Abstract
Background
Substance use frequently goes undetected in primary care. Though barriers to implementing systematic screening for alcohol and drug use have been examined in urban settings, less is known about screening in rural primary care.
Objective
To identify current screening practices, barriers, facilitators, and recommendations for the implementation of substance use screening in rural federally qualified health centers (FQHCs).
Design
As part of a multi-phase study implementing electronic health record–integrated screening, focus groups (n = 60: all stakeholder groups) and individual interviews (n = 10 primary care providers (PCPs)) were conducted.
Participants
Three stakeholder groups (PCPs, medical assistants (MAs), and patients) at three rural FQHCs in Maine.
Approach
Focus groups and interviews were recorded, transcribed, and content analyzed. Themes surrounding current substance use screening practices, barriers to screening, and recommendations for implementation were identified and organized by the Knowledge to Action (KTA) Framework.
Key Results
Identifying the problem: Stakeholders unanimously agreed that screening is important, and that universal screening is preferred to targeted approaches. Adapting to the local context: PCPs and MAs agreed that screening should be done annually. Views were mixed regarding the delivery of screening; patients preferred self-administered, tablet-based screening, while MAs and PCPs were divided between self-administered and face-to-face approaches. Assessing barriers: For patients, barriers to screening centered around a perceived lack of rapport with providers, which contributed to concerns about trust, judgment, and privacy. For PCPs and MAs, barriers included lack of comfort, training, and preparedness to address screening results and offer treatment.
Conclusions
Though stakeholders agree on the importance of implementing universal screening, concerns about the patient-provider relationship, the consequences of disclosure, and privacy appear heightened by the rural context. Findings highlight that strong relationships with providers are critical for patients, while in-clinic resources and training are needed to increase provider comfort and preparedness to address substance use.
Electronic supplementary material
The online version of this article (10.1007/s11606-019-05232-y) contains supplementary material, which is available to authorized users.
KEY WORDS: substance use disorder, screening, primary care, rural
INTRODUCTION
Substance use is a leading cause of preventable death in the United States (US) that is rarely identified in primary care. In 2017, almost 25% of the US population over age 12 reported past month binge alcohol use, and approximately 11% reported past month illicit drug use.1 The highest number of annual US overdose deaths, nearly 72,000, was recorded in 2017.2 Despite these staggering figures, the substance use treatment admission rate decreased from 756 to 557 per 100,000 people in the past decade.3 Of the 21.0 million people estimated to have a substance use disorder (SUD) in 2017, under 12% received any treatment from an addiction treatment program.4
Although substance use is one of the top 10 priorities of Rural Healthy People 2020,5 accessing treatment is particularly challenging in rural regions,6 which have been disproportionately impacted by the opioid epidemic.7 Patients in rural settings may rely on primary care providers (PCPs) for SUD treatment and prevention, and yet are less likely to be screened for substance use than those in suburban or urban settings.8 Failure to identify those at risk for SUDs represents a missed opportunity to intervene and potentially prevent or reverse the health consequences of these conditions.
Adopted in 2017 as a Healthcare Effectiveness Data and Information Set (HEDIS) measure,9 the United States Preventative Services Task Force (USPSTF) recommends that primary care clinicians routinely screen adults for risky alcohol use and provide brief behavioral counseling.10 While the USPSTF found insufficient evidence to support screening for drug use in adults or adolescent primary care patients,10, 11 others,12, 13 including the US Surgeon General,14 recommend screening for drug use in primary care settings. Despite these guidelines, screening rates for alcohol and drug use remain low. Within federally qualified health centers (FQHCs), a 2011 survey estimated that while 53% routinely screen all patients for depression, only 39% routinely screen for alcohol or drug use.15, 16 Well-documented barriers to screening in primary care include time, a lack of training, and provider discomfort.8, 17
Though some barriers to implementing screening in primary care appear consistent across urban and rural settings, several rural community characteristics may exacerbate these challenges. The rural primary care workforce is struggling to meet the demands for service.18 Rural patients have less access to primary care, as the PCP-to-patient ratio in rural areas is 39.8 compared with 53.3/100,000 in urban settings.19 In these regions, FQHCs are a critical healthcare access point, serving one in seven rural residents and a patient population with high rates of substance use.20 Rural regions also have limited behavioral health services,21–24 and less than 10% of patients with SUDs access treatment.25
To gain an understanding about the feasibility of integrating substance use screening into rural primary care and collecting this information in electronic health records (EHRs), our team launched a multi-site study through the National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN). This study is guided by the “Knowledge-to-Action” (KTA) Framework which informs the implementation of new clinical practices.26 Developed from a synthesis of 31 theories of planned action,26 the KTA Framework is a process model useful for evaluating the development of implementation projects.27
This phased feasibility and proof-of-concept study is designed to examine the implementation of screening using EHR common data elements to deliver validated substance use screening in primary care. A parent NIDA CTN study was launched to study the implementation of screening for substance use in two urban academic health systems in New York and Massachusetts.28 This paper presents findings from the ancillary study, an expansion of the parent study examining the implementation of screening within a network of rural FQHCs in Maine, a state with the highest percentage of rural residents in the US.29, 30 This ancillary study was designed to inform screening implementation in a common rural practice setting, using the validated Tobacco, Alcohol, and Prescription Medication Screening Tool (TAPS Tool).31
METHODS
Design
We solicited patient, PCP, and medical assistant (MA) input regarding implementation strategies for tobacco, alcohol, and drug screening through a combination of focus groups and individual interviews. Interview guides (Appendixes 1–3) were adapted from those used in the parent study and followed the themes from the knowledge implementation (“Action Cycle”) component of the KTA Framework: identifying the problem, adapting to the local context, and assessing barriers.26 In addition to collecting data on attitudes and group norms regarding the feasibility and preferences for screening approaches using focus groups, individual interviews explored PCPs’ workflow and comfort treating substance use. Interviews also offered an opportunity for PCPs who could not attend focus groups to participate. The study was approved by the Institutional Review Boards (IRBs) of New York University School of Medicine and Dartmouth College.
