Abstract
Proliferative verrucous leukoplakia (PVL) is a premalignant condition of the oral mucosa with > 70% chance of progression to squamous cell carcinoma (SCC), while lacking the common risks and behavior seen in non-PVL oral squamous carcinogenesis. PVL follows a multi-stage slow, relentless and usually multifocal expansion of surface epithelial thickening that over time takes on a verrucous architecture, eventually leading to verrucous carcinoma and/or dysplasia followed by “conventional” SCC, a process that takes years and is notoriously difficult to manage. As mucosal surfaces and carcinomas arising at these sites, are colonized by microorganisms, host receptors for microbial products have received attention as potential contributors to carcinogenesis. Studies show that microbial pattern recognition toll-like receptor (TLR)2 in various epithelial cells is upregulated in premalignant lesions and in malignant cells and can activate oncogenic pathways. Because of the highly progressive nature of PVL, we examined TLR2 expression in well-characterized PVL samples by immunohistochemistry. We found that, similar to epithelial dysplasia and SCC, PVL keratinocytes throughout the epithelial thickness showed diffuse TLR2 expression even in early stage lesions prior to onset of dysplasia. In contrast, oral mucosal samples in the absence of hyperorothokeratosis or dysplasia, expressed TLR2 primarily in the basal and parabasal layers. Given the high rates of PVL transformation and the previously established pro-cancer role of high TLR2 expression in malignant oral squamous cells, it is important to determine how its’ expression and functions are regulated in the oral squamous epithelium, and what is the specific TLR2 role in carcinogenesis.
Keywords: Proliferative verrucous leukoplakia, PVL, Verrucous hyperplasia, Toll-like receptor 2, TLR2, Squamous epithelium, Squamous cell carcinoma, Oral cancer
Introduction
The stratified squamous epithelium of the oral mucosa, similar to other skin and mucosal surfaces, is susceptible to carcinogenesis. While the oropharyngeal and base of tongue epithelium overlying mucosa-associated lymphoid tissue is more likely to develop squamous cell carcinoma (SCC) because of high-risk Human papillomavirus (HPV) infection, SCC of other oral cavity surfaces are usually unrelated to HPV [1, 2]. Several subtypes of oral SCC are recognized, including the so-called “conventional”, verrucous and papillary, among others, and the “conventional” SCC type is the most common [1, 3]. The “conventional” SCC is preceded by specific morphologic and molecular intraepithelial changes defined as epithelial dysplasia, also known as pre-cancer, with a direct relationship between the amount of dysplasia and the probability of SCC [1, 3].
In the past several decades, many clinical-pathologic studies have described an oral epithelial abnormality called proliferative verrucous leukoplakia (PVL) as a premalignant condition with a particularly high (> 70%) chance of progression to SCC [1], while lacking the most common risks and behavior seen in non-PVL oral squamous carcinogenesis. The diagnostic criteria and definitions vary in the literature, but there is general agreement that PVL exhibits slow, relentless and usually multifocal proliferation of the oral surface epithelium that over time takes on a verrucous architecture, eventually leading to verrucous carcinoma and/or dysplasia followed by “conventional” SCC, a process that takes years [1, 4, 5]. Histopathologic diagnosis at the earliest stages is variable and generally non-specific. Biopsies of the verrucous lesions are often diagnosed as “hyperkeratosis with verrucous architecture”, “verrucous hyperplasia”, or “atypical verrucous hyperplasia”, and pathologists familiar with this condition usually include a comment about the possibility of PVL.
Oral SCC develop in the context of surface-associated microbes, potentially contributing to disease pathogenesis. Various commensal and pathogenic Gram-positive (Gpos) and Gram-negative (Gneg) bacteria colonize oral cancer tissues [6–11], and oral SCC cells tested in vitro are deficient in the production of several anti-microbial peptides [12]. Professional innate immune system cells are the primary responders to microbes, because common microbe-associated molecular patterns (MAMP) activate cell surface and intracellular pattern recognition receptors, among which toll-like receptors (TLR) are the best characterized (reviewed in [13, 14]). Much remains unknown about the role of microorganisms and TLR in the pathogenesis of oral cancer. However, studies show that inflammation induced by TLR activities in innate immune system monocytes, macrophages, and dendritic cells provides soluble factors that can support oncogenesis thanks to potent activation of NF-kB [15–19]. Among the induced factors are interleukin (IL-6), which activates an important pro-survival transcription factor STAT3 in SCC cells [15], hypoxia-inducible factor (HIF)-1alpha important for tumor growth and metastasis [16, 19], vascular endothelial growth factor (VEGF-A) important for tumor angiogenesis, which is induced via STAT3 activity [15, 16], and other factors. Moreover, the induction of CCL-2 and other chemokines [15, 16] further amplifies the inflammation by attracting more monocytes into the tumor environment.
