Abstract
SOX10 immunoexpression is increasingly recognized in salivary gland tumors, including but not limited to those with myoepithelial, serous acinar, and intercalated duct differentiation. However, SOX10 expression has not been extensively evaluated in other epithelial tumors that can mimic salivary origin. Basaloid squamous cell carcinoma (SCC) is a unique variant of SCC that shows morphologic overlap with several salivary tumors, including adenoid cystic carcinoma, basal cell adenocarcinoma, and myoepithelial carcinoma. We performed SOX10 immunohistochemistry on 22 basaloid SCCs and 280 non-basaloid SCCs. If tissue was available, we also performed immunohistochemistry for S100 and p16, and in-situ hybridization for high-risk HPV RNA. SOX10 was positive in 13/22 basaloid SCCs (59%), including 5/6 (83%) that were HPV-positive and 6/12 (50%) that were HPV-negative. Only 2/12 basaloid SCC (17%) demonstrated focal S100 expression. All non-basaloid SCCs were SOX10 negative. Frequent positivity for SOX10 in basaloid SCC presents a significant diagnostic pitfall for distinguishing these tumors from various basaloid salivary carcinomas. The preponderance of SOX10 expression in the basaloid variant of HPV-positive SCC also presents a diagnostic challenge in separating it from HPV-related multiphenotypic sinonasal carcinoma. SOX10 may be more broadly considered a marker of basal differentiation and should not be assumed to be specific for salivary origin in epithelial head and neck tumors.
Keywords: Basaloid squamous cell carcinoma, Squamous cell carcinoma, SOX10, Human papillomavirus, Immunohistochemistry
Background
SOX10 is a transcription factor that that promotes differentiation in neural crest-derived cells and initially found diagnostic use as a sensitive and specific nuclear marker of melanocytic and nerve sheath tumors [1–4]. More recently, SOX10 expression has also been reported in a broad range of benign and malignant salivary gland neoplasms, including but not limited to those of myoepithelial, serous acinar, and intercalated duct differentiation. To date, acinic cell carcinoma, adenoid cystic carcinoma, basal cell adenocarcinoma, basal cell adenoma, epithelial-myoepithelial carcinoma, low grade intraductal carcinoma, pleomorphic adenoma, secretory carcinoma, and sialoblastoma have all shown SOX10 expression [5–9]. Given this widespread positivity in salivary neoplasms, it is tempting to regard SOX10 as a marker of salivary origin in epithelial head and neck tumors. However, SOX10 expression has not been comprehensively evaluated in common mimics of salivary carcinoma.
The basaloid variant of squamous cell carcinoma (SCC) is a unique subtype of head and neck SCC that has been recognized in both HPV-negative and HPV-positive tumors [10, 11]. It is defined by hyperchromatic tumor cells with minimal cytoplasm, adenoidal architecture with formation of small pseudoglandular spaces containing myxoid material, and variable stromal hyalinosis or comedo-type necrosis [12–14]. These features can cause basaloid SCC to be misclassified as one of several basaloid salivary carcinomas, including adenoid cystic carcinoma, basal cell adenocarcinoma, or myoepithelial carcinoma. Historically, minimal reactivity for myoepithelial markers such as S100 and SMA has facilitated the distinction of basaloid SCC from tumors that demonstrate true myoepithelial differentiation [15–19]. However, we have recently observed several cases where positivity for SOX10 in basaloid SCC created diagnostic confusion. Although SOX10 expression has been reported in just 6% of head and neck SCC overall [7], distribution of expression across histologic variants has never been documented. This study aims to evaluate the frequency of SOX10 expression in basaloid SCC.
Methods
Twenty-two cases of basaloid SCC occurring in head and neck sites were identified from the surgical pathology archives of The Johns Hopkins Hospital between January 1998 and October 2018. Squamous differentiation was confirmed on the basis of at least focal overt keratinization or associated surface dysplasia in all tumors, while classification as basaloid variant was strictly defined to include not only a high nuclear:cytoplasmic ratio and nuclear hyperchromasia but also peripheral palisading, adenoidal architecture, comedo-pattern necrosis, or stromal hyalinosis [12–14]. Although cases with basaloid morphology were selected for this study regardless of HPV status, this group excluded non-keratinizing HPV-related SCC that lacked these more specific basaloid features [10, 11]. Sixteen cases were evaluated on whole slide sections, and six were part of previously constructed tissue microarrays that also contained 280 non-basaloid SCC; these included 236 conventional and 44 non-keratinizing SCC [20].