Setting
Data were collected prior to the introduction of screening at three primary care clinics affiliated with Penobscot Community Healthcare (PCH), a rural FQHC based in Bangor, ME. PCH is one of the largest FQHCs in New England, with a network of seven primary care practices across the region. Participating clinics were selected by health system leadership based on patient census, use of a common Centricity EHR, and presence of multiple providers serving adult primary care patients.
Participants
Focus groups and interviews (n = 65 participants) were conducted between October 2017 and April 2018. Patients (n = 22) were English-speaking adults currently receiving care in a participating clinic, while PCPs (n = 21) and MAs (n = 22) were currently working in the clinic (Table 1). Twelve focus groups (6 with patients, 3 with PCPs, 3 with MAs) and ten PCP interviews were conducted. All participants were given a study information sheet and provided verbal consent.
Table 1.
Stakeholder Demographics (n= 65)
| Patients | Providers* | MAs | ||
|---|---|---|---|---|
| Focus groups (n = 22) |
Focus groups (n = 14) |
Interviews (n = 10)* |
Focus groups (n = 22) |
|
| Age in years m(sd) | 44.0 (16.5) | 44.9 (13.9) | 40.4 (10.7) | 42.5 (9.98) |
| Gender n(%) | ||||
| Female | 13 (59.1%) | 10 (71.4%) | 4 (40.0%) | 21 (95.5%) |
| Race and ethnicity n(%) | ||||
|
White Not Hispanic or Latino |
19 (86.4%) 22 (100%) |
14 (100%) 14 (100%) |
9 (90.0%) 10 (100%) |
22 (100%) 22 (100%) |
| Clinic n(%) | ||||
| Clinic 1 | 7 (31.8%) | 2 (14.3%) | 3 (30.0%) | 4 (18.2%) |
| Clinic 2 | 10 (45.5%) | 8 (57.1%) | 4 (40.0%) | 7 (31.8%) |
| Clinic 3 | 5 (22.7%) | 6 (42.9%) | 3 (30.0%) | 11 (50.0%) |
| Years in practice m(sd) | – | 15.3 (14.2) | 10.2 (11.9) | 11.6 (11.1) |
| Role n(%) | ||||
|
Doctor of osteopathy Medical doctor Nurse practitioner Medical assistant Physician’s assistant Other |
– – – – – – |
1 (7.1%) 3 (21.4%) 5 (35.7%) 0 (0%) 5 (35.7%) 2 (12.5%) |
0 (0%) 3 (30.0%) 4 (40.0%) 0 (0%) 3 (30.0%) 0 (%) |
0 (0%) 0 (0%) 0 (0%) 22 (100%) 0 (0%) 0 (0%) |
| Specialty n(%) | ||||
|
Family medicine Internal medicine Other |
– – – |
12 (85.7%) 0 (0%) 2 (14.3%) |
10 (100%) 0 (0%) 0 (0%) |
13 (72.2%) 2 (11.1%) 3 (16.7%) |
| Number of patients per week n (%) | ||||
|
0–50 patients 51–100 patients 101–150 patients 150+ patients |
– – – – |
1 (7.1%) 11 (78.6%) 2 (14.3%) 0 0%) |
2 (20.0%) 7 (70.0%) 1 (1.0%) 0 (0%) |
6 (25.0%) 10 (41.7%) 5 (20.8%) 3 (12.5%) |
*Five PCPs participated in both a focus group and an interview but were included in enrollment totals once
Focus group recruitment flyers advertising opportunities to share opinions of a new substance use screening process were posted in clinics and given to patients in waiting rooms. PCPs and MAs learned about focus groups by email. Focus groups were led by a research team member (JM or ES) and lasted approximately 45 minutes. Participants were incentivized with gift cards ($25: patients/$50: MAs, PCPs). Individual interviewees were PCPs selected for participation by health system leadership due to their roles as opinion leaders within their respective clinics. Participant interviews lasted 60 minutes, were conducted by phone by research staff (ES), and incentivized with $100 gift cards.
Analysis
Focus groups and interviews were audio recorded and transcribed verbatim. Provider and patient interview and focus group transcripts were uploaded to Atlas.ti (8.0)32 software for organization as distinct analyses. A researcher (SM) coded provider (interview and focus group) and patient (focus group) transcripts using unique codebooks, generated by researchers from the parent study, guided by the KTA Framework and adapted for this new dataset. All additional, inductively generated codes were identified and discussed with another researcher (ES) before being added to the codebooks and used to update coding across transcripts. Researchers (ES and SM) met to discuss randomly selected coded transcripts from each stakeholder group to establish consensus. They then randomly selected an additional transcript from each stakeholder group to code independently and estimate inter-rater reliability (IRR). To measure IRR, the researchers uploaded dually coded transcripts to the Coding Analysis Toolkit (CAT)33 and computed Cohen’s kappa. The kappa coefficient averaged across the stakeholder transcripts was 0.74, which is considered good in qualitative research.34 The researchers (SM, ES) exported text segments by code and conducted subtheme analyses by KTA domain, stakeholder group, and data collection method. To stay focused on the research question,35 and enable both within- and across-stakeholder’ group comparisons,36 they created stakeholder summaries by data collection method which supported the creation of a systematic matrix,36 organizing the findings by KTA domain and stakeholder groups.
RESULTS
Qualitative results (Table 2) are presented within the following KTA action cycle domains: (1) identifying the problem (current screening practices and the importance of screening), (2) adapting to the local context (recommendations about implementation within the rural FQHC setting), and (3) assessing barriers (individual- and system-level).