Recent reports have revealed interesting functions of TLR in various epithelial cells, and TLR2 (also known as CD282), a cell-surface receptor for mainly Gpos bacterial products, has emerged as an important regulator of epithelial cells [18, 20–24]. A unique feature of TLR2 is the broad specificity, as it dimerizes with TLR1 or TLR6 [14]. Our previous studies showed an important intrinsic role for TLR2 (both TLR2/1 and TLR2/6 dimers) in squamous epithelial cells [24]. Established oral SCC cell lines expressed high levels of TLR2 > 80% of the time (5/6 cell lines), and TLR2-high SCC cells activated the oncogenic MAPK ERK1/2 pathway in response to TLR2 ligands, which protected the cells from apoptosis and stimulated proliferation. Moreover, in oral SCC specimens, we noticed that transition from hyperplastic to dysplastic squamous epithelium was marked by increase in TLR2 expression, suggesting that TLR2 may play a role in the early stages of squamous carcinogenesis, at least with respect to conventional SCC.
In the current study, we analyzed samples of human oral mucosal PVL to test if TLR2 is associated with this high-risk premalignant condition before the development of dysplasia or SCC. We show evidence that supports our hypothesis. Moreover, we evaluated additional hyperkeratotic lesions to determine if oral mucosal subsite or morphologic features may predict the pattern of TLR2 expression in epithelial cells.
Materials and Methods
Cases
The use of archival specimens was approved by Augusta University IRB (#964456). All cases were reviewed and selected by a board-certified oral and maxillofacial pathologist (ZK). Ten cases of well-documented PVL were selected on the basis of clinical features and multiple biopsy specimens (Table 1). The following PVL criteria accepted in the literature were applied: (1) large and multifocal white plaque-like lesions; (2) progressive spread and recurrences over months or years; (3) multiple biopsy samples showing hyperorthokeratosis and/or verrucous hyperplasia with or without dysplasia. The lesions involved multiple vestibular, buccal and gingival sites, and occasionally, tongue; 4 out of 10 cases were documented to progress to verrucous, papillary or conventional squamous cell carcinoma.
Table 1.
Summary of PVL cases
| Case # | Patient age at first biopsy, sex, smoking hx (+/−) | Years of follow-upa (N of biopsies) | Location of lesions/biopsy samples | Pathologic features | |||
|---|---|---|---|---|---|---|---|
| Dysplasia | Verrucous hyperplasia | Verrucous or papillary carcinoma | Conventional SCC | ||||
| 1 | 60M(ST) | 1 (3) | R&L tongue, L vestibule | + | + | ||
| 2 | 62F(ST) | 5 (4) | R&L FOM, ventral tongue, R BM | + | + | + | |
| 3 | 53M(ST) | 2 (4) | R&L mand gingiva, R vestibule | + | |||
| 4 | 82F(?) | 4 (5) | entire mand gingiva | + | + | + | + |
| 5 | 46M(ST) | 3 (4) | R anterior and posterior mand gingiva | + | + | ? | |
| 6 | 67F(−) | 3 (7) | anterior vestibule, R BM, tongue, entire palate | + | + | + | |
| 7 | 41M(+) | 1 (6) | R max alveolar mucosa, palate, L BM | + | + | ||
| 8 | 71F(−) | 2 (5) | R and L mand gingiva, lip | + | + | + | |
| 9 | 65F(?) | 7 (7) | tongue, FOM, R mand alveolar mucosa | + | + | ||
| 10 | 60M (+) | 5 (2) | R BM | + | |||
Most cases had some areas of low-grade dysplasia
hx history, L left, R right, BM buccal mucosa, mand mandibular, max maxillary, “?” no specimen, ST smokeless tobacco
aMost follow-up data are based upon the dates of submitted specimens
We selected 38 hyperkeratosis (HK) cases, presenting clinically as single white lesions of any oral mucosal surface, and diagnosed as hyperkeratosis with or without dysplasia, and not associated with PVL. All sites involved in PVL cases were represented in HK samples: buccal mucosa and vestibule (17 cases), tongue and floor of mouth (14 cases), gingiva (7 cases).
