (Leica), as per the manufacturer's guidelines. Ki67 and Caspase 3 proteins were targeted with the respective antibodies, MIB1 and ASP175. All H&E and IHC slides were viewed using an Eclipse 80i microscope (Nikon), and digital images were acquired using an Infinity 3 Microscope Camera (Roper Technologies) along with Image Pro Premier Software (Media Cybernetics). Digital images of the IHC slides were analysed using ImmunoRatio, a free Web-based application for automated image analysis of stained tissue sections [[21], [22], [23]]. Proliferation and apoptotic indices were calculated as the number of positively stained cells divided by the total number of cells, multiplied by 100. For each slide, this was performed on 2–3 randomly chosen high-power field images that contained positively stained cells.
Flow Cytometry
Tissue processed for testing was dissociated into single cells by initially mincing the tissue using a scalpel in a criss-cross cutting action. The minced tissue was added to a 1-ml tissue disaggregation solution (Sigma) and kept in an incubator at 37°C for 2 hours on a tube rotator. The filtered disaggregated solution was centrifuged at 400 x g for 5 min and the pellet mixed with 1 ml of HEPES buffer. Both Annexin V (AV) and propidium iodide (PI) were then added to 500 μl of the cell suspension, allowed to mix for 15 min in the dark, and the solution was analysed within a BD FACSCalibur flow cytometer (BD) to stratify cells according to AV and PI staining. Data acquisition was performed using the BD CellQuest Pro software which used the clustering of cell events, into four distinct populations: cells with minimal to no AV and PI staining (healthy); cells positively stained for AV but with the cell membrane still intact, hence excluding PI (early apoptosis); cells that have undergone irreversible apoptotic changes that mean the cell membrane has lost its integrity and is permeable to PI (late apoptosis); and finally, cells that have not gone through the apoptotic pathway and died via necrosis (only positive for PI, necrotic).
Trypan Blue Staining and Cell Counts
The disaggregated tissue cell suspension (10 μl) was mixed with 10 μl of 0.4% trypan blue solution. The mixture was pipetted onto a haemocytometer, and the viable percentage was calculated by dividing the number of viable cells (cells that excluded the dye/unstained) by the total number of cells (stained and unstained) multiplied by 100 [24].
Lactate dehydrogenase Analysis
Cell viability was also assessed using a colorimetric cytotoxic assay, lactate dehydrogenase (LDH) Cytotoxic Kit (Sigma Aldrich). The LDH assay was used as per manufacturer's protocol on the effluent collected from the MF device to quantify LDH release via absorption using the colorimetric assay using the Synergy HT Plate Reader (BioTek) at 490 nm.
Statistical Analysis
Statistical analysis was carried out using Graph Pad Prism (Graph Pad Software). A combination of paired and unpaired t-tests was used to determine statistical significance between fresh tissue assays and assays within the MF device. A P value of ≤0.05 was considered to be significant, and thus, the null hypothesis that the differences in the results were due to chance was rejected. All graph error bars display the standard error of the mean.
Results
A total of 128 human GBM tumour specimens (from 33 patients) were obtained for tissue maintenance within a MF device (Table 2). Tissues maintained within the device were cultured for an average of 70.2 hours (±6). The nonperfusion time (length of time from sample acquisition to perfusing the tissue within the MF device) was 69 min (range of 40–120 min) (Table 3), with no correlation between the nonperfused time and viability of samples, when analysed (n = 28) using Pearson's correlation coefficient, r2 value of 0.027 and P value of 0.41. After the tissue maintenance period ended, samples were removed from the device, and the tissues and effluent were analysed.
Table 2.
Histological Types and Demographics of Patients Included in the Study
| Patients Recruited (n = 33) | No. |
|---|---|
| Age | 59.2 |
| Sex (M:F) | 2.3:1 |
| WHO Grade Tumour | |
| GBM IDH1 Wild Type | 23 |
| GBM IDH Status Unknown | 1 |
| Gliosarcoma | 1 |
| Anaplastic/GBM IDH1 mutant | 3 |
| Anaplastic oligoastrocytoma IDH1 mutant∗ | 1 |
| Schwannoma Grade 1∗ | 1 |
| Anaplastic astrocytoma IDH1 wild type∗ | 2 |
| Gemistocytic astrocytoma IDH1 mutant∗ | 1 |
| Primary tumours | 29 |
| Recurrent tumours | 4 |
| Surgical Resection | |
| Debulking | 31 |
| Biopsy | 2 |
Non-GBM tumours.
Table 3.