Immunohistochemistry (IHC) was performed on all cases using a monoclonal antibody for SOX10 (clone N-20; Biocare Medical, Concord, CA). If tissue was available, tumors were also evaluated using IHC for S100 (clone 4C4.9; Ventana Medical Systems, Tucson AZ) and p16 (clone INK4a; Ventana) and mRNA in-situ hybridization (ISH) for 18 high-risk HPV genotypes (Advanced Cell Diagnostics, Hayward, CA). All staining was performed on a Ventana Benchmark XT autostainer according to manufacturers’ instructions and in the presence of appropriate controls. Nuclear staining for SOX10 or nuclear and cytoplasmic staining for S100 was defined as diffuse if present in > 50% of tumor cells and focal if present in < 50% of tumor cells. P16 positivity required > 70% of cells with nuclear and cytoplasmic staining and HPV RNA ISH positivity was defined as multiple punctate nuclear and cytoplasmic signals.
Results
The 22 basaloid SCC represented 22 patients, including 18 men and 4 women, with a median age of 60 years (range 38–82). Primary tumor sites included the oropharynx (n = 8), larynx (n = 6), oral cavity (n = 4), hypopharynx (n = 3) and sinonasal tract (n = 1). Staining results are summarized in Table 1. SOX10 was positive in 13 basaloid SCC (59%), with diffuse staining in 11 cases (Fig. 1) and focal staining in two cases. All cases that showed less than 100% expression of SOX10 lacked a clear biphasic staining pattern, showing either patchy heterogeneous reactivity throughout the tumor or accentuation of staining in the basilar half to two-thirds of the epithelium (Fig. 1). In contrast, focal S100 positivity was only seen in 2 of 12 cases stained (17%). Of the 18 basaloid SCC tested for HPV, 6 oropharyngeal cases were positive for both p16 IHC and HPV RNA ISH; 5 of these (83%) were also positive for SOX10 (Fig. 2). The remaining 12 cases were all HPV RNA ISH negative, of which 6 (50%) were positive for SOX10. All non-basaloid SCC were entirely negative for SOX10 (0%), including 236 HPV-negative and 44 HPV-positive tumors. Including both basaloid and non-basaloid cases, SOX10 was positive in 13 of 302 head and neck SCC (4%).
Table 1.
SOX10 expression in head and neck squamous cell carcinomas
| Tumor type | SOX10 positive |
|---|---|
| Basaloid | |
| HPV-positive | 5/6 (83%) |
| HPV-negative | 6/12 (50%) |
| Total | 13/22 (59%) |
| Non-basaloid | |
| HPV-positive | 0/44 (0%) |
| HPV-negative | 0/236 (0%) |
| Total | 0/280 (0%) |
| All SCC | |
| HPV-positive | 5/50 (10%) |
| HPV-negative | 6/242 (2%) |
| Total | 13/302 (4%) |
HPV Human papillomavirus, SCC squamous cell carcinoma
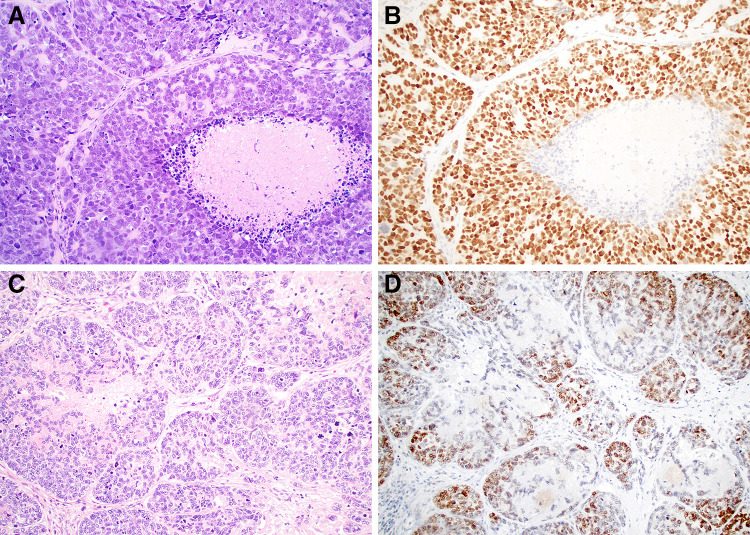
Fig. 1.