Immunohistochemistry (IHC) and Analysis of TLR2 Expression
Two to four separate 5-µm sections from each of the formalin-fixed, paraffin-embedded tissue blocks were stained by IHC using anti-TLR2 and negative control rabbit antibodies (see below) along with reagents and rabbit-specific Pierce Ultra-Sensitive ABC Staining Kit (Thermo Scientific) according to manufacturer instructions. Briefly, deparaffinized sections were heated to retrieve antigen for 20 min in citrate buffer, pH 6.0 (Thermo Scientific), and then re-equilibrated in phosphate-buffered saline (PBS). Endogenous peroxidase and non-specific antibody binding were sequentially blocked using Hydrogen Peroxide and normal goat serum, respectively (Thermo Scientific). The sections were then incubated at 4 °C overnight in a humidity chamber with 2 µg/mL polyclonal rabbit anti-TLR2 antibody (Ab) (#2229, Cell Signaling Technology), or 2ug/ml non-specific polyclonal rabbit Ab (Abcam, Cambidge MA). To detect the primary Ab binding, sections were sequentially incubated at room temperature for 30 min, each, with Biotinylated Affinity Purified Goat Anti-Rabbit IgG and ABC Complex (Ultra-Sensitive ABC Staining Kit, Thermo Scientific), followed by diaminobenzidine tetrahydrochloride (DAB) substrate (Thermo Scientific). Sections were counterstained using Mayer’s hematoxylin. All incubations, except the non-specific antibody binding block, were followed by washing with PBS. Coverslips were applied with aqueous mounting medium (Thermo-Fisher). All H&E and IHC-stained sections were photographed at 50–200× magnifications using microscope-mounted digital camera (Olympus Corp., Tokyo, Japan). The H&E and IHC features were tabulated and patterns were identified, followed by adding the clinical and diagnostic information.
Results
Squamous Epithelium in PVL Shows Full-Thickness Expression of TLR2
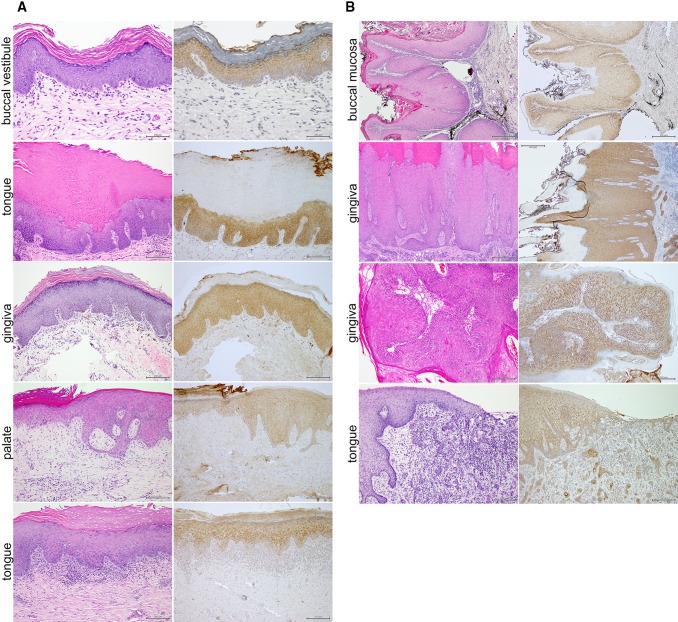
The available clinical information for the PVL cases is summarized in Table 1. As seen in representative examples, the common thread was the diffuse cytoplasmic TLR2 expression throughout the epithelial thickness from the basal layer up to the stratum corneum (Figs. 1a, 3c). This pattern was independent of the presence or absence of inflammation or dysplasia. In addition, four out of 10 PVL had progressed to carcinomas, including verrucous, papillary and so-called conventional. Malignant epithelial cells in the carcinomas usually retained diffuse cytoplasimic TLR2 expression, with more intense perinuclear distribution in the verrucous and papillary subtypes (Fig. 1b). As most of the PVL samples in our set were from the buccal mucosa/vestibule and the gingiva, additional analysis of the oral mucosa was needed to assess whether TLR2 distribution was independent of the subsite and/or the morphologic features of the epithelium.
Fig. 1.
