Abstract
Background
Currently, there are no accepted FDA-approved pharmacotherapies for cocaine use disorder, though numerous medications have been tested in clinical trials. We conducted a systematic review and meta-analysis to better understand the effectiveness of pharmacotherapy for cocaine use disorder.
Methods
We searched multiple data sources (MEDLINE, PsycINFO, and Cochrane Library) through November 2017 for systematic reviews and randomized controlled trials (RCTs) of pharmacological interventions in adults with cocaine use disorder. When possible, we combined the findings of trials with comparable interventions and outcome measures in random-effects meta-analyses. We assessed the risk of bias of individual trials and the strength of evidence for each outcome using standardized criteria. Outcomes included continuous abstinence (3+ consecutive weeks); cocaine use; harms; and study retention. For relapse prevention studies (participants abstinent at baseline), we examined lapse (first cocaine positive or missing UDS) and relapse (two consecutive cocaine positive or missed UDS′).
Results
Sixty-six different drugs or drug combinations were studied in seven systematic reviews and 48 RCTs that met inclusion criteria. Antidepressants were the most widely studied drug class (38 RCTs) but appear to have no effect on cocaine use or treatment retention. Increased abstinence was found with bupropion (2 RCTs: RR 1.63, 95% CI 1.02 to 2.59), topiramate (2 RCTs: RR 2.56, 95% CI 1.39 to 4.73), and psychostimulants (14 RCTs: RR 1.36, 95% CI 1.05 to 1.77), though the strength of evidence for these findings was low. We found moderate strength of evidence that antipsychotics improved treatment retention (8 RCTs: RR 1.33, 95% CI 1.03 to 1.75).
Discussion
Most of the pharmacotherapies studied were not effective for treating cocaine use disorder. Bupropion, psychostimulants, and topiramate may improve abstinence, and antipsychotics may improve retention. Contingency management and behavioral interventions along with pharmacotherapy should continue to be explored.
SR Registration
Prospero CRD42018085667
Electronic supplementary material
The online version of this article (10.1007/s11606-019-05074-8) contains supplementary material, which is available to authorized users.
KEY WORDS: substance use, pharmacotherapy, systematic review, cocaine
INTRODUCTION
Cocaine use disorder remains a serious problem in the USA and worldwide. In the USA, 900,000 adults met criteria for cocaine use disorder in 2014 and 40% of visits to emergency departments for drug misuse or abuse involved cocaine.1 Cocaine use is associated with cardiovascular and neurologic effects, and chronic repeated exposure leads to tolerance, adverse psychological and behavioral effects, and complications including infections, stroke, and seizure.2, 3
Psychosocial and behavioral therapies, including cognitive behavioral therapy (CBT) and contingency management (CM) interventions, are the primary treatments for cocaine use disorder. However, they are time-consuming, not universally accessible, and suffer from low treatment retention. Currently, there are no Food and Drug Administration (FDA)-approved medications to treat cocaine use disorder. One challenge in establishing the evidence base for pharmacotherapy of cocaine use disorder is the sheer number of drug classes that have been studied. Prior systematic reviews (SRs) have largely focused on single drugs4 or drug classes (anticonvulsants/carbamazepine,5 dopamine agonists,6 psychostimulants,7 and antipsychotics8). To our knowledge, none have examined the treatment of cocaine use disorder across drug classes. This SR examines the benefits and harms of pharmacological interventions for cocaine use disorder, and was part of a larger report of stimulant use disorders commissioned by the Veterans Health Administration (VHA).
METHODS
Data Sources and Search Strategies
We searched MEDLINE, PsycINFO, and EBM Reviews Cochrane Database of Systematic Reviews through November 2017 (Online Appendix Table 1). We reviewed the bibliographies of relevant articles and contacted experts to identify additional studies. To identify in-progress or unpublished studies, we searched ClinicalTrials.gov, OpenTrials, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). The review protocol was registered to PROSPERO before we initiated the study (CRD42018085667). Our methods and reporting follow PRISMA guidelines.9
Study Selection
Study selection was guided by an analytic framework (Online Appendix Figure 1). We included randomized controlled trials (RCTs) of adults with cocaine use disorder that compared pharmacotherapies (head to head), to placebo, usual care, or psychotherapy. We also included RCTs that had been included in existing good quality SRs. We excluded studies examining patients with comorbid psychotic spectrum or bipolar disorders. We excluded studies that did not perform urine drug screens (UDS) at least once per week. PICOTS and study selection criteria are specified in Online Appendix Tables 2 and 3.