Basaloid SCC demonstrates an adenoidal growth pattern with peripheral palisading and comedo-type necrosis that can mimic salivary tumors (a and c; × 20). These tumors frequently show diffuse SOX10 expression (b; × 20). Although the basaloid SCC uniformly lack a true biphasic staining pattern, some cases do show accentuation of staining in the basal half to two-thirds of the epithelium (d; × 20)
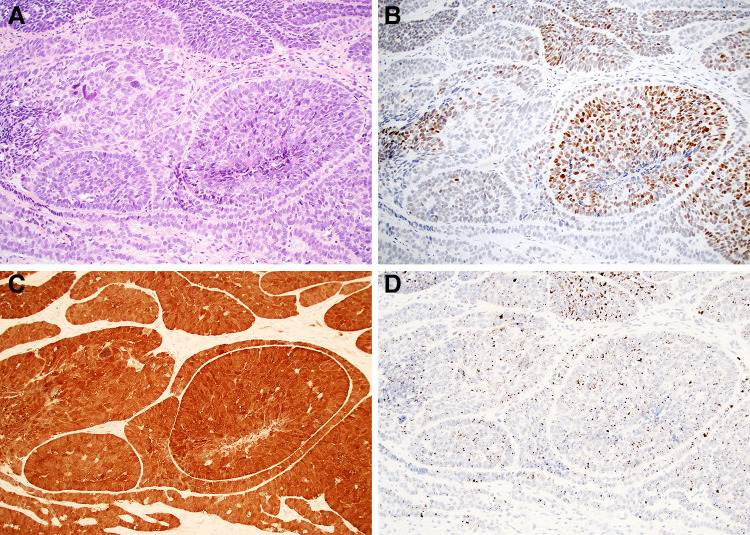
Fig. 2.
The subset of basaloid SCC that are HPV-positive can also mimic HPV-related multiphenotypic sinonasal carcinoma due to the presence of pseudoglandular elements (a; × 20). Prominent SOX10 positivity (b; × 20) in these cases, which are also positive for p16 (c; × 20) and high-risk HPV RNA in-situ hybridization (d; × 20) can add to the diagnostic confusion
Conclusions
While SOX10 was originally described as a diagnostic marker of melanocytic and nerve sheath differentiation, it has recently garnered increasing recognition for expression in many salivary neoplasms, including those with myoepithelial, serous acinar, and intercalated duct differentiation [5–9]. Indeed, the spectrum of salivary tumors that express SOX10 is so broad that it is tempting to regard it as a marker of salivary differentiation in epithelial tumors of the head and neck. However, SOX10 expression has not been extensively evaluated in common mimics of salivary tumors. After observing diagnostic confusion when SOX10 expression was unexpectedly encountered in basaloid SCC, we aimed to evaluate the frequency of this phenomenon.
This study demonstrates that 59% of basaloid SCC are also SOX10 positive, compounding its considerable morphologic overlap with certain salivary gland carcinomas. Due to its unique adenoidal architecture, stromal hyalinosis, and comedo-pattern necrosis, basaloid SCC can raise histologic concern for various basaloid salivary neoplasms, including adenoid cystic carcinoma, basal cell adenocarcinoma, or myoepithelial carcinoma [12, 13, 21]. Although negativity for other myoepithelial markers such as S100 or SMA has previously facilitated this distinction, frequent diffuse SOX10 positivity in basaloid SCC presents a significant diagnostic pitfall. Furthermore, while biphasic p63 or p40 reactivity that is restricted to the myoepithelial component of adenoid cystic carcinoma and basal cell adenocarcinoma can help distinguish them from basaloid SCC, SOX10 generally stains both ductal and myoepithelial cells in these mimics [5–8], producing a diffuse staining pattern that is identical to that seen in basaloid SCC. As such, the usefulness of SOX10 in differentiating basaloid salivary tumors from SCC is extremely limited.
This study also highlights that SOX10 reactivity is especially common in those basaloid SCC that are also HPV-positive, creating additional potential diagnostic confusion. HPV testing has revealed that basaloid SCC as traditionally defined is a mixed category, comprised of highly aggressive HPV-negative tumors and less aggressive HPV-positive tumors [10, 11]. The frequent SOX10 positivity in the basaloid variant of HPV-related SCC in particular raises concern for additional diagnostic overlap with HPV-related multiphenotypic sinonasal carcinoma (HMSC). HMSC is an unusual HPV-driven tumor that also demonstrates a basaloid appearance and shows both squamous and salivary differentiation; it has been exclusively reported in the sinonasal tract to date [22, 23]. Similar to true salivary gland neoplasms, HMSC is characterized by biphasic cell populations that demonstrate restricted reactivity for most ductal and myoepithelial immunohistochemical markers, but SOX10 positivity has been reported in both components [24]. As such, SOX10 staining cannot be reliably used to distinguish basaloid variant of HPV-related SCC and HMSC. SOX10 staining alone in a basaloid HPV-positive tumor should not be considered sufficient evidence to classify it as HMSC, particularly in the oropharynx where this distinctive tumor type has not yet been documented to occur.