Representative histopathologic features and TLR2 expression in early PVL lesions and in associated carcinomas. a Four representative examples of PVL cases showing the recognizable morphologic features. The two top panels represent two different sites of the same case. Note the diffuse cytoplasmic TLR2 expression in all epithelial layers, irrespective of the specific site and variation in morphology. b Examples of squamous carcinomas associated with four of the evaluated PVL cases (top to bottom, respectively): VC of buccal mucosa, VC of gingiva, papillary SCC of gingiva, and poorly differentiated SCC of the tongue
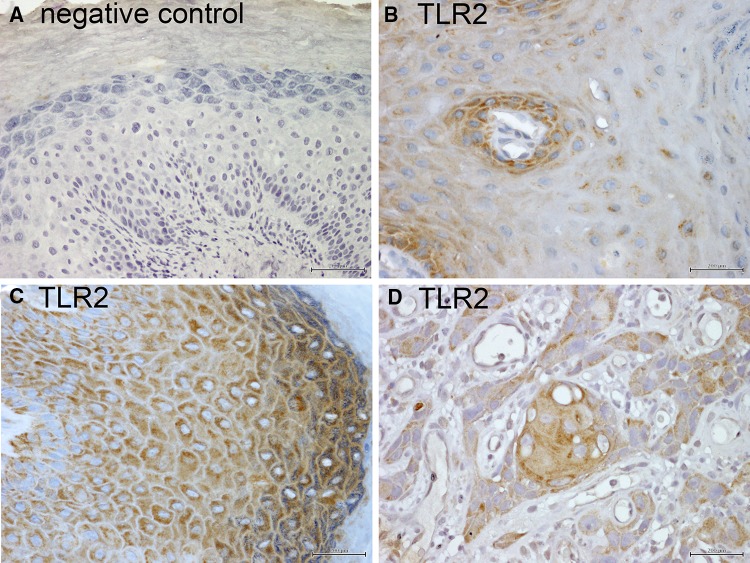
Fig. 3.
Close-up of patterns of TLR2 expression in oral keratinocytes. IHC for TLR2 was performed as described in “Materials and Methods”. All images are acquired at 200×. Brown: TLR2 stain; blue = hematoxylin counterstain. a Negative control. b Diffuse cytoplasmic TLR2 expression in basal keratinocytes, rapidly decreasing to occasional small perinuclear foci in the spinous layer. c Diffuse cytoplasmic expression of TLR2 in keratinocytes of all squamous epithelial layers. d Diffuse cytoplasmic TLR2 expression in malignant squamous cells of oral SCC
Samples of Hyperkeratosis Exhibit Variable Patterns of TLR2 Expression
The samples diagnosed as “hyperkeratosis” were selected based upon presentation as single lesions with or without dysplasia, and included all oral mucosal subsites. We found a significant variation in TLR2 expression, sometimes within the individual samples. One common pattern was diffuse cytoplasmic expression limited to keratinocytes in the basal and parabasal layer, with only occasional small perinuclear foci remaining in the spinous layer of some keratinocytes (Figs. 2, 3b). This pattern was prevalent in the absence of orthokeratosis, dysplasia or inflammation, irrespective of subsite, as seen in examples from buccal mucosa/vestibule (Fig. 2a), gingiva (Fig. 2b), and tongue (Fig. 2c). However, a TLR2 expression pattern similar to that in PVL was noted in specific conditions. In the presence of dysplasia, inflammation or orthokeratosis, diffuse cytoplasmic TLR2 expression increased in the spinous layers at all subsites (Fig. 2).
Fig. 2.