Characteristics of the Tissues Placed in the Microfluidic Devices
| GBM Samples | No. |
|---|---|
| Weight | 12.1 mg (±2.2) |
| Nonperfusion time | 69 min (range 40–120 min) |
| Length of perfusion | 70.2 hours (±6) |
| Blocked devices | 20 |
Tumour Morphology
On inspection of the tissues at the end of experimentation, there was little difference in the macroscopic appearance of the tumour samples when compared with tissue samples resected before the commencement of the experiment. Tissue maintained within the MF device for 72 hours did not disrupt the tissue architecture when H&E sections were compared with fresh tissue samples (Figure 2). Characteristic features of GBM, including pleomorphic nuclei, vascular proliferation, pseudopalisading, and areas of necrosis were equally visible in both tissue samples maintained on chip, as well as those processed immediately after sample acquisition. In particular, there were no differences in cellular density and architecture within peripheral portions of the tissue sections compared with the central portions.
Figure 2.
Representative images of Ki67 IHC (n = 14) (A, fresh tissue; B, chip tissue); H&E sections (n = 28) (C, fresh tissue; D, chip tissue); and Caspase 3 IHC (n = 13) (E, fresh tissue; F, chip tissue) of GBM tissue. All images are taken at ×400 magnification.
Immunohistochemistry
Similarly, immunohistochemical markers were relatively well preserved in tissue within the chip over the 72-hours period when compared with fresh tissue (Figure 2). The average proliferation index (Ki67 staining) among 14 matched pairs (fresh and chip tissue) was 16.6% (±15.5) for fresh tissue compared with 15.9% (±17.7) for tissue maintained on chip (P = 0.74). Caspase 3 staining of tissue (13 matched pairs) did not show any significant difference between fresh tissue and chip tissue with an average apoptotic index of 38.9% (±23.9) and 44.4% (±21.3), respectively (Figure 3).
Figure 3.
Average proliferation (Ki67) index for fresh tissue (stained immediately) and chip tissue (stained after 72 hours in the microfluidic device) n = 14 paired tissue samples (P = 0.74); Average apoptotic index (Caspase 3) for fresh tissue (stained immediately) and chip tissue (stained after 72 hours in the microfluidic device); n = 13 paired tissue samples (P = 0.14).
AV and PI
AV is a protein with a high affinity for translocated phosphatidylserine in the outer membrane of cells, which occurs during apoptosis [25]. The measurement of AV is coupled with a dye exclusion test—PI, intercalates with DNA, is unable to cross through intact cell membranes, making it a valuable stain for identifying necrotic cells, as well as those at the end stages of apoptosis. While AV staining only occurs in apoptotic cells, PI staining will be seen in any cell with a disrupted cell membrane. Viability of disassociated cells procured from lysed tissue sections was analysed using flow cytometric AV and PI assays. Estimates of tissue viability, as well as measures of apoptosis and necrosis, were calculated (Figure 4A). Mean viability of fresh tissue controls was 68.9% (±29.8) compared with 61.1% (±28.3) of tissue maintained on chip. Despite a mean difference of less than 8%, the difference was statistically significant when analysed with a paired t-test, P = 0.01 (Figure 4C). Stratifying results according to histological diagnosis showed that GBM tissue viability (n = 27) was greater than lower grade tumours (n = 5), with viability of grade 1–3 tumours of 31.8% (±17.2), compared with GBM tissue which had a mean viability of 60.1% (±28.8) P = 0.01.
Figure 4.
Flow cytometric analysis of matched pairs (fresh tissue and chip tissue) used to quantify proportion of healthy cells (AV-/PI-). (A) Dot plot produced on CellQuest Pro software displaying how the flow cytometric analysis of Annexin V and PI is quantified into four quadrants. (B) Converting the flow cytometric analysis to a Histogram. (C) Histogram of 26 matched pairs of tissue (fresh tissue vs. chip tissue) to show the fate of disaggregated cells after Annexin V and PI staining and stratification with the flow cytometer. The proportion of healthy cells (cells with minimal Annexin V and PI staining) was 68.9% in Fresh GBM samples compared with 61.1% in tissue maintained in the microfluidic device. This difference was statistically significant when tested with a paired t-test; P = 0.01.
Trypan Blue
Trypan blue staining of disassociated cells allowed visual confirmation of cell viability via dye exclusion. This was performed on the disaggregated cells from fresh tissue controls and chip tissue (n = 19). The results from the 6 matched pair samples revealed mean cell viability of 73% (±14.2) for fresh tissue and 61% (±26.4) in tissue that had been maintained within the MF device for 72 hours (P = 0.16). Trypan blue assays were concordant with AV/PI assays with strong linear relationship between the two viability assays (r2 value of 0.78).