Finally, the frequent positivity for SOX10 in basaloid SCC documented in this study adds to mounting evidence that SOX10 should be more broadly considered a marker of basal differentiation. Of course, the phenotypic spectrum between basal and myoepithelial lineages is well-established in the salivary gland, with SOX10 reactivity noted in tumors thought to recapitulate both cell types [5, 8]. But expression of SOX10 in basaloid SCC suggests that this marker may recognize cells that differentiate toward the basal aspect of the epithelium in other organs. An analogous phenomenon has been documented in the breast, where SOX10 expression has been reported in approximately 60% of basal-like breast cancers [6, 25–27]; this finding correlates with a gene expression profile that carries parallels to normal myoepithelial cells [28]. Regardless, as understanding of the breadth of SOX10 expression evolves, it should not be considered specific for salivary origin among head and neck epithelial tumors.
Compliance with Ethical Standards
Conflict of interest
All authors have no potential conflicts of interest to declare. This work was performed under Johns Hopkins IRB00176170 with consent waiver.
References
- 1.Karamchandani JR, Nielsen TO, van de Rijn M, West RB. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol. 2012;20(5):445–450. doi: 10.1097/PAI.0b013e318244ff4b. [DOI] [PubMed] [Google Scholar]
- 2.Mohamed A, Gonzalez RS, Lawson D, Wang J, Cohen C. SOX10 expression in malignant melanoma, carcinoma, and normal tissues. Appl Immunohistochem Mol Morphol. 2013;21(6):506–510. doi: 10.1097/PAI.0b013e318279bc0a. [DOI] [PubMed] [Google Scholar]
- 3.Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol. 2008;32(9):1291–1298. doi: 10.1097/PAS.0b013e3181658c14. [DOI] [PubMed] [Google Scholar]
- 4.Shin J, Vincent JG, Cuda JD, Xu H, Kang S, Kim J, et al. Sox10 is expressed in primary melanocytic neoplasms of various histologies but not in fibrohistiocytic proliferations and histiocytoses. J Am Acad Dermatol. 2012;67(4):717–726. doi: 10.1016/j.jaad.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh MS, Lee YH, Chang YL. SOX10-positive salivary gland tumors: a growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. Hum Pathol. 2016;56:134–142. doi: 10.1016/j.humpath.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Ivanov SV, Panaccione A, Nonaka D, Prasad ML, Boyd KL, Brown B, et al. Diagnostic SOX10 gene signatures in salivary adenoid cystic and breast basal-like carcinomas. Br J Cancer. 2013;109(2):444–451. doi: 10.1038/bjc.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miettinen M, McCue PA, Sarlomo-Rikala M, Biernat W, Czapiewski P, Kopczynski J, et al. Sox10–a marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: a systematic analysis of 5134 tumors. Am J Surg Pathol. 2015;39(6):826–835. doi: 10.1097/PAS.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohtomo R, Mori T, Shibata S, Tsuta K, Maeshima AM, Akazawa C, et al. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol. 2013;26(8):1041–1050. doi: 10.1038/modpathol.2013.54. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt AC, Cohen C, Siddiqui MT. Expression of SOX10 in salivary gland oncocytic neoplasms: a review and a comparative analysis with other immunohistochemical markers. Acta Cytol. 2015;59(5):384–390. doi: 10.1159/000441890. [DOI] [PubMed] [Google Scholar]
- 10.Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol. 2008;32(7):1044–1050. doi: 10.1097/PAS.0b013e31816380ec. [DOI] [PubMed] [Google Scholar]
- 11.Chernock RD, Lewis JS, Jr, Zhang Q, El-Mofty SK. Human papillomavirus-positive basaloid squamous cell carcinomas of the upper aerodigestive tract: a distinct clinicopathologic and molecular subtype of basaloid squamous cell carcinoma. Hum Pathol. 2010;41(7):1016–1023. doi: 10.1016/j.humpath.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes L, Ferlito A, Altavilla G, MacMillan C, Rinaldo A, Doglioni C. Basaloid squamous cell carcinoma of the head and neck: clinicopathological features and differential diagnosis. Ann Otol Rhinol Laryngol. 1996;105(1):75–82. doi: 10.1177/000348949610500115. [DOI] [PubMed] [Google Scholar]