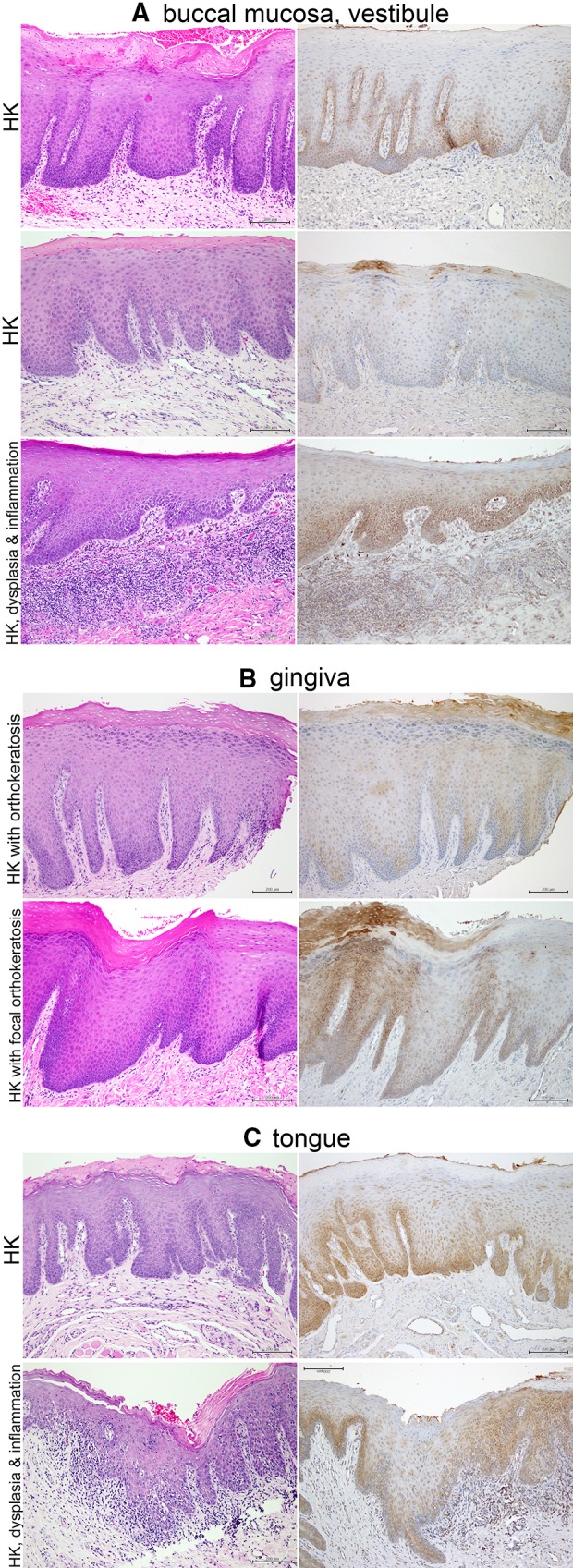
Representative examples of hyperkeratosis in the buccal mucosa or vestibule, gingiva and tongue (mag. ×100). a Buccal mucosa and vestibule. Left column—H&E, right column—TLR2. Note acanthosis and hyperparakeratosis (top two examples) and focal orthokeratosis (middle), with primarily basal and parabasal TLR2 expression. Small foci of perinuclear TLR2 expression are present in the keratinocytes of the spinous layer (middle). A case of hypeparakeratosis with focal acanthosis and with mild dysplasia (lower panel) shows retention of diffuse cytoplasmic TLR2 expression above the basal/parabasal layers, following the pattern of dysplasia. b Gingiva. Acanthosis, hyperpara- and orthokeratosis, and variable TLR2 expression. c Tongue. Acanthosis and hyperparakeratosis with TLR2 predominantly in basal and parabasal layers. Only small perinuclear foci of TLR2 appear to be present in the spinous layers. However, in the case of transition to moderate dysplasia (lower panel), note retention of diffuse cytoplasmic TLR2 expression in the spinous layers, following the dysplasia. Note: same portions of the specimens were photographed, although the H&E-stained and IHC-stained sections were not serial
Discussion
Overall, our current study revealed that the oral squamous epithelium constitutively expresses TLR2 in the basal and parabasal layers at any subsite. The marked expansion of TLR2 expression towards the cornified layer of the epithelial surface was characteristic of early-intermediate PVL lesions, and was also present in hyperorthokeratosis and epithelial dysplasia, as well as inflammation.
The patterns of TLR2 expression in oral squamous epithelium are of interest for several reasons related to TLR2 functions at host surfaces and to the apparent contributions of this pattern recognition receptor to carcinogenesis. Relevant to the former, oral mucosa, like other host surfaces, is colonized by microorganisms, and the surface epithelium serves as a barrier. In the epidermis, TLR2 activity was shown to contribute to the function of tight junctions between skin keratinocytes by strengthening the barrier, as well as facilitating wound repair [21, 25]. The activation of epidermal keratinocyte TLR2 increased epithelial resistance and decreased its permeability at least in part due to the impact on desmosomal proteins [21, 25]. Moreover, barrier function of the glandular epithelium that covers most of the gastrointestinal tract is also enhanced by TLR2 activity [26, 27]. Intestinal epithelial cells (IEC) stimulated through TLR produce anti-bacterial proteins that enhance barrier function [27], while some commensal microorganisms in the intestines interact with TLR2 on IEC and other mucosal cells, which suppresses inflammation. These data support the possibility that activation of TLR2 in oral squamous cells could also enhance barrier function. Consistent with such a possibility is the observation that TLR2-high hyperkeratotic lesions of PVL typically contained little or no inflammatory infiltrates, suggesting potent barrier function, although thick surface keratin was a likely contributor, as well. On the other hand we noted an association between inflammation and possible increase in TLR2 expression. Inflammatory factors, such as IL-6, can stimulate TLR2 expression by activating the transcription factor STAT3 at least in gastric epithelial cells [28]. However, most of the samples with inflammation also showed epithelial dysplasia, so it is not clear if other cell-intrinsic abnormalities in epithelial dysplasia alone or the associated inflammation, was the key factor responsible for increased TLR2 expression.