Analysis of the effluent (LDH Absorbance)
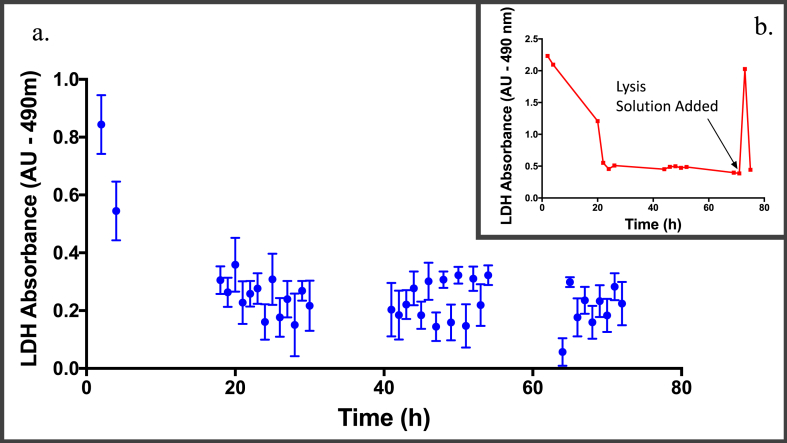
The pattern of LDH release (measured in terms of LDH absorbance) into the effluent from chip tissue was distinctly similar in all samples maintained on chip. This is primarily one in which there is an initial peak in LDH absorbance (thought to be attributed to tissue dissection) followed by a low and steady state of absorbance [20]. Figure 5A summarises the average LDH released at set time points during all experiments and confirms this pattern of LDH release. The injection of a tissue lysis solution to the tissues on chip at the end of the maintenance period (performed on 8 GBM samples at the 71st hour) was associated with an increase in LDH release (measured by absorbance). Figure 5B displays the LDH absorbance in the 6th patient, where there was a 4-fold increase in the amount of LDH released after the introduction of the lysis solution. Analysis of all the effluent samples collected immediately after introduction of the lysis solution revealed, on average, a 3-fold increase in LDH.
Figure 5.
Average LDH absorbance from effluent samples collected over the 72-hours time period. (A) Graph displaying the average absorbance values for each time point from all experiments, e.g., the average absorbance of all effluent samples collected at 2 hours (first value on graph) after the start of the experiments was 0.84 AU. Four hours after the start of the experiments, the average absorbance from the effluents collected was 0.54 AU. The graph confirms the trend of an initially high LDH absorbance, followed by a low and stable absorbance value. (B) Representative graph (n = 8) displaying LDH absorbance over time in one of the patient's GBM sample, where there was a 4-fold increase in LDH absorbance after introduction of the lysis solution.
Correlation with Clinical Outcomes
There was no clear congruity between patient outcome and viability or performance of tumour on chip. However, there was a disparity in confounding factors known to have an impact on patient survival, such as age [26], postoperative therapy [5], extent of tumour resection [27] and number of previous operations [28]. One of the more sensitive markers of GBM prognosis is the extent of tumour resection [27,[29], [30], [31], [32], [33]], and it was possible to stratify the performance of tissue on chip according to the extent of resection. When evaluating only patients that had greater than 90% of their tumours resected, there was a trend towards reduced viability in tumours from patients who survived beyond 250 days (n = 5), compared with patients who died within 250 days (n = 5). When comparing the performance of recurrent tumours (n = 4) with that of primary tumours (n = 22), there was a trend towards improved viability (79% ±15.3 vs. 57.5% ±29.9) as measured with AV/PI flow cytometry. Similarly, Isocitrate Dehydrogenase (IDH) wild type tumours retained greater viability on chip than the IDH mutant tumours (76.7% ±22 vs. 55.7% ±13.4). However, there was no statistically significant difference when the groups were compared with a t-test (Figure 6).
Figure 6.
A) Bar graph comparing the viability (determined by Annexin V and PI) of GBM (n = 27) tissues on chip with grade 1–3 gliomas (n = 5), P = 0.01. (B) Comparing viability (with Annexin V and PI assay) of IDH wild type (n = 20) with IDH mutant tumours (n = 4). Results were not significantly different; P = 0.08 as analysed with unpaired t-test. (C) Comparing viability (measured with Annexin V and PI assay) of recurrent tumours (n = 4) with that of primary tumours (n = 22). No statistically significant difference was found (unpaired t-test; P = 0.017).
Discussion
The present study maintained 128 GBM tissues from 33 patients within MF devices for 3 days. The bespoke device was made from two layers of glass thermally bonded to incorporate connected microchannels and a tissue chamber, facilitating the continuous perfusion of GBM tissue with nutrient media. Cell viability assays, including AV and PI flow cytometry, LDH release, trypan blue dye exclusion, and histological and immunohistochemical analyses confirm that viable tissue was maintained during the assigned time. Results of tissue viability and morphological appearances were comparable to those of fresh tissue that was analysed immediately after excision from the patient.