We dual reviewed and evaluated titles and abstracts for 18.6% of the search yield to ensure reliability. Two investigators independently reviewed the full text of all potentially relevant articles for inclusion. All discordant results were resolved through consensus or consultation with a third reviewer.
Data Abstraction and Quality Assessment
One investigator abstracted, and a second investigator confirmed details related to study design, setting, population, intervention and follow-up, co-interventions, outcomes, and harms. Two reviewers independently assessed the quality of each RCT using a tool developed by the Cochrane Collaboration10 (Online Appendix Table 8). We directly report the findings from previous SRs as well as their assessments of study quality.
Our outcomes of interest were sustained abstinence (three or more weeks of negative UDS′),11 cocaine use, treatment retention, serious adverse events (SAEs), and treatment dropouts due to adverse events (AEs). For relapse prevention studies of participants abstinent at baseline, we examined lapse (first cocaine positive or missed UDS) and relapse (two consecutive cocaine positive or missed UDS′). For outcomes related to abstinence and use, we excluded studies relying on self-reported drug use, with the exception of findings from previous SRs.
Data Synthesis and Analysis
We qualitatively synthesized the evidence and separately examined the findings in patients with comorbid opioid use disorder. When possible, we combined data from trials as they were reported in previous SRs with data we abstracted directly from newer RCTs identified in our search in random-effects meta-analyses.12 We used RevMan 5.313 to calculate the overall relative risk (RR) and 95% CI of each outcome in the active treatment group compared with placebo. We assessed statistical heterogeneity among the pooled studies using the I2 statistic.14, 15
We assessed the overall strength of evidence (SOE) for each outcome using an established method, and classified SOE as high, moderate, low, or insufficient.16
RESULTS
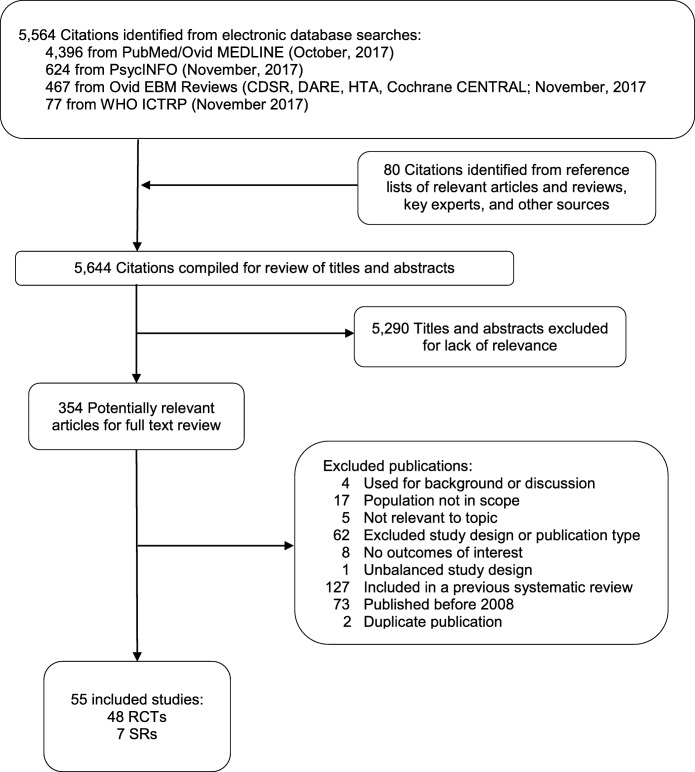
Our larger search for stimulant use disorders yielded 5564 citations. After reviewing the full text of 354 studies, we included seven systematic reviews and 48 RCTs specific to cocaine use disorder (Fig. 1). The included SRs and RCTs examined 66 different drugs including antidepressants, antipsychotics, anxiolytics, cognitive enhancing drugs, dopamine agonists, muscle relaxants, anticonvulsants, medications approved by the FDA for other substance use disorders, and a wide range of other pharmacotherapies (Online Appendix Table 4).