Table 2.
KTA Results
| KTA domain | Patient quotes | Primary care provider (PCP) quotes | Medical assistant (MA) quotes |
|---|---|---|---|
| Identifying the problem | |||
|
Current screening practices |
DNE | Implementation definitely varies widely from one provider to the next. | I feel like it should be brought up more… I only have the first encounter with the patient, after that it’s not mentioned. |
| Importance of substance use (SU) screening | I do think it could be important to talk about these risks…to help [patients] live longer. | It’s a top priority. | It’s very important. |
| Adapting to the local context | |||
| Universal or targeted | Everybody should be screened. | We ask other preventative health questions to everybody, so I think SU disorder should be the same. | I do not think you can judge a book by its cover. You cannot always tell who needs to be asked. |
| Frequency | DNE | I always screen at least annually, or if there’s a compelling reason. | No less than every six months |
| Format | It might be easier for some to do it on a computer, or paper unless you have a connection with your doctor… which is not happening in this practice. | I think there are great advantages to self-administered. [Patients] have a little more time to reflect on their own rather than face-to-face where it can be a little more tempting not to share. | If you are filling out a piece of paper, you do not feel judged. |
| Assessing barriers | |||
| Patient/provider relationship | [To share SU] you have to know that your provider really does care for you and is not just taking care of you because it’s their job. | I would think that…as we see them, and start to develop rapport, that the percent starts to increase in terms of the number of patients we are identifying that do have SU issues. | It takes them longer to trust us before the provider; they have way more respect for the provider. |
| Comfort/preparedness discussing and treating SU | SU counselors are trained to look for SU. A medical doctor is trained to look for problems with the body. Sometimes they go together. Sometimes they do not. | I’m prepared, but yes, I could be better prepared…I’m very comfortable as long as I’m familiar with the patient. | DNE |
| Consequences of disclosing SU | I went to the ER with a broken leg and because I said something about SU I got no pain medication. | Somebody might decide not to use any kind of pain med for their broken leg… They’re afraid that’s going to happen, so they do not disclose. | If they are already on some type of pain med and they fess up to [SU], then they are going to think, “oh great, I’m gonna be cut off”. |
| Privacy | The information really does not stay private… that information flies all over. | The feedback I get from patients is they felt that their privacy was being invaded… They were not sure who that information would be going to. | They do not want to government to know. That’s exactly what they say. “It’s none of the government’s business.” |
| Lack of time | Unless the person is ready to say they need help… there’s just no way a doctor can do a complete study of you in a 15-minute period. | Time. That could be probably everyone’s answer. | We have no time. We’re trying to get through our part so fast that we are not cutting into the provider’s time. |
DNE Data did not emerge in substantive way on this topic
Identifying the Problem
Current Screening Practices
The majority of MAs and PCPs agreed that current screening practices were not systematic. Tobacco and alcohol use were assessed by some providers at new patient visits and annual physicals, but without consistent use of a validated screening tool.
Importance of Substance Use Screening in Rural Primary Care
Patients, PCPs, and MAs unanimously agreed that identifying and addressing substance use in primary care was important due to its negative impact on overall health, co-occurring conditions, and treatment adherence. One patient emphatically stated “you need to let your doctor know if you’re taking drugs or alcohol.” A PCP explained that, aside from the direct health effects, “patients who are addicted are less apt to be compliant with other treatments,” and MAs worried about potential medication contraindications. All stakeholders viewed screening for cannabis as important. Patients noted that “people are more likely to talk about marijuana now with no issue”, presenting an opportunity for more honest conversations because, “here marijuana’s legal” and “less demonized.” MAs and PCPs felt patients no longer viewed cannabis as a drug, and worried that this may lead patients to ignore problematic use.
PCPs and MAs also noted that screening would provide an opportunity for discussion with patients who were unlikely to bring up substance use, and increase patient awareness, particularly for those who may be uninformed of what constitutes risky use: “[a patient] talked about having two or three drinks every night… for three or four years but didn’t realize [the risk] until the provider brought it up.” [MA] The PCPs and MAs suggested that regular screening and discussion could help patients monitor and moderate their use. In addition, several PCPs and MAs drew a connection between screening and the expansion of addiction treatment. “We’re developing a [buprenorphine treatment] program here …there’s a lot of collaboration being done in the Bangor area, so we are all sort of on the same page about screening and treating SUD” [PCP]. Paralleling changes in the broader community, providers felt that all stakeholders need to view substance use as a medical “primary illness” for it to be effectively addressed.
Adapting to the Local Context
Despite some mixed opinions on screening frequency and format, stakeholders generally recommended universal screening at annual visits in a self-administered, tablet-based format, and reviewed by PCPs. Additionally, they agreed that a non-judgmental approach focused on establishing patient-provider rapport would optimize screening.
Universal vs Targeted Screening
Universal screening of all adult patients was strongly preferred to targeted approaches by all stakeholders. Patients believed that universal screening was less “accusatory,” and PCPs felt that it was critical to identifying substance use that targeted approaches would likely miss. Some noted that it is exceedingly challenging to identify which patients may be using substances, “...even little old ladies have surprised me.” [PCP]
Screening Frequency
PCPs and MAs agreed that screening should be conducted annually, during the physical exam, or as indicated (e.g., based on changes in patient presentation, lifestyle, or prescriptions). There was consensus that screening at all visits, particularly problem-focused visits, would frustrate patients. Some felt that new patients should be screened, yet most felt that since a relationship with the provider has yet to be formed, this would be a poor time to engage them.