The MF device used in the present study has been established in the maintenance of a variety of tissues including animal liver biopsies [34], human colorectal cancer specimens [35,36] and head and neck cancers [20,37,38]. Hattersley et al. kept liver tissue from rats in a viable state within the device for 70 hours and showed that tissues retained normal morphology on H&E sections as well as viability, measured with LDH release and urea and albumin synthesis [39]. The group expanded on the applications of the device by maintaining head and neck squamous cell carcinomas for 7 days, proving tissue was viable with LDH and a tetrazolium proliferation assay [20]. Similarly, Bower et al. found that PI and trypan blue staining in head and neck cancer tissues maintained on chip for 48 hours was comparable to tissue that was processed immediately from the patient [37]. Here, for the first time, human GBM tissue biopsies have been maintained in a MF device. Previous studies focused on maintaining human GBM tissue, date back to the 1970s, and using static culture techniques in culture dishes that required the culture media to be replenished every 1–4 days [[40], [41], [42], [43]]. More recently, a number of groups have maintained human GBM tissues on collagen gel matrices [44] and on membrane culture inserts (Millipore) [45,46] but none of these experimental models have applied continuous tissue flow. Compared with previous static culture experiments, the length of tissue culture in the present study is considerably shorter, with only 2 tissue sections cultured for more than 3 days, one for 5 days and another for a week. Rubenstein and Herman were able to culture GBM explants on a sponge foam matrix, changing the culture media twice a week, for up to 4 months [40,47]. They showed that tissue architecture and cytological features were conserved over the period; however, viability, cell death, or apoptosis were not measured nor correlated with the histological data. Ono et al. cultured 22 GBM tissues on collagen gel matrix assays for 7 days and treated the tissues with a variety of chemotherapeutic agents, and although the group were able to assess the inhibitory effects of the drugs, they did not report on the viability of the untreated tissues [44]. Merz et al. maintained 12 GBM tissues for over 2 weeks and showed that histological appearances were maintained over that time period; however, the group did not report on how viability assays of tissue slice cultures compared to fresh tissue [46]. To our knowledge, the present study is the largest series of human GBM tumours maintained as whole tissue pieces as well as represents the first time that the combined analysis of histology, IHC, and cell viability assays has been used to gain a comprehensive understanding of the fate of GBM tissue cultured, ex vivo. It provides an accurate estimate of how well maintained the tissue is over a 72-hours period and the feasibility of using the method as a preclinical model for GBM testing.
Earlier iterations of this device had maintained human tissues successfully with a flow rate of 2 μl per min. Studies on animal brain slices maintained smaller tissue sections (350- to 500-μm thick) within a range of flow rates between 0.3 μl and 17 ml per min [[48], [49], [50], [51]]. The rate of perfusion is specific for each device and is adjusted to account for the nutrient media used, as well as the tissue type and thickness. Normal cerebral blood flow is approximately 50 ml per 100 g of tissue per minute, but in GBM, the cerebral blood flow can range between 39 and 82 ml/100 g/min [52]. This equates to a rate of 4–8 μl/min per 10 mg of tissue, a rate matched during our experiments with flow of media at 4 μl per min for GBM tissues of an average wet weight of 12.1 mg. Benefits of continuous flow of nutrient media over static brain tissue cultures have been stressed in previous studies. Reid et al. found failure of electrical activity within hippocampal brain slices if nutrient flow stopped for over a minute [53]. Rambani et al. proved that viability of their brain slices was significantly better if the tissue was perfused at 20 μl per hour compared with nonperfused tissue slices [49] and recently Killian et al. were able to show that perfusion of brain tissue correlated with an improvement in tissue cell viability, as well as the thickness of the tissue mantle, which thinned significantly with nonperfused tissue [54]. Optimisation experiments in the present study showed that tissue maintained with MF flow outperformed static tissue cultures in terms of cell viability, LDH release, and morphological appearances (Figure 5). The benefits of tissue flow likely arise from the mimicry of normal in vivo conditions where there is continuous influx of nutrients as well as removal of waste products at a constant rate, recapitulated within a MF culture paradigm.
Strengths of the study include the reliability of the method, with an inexpensive and efficient model for GBM tissue maintenance. Compared with other GBM models such as cell spheroids and PDX, a MF tissue chip can be set up in less than 2 hours of the tissue being resected. A total of 97% of GBM tissues were successfully cultured for the duration of the experiments without any disruption to the continuous inflow of nutrients and removal of waste, all with minimal hands-on input required once the system had been set up. Such reliability in a preclinical model can challenge current options such as PDX, which currently have an engraftment rate that can be as low as 22% [11] and require several weeks to months to develop before they can be tested [55].