Figure 1.
Literature flow diagram. RCT, randomized controlled trial; SR, systematic review.
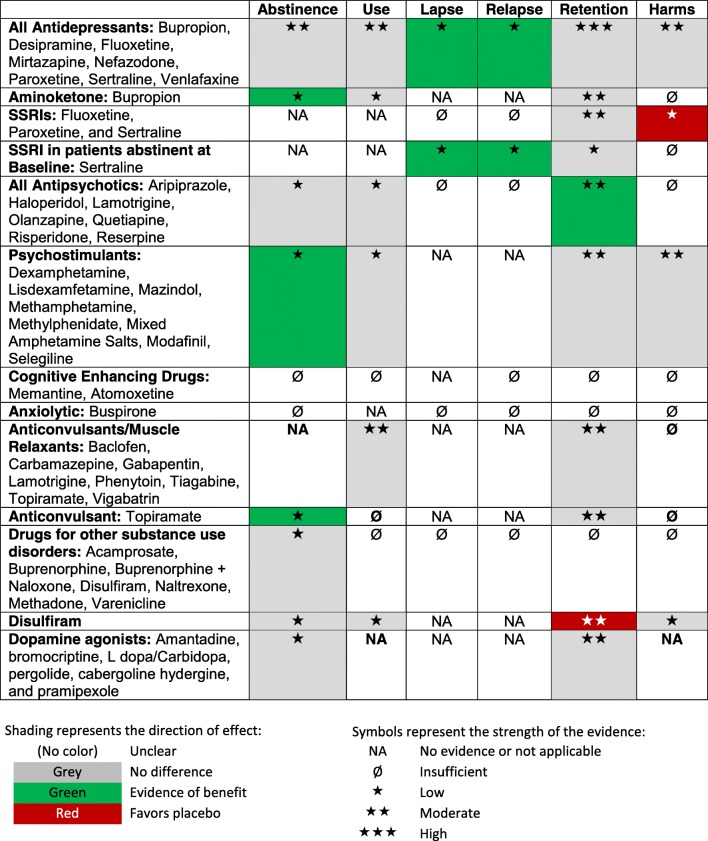
Table 1 presents a brief summary of findings for all drug classes. Table 2 provides a more detailed summary of the evidence on all pharmacotherapies for cocaine use disorder, stratified by drug class. The characteristics and findings of individual studies are provided in Online Appendix Tables 5 and 6.
Table 1.
Brief Summary of Findings
Table 2.