- 13.Lewis JS, Gillison ML, Westra WH, Zidar N. Basaloid squamous cell carcinoma. In: El-Naggar A, Chan JK, Grandis JR, Takata T, Slootweg PJ, editors. WHO Classification of Head and Neck Tumours. Lyon: International Agency for Research on Cancer; 2017. pp. 85–86. [Google Scholar]
- 14.Wain SL, Kier R, Vollmer RT, Bossen EH. Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx: report of 10 cases. Hum Pathol. 1986;17(11):1158–1166. doi: 10.1016/S0046-8177(86)80422-1. [DOI] [PubMed] [Google Scholar]
- 15.Banks ER, Frierson HF, Jr, Mills SE, George E, Zarbo RJ, Swanson PE. Basaloid squamous cell carcinoma of the head and neck. A clinicopathologic and immunohistochemical study of 40 cases. Am J Surg Pathol. 1992;16(10):939–946. doi: 10.1097/00000478-199210000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Emanuel P, Wang B, Wu M, Burstein DE. p63 Immunohistochemistry in the distinction of adenoid cystic carcinoma from basaloid squamous cell carcinoma. Mod Pathol. 2005;18(5):645–650. doi: 10.1038/modpathol.3800329. [DOI] [PubMed] [Google Scholar]
- 17.Klijanienko J, el-Naggar A, Ponzio-Prion A, Marandas P, Micheau C, Caillaud JM. Basaloid squamous carcinoma of the head and neck. Immunohistochemical comparison with adenoid cystic carcinoma and squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1993;119(8):887–890. doi: 10.1001/archotol.1993.01880200093013. [DOI] [PubMed] [Google Scholar]
- 18.Morice WG, Ferreiro JA. Distinction of basaloid squamous cell carcinoma from adenoid cystic and small cell undifferentiated carcinoma by immunohistochemistry. Hum Pathol. 1998;29(6):609–612. doi: 10.1016/S0046-8177(98)80011-7. [DOI] [PubMed] [Google Scholar]
- 19.Serrano MF, El-Mofty SK, Gnepp DR, Lewis JS., Jr Utility of high molecular weight cytokeratins, but not p63, in the differential diagnosis of neuroendocrine and basaloid carcinomas of the head and neck. Hum Pathol. 2008;39(4):591–598. doi: 10.1016/j.humpath.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Westra WH, Taube JM, Poeta ML, Begum S, Sidransky D, Koch WM. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14(2):366–369. doi: 10.1158/1078-0432.CCR-07-1402. [DOI] [PubMed] [Google Scholar]
- 21.Ereno C, Gaafar A, Garmendia M, Etxezarraga C, Bilbao FJ, Lopez JI. Basaloid squamous cell carcinoma of the head and neck: a clinicopathological and follow-up study of 40 cases and review of the literature. Head Neck Pathol. 2008;2(2):83–91. doi: 10.1007/s12105-008-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bishop JA, Andreasen S, Hang JF, Bullock MJ, Chen TY, Franchi A, et al. HPV-related multiphenotypic sinonasal carcinoma: an expanded series of 49 cases of the tumor formerly known as hpv-related carcinoma with adenoid cystic carcinoma-like features. Am J Surg Pathol. 2017;41(12):1690–1701. doi: 10.1097/PAS.0000000000000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bishop JA, Ogawa T, Stelow EB, Moskaluk CA, Koch WM, Pai SI, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol. 2013;37(6):836–844. doi: 10.1097/PAS.0b013e31827b1cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh MS, Lee YH, Jin YT, Huang WC. Strong SOX10 expression in HPV-related multiphenotypic sinonasal carcinoma: report of six new cases validated by high-risk HPV mRNA in situ hybridization test. Hum Pathol. 2018 doi: 10.1016/j.humpath.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Cimino-Mathews A, Subhawong AP, Elwood H, Warzecha HN, Sharma R, Park BH, et al. Neural crest transcription factor Sox10 is preferentially expressed in triple-negative and metaplastic breast carcinomas. Hum Pathol. 2013;44(6):959–965. doi: 10.1016/j.humpath.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harbhajanka A, Chahar S, Miskimen K, Silverman P, Harris L, Williams N, et al. Clinicopathological, immunohistochemical and molecular correlation of neural crest transcription factor SOX10 expression in triple-negative breast carcinoma. Hum Pathol. 2018;80:163–169. doi: 10.1016/j.humpath.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson ER, Sharma R, Argani P, Cimino-Mathews A. Utility of Sox10 labeling in metastatic breast carcinomas. Hum Pathol. 2017;67:205–210. doi: 10.1016/j.humpath.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Fadare O, Tavassoli FA. The phenotypic spectrum of basal-like breast cancers: a critical appraisal. Adv Anat Pathol. 2007;14(5):358–373. doi: 10.1097/PAP.0b013e31814b26fe. [DOI] [PubMed] [Google Scholar]