There was no correlation between tissue viability on chip and patient survival. Validity of the model requires the performance of the tissue on chip to have some resemblance to the in vivo behaviour of the tumour. There remains, however, a complex relationship between tumour behaviour and survival, with factors including patient age, performance status, the extent of tumour debulked, and the treatment provided all having an impact on survival. Patient survival is determined by the difference between the patient's risk factor profile (determines degree to which the patient can defend against deleterious tumour effects) and the inherent tumour biology (as well as treatment resistance). Patient survival is probably not the best correlate to tissue viability on chip because survival is so multifactorial.
Instead, the performance of the tissue on chip can be used to deduce the inherent tumour behaviour or aggressiveness. When patient risk factors are controlled for, the performance of the tumour on chip does bore out some association with patient survival in terms of viability. Evidence suggests that recurrent GBMs after treatment display phenotypic transition from proneural to mesenchymal subtype, leading to disease recurrence and a more aggressive tumour [56]. In our study, recurrent tumours were the more sustainable and viable tumours on chip compared with primary tumours. Also, in keeping with the trend was the finding that GBM tumours had higher cell viability than grade 1–3 tumours. There was also improved viability in the IDH wild-type tumours, which generally have a worse prognosis than IDH mutants [57]. Although these differences did not reach statistical significance, the trend was commonplace on testing. Further experiments with a larger cohort are required to clearly elucidate the associations and clarify the usefulness of the method in predicting the aggressiveness of GBM tumours.
With such promising results on the assessment of tissue viability of samples maintained on chip, the response of tissues on chip to drugs is essential to ensure the model is fit for purpose. Early results are yet to show any significant effects, but appropriate dosing, delivery methods, and duration of treatment are yet to be established. Further work is required to better understand the method and explore the benefits of different methodological iterations, including the effects of changing the direction of flow within the chip to one that is perpendicular, which is known to improve viability by enhancing the diffusion of nutrients. Also necessary is more intensive testing by prolonging the length of time the tissue is maintained, which would support more detailed drug testing, and testing experimental and established chemotherapeutic drugs such as temozolomide. The effect of radiotherapy is required to ensure that current treatment standards can be simulated on GBM tissue on chip. Carr et al. were able to continue tissue maintenance within their MF device while irradiation of the tissue on chip took place using a 6-MV photon beam from a Varian Linear Accelerator [38]. All this will allow a more robust examination of the MF culture paradigm as a GBM ex vivo model.
Conclusion
A diagnosis with glioblastoma equates to a poor prognosis regardless of treatment. Conventional therapy (surgery, radiotherapy, and chemotherapy) affords a modest survival benefit of approximately 12 months compared with that of patients who are left untreated [58]. This pilot study is the first time that a MF device has been used to maintain human GBM tissue ex vivo and is also the largest series of human GBM tumours "cultured" ex vivo. Although still in its infancy, the use of MF principles to study whole tissues is improving our understanding of complex diseases. Analysis of experiments with a larger number of patients will allow a more definitive exploration of the method and its potential as a preclinical disease model, a model with the prospect of enabling more detailed inspections of GBMs, more accurate predictions of treatment responses, and better outcomes for patients. GBM treatment starts with maximal safe resection, and there is, on average, 4–6 weeks between surgical resection and adjuvant therapy [59]. There is a potential to use this time to study individual characteristics of a tumour biopsy in a MF device and to tailor adjuvant therapy based on biological testing. More enticing is the possibility of a new preclinical drug model for the investigation of novel therapies to treat GBM.
Disclosure
The authors have no conflicts of interest to disclose.