Summary of the Evidence on Pharmacotherapies for Cocaine Use Disorder, Stratified by Drug Class
| Outcomes | N studies per outcome; ROB (N = combined participants) | Summary of findings by outcome | Strength of evidence* | Comments and rationale for strength of evidence rating |
|---|---|---|---|---|
| Psychopharmacotherapies (antidepressants, antipsychotics, anxiolytics, cognitive enhancing drugs, and psychostimulants) | ||||
| Antidepressants (all) | ||||
| Abstinence |
1 SR of 8 RCTs17 (N = 942) 1 low-ROB RCT18 (N = 130) |
No difference. Meta-analysis of 10 RCTs, N = 1226, RR 1.27 (95% CI 0.99 to 1.63) |
Moderate | Inconsistent findings. Trend toward benefit disappeared when restricted to studies using strict criteria for cocaine dependence. |
| Use |
1 SR of 4 RCTs17 (N = 251) 1 low-ROB RCT18 (N = 130) 1 high-ROB RCT19 (N = 24) |
No difference. 1 SR reported a combined use of cocaine (self-reported or objective) RR of 1.05 (95% CI 0.91 to 1.21). Similar findings were reported in both more recent low-ROB and high-ROB RCTs. | Moderate | Indirectness (of outcome) |
| Lapse | 2 low-ROB RCTs20, 21 (N = 116) | Favors antidepressants. Participants abstinent at baseline with 1 cocaine positive UDS, combined RR 0.79 (95% CI 0.62 to 1.00). | Low |
Small body of evidence Indirectness (of results to general population—participants had achieved abstinence prior to the outpatient phase). Lapse is defined as the first cocaine positive or missing UDS; relapse is 2 consecutive cocaine positive or missing UDS′. |
| Relapse | Favors antidepressants. Participants abstinent at baseline with 2 consecutive cocaine positive UDS′, combined RR 0.74 (95% CI 0.57 to 0.96). | Low | ||
| Retention |
1 SR of 27 RCTs17 (N = 2417) |
No difference. Meta-analysis of 33 RCTs N = 2918, RR 0.95 (95% CI 0.87 to 1.03) | High | Findings were similar in analyses limited to RCTs specifying DSM cocaine dependence criteria for inclusion. |
| Harms |
1 SR of 13 RCTs17 (N = 1396) 1 low-ROB RCT18 (N = 130) 1 high-ROB RCT19 (N = 24) |
No difference. 1 SR reported a combined withdrawal due to an adverse event RR of 1.39 (95% CI 0.91 to 2.12). Two more recent RCTs (1 low-ROB, 1 high-ROB) reported consistent findings. No difference. Two RCTs found no difference in severe adverse events by group. |
Withdrawal due to AEs: Moderate Severe AEs: low |
Treatment withdrawal findings are from 1 low and 1 high RCT and a SR/meta-analysis of 37 RCTs. The SR included studies with any definition of cocaine dependence or abuse. Findings of SAEs are from a small body of evidence. |
| Antidepressants (tricyclics) | ||||
| Abstinence | 1 SR of 5 RCTs17 (N = 367) | No difference. 3+ week abstinence, combined RR 1.55 (95% CI 1.10 to 2.17). Limited to DSM criteria for cocaine dependence (3 studies, N = 234): combined RR 1.41, (95% CI 0.93 to 2.14). | Low | 4/5 studies are of desipramine. |
| Use | 1 SR of 2 RCTs17 (N = 37) | No difference. Use of cocaine (self-reported or objective), combined RR 0.85 (95% CI 0.34 to 2.11) | Insufficient | Small body of evidence. Imprecise estimate. Indirectness (of outcome) |
| Retention | 1 SR of 15 RCTs17 (N = 1141) | No difference. Number of participants who did not complete the trial, combined RR 1.00 (95% CI 0.85 to 1.18) | High | Findings were similar in an analysis limited to RCTs specifying DSM cocaine dependence criteria for inclusion and in an analysis excluding high-ROB trials. 13/15 studies are of desipramine. |
| Harms | 1 SR of 5 RCTs17 (N = 381) |
No difference. Withdrawal due to an adverse event, combined RR 1.24 (95% CI 0.64 to 2.43) SAE: NA |
Moderate No evidence: SAE |
Findings were similar in analyses limited to RCTs specifying DSM cocaine dependence criteria for inclusion. Imprecise estimate. 4/5 studies are of desipramine. |