Screening Format
Patients preferred self-administered to interviewer-administered screening due to reduced stigma, greater perceived data security, increased validity, and increased efficiency. The majority of patients also preferred tablet- to paper-based screening. However, for some patients—especially older patients—having this information in an electronic format raised concerns about the potential for results to be viewed by individuals outside the medical system: “A lot of things will be hacked…You can take this [paper form] home… and then you can throw it in the wood stove or feed it to your mom or rabbit. Well, not your mom, but your rabbit.” [Patient] Even with these concerns, most patients still preferred tablet- to paper-based screening because of similar worries about the privacy of paper screeners: “Electronically, I could see it going straight to a file, but those paper charts…how do I know they’re gonna be kept safe?” [Patient].
MAs generally agreed that a self-administered approach is superior because of putting patients at ease: “[Patients] could take how you ask the question the wrong way, the tone of voice or [how] you’re looking at them.” [MA] The MAs noted trade-offs between tablet- and paper-based screeners. While MAs believed paper screeners may be more attractive to patients who mistrusted or lacked technology literacy, they were also concerned that paper screeners could be lost or that results may not be seen by providers at the point of care. PCPs expressed mixed opinions. Some felt that a self-administered approach is standard, while others cited patients’ dislike for filling out forms, poor reading comprehension, lack of honesty, and privacy concerns. Other PCPs worried that the collected data may not be reviewed or addressed if screening is not conducted as an interview.
Assessing Barriers
Stakeholders identified individual-level (patient-provider relationship, confidentiality, judgment; comfort and preparedness discussing and treating substance use; consequences of disclosing substance use) and system-level (privacy, lack of time) barriers to screening.
Individual-Level
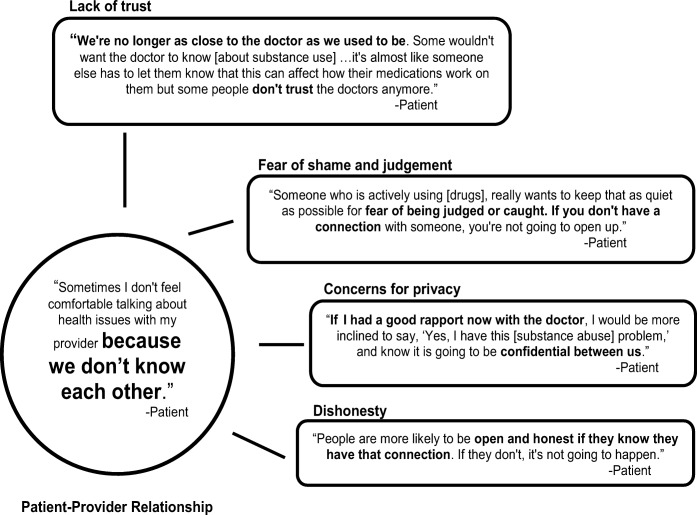
While all stakeholders acknowledged the importance of the patient-provider relationship, patients distinctly viewed most barriers through the lens of this relationship (Fig. 1). Patient concerns about trust and privacy, fear of shame and judgment, and likelihood of honestly disclosing substance use were all influenced by this relationship. Though PCPs and MAs were less focused on this relationship as a barrier, some providers echoed patient sentiment that familiarity and connectedness are critical to promote the honest disclosure of substance use. “When they feel comfortable, know you’re not judging… that is your best way of getting in with patients.” [PCP]
Figure 1.
Model demonstrating how patients view a weak patient-provider relationship as a fundamental barrier to screening.
Comfort and Preparedness Discussing and Treating Substance Use
Discomfort and lack of preparedness were identified as barriers by MAs and PCPs. While MAs viewed addressing substance use as primarily the role of the PCP, they felt accountable for responding to patients in the moment and did not feel equipped to do so. PCPs were compelled to treat or refer patients screening positive and were, therefore, uncomfortable asking about substance use unless they could offer a treatment plan. Few felt adequately prepared to discuss or offer treatment: “It’s hard when you’re not comfortable and the patient’s not comfortable and you don’t know what to do. And then you have two of you in the room not knowing what to do or say.” [PCP] Very few PCPs or MAs had training in screening or treating SUDs. “Education is the main thing…If I knew what questions to ask, I would feel comfortable asking them.” [PCP] Several patients concurred, noting that “some doctors don’t know how to help you.”
Consequences of Disclosing Substance Use
Concerns about the impact of disclosure on care were another barrier cited by all stakeholders. Patients feared disclosing substance use would have consequences on medical care and future pain management. “I think that’s why people don’t want a flag on their record… if they get hurt, they won’t get any help.” [Patient] Patients were also concerned that information about substance use could impact employment, trigger legal consequences, or increase health insurance rates. Both PCPs and MAs expressed awareness of these concerns. “Many [patients] think that depending on what they say to their provider, it’s going to alter the way they are cared for… [and] treated in general.” [MA] All groups acknowledged that these concerns may impact whether patients choose to report substance use.
Privacy
Worries about EHR privacy surfaced as a barrier for all stakeholders. Though many patients were unclear about who could see their medical record, they were generally aware that information is accessible to providers through the Maine Health Information Exchange (HIE), a state-wide system combining information from separate healthcare sites to create a single EHR for each patient. Concerns about who could access screening results arose across patient focus groups and made them wary. “Patient 1: Does [my PCP] have to put on that computer thing everything you say? And then everybody else in Bangor gets to read it, other medical facilities. I don’t know where it goes. Patient 2: It goes to the moon. That’s what it feels like.” PCPs and MAs were also acutely aware of these privacy concerns. One PCP explained: “If it’s a mental health diagnosis diagnosed by a psychiatrist it is protected under special rules, however it’s not if it’s diagnosed and treated by a primary care provider, and that data gets uploaded so that all people can see that who work in the health system.”