The causes and consequences of TLR2 expression throughout the basal, spinous and granular layers in PVL and in other hyperorthokeratotic lesions of the oral mucosa remain to be determined. As already mentioned, the pattern of TLR2 staining is consistent with intracellular distribution. Studies have shown that in immune system cells, TLR2 is predominantly a plasma membrane protein that can translocate to the endosomes upon stimulation with TLR2-specific bacterial products [29], which may also be true of keratinocytes. If so, this would suggest that PVL and hyperorthokeratotic lesions may contain a source of TLR2 agonists. It would be of interest to further characterize hyperorthokeratotic lesions and PVL to determine the significance of diffuse and full-thickness TLR2 expression.
Another important reason to investigate TLR2 in oral epithelium is that epithelial cell-intrinsic TLR2 can contribute to carcinogenesis via oncogenic pathways in squamous carcinomas and adenocarcinomas [24, 28, 30]. Moreover, the expression of TLR2 increases in premalignant and malignant oral and GI epithelial lesions of squamous and glandular differentiation [22, 24, 31–33], suggesting that TLR2 may be important early in carcinogenesis. Early PVL lesions are yet another example of a highly-progressive premalignant condition that implicates TLR2 in carcinogenesis.
Although patients with PVL may use tobacco and/or alcohol, in general, PVL lacks consistent associations with the well-known oral cancer risk factors, which leaves open the question of initiating and promoting factors. If TLR2 is important in the progression of PVL, then TLR2 ligands including products of bacteria, would be of significant interest. The slow, but relentless progressive spread of PVL lesions along mucosal surfaces even prior to overt dysplastic characteristics is particularly interesting from the pathogenesis standpoint, as it sets PVL apart from other oral premalignant conditions. Our study adds TLR2 to the increasing list of molecular changes in the affected epithelial cells.
Strengths and Limitations of the Study
The strengths of this study include: (1) Careful selection of well-documented PVL cases that involved multiple sites, and prolonged patient follow-up; (2) the use of multiple sections and all available biopsies; (3) comparison to other white lesions from the same sites; (4) identification of epithelial disturbances that are associated with increased TLR2 expression.
The limitations of this study are the small number of PVL cases and the use of immunohistochemistry alone to identify TLR2 expression. However, the limitations are minimized by the use of a significant number of hyperkeratotic lesions that varied by subsite and morphologic changes, which allowed to identify epithelial disturbances most likely to be associated with high TLR2 expression. Moreover, the selected method showed clear differences in the patterns of TLR2 expression, suggesting that some epithelial disturbances may lead to the retention of diffuse cytoplasmic TLR2 expression well above the basal cells. This mapping would not be possible by other methods.
Conclusions
In summary, the diffuse cytoplasmic TLR2 expression throughout the epithelium in early to intermediate stages of PVL and in epithelial dysplasia suggests that this microbial pattern recognition receptor may play a role in oral carcinogenesis. Given the high rates of PVL transformation and the previously established pro-cancer role of high TLR2 expression in malignant oral squamous cells, it is important to determine how its’ expression and functions are regulated in the oral squamous epithelium, and what is the specific TLR2 role in carcinogenesis. Such studies could lead to strategies that prevent or control the progression of a notoriously difficult oral health problem.
Acknowledgements
We thank Ms. Regina Hand, histotechnologist at the Dental College of Georgia, for providing sections of archival specimens for the study. This study was supported by Augusta University Pilot Project Grant.