| Antidepressants (SSRIs): fluoxetine and sertraline | ||||
| Abstinence | NA | NA | No evidence | NA |
| Use | NA | NA | No evidence | NA |
| Relapse | 2 low-ROB RCTs20, 21 (N = 133) | Favors sertraline. Participants abstinent at baseline with 2 consecutive cocaine positive UDS′, combined RR 0.74 (95% CI 0.57 to 0.96). | Low |
Small body of evidence Indirectness (of results to general population—participants had achieved abstinence prior to the outpatient phase). Lapse is defined as the first cocaine positive UDS, relapse is 2 consecutive cocaine positive UDS’. |
| Lapse | Favors sertraline. Abstinent at baseline participants with 1 cocaine positive UDS, combined RR 0.79 (95% CI 0.62 to 1.00). | Low | ||
| Retention |
1 SR of 7 RCTs17 (N = 527) |
No difference. The SR’s combined RR for participants not completing the trial was 0.99 (95% CI 0.70 to 1.71). No difference in 2 more recent RCTs. | Moderate | Inconsistent results. Findings favored placebo when excluding 1 outlier, and no difference was found when further excluding 1 high-ROB RCT. Indirectness (of population)—2 more recent RCTs enrolled only patients who had achieved abstinence. |
| Harms | 1 SR of 3 RCTs17 (N = 251) |
Favors placebo. Withdrawal due to an adverse event, combined RR 3.55 (95% CI 1.11 to 11.34). SAE: NA |
Low No evidence: SAE |
Imprecise estimate, small body of evidence |
| Antidepressant (SNRI): venlafaxine | ||||
| Abstinence | 1 low-ROB RCT18 (N = 130) | No difference. 1 RCT found no difference in 3+ week abstinence between groups (P = 0.94). | Insufficient | 1 single site study. |
| Use | No difference. 1 RCT found no difference in negative UDS′ between groups (P = 0.74). | Insufficient | ||
| Retention | No difference. 1 RCT found no difference in retention between groups. | Insufficient | ||
| Harms |
No difference. 1 RCT found no difference in withdrawals due to adverse events by group. No difference. 1 RCT found no difference in severe AEs between groups. |
Insufficient | ||
| Antidepressant (Atypical): mirtazapine | ||||
| Abstinence | NA | NA | No evidence | NA |
| Use | 1 high-ROB RCT19 (N = 24) | No difference. 1 RCT found no difference in study period use between groups. | Insufficient | 1 very small underpowered study. Details regarding randomization and allocation concealment NR. |
| Retention | NA | NA | No evidence | NA |
| Harms | 1 high-ROB RCT19 (N = 24) |
No difference. 1 RCT found no difference in withdrawals due to AEs between groups (none). No difference. 1 RCT found no difference in severe AEs between groups (because there were none). |
Insufficient | 1 very small underpowered study. Details regarding randomization and allocation concealment NR. |
| Antidepressant (aminoketone): bupropion | ||||
| Abstinence | 1 SR of 2 RCTs7 (N = 176) | Favors bupropion. 1 SR reported a combined 3+ week abstinence RR of 1.63 (95% CI 1.02 to 2.59). | Low |
Small body of evidence Imprecise estimates |
| Use | No difference. Use of cocaine, combined SMD 0.24 (95% CI − 0.06 to 0.54). | Low | ||
| Retention | 1 SR of 3 RCTs17 (N = 325) | No difference. The SR’s combined RR for participants not completing the trial was 0.99 (95% CI 0.79 to 1.25). | Moderate | Inconsistent results |
| Harms | 1 SR of 1 RCT7 |
No difference. Mean withdrawals due to AEs RD 0.00 (95% CI − 0.05 to. 0.05) SAE: NA |
Insufficient No evidence: SAE |
Small body of evidence |
| Antipsychotics (all) | ||||
| Abstinence | 1 SR of 3 RCTs8 (N = 139) | No difference. 1 SR reported a combined 3+ week abstinence RR of 1.30 (95% CI 0.73 to 2.32). | Low |
Small body of evidence Imprecise estimate |
| Use |
1 SR of 2 RCTs8 (N = 150) 1 high-ROB RCT22 (N = 18 opioid randomized, 41 enrolled opioid-dependent participants) |