Lack of Time
PCPs and MAs unanimously identified lack of time with the patient to properly address substance use as a system-level barrier. They reported feeling overburdened by competing priorities during clinic visits: “What am I going to do if the answer is something I don’t have time to deal with today?” [PCP] Some patients were aware of these time constraints, noting that PCPs sometimes appeared overwhelmed: “I think screening would be important, but as the system’s set up, I don’t think [PCPs] would be capable of handling that extra information. They barely know you as it is, it’s just more information,” [Patient] and doubted that problematic substance use could be effectively addressed during 15- or 30-minute visits.
DISCUSSION
This study adds a novel perspective to research characterizing screening practices, identifying barriers and facilitators, and informing optimal implementation of substance use screening in primary care by examining these issues in a rural context. The results reinforce previous findings, add variability related to the rural FQHC setting, and offer guidance on how best to implement screening in this setting. Results also suggest that rural PCPs face exacerbated challenges compared with urban counterparts.
Consistent with the parent study and other research conducted in urban regions,28, 37 all participants agreed on the importance of screening in primary care,38, 39 yet acknowledged that screening is not done systematically.15 Stakeholders unanimously believed that knowledge of a patient’s substance use is important due to its impact on health and medical care. Notably, Maine is an epicenter for the US overdose epidemic with a 2017 drug overdose death rate of 34.4 persons per 100,000 compared with 21.7 nationally.40 Providers viewed screening as an important counterpart to the recent expansion of MOUD in rural Maine, in response to the opioid crisis, and emphasized the importance of treating substance use as a medical condition. In addition, MAs and PCPs believed that it was important to identify risky substance use and intervene before it becomes severe. Early diagnosis and treatment of opioid use within primary care settings may be a crucial secondary prevention strategy to reduce substance-related death.41
Screening for all substances was viewed as especially important in the context of legalized medical and recreational cannabis. There was consensus among stakeholders that open dialogue about cannabis is occurring. Cannabis legalization could have a positive impact on screening by making patients more comfortable disclosing use, but increased perceptions that legality is a proxy for safety may be detrimental to efforts to reduce substance use.42, 43 Providers felt that educating patients on the risks of cannabis use, similar to tobacco and alcohol, was critical. Cannabis-specific guidelines may assist providers in the discussion of legalized cannabis.44 Additionally, while state and federal cannabis legislation remains incongruous, education for providers and patients on the complexities and consequences of cannabis use within the FQHC setting is necessary.
Participants agreed that potential consequences of disclosing substance use were pertinent barriers, and patients particularly underscored the pivotal patient-provider relationship. Barriers identified by providers focused on a lack of comfort borne of insufficient knowledge,45 a lack of preparedness,46 and a need for training.47 There is ample evidence that medical providers feel unprepared to screen for substance use, let alone diagnose and treat it.48, 49 New recommendations include integrating treatment for SUDs into routine care, rather than relying on referrals to specialty treatment providers.50 To support this effort, additional training and support for providers is needed, especially for rural providers who have fewer opportunities than urban counterparts to engage in consultation and training.51, 52 Recent Maine legislation for medical re-licensure requires that physicians who prescribe controlled substances complete Continuing Medical Education (CME) related to the state’s drug monitoring program, pain management, and/or SUDs, could address lack of training, but is unlikely to be sufficient.51–53 Remote education and mentoring programs like Project ECHO (Extension for Community Health Outcomes) may better facilitate practice-level changes, and can be deployed in rural settings.54–56 These programs must also be accompanied by additional resources within the clinics, including expanded access to behavioral health providers.57–59
Three barriers have important implications for screening in rural settings. First is the concern among rural stakeholders that disclosing substance use will lead to the under-management of pain. Patients worried that disclosure of substance use would preclude them from receiving opioids for pain. In 2016, Maine passed legislation limiting opioid prescriptions to a daily maximum of 100 milligrams of morphine and capping prescription duration.53 Prescription opioid sales in Maine decreased by 32% from 2013 to 2017, one of the largest declines in the nation.60 As these legislative changes and resulting prescribing trends were highly publicized,61–64 patient awareness and concern about the potential impact of disclosure on pain management may have been heightened. Similar legislation and subsequent practice change in other rural regions may similarly contribute to increased patient apprehension about disclosure.
An intensified focus on the patient-provider relationship also distinguishes these findings from urban studies, where this barrier was less prominent.28, 65 Weak rapport with PCPs was viewed by patients as a barrier to honest disclosure of substance use, possibly due to differing expectations of patient-provider relationships in rural versus urban settings. Rural patients may expect a closer relationship than urban patients.66 While close, trusting relationships can encourage disclosure, they may also amplify patient concerns about stigma. Worry about stigma prevents rural patients from seeking healthcare, especially in small, tight-knit communities where anonymity is nearly impossible.52 Additionally, PCPs report that embarrassment about discussing stigmatized illnesses is a more significant barrier to seeking care for rural patients.67 Strengthening the patient-provider relationship combined with the normalization of substance use discussions may be especially important to optimize honest disclosure in rural settings. Preliminary evidence suggests that approaches like the Comprehensive Care Physician (CCP) model, which focus on strengthening patient-provider relationships through continuity of physician care,68 may facilitate conversations about substance use.
A third barrier is heightened privacy concerns. Patients’ deep convictions about the potential for privacy breaches are well described in other literature16, 69, 70 and are potentially greater barriers to healthcare utilization in rural compared with urban regions.52 Privacy may be a heightened concern in rural settings when patients and providers sometimes have dual relationships.52, 71, 72 Concerns about confidentiality may be especially relevant when discussing stigmatized behaviors, like substance use. Expanded use of HIE systems may compound these concerns. Study patients were acutely aware that their medical information could be accessed by providers across the HIE. In the presence of a state-wide HIE, a strong patient-provider relationship may be critical to mitigate the impact of privacy concerns on disclosure of sensitive information.73, 74 Documenting screening results in a section of the EHR that does not synchronize with the HIE may also assuage this fear.