Abbreviations
- CD282
Cluster of differentiation 282
- DAB
Diaminobenzidine
- ERK
Extracellular signal regulated kinase
- Gneg
Gram-negative
- Gpos
Gram-positive
- H&E
Hematoxylin and eosin
- HPV
Human papillomavirus
- IHC
Immunohistochemistry
- MAPK
Mitogen-activated protein kinase
- PBS
Phosphate buffered saline
- PVL
Proliferative verrucous leukoplakia
- SCC
Squamous cell carcinoma
- TLR
Toll-like receptor
Compliance with Ethical Standards
Conflict of interest
Authors have no conflicts of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Agency for Research on Cancer . WHO classification of head and neck tumours. 4. Lyon: IARC; 2017. [Google Scholar]
- 2.Castellsague X, Alemany L, Quer M, Halec G, Quiros B, Tous S, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108(6):djv403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 3.Neville BWDD, Allen CM, Chi AC. Oral and maxillofacial pathology. 4. Amsterdam: Elsevier; 2016. [Google Scholar]
- 4.Hansen LS, Olson JA, Silverman S. Proliferative verrucous leukoplakia. A long-term study of thirty patients. Oral Surg Oral Med Oral Pathol. 1985;60(3):285–298. doi: 10.1016/0030-4220(85)90313-5. [DOI] [PubMed] [Google Scholar]
- 5.Gillenwater AM, Vigneswaran N, Fatani H, Saintigny P, El-Naggar AK. Proliferative verrucous leukoplakia: recognition and differentiation from conventional leukoplakia and mimics. Head Neck. 2014;36(11):1662–1668. doi: 10.1002/hed.23505. [DOI] [PubMed] [Google Scholar]
- 6.Hooper SJ, Crean SJ, Fardy MJ, Lewis MA, Spratt DA, Wade WG, et al. A molecular analysis of the bacteria present within oral squamous cell carcinoma. J Med Microbiol. 2007;56(Pt 12):1651–1659. doi: 10.1099/jmm.0.46918-0. [DOI] [PubMed] [Google Scholar]
- 7.Hooper SJ, Wilson MJ, Crean SJ. Exploring the link between microorganisms and oral cancer: a systematic review of the literature. Head Neck. 2009;31(9):1228–1239. doi: 10.1002/hed.21140. [DOI] [PubMed] [Google Scholar]
- 8.Pushalkar S, Ji X, Li Y, Estilo C, Yegnanarayana R, Singh B, et al. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012;12:144. doi: 10.1186/1471-2180-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pushalkar S, Mane SP, Ji X, Li Y, Evans C, Crasta OR, et al. Microbial diversity in saliva of oral squamous cell carcinoma. FEMS Immunol Med Microbiol. 2011;61(3):269–277. doi: 10.1111/j.1574-695X.2010.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt BL, Kuczynski J, Bhattacharya A, Huey B, Corby PM, Queiroz EL, et al. Changes in abundance of oral microbiota associated with oral cancer. PLoS ONE. 2014;9(6):e98741. doi: 10.1371/journal.pone.0098741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Funchain P, Bebek G, Altemus J, Zhang H, Niazi F, et al. Microbiomic differences in tumor and paired-normal tissue in head and neck squamous cell carcinomas. Genome Med. 2017;9(1):14. doi: 10.1186/s13073-017-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joly S, Compton LM, Pujol C, Kurago ZB, Guthmiller JM. Loss of human beta-defensin 1, 2, and 3 expression in oral squamous cell carcinoma. Oral Microbiol Immunol. 2009;24(5):353–360. doi: 10.1111/j.1399-302X.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- 13.De Nardo D. Toll-like receptors: activation, signalling and transcriptional modulation. Cytokine. 2015;74(2):181–189. doi: 10.1016/j.cyto.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 15.Kurago ZB, Lam-ubol A, Stetsenko A, De La Mater C, Chen Y, Dawson DV. Lipopolysaccharide-squamous cell carcinoma-monocyte interactions induce cancer-supporting factors leading to rapid STAT3 activation. Head Neck Pathol. 2008;2(1):1–12. doi: 10.1007/s12105-007-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atretkhany KN, Drutskaya MS, Nedospasov SA, Grivennikov SI, Kuprash DV. Chemokines, cytokines and exosomes help tumors to shape inflammatory microenvironment. Pharmacol Ther. 2016;168:98–112. doi: 10.1016/j.pharmthera.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Arjunan P, Meghil MM, Pi W, Xu J, Lang L, El-Awady A, et al. Oral pathobiont activates anti-apoptotic pathway, promoting both immune suppression and oncogenic cell proliferation. Sci Rep. 2018;8(1):16607. doi: 10.1038/s41598-018-35126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dajon M, Iribarren K, Cremer I. Toll-like receptor stimulation in cancer: a pro- and anti-tumor double-edged sword. Immunobiology. 2017;222(1):89–100. doi: 10.1016/j.imbio.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Beasley NJ, Leek R, Alam M, Turley H, Cox GJ, Gatter K, et al. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res. 2002;62(9):2493–2497. [PubMed] [Google Scholar]