No difference. | Low |
Small body of evidence Methodological limitations of studies. Indirectness of population. |
| Relapse | 1 high-ROB RCT22 (N = 18 opioid randomized, 41 enrolled opioid-dependent participants) | No difference. | Insufficient | Small, methodologically limited single trial. Indirectness (of results to general population—participants had achieved abstinence prior to the outpatient phase). Lapse is defined as the first cocaine positive UDS, relapse is 2 consecutive cocaine positive UDS′. |
| Lapse | No difference. | Insufficient | ||
| Retention |
1 SR of 8 RCTs8 (N = 397) 1 high-ROB RCT22 (N = 18 randomized, 41 enrolled opioid-dependent participants) |
Favors any antipsychotic. 1 SR reported dropouts RR 0.75 (95% CI 0.57 to 0.97). 1 high-ROB RCT of comorbid cocaine and opioid-dependent methadone-maintained participants found no difference in retention between groups. |
Moderate | Newer trial found no difference (indirectness of population). |
| Harms | 1 high-ROB RCT22 (N = 18 randomized, 41 enrolled opioid-dependent participants) |
Withdrawals: no difference. SAE: NA |
Insufficient No evidence: SAE |
Small, methodologically limited single trial. Indirectness (of population) |
| Antipsychotics (first generation): haloperidol | ||||
| Abstinence | NA | NA | No evidence | NA |
| Use | NA | NA | No evidence | NA |
| Retention | 1 SR of 1 RCT8 (N = 31) | No difference. 1 SR reported a RR for participants not completing the trial of 1.50 (95% CI 0.63 to 3.57). 1 head to head trial found no difference between haloperidol and olanzapine (N = 31; RR 1.50, 95% CI 0.63 to 3.57). | Insufficient | Findings are from a single study in a SR/meta-analysis of 14 RCTs. |
| Harms | NA | NA | No evidence | NA |
| Antipsychotics (second generation): aripiprazole, olanzapine, risperidone, quetiapine | ||||
| Abstinence | 1 SR of 3 RCTs8 (N = 139) | No difference. Three studies in a SR found no difference between an atypical antipsychotic and placebo on sustained abstinence. | Low |
Small body of evidence Imprecise estimate |
| Use |
1 SR of 1 RCT8 (N = 31) 1 high-ROB RCT22 (N = 18 randomized, 41 enrolled opioid-dependent participants) |
No difference. 1 RCT from 1 SR and 1 high-ROB RCT of opioid-dependent participants found no difference between groups. | Insufficient |
Small body of evidence Methodological limitations of studies. Indirectness of population. |
| Relapse | 1 high-ROB RCT22 (N = 18 randomized, 41 enrolled opioid-dependent participants) | No difference. 1 high-ROB found no difference in relapse by group. | Insufficient | Small, methodologically limited single trial. Indirectness (of results to general population—participants had achieved abstinence prior to the outpatient phase). Lapse is defined as the first cocaine positive UDS, relapse is 2 consecutive cocaine positive UDS′. |
| Lapse | No difference. 1 high-ROB found no difference in lapse by group. | Insufficient | ||
| Retention |
1 SR of 7 RCT8 (N = 365) 1 high-ROB RCT22 (N = 18 randomized, 41 enrolled opioid-dependent participants) |
No difference. Seven studies in 1 SR and 1 high-ROB RCT of comorbid cocaine and opioid-dependent methadone-maintained participants found no benefit of atypical antipsychotics on study retention | Moderate | Newer trial found no difference (indirectness of population). |
| Harms | 1 high-ROB RCT22 (N = 18 randomized, 41 enrolled opioid-dependent participants) | No difference. 1 high-ROB RCT of comorbid cocaine and opioid-dependent methadone-maintained participants found no difference in withdrawals due to AEs by group. SAE: NA |
Insufficient No evidence: SAE |
Small, methodologically limited single trial. Indirectness (of results to general population—participants had achieved abstinence prior to the outpatient phase). |