This study has several limitations. Participants were recruited from the Bangor, ME, area. The opinions of stakeholders on screening may differ in other rural regions. This study was conducted in a state that recently legalized recreational cannabis and the impact of this legislation on screening may change with time. The restricted geographic region as well as the legalized recreational cannabis landscape combines to potentially impact the generalizability of the findings. Also, some participants may have felt uncomfortable speaking in focus groups. While these stakeholders expressed diverse opinions, social desirability bias may have influenced outcomes.
CONCLUSIONS
This study offers insights into the acceptability, and informs the implementation, of substance use screening in a rural FQHC. With rural regions being significantly impacted by the opioid overdose crisis, timely identification and treatment of substance use among FQHC patients is critical. Stakeholder agreement that screening has value and should be routinely completed, as well as readiness to integrate screening and treatment into this setting, is encouraging. However, barriers present in the rural FQHC setting, including sensitivity to the patient-provider relationship, concerns about the consequences of disclosure, and privacy concerns warrant attention as they appear heightened. Strengthening patient-provider relationships, increasing provider training and in-clinic resources, and educating stakeholders about privacy in the context of tablet-based screening and HIEs may be necessary first steps.
Electronic supplementary material
(DOCX 61 kb)
Acknowledgments
The authors would like to thank Kimberly Clark, Alison Carter, and Seamus Higgins, patients, providers, and staff at Penobscot Community Health Center, Brewer Medical System, and Helen Hunt Health Center who participated in the research.
Funding
This research was financially supported by grants from the National Institute on Drug Abuse Treatment Clinical Trials Network (UG1DA013035 [PIs John Rotrosen and Edward Nunes], UG1DA040309 [PI Lisa Marsch]), and T32-DA037202 (PI Alan Budney).
Compliance with Ethical Standards
The study was approved by the Institutional Review Boards (IRBs) of New York University School of Medicine and Dartmouth College.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Prior Presentations
McLeman, B., McNeely, J., Saunders, E.C., Farkas, S., Walsh, O., Meier, A., Gardner, T., Nesin, N., Higgins, S., and Marsch. L.A. (June 2018). Implementing substance use screening in rural federally-qualified health centers: Results from focus groups. Poster presentation at the annual meeting of the College of Problems on Drug Dependence (CPDD), San Diego, CA.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Substance Abuse and Mental Health Services Administration (SAMHSA). Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (NSDUH). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration (SAMHSA);2018. HHS Publication No. SMA 18–5068, NSDUH Series H-53.
- 2.Ahmad FB, Rossen LM, Spencer MR, Warner M, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics: Atlanta; 2018. [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration (SAMHSA). State Admissions to Substance Abuse Treatment Services: TEDS 2005–2015. Rockville, MD: Center for Behavioral Health Statistics and Quality;2017. BHSIS Series S-95, HHS Publication No. SMA 17–4360.
- 4.Substance Abuse and Mental Health Services Administration (SAMHSA). Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (NSDUH). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration (SAMHSA); 2017. HHS Publication No. SMA 17–5044.
- 5.Bolin JN, Bellamy GR, Ferdinand AO, et al. Rural Healthy People 2020: New decade, same challenges. J Rural Health. 2015;31(3):326–333. doi: 10.1111/jrh.12116. [DOI] [PubMed] [Google Scholar]
- 6.Rural Assistance Center. Substance Abuse. 2018; https://www.ruralhealthinfo.org/topics/substance-abuse. Accessed 20 Dec 2018.
- 7.Palombi LC, St Hill CA, Lipsky MS, Swanoski MT, Lutfiyya MN. A scoping review of opioid misuse in the rural United States. Ann Epidemiol. 2018. [DOI] [PubMed]
- 8.Chan YF, Lu SE, Howe B, Tieben H, Hoeft T, Unutzer J. Screening and follow-up monitoring for substance use in primary care: An exploration of rural-urban variations. J Gen Intern Med. 2016;31(2):215–222. doi: 10.1007/s11606-015-3488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Quality Assurance (NCQA). HEDIS Measure: Unhealthy alcohol use screening and follow-up. Washington, DC; 2018.
- 10.Curry SJ, Krist AH, Owens DK, et al. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. J Am Med Assoc. 2018;320(18):1899–1909. doi: 10.1001/jama.2018.16789. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Preventive Services Task Force (USPSTF). Final Recommendation Statement: Drug Use, Illicit: Primary Care Interventions for Children and Adolescents. Rockville, MD; March 2015.
- 12.The American College of Obstetricians and Gynecologists (ACOG) ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070–1076. doi: 10.1097/AOG.0b013e318256496e. [DOI] [PubMed] [Google Scholar]
- 13.The American Academy of Pediatrics (AAP). Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138(1). [DOI] [PubMed]
- 14.U.S. Department of Health & Human Services. Facing addiction in America: The Surgeon General’s Report on Alcohol, Drugs and Health. Washington, DC; 2016. [PubMed]
- 15.Lardiere MR, Jones E, Perez M. NACHC 2010 assessment of behavioral health services provided in federally qualified health centers. Bethesda: National Association of Community Health Centers; 2011. [Google Scholar]
- 16.Hutchison L, Blakely C. Substance abuse trends in rural areas: A literature review. College Station: Southwest Rural Health Research Center School of Rural Public Health, The Texas A&M University System Health Science Center; 2010. [Google Scholar]
- 17.Venner KL, Sanchez V, Garcia J, Williams RL, Sussman AL. Moving away from the tip of the pyramid: Screening and brief intervention for risky alcohol and opioid use in underserved patients. J Am Board Fam Med. 2018;31(2):243–251. doi: 10.3122/jabfm.2018.02.170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakhan SE, Laird C. Addressing the primary care physician shortage in an evolving medical workforce. Int Arch Med. 2009;2(1):14. doi: 10.1186/1755-7682-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hing E, Hsiao CJ. State variability in supply of office-based primary care providers: United States, 2012. NCHS data brief. 2014;151:1–8. [PubMed] [Google Scholar]
- 20.National Association of Community Health Centers (NACHC) I. Removing barriers to care: Community health centers in rural areas. 2013.