- 20.Chefetz I, Alvero AB, Holmberg JC, Lebowitz N, Craveiro V, Yang-Hartwich Y, et al. TLR2 enhances ovarian cancer stem cell self-renewal and promotes tumor repair and recurrence. Cell Cycle. 2013;12(3):511–521. doi: 10.4161/cc.23406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo IH, Carpenter-Mendini A, Yoshida T, McGirt LY, Ivanov AI, Barnes KC, et al. Activation of epidermal toll-like receptor 2 enhances tight junction function: implications for atopic dermatitis and skin barrier repair. J Invest Dermatol. 2013;133(4):988–998. doi: 10.1038/jid.2012.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huhta H, Helminen O, Lehenkari PP, Saarnio J, Karttunen TJ, Kauppila JH. Toll-like receptors 1, 2, 4 and 6 in esophageal epithelium, Barrett’s esophagus, dysplasia and adenocarcinoma. Oncotarget. 2016;7(17):23658–23667. doi: 10.18632/oncotarget.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chavarria-Velazquez CO, Torres-Martinez AC, Montano LF, Rendon-Huerta EP. TLR2 activation induced by H. pylori LPS promotes the differential expression of claudin-4, -6, -7 and -9 via either STAT3 and ERK1/2 in AGS cells. Immunobiology. 2018;223(1):38–48. doi: 10.1016/j.imbio.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Palani CD, Ramanathapuram L, Lam-Ubol A, Kurago ZB. Toll-like receptor 2 induces adenosine receptor A2a and promotes human squamous carcinoma cell growth via extracellular signal regulated kinases (1/2) Oncotarget. 2018;9(6):6814–6829. doi: 10.18632/oncotarget.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basler K, Bergmann S, Heisig M, Naegel A, Zorn-Kruppa M, Brandner JM. The role of tight junctions in skin barrier function and dermal absorption. J Control Release. 2016;242:105–118. doi: 10.1016/j.jconrel.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Hanson PJ, Moran AP, Butler K. Paracellular permeability is increased by basal lipopolysaccharide in a primary culture of colonic epithelial cells; an effect prevented by an activator of Toll-like receptor-2. Innate Immun. 2011;17(3):269–282. doi: 10.1177/1753425910367813. [DOI] [PubMed] [Google Scholar]
- 27.Kubinak JL, Round JL. Toll-like receptors promote mutually beneficial commensal-host interactions. PLoS Pathog. 2012;8(7):e1002785. doi: 10.1371/journal.ppat.1002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tye H, Kennedy CL, Najdovska M, McLeod L, McCormack W, Hughes N, et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell. 2012;22(4):466–478. doi: 10.1016/j.ccr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Stack J, Doyle SL, Connolly DJ, Reinert LS, O’Keeffe KM, McLoughlin RM, et al. TRAM is required for TLR2 endosomal signaling to type I IFN induction. J Immunol. 2014;193(12):6090–6102. doi: 10.4049/jimmunol.1401605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheeren FA, Kuo AH, van Weele LJ, Cai S, Glykofridis I, Sikandar SS, et al. A cell-intrinsic role for TLR2-MYD88 in intestinal and breast epithelia and oncogenesis. Nat Cell Biol. 2014;16(12):1238–1248. doi: 10.1038/ncb3058. [DOI] [PubMed] [Google Scholar]
- 31.Pimentel-Nunes P, Afonso L, Lopes P, Roncon-Albuquerque R, Jr, Goncalves N, Henrique R, et al. Increased expression of toll-like receptors (TLR) 2, 4 and 5 in gastric dysplasia. Pathol Oncol Res. 2011;17(3):677–683. doi: 10.1007/s12253-011-9368-9. [DOI] [PubMed] [Google Scholar]
- 32.West AC, Tang K, Tye H, Yu L, Deng N, Najdovska M, et al. Identification of a TLR2-regulated gene signature associated with tumor cell growth in gastric cancer. Oncogene. 2017;36(36):5134–5144. doi: 10.1038/onc.2017.121. [DOI] [PubMed] [Google Scholar]
- 33.Paarnio K, Vayrynen S, Klintrup K, Ohtonen P, Makinen MJ, Makela J, et al. Divergent expression of bacterial wall sensing Toll-like receptors 2 and 4 in colorectal cancer. World J Gastroenterol. 2017;23(26):4831–4838. doi: 10.3748/wjg.v23.i26.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]