| Antipsychotics (other): reserpine | ||||
| Abstinence | NA | NA | No evidence | NA |
| Use | 1 SR of 1 RCT8 (N = 119) | No difference. 1 study in the SR found a no difference in use between groups. | Insufficient | Small body of evidence. Imprecise estimate. |
| Retention | NA | NA | No evidence | NA |
| Harms | NA | NA | No evidence | NA |
| Anxiolytics: buspirone | ||||
| Abstinence | 1 High-ROB RCT26 (N = 62) | No difference. 1 RCT found no difference between groups in the mean number of days of (post-discharge) abstinence. | Insufficient | Small, methodologically limited single trial. Indirectness (of results to general population—participants had achieved abstinence prior to the outpatient phase). Lapse is defined as the first cocaine positive UDS, relapse is 2 consecutive cocaine positive UDS′. |
| Use | NA | No evidence | ||
| Lapse | No difference. 1 RCT found no difference between groups in number of days to lapse. | Insufficient | ||
| Retention | No difference. 1 RCT reported high rates of retention (94% buspirone vs 93% placebo), but no difference between groups. | Insufficient | ||
| Withdrawal due to AE | No difference. In 1 RCT there were no withdrawals due to AEs. | Insufficient | ||
| Severe AE | Favors placebo. In 1 RCT there were 3 SAEs in participants receiving buspirone vs 0 receiving placebo. | Insufficient | ||
| Cognitive enhancing drugs: memantine, atomoxetine | ||||
| Abstinence | 1 low-ROB RCT24 (N = 81) | No difference. Participants who did not achieve abstinence at baseline (N = 45), there was no difference between groups in the achievement of sustained abstinence (3+ weeks). | Insufficient | Single small RCT with a 2-week placebo lead-in to encourage abstinence after randomization. |
| Use |
1 low-ROB RCT24 (N = 81) 1 unclear-ROB RCT25 (N = 50) |
No difference. There was no difference in cocaine negative UDS′ between groups. | Insufficient | Small body of evidence. Methodological limitations of studies. |
| Relapse | 1 low-ROB RCT24 (N = 81) | No difference. Among participants who achieved abstinence at baseline (N = 36), there was no difference between groups in relapse or time to relapse. | Insufficient | Small body of evidence. Indirectness (of results to general population—participants had achieved abstinence prior to the outpatient phase). Relapse is defined as 2 consecutive cocaine positive UDS′. |
| Retention | 1 low-ROB RCT24 (N = 81) | No difference. There was no difference in retention by group. | Insufficient | Small body of evidence. Methodological limitations of studies. |
| Harms | 1 unclear-ROB RCT25 (N = 50) |
No difference. There was no difference in retention by group. No difference. 0 participants receiving memantine experienced a SAE compared with 2 who received placebo. |
Insufficient | |
| Psychostimulants: dexamphetamine, mazindol, methamphetamine, methylphenidate, mixed amphetamine salts, modafinil, lisdexamphetamine, selegiline | ||||
| Abstinence | 1 SR of 14 studies7 (N = 1549) | Favors psychostimulants. 1 SR reported a combined 3+ week abstinence RR of 1.36 (95% CI 1.05 to 1.77). | Low | Large body of evidence and consistent results even after removing bupropion studies, but many trials were methodologically flawed. Findings from individual drugs favor dexamphetamine (small body of evidence) and mixed amphetamine salts (single study). |
| Use | 1 SR of 8 RCTs7 (N = 526) | No difference. Use of cocaine, combined SMD 0.16 (95% CI − 0.02 to 0.33). | Low | Trend toward small benefit, inconsistent results |
| Retention | 1 SR of 24 studies7 (N = 2205) | No difference. Number of participants who did not complete the trial, combined RR 1.00 (95% CI 0.93 to 1.06) | Moderate |