- 21.Booth BM, Kirchner J, Fortney J, Ross R, Rost K. Rural at-risk drinkers: Correlates and one-year use of alcoholism treatment services. J Stud Alcohol. 2000;61(2):267–277. doi: 10.15288/jsa.2000.61.267. [DOI] [PubMed] [Google Scholar]
- 22.Borders TF, Booth BM. Research on rural residence and access to drug abuse services: Where are we and where do we go? J Rural Health. 2007;23(Suppl):79–83. doi: 10.1111/j.1748-0361.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- 23.Lenardson J, Gale JA. Distribution of substance abuse treatment facilities across the rural-urban continuum. Portland: Maine Rural Health Research Center, Muskie School of Public Health, University of Southern Maine; 2008. [Google Scholar]
- 24.Pullen E, Oser C. Barriers to substance abuse treatment in rural and urban communities: Counselor perspectives. Subst Use Misuse. 2014;49(7):891–901. doi: 10.3109/10826084.2014.891615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyiewuare PO, McCullough C, Ober A, Becker K, Osilla K, Watkins KE. Demographic and mental health characteristics of individuals who present to community health clinics with substance misuse. Health Services Research and Managerial Epidemiology. 2017;4:2333392817734523. doi: 10.1177/2333392817734523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: Time for a map? J Contin Educ Heal Prof. 2006;26(1):13–24. doi: 10.1002/chp.47. [DOI] [PubMed] [Google Scholar]
- 27.Lynch EA, Mudge A, Knowles S, Kitson AL, Hunter SC, Harvey G. “There is nothing so practical as a good theory”: A pragmatic guide for selecting theoretical approaches for implementation projects. BMC Health Serv Res. 2018;18(1):857. doi: 10.1186/s12913-018-3671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNeely J, Kumar PC, Rieckmann T, et al. Barriers and facilitators affecting the implementation of substance use screening in primary care clinics: A qualitative study of patients, providers, and staff. Addict Sci Clin Pract. 2018;13(1):8. doi: 10.1186/s13722-018-0110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ringelstein Z. Medicaid is great, but rural Maine needs hospitals, too. The New York Times. 2017;Opinion.
- 30.Wickenheiser M. Census: Maine most rural state in 2010 as urban centers grow nationwide. Bangor Daily News. 2012.
- 31.McNeely J, Wu LT, Subramaniam G, et al. Performance of the Tobacco, Alcohol, Prescription Medication, and Other Substance Use (TAPS) Tool for substance use screening in primary care patients. Ann Intern Med. 2016;165(10):690–699. doi: 10.7326/M16-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atlas.ti, Version 7 [computer program]. Berlin, GER; 2013.
- 33.Lu CJ, Shulman SW. Rigor and flexibility in computer-based qualitative research: Introducing the Coding Analysis Toolkit (CAT) Int J Mult Res Approaches. 2008;2:105–117. [Google Scholar]
- 34.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 35.Elliott V. Thinking about the coding process in qualitative data analysis. Qual Rep. 2018;23(11):2850–2861. [Google Scholar]
- 36.Miles MB, Huberman AM. Qualitative data analysis: An expanded sourcebook. 2nd ed. Thousand Oaks: Sage Publications; 1994. [Google Scholar]
- 37.Rahm AK, Boggs JM, Martin C, et al. Facilitators and Barriers to Implementing SBIRT in Primary Care in Integrated Health Care Settings. Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse. 2014:0. [DOI] [PubMed]
- 38.Berger D, Bradley KA. Primary care management of alcohol misuse. Med Clin N Am. 2015;99(5):989–1016. doi: 10.1016/j.mcna.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Townley C, Dorr H. Integrating substance use disorder treatment into primary care: A publication of the National Academy for State Health Policy. National Academy for State Health Policy: Washington, D.C; 2017. [Google Scholar]
- 40.National Center for Health Statistics (NCHS). Drug poisoning mortality by state: United States. Rockville, MD; 2018.
- 41.Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: A public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–574. doi: 10.1146/annurev-publhealth-031914-122957. [DOI] [PubMed] [Google Scholar]
- 42.Center for Study of Health and Risk Behaviors. Young adult health survey: Marijuana. University of Washington; 2015.
- 43.Pacek LR, Mauro PM, Martins SS. Perceived risk of regular cannabis use in the United States from 2002 to 2012: Differences by sex, age, and race/ethnicity. Drug Alcohol Depend. 2015;149:232–244. doi: 10.1016/j.drugalcdep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nunes AP, Richmond MK, Marzano K, Swenson CJ, Lockhart J. Ten years of implementing screening, brief intervention, and referral to treatment (SBIRT): Lessons learned. Subst Abus. 2017;38(4):508–512. doi: 10.1080/08897077.2017.1362369. [DOI] [PubMed] [Google Scholar]
- 45.Ghitza UE, Tai B. Challenges and opportunities for integrating preventive substance-use-care services in primary care through the Affordable Care Act. J Health Care Poor Underserved. 2014;25(1 Suppl):36–45. doi: 10.1353/hpu.2014.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wakeman SE, Pham-Kanter G, Donelan K. Attitudes, practices, and preparedness to care for patients with substance use disorder: Results from a survey of general internists. Subst Abus. 2016;37(4):635–641. doi: 10.1080/08897077.2016.1187240. [DOI] [PubMed] [Google Scholar]
- 47.CASA Columbia . Missed opportunity: National survey of primary care physicians and patients on substance abuse. New York: Columbia University; 2000. [Google Scholar]
- 48.Yoast RA, Wilford BB, Hayashi SW. Encouraging physicians to screen for and intervene in substance use disorders: Obstacles and strategies for change. J Addict Dis. 2008;27(3):77–97. doi: 10.1080/10550880802122687. [DOI] [PubMed] [Google Scholar]
- 49.Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: A qualitative study in five VA clinics. J Gen Intern Med. 2018;33(3):258–267. doi: 10.1007/s11606-017-4202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Council for Behavioral Health. Implementing care for alcohol and other drug use in medical settings: An extension of SBIRT. February 2018.