Acknowledgements
F.O., under the supervision of J.G. and S.A., designed and carried out the study and collected and interpreted the data; all authors contributed to, read, and approved the final manuscript. The authors would like to thank Naomi Guppy and the University College London Advanced Diagnostics Laboratory for histological and immunohistochemical processing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2019.09.002.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Ostrom Q.T., Bauchet L., Davis F.G., Deltour I., Fisher J.L., Langer C.E. The epidemiology of glioma in adults: a "state of the science" review. Neuro Oncol. 2014;16(7):896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldape K.D., Okcu M.F., Bondy M.L., Wrensch M. Molecular epidemiology of glioblastoma. Cancer J. 2003;9(2):99. doi: 10.1097/00130404-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):1–18. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 4.Chang K., Creighton C.J., Davis C., Donehower L., Drummond J., Wheeler D. The cancer Genome Atlas Pan-cancer analysis project. Nat Genet. 2013;45(10):1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stupp R., Mason W.P., Bent van den M.J., Weller M., Fisher B., Taphoorn M.J.B. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Masui K., Cloughesy T.F., Mischel P.S. Molecular pathology in adult high-grade gliomas: from molecular diagnostics to target therapies. Neuropathol Appl Neurobiol. 2012;38(3):271–291. doi: 10.1111/j.1365-2990.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldape Kenneth, Zadeh Gelareh, Mansouri Sheila, Guido Reifenberger A. von D. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129:829–848. doi: 10.1007/s00401-015-1432-1. [DOI] [PubMed] [Google Scholar]
- 8.Sant M., Minicozzi P., Lagorio S., Børge Johannesen T., Marcos-Gragera R., Francisci S. Survival of European patients with central nervous system tumors. Int J Cancer. 2012;131(1):173–185. doi: 10.1002/ijc.26335. [DOI] [PubMed] [Google Scholar]
- 9.Ding H., Nagy A., Gutmann D.H., Guha A., Gurmann D. A review of astrocytoma models. Neurosurg Focus. 2000;8(4):1–8. [Google Scholar]
- 10.El Meskini R., Iacovelli A.J., Kulaga A., Gumprecht M., Martin P.L., Baran M. A preclinical orthotopic model for glioblastoma recapitulates key features of human tumors and demonstrates sensitivity to a combination of MEK and PI3K pathway inhibitors. Dis Model Mech. 2015;8(1):45–56. doi: 10.1242/dmm.018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.William D., Mullins C.S., Schneider B., Orthmann A., Lamp N., Krohn M. Optimized creation of glioblastoma patient derived xenografts for use in preclinical studies. J Transl Med. 2017;15(1):27. doi: 10.1186/s12967-017-1128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziółkowska K., Kwapiszewski R., Brzózka Z. Microfluidic devices as tools for mimicking the in vivo environment. New J Chem. 2011;35(5):979. [Google Scholar]
- 13.Huh D., Matthews B.D., Mammoto A., Montoya-Zavala M., Hsin H.Y., Ingber D.E. Reconstituting organ-level lung functions on a chip. Science. 2010;328(5986):1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez F.A. The future of microfluidic point-of-care diagnostic devices. Bioanalysis. 2013;5(1):1–3. doi: 10.4155/bio.12.307. [DOI] [PubMed] [Google Scholar]
- 15.Sylvester D.C., Hattersley S.M., Stafford N.D., Haswell S.J., Greenman J. Development of microfluidic-based analytical methodology for studying the effects of chemotherapy agents on cancer tissue. Curr Anal Chem. 2013;9(1):2–8. [Google Scholar]
- 16.Cheah L.-T., Dou Y.-H., Seymour A.-M.L., Dyer C.E., Haswell S.J., Wadhawan J.D. Microfluidic perfusion system for maintaining viable heart tissue with real-time electrochemical monitoring of reactive oxygen species. Lab Chip. 2010;10(20):2720. doi: 10.1039/c004910g. [DOI] [PubMed] [Google Scholar]
- 17.Ataç B., Wagner I., Horland R., Lauster R., Marx U., Tonevitsky A.G. Skin and hair on-a-chip: in vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip. 2013;13(18):3555. doi: 10.1039/c3lc50227a. [DOI] [PubMed] [Google Scholar]
- 18.Dawson A., Dyer C., Macfie J., Davies J., Karsai L., Greenman J. A microfluidic chip based model for the study of full thickness human intestinal tissue using dual flow. Biomicrofluidics. 2016;10(6) doi: 10.1063/1.4964813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astolfi M., Péant B., Lateef M.A., Rousset N., Kendall-Dupont J., Carmona E. Micro-dissected tumor tissues on chip: an ex vivo method for drug testing and personalized therapy. Lab Chip. 2015;16(2):312–325. doi: 10.1039/c5lc01108f. [DOI] [PubMed] [Google Scholar]
- 20.Hattersley S.M., Sylvester D.C., Dyer C.E., Stafford N.D., Haswell S.J., Greenman J. A microfluidic system for testing the responses of head and neck squamous cell carcinoma tissue biopsies to treatment with chemotherapy drugs. Ann Biomed Eng. 2012;40(6):1277–1288. doi: 10.1007/s10439-011-0428-9. [DOI] [PubMed] [Google Scholar]
- 21.Fulawka L., Halon A. Proliferation index evaluation in breast cancer using ImageJ and ImmunoRatio applications. Anticancer Res [Internet] 2016;36(8):3965–3972. [PubMed] [Google Scholar]
- 22.Tuominen V.J., Ruotoistenmäki S., Viitanen A., Jumppanen M., Isola J. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res. 2010;12(4):R56. doi: 10.1186/bcr2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parwani A.V., Almeida J.S., Iriabho E.E., Gorrepati V.L., Wilkinson S.R., Grüneberg A. ImageJS: personalized, participated, pervasive, and reproducible image bioinformatics in the web browser. J Pathol Inform. 2012;3 doi: 10.4103/2153-3539.98813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornicek F.J., Malinin G.I. Encyclopedia of immunology. 1998. Viability, methods of assessing leukocyte; pp. 2474–2475. [Google Scholar]
- 25.Mariño G., Kroemer G. Mechanisms of apoptotic phosphatidylserine exposure. Cell Res. 2013;23(11):1247–1248. doi: 10.1038/cr.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis F.G., Freels S., Grutsch J., Barlas S., Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973-1991. J Neurosurg. 1998;88(1):1–10. doi: 10.3171/jns.1998.88.1.0001. [DOI] [PubMed] [Google Scholar]
- 27.Li Y.M., Suki D., Hess K., Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg. 2016;124(4):977–988. doi: 10.3171/2015.5.JNS142087. [DOI] [PubMed] [Google Scholar]
- 28.van Linde M.E., Brahm C.G., de Witt Hamer P.C., Reijneveld J.C., Bruynzeel A.M.E., Vandertop W.P. Treatment outcome of patients with recurrent glioblastoma multiforme: a retrospective multicenter analysis. J Neuro Oncol. 2017;135(1):183–192. doi: 10.1007/s11060-017-2564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almenawer S.A., Badhiwala J.H., Alhazzani W., Greenspoon J., Farrokhyar F., Yarascavitch B. Biopsy versus partial versus gross total resection in older patients with high-grade glioma: a systematic review and meta-analysis. Neuro Oncol. 2015;17(6):868–881. doi: 10.1093/neuonc/nou349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanai N., Polley M.-Y., McDermott M.W., Parsa A.T., Berger M.S. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 31.Yong R.L., Lonser R.R. Surgery for glioblastoma multiforme: striking a balance. World Neurosurg. 2011;76(6):528–530. doi: 10.1016/j.wneu.2011.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan J.-L., van der Hoorn A., Larkin T.J., Boonzaier N.R., Matys T., Price S.J. Extent of resection of peritumoral diffusion tensor imaging-detected abnormality as a predictor of survival in adult glioblastoma patients. J Neurosurg. 2016;1–8 doi: 10.3171/2016.1.JNS152153. [DOI] [PubMed] [Google Scholar]
- 33.Hervey-Jumper S.L., Berger M.S. Maximizing safe resection of low- and high-grade glioma. J Neuro Oncol. 2016;130(2):269–282. doi: 10.1007/s11060-016-2110-4. [DOI] [PubMed] [Google Scholar]
- 34.Hattersley S.M., Greenman J., Haswell S.J. Study of ethanol induced toxicity in liver explants using microfluidic devices. Biomed Microdevices. 2011;13(6):1005–1014. doi: 10.1007/s10544-011-9570-2. [DOI] [PubMed] [Google Scholar]
- 35.Webster A., Dyer C.E., Haswell S.J., Greenman J. A microfluidic device for tissue biopsy culture and interrogation. Anal Methods. 2010;2(8):1005. [Google Scholar]
- 36.Patel R., Green V., Hunter I., Greenman J. Apoptosis in rectal cancer biopsies following irradiation in a microfluidic device: a predictor of response? Br J Surg. 2014;101(Supplement 4):12. [Google Scholar]
- 37.Bower R., Green V.L., Kuvshinova E., Kuvshinov D., Karsai L., Crank S.T. Maintenance of head and neck tumor on- chip: gateway to personalized treatment? Aim: Futur Sci OA. 2017;3(2) doi: 10.4155/fsoa-2016-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carr S.D., Green V.L., Stafford N.D., Greenman J. Analysis of radiation-induced cell death in head and neck squamous cell carcinoma and rat liver maintained in microfluidic devices. Otolaryngol Head Neck Surg. 2014;150(1):73–80. doi: 10.1177/0194599813507427. [DOI] [PubMed] [Google Scholar]
- 39.Hattersley S.M., Dyer C.E., Greenman J., Haswell S.J. Development of a microfluidic device for the maintenance and interrogation of viable tissue biopsies. Lab Chip. 2008;8(11):1842–1846. doi: 10.1039/b809345h. [DOI] [PubMed] [Google Scholar]
- 40.Rubinstein L.J., Herman M.M. In vitro characteristics of human glioblastomas maintained in organ culture systems. Am J Pathol. 1973;71(April) [PMC free article] [PubMed] [Google Scholar]
- 41.Lyser K.M. Organ culture of human nervous system tumors. In Vitro. 1976;12(1):48–56. doi: 10.1007/BF02832793. [DOI] [PubMed] [Google Scholar]