- 51.Kirchhoff AC, Hart G, Campbell EG. Rural and urban primary care physician professional beliefs and quality improvement behaviors. J Rural Health. 2014;30(3):235–243. doi: 10.1111/jrh.12067. [DOI] [PubMed] [Google Scholar]
- 52.Brems C, Johnson ME, Warner TD, Roberts LW. Barriers to healthcare as reported by rural and urban interprofessional providers. Journal of Interprofessional Care. 2006;20(2):105–118. doi: 10.1080/13561820600622208. [DOI] [PubMed] [Google Scholar]
- 53.127th Maine Legislature. An act to prevent opiate abuse by strengthening the controlled substances prescription monitoring program, Maine Public Law 2015 Chapter 488. 2015.
- 54.Agley J, Adams ZW, Hulvershorn LA. Extension for Community Healthcare Outcomes (ECHO) as a tool for continuing medical education on opioid use disorder and comorbidities. Addiction. 2019;114(3):573–574. doi: 10.1111/add.14494. [DOI] [PubMed] [Google Scholar]
- 55.Komaromy M, Duhigg D, Metcalf A, et al. Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Subst Abus. 2016;37(1):20–24. doi: 10.1080/08897077.2015.1129388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Komaromy M, Bartlett J, Manis K, Arora S. Enhanced primary care treatment of behavioral disorders with ECHO case-based learning. Psychiatr Serv. 2017;68(9):873–875. doi: 10.1176/appi.ps.201600471. [DOI] [PubMed] [Google Scholar]
- 57.Watkins KE, Ober AJ, Lamp K, et al. Collaborative care for opioid and alcohol use disorders in primary care: The SUMMIT randomized clinical trial. JAMA Intern Med. 2017;177(10):1480–1488. doi: 10.1001/jamainternmed.2017.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korthuis PT, McCarty D, Weimer M, et al. Primary care-based models for the treatment of opioid use disorder: A scoping review. Ann Intern Med. 2017;166(4):268–278. doi: 10.7326/M16-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: A cluster-randomized clinical trial. JAMA Intern Med. 2017;177(9):1265–1272. doi: 10.1001/jamainternmed.2017.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.IQVIA Xponent. State and national totals of retail filled prescriptions: All opioid analgesics, 2013–2017. Danbury, CT; 2018.
- 61.Haskell M. Opioid prescribing declines steeply in Maine, data show. Bangor Daily News; 2018.
- 62.Lawlor J. Number of opioids prescribed in Maine in 2017 fell 13 percent. Portland Press Herald; 2018.
- 63.Ferguson C. Maine leads nation in decline of prescription opioid sales, report finds. Bangor Daily News; 2018.
- 64.Wight, P. Intent on reversing its opioid epidemic, a state limits prescriptions. NHPR Morning Edition; 2017.
- 65.Palmer A, Karakus M, Mark T. Barriers faced by physicians in screening for substance use disorders among adolescents. Psychiatr Serv. 2019;70(5):409–412. doi: 10.1176/appi.ps.201800427. [DOI] [PubMed] [Google Scholar]
- 66.Desjarlais-deKlerk K, Wallace JE. Instrumental and socioemotional communications in doctor-patient interactions in urban and rural clinics. BMC Health Serv Res. 2013;13:261. doi: 10.1186/1472-6963-13-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warner TD, Monaghan-Geernaert P, Battaglia J, Brems C, Johnson ME, Roberts LW. Ethical considerations in rural health care: A pilot study of clinicians in Alaska and New Mexico. Community Ment Health J. 2005;41(1):21–33. doi: 10.1007/s10597-006-2597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meltzer DO, Ruhnke GW. Redesigning care for patients at increased hospitalization risk: The Comprehensive Care Physician model. Health Aff. 2014;33(5):770–777. doi: 10.1377/hlthaff.2014.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fortney J, Mukherjee S, Curran G, Fortney S, Han X, Booth BM. Factors associated with perceived stigma for alcohol use and treatment among at-risk drinkers. J Behav Health Serv Res. 2004;31(4):418–429. doi: 10.1007/BF02287693. [DOI] [PubMed] [Google Scholar]
- 70.Hargrove DS. Ethical issues in rural mental health practice. Prof Psychol Res Pract. 1986;17(1):20–23. [Google Scholar]
- 71.Nelson WA. Boundary issues in rural America. Overlapping relationships create ethical challenges for rural healthcare professionals. Healthc Exec. 2010;25(2):56–57. [PubMed] [Google Scholar]
- 72.Graber MA. The overlapping roles of the rural doctor. AMA Journal of Ethics: Virtual Mentor. 2011;13(5):273–277. doi: 10.1001/virtualmentor.2011.13.5.ccas1-1105. [DOI] [PubMed] [Google Scholar]
- 73.Unertl KM, Johnson KB, Lorenzi NM. Health information exchange technology on the front lines of healthcare: Workflow factors and patterns of use. J Am Med Inform Assoc. 2012;19(3):392–400. doi: 10.1136/amiajnl-2011-000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Esmaeilzadeh P, Sambasivan M. Patients’ support for health information exchange: A literature review and classification of key factors. BMC Medical Informatics and Decision Making. 2017;17(1):33. doi: 10.1186/s12911-017-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 61 kb)