- 42.Sipe J.C., Herman M.M., Rubinstein L.J. Electron microscopic observations on human glioblastomas and astrocytomas maintained in organ culture systems. Am J Pathol. 1973;73(3):589–606. [PMC free article] [PubMed] [Google Scholar]
- 43.Holmström T., Saksela E. Growth of human brain tumour explants in matrix cultures in different human sera. Acta Pathol Microbiol Scand Sect A Pathol. 1971;79 A(4):399–406. doi: 10.1111/j.1699-0463.1971.tb01837.x. [DOI] [PubMed] [Google Scholar]
- 44.Ono A., Kanno H., Hayashi A., Nishimura S., Kyuma Y., Sato H. Collagen gel matrix assay as an in vitro chemosensitivity test for malignant astrocytic tumors. Int J Clin Oncol. 2007;12(2):125–130. doi: 10.1007/s10147-006-0636-8. [DOI] [PubMed] [Google Scholar]
- 45.Bayin N.S., Ma L., Thomas C., Baitalmal R., Sure A., Fansiwala K. Patient-specific screening using high-grade glioma explants to determine potential radiosensitization by a TGF-β small molecule inhibitor. Neoplasia (United States) 2016;18(12):795–805. doi: 10.1016/j.neo.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merz F., Gaunitz F., Dehghani F., Renner C., Meixensberger J., Gutenberg A. Organotypic slice cultures of human glioblastoma reveal different susceptibility to treatments. Neuro-oncology. 2013;49(6):8352. doi: 10.1093/neuonc/not003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hess J.R., Michaud J., Sobel R.A., Herman M.M., Rubinstein L.J. The kinetics of human glioblastomas maintained in an organ culture system - an in vitro autoradiographic study. Acta Neuropathol. 1983;61(1):1–9. doi: 10.1007/BF00688379. [DOI] [PubMed] [Google Scholar]
- 48.Blake A., Pearce T.M., Rao N.S., Johnson S.M., Williams J.C. Multilayer PDMS microfluidic chamber for controlling brain slice microenvironment. Lab Chip. 2007;7(7):842–849. doi: 10.1039/b704754a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rambani K., Vukasinovic J., Glezer A., Potter S.M. Culturing thick brain slices: an interstitial 3D microperfusion system for enhanced viability. J Neurosci Methods. 2009;180(2):243–254. doi: 10.1016/j.jneumeth.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi Y., McClain M.A., LaPlaca M.C., Frazier A.B., Allen M.G. Three dimensional MEMS microfluidic perfusion system for thick brain slice cultures. Biomed Microdevices. 2007;9(1):7–13. doi: 10.1007/s10544-006-9004-8. [DOI] [PubMed] [Google Scholar]
- 51.Hill M.R.H., Greenfield S.A. The membrane chamber: a new type of in vitro recording chamber. J Neurosci Methods. 2011;195(1):15–23. doi: 10.1016/j.jneumeth.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 52.Kim C., Kim H.S., Shim W.H., Choi C.G., Kim S.J., Kim J.H. Recurrent glioblastoma: combination of high cerebral blood flow with MGMT promoter methylation is associated with benefit from low-dose temozolomide rechallenge at first recurrence. Radiology. 2016;000(0):152152. doi: 10.1148/radiol.2016152152. [DOI] [PubMed] [Google Scholar]
- 53.Reid K.H., Schurr A., Tseng M.T., Edmonds H.L., Jr. Resistance to hypoxia in the rat hippocampal slice. Brain Res. 1984;302(2):387–391. doi: 10.1016/0006-8993(84)90255-5. [DOI] [PubMed] [Google Scholar]
- 54.Killian N.J., Vernekar V.N., Potter S.M., Vukasinovic J. A device for long-term perfusion, imaging, and electrical interfacing of brain tissue in vitro. Front Neurosci. 2016;10(MAR):1–14. doi: 10.3389/fnins.2016.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Z., Kader M., Sen R., Placantonakis D.G. Orthotopic patient-derived glioblastoma xenografts in mice. Methods Mol Biol. 2018;1741:183–190. doi: 10.1007/978-1-4939-7659-1_14. [DOI] [PubMed] [Google Scholar]
- 56.Mondal A., Kumari Singh D., Panda S., Shiras A. Extracellular vesicles as modulators of tumor microenvironment and disease progression in glioma. Front Oncol. 2017;7(July):1–8. doi: 10.3389/fonc.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanson M., Marie Y., Paris S., Idbaih A., Laffaire J., Ducray F. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 58.Delgado-López P.D., Corrales-García E.M. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;18(11):1062–1071. doi: 10.1007/s12094-016-1497-x. [DOI] [PubMed] [Google Scholar]
- 59.Louvel G., Metellus P., Noel G., Peeters S., Guyotat J., Duntze J. Delaying standard combined chemoradiotherapy after surgical resection does not impact survival in newly diagnosed glioblastoma patients. Radiother Oncol. 2016;118(1):9–15. doi: 10.1016/j.radonc.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Dawson A., Green V., Bower R., Greenman J. Microfluidics: the Fur-free way towards personalised medicine in cancer therapy. Drug Target Rev. 2016;3:12–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.