Methodological limitations of many included studies. Heterogeneous population. No bupropion studies are included in findings of SAEs. |
| Harms |
Withdrawal: 1 SR of 19 RCTs7 (N = 1601) Serious AEs: 1 SR of 6 RCTs7 (N = 444) |
No difference. Number of participants who withdrew due to AEs, combined mean RD 0.00 (95% CI − 0.01 to 0.01). No difference. Number of participants who reported severe AEs, combined mean RD − 0.02 (95% CI − 0.06 to 0.01). |
Moderate | |
| Anticonvulsants and muscle relaxants | ||||
| Baclofen | ||||
| Abstinence | 2 unclear-ROB RCTs27, 28 (N = 230) | No difference. | Low | |
| Use | 2 unclear-ROB RCTs27, 28 (N = 230) | No difference. | Low | |
| Retention | 2 unclear-ROB RCTs27, 28 (N = 230) | No difference. | Low | |
| Withdrawal due to AE | 1 unclear-ROB RCT27 (N = 70) | No difference. | Insufficient | |
| Severe AE | 2 unclear-ROB RCTs27, 28 (N = 230) | No difference. | Low | |
| Carbamazepine, gabapentin, lamotrigine, phenytoin, tiagabine, topiramate, and vigabatrin (drugs combined in analysis) | ||||
| Abstinence | 1 SR5 | NR | No evidence |
These represent the combined results for all drug classes included in the SR.5 SOE was determined by the SR authors |
| Use | 1 SR of 9 RCTs5 (N = 867) | No difference. Use of cocaine (self-reported or objective), combined RR 0.92 (95% CI 0.84 to 1.02)5 | Moderate5 | |
| Retention | 1 SR that included 17 RCTs5 (N = 1695) | No difference. RR 0.95 (95% CI 0.86 to 1.05)5 | Moderate5 | |
| Topiramate | ||||
| Abstinence |
1 low-ROB RCT29 (N = 60) |
Favors topiramate (3 RCTs). Relapse prevention RCTs: combined findings from 2 unclear-ROB RCTs31, 32 (RR 2.56 [95% CI 1.39 to 4.73]) for 3 or more weeks of continuous abstinence |
Low | |
| Use | 1 low-ROB RCT29 (N = 60) | Favors topiramate. | Insufficient | Only 1 small trial |
| Retention | 5 RCTs: 1 high-ROB33; 2 unclear-ROB30, 32; 2 low-ROB29, 34 (N = 617) | No difference. Combined RR 1.01 (95% CI: 0.93 to 1.10). | Moderate | Methodological limitations of several trials. |
| Harms | 1 low-ROB RCT29 (N = 60) | No withdrawals occurred due to AE. No severe AEs occurred. | Insufficient | Only 1 small RCT |
| Vigabatrin | ||||
| Abstinence | 1 high-ROB RCT83 (N = 103) | Favors vigabatrin. Full 3-week end-of-trial abstinence 28% vs 7.5% P ≤ 0.01 | Insufficient | Incomplete data was reported for the full trial period. |
| Use |
1 unclear-ROB RCT84 (N = 186) 1 high-ROB RCT83 (N = 103) |
No difference. Total events: 76 (treatment), 86 (placebo). RR 0.88; 95% CI 0.69 to 1.13 | Low | Analysis from SR5 |
| Retention |
1 unclear-ROB RCT84 (N = 186) 1 high-ROB RCT83 (N = 103) |
No difference. Total events: 98 (treatment), 108 (placebo). RR 0.74; 95% CI 0.53 to 1.02. | Low | |
| Harms | 1 unclear-ROB RCT84 (N = 186) | No difference. RR 0.97; 95% CI 0.88 to 1.08 | Insufficient | |
| Medications FDA-approved for other substance use disorders | ||||
| Acamprosate | ||||
| Abstinence | – | No evidence | – | |
| Use | 1 low-ROB RCT50 (N = 60) | No difference. % UDS(−): 22% vs 23%, P = 0.44 | Insufficient | Only 1 small RCT |
| Retention | 1 low-ROB RCT50 (N = 60) | No difference. 18/34 (53%) vs 18/26 (69%), P = NS | Insufficient | Only 1 small RCT |
| Harms | – | No evidence | – | |
| Buprenorphine plus naloxone, 2 doses | ||||
| Abstinence | 1 low-ROB RCT49 (N = 302) | No difference. Rates of abstinence during weeks 5–8 similar between placebo group (16%) and Bup 4 mg 17.9%, (P = 0.36) and Bup 16 mg 18.6%, (P = 0.32) | Insufficient | Only 1 trial |
| Use | 1 low-ROB RCT49 (N = 302) | Mixed findings. Significantly less use with Bup 16 mg + naloxone 4 mg vs placebo. No difference with lower dose | Insufficient | |
| Retention | 1 low-ROB RCT49 (N = 302) | No difference. Rates of retention similar between placebo (87.3%) vs Bup 4 mg (86.0%) vs Bup 16 mg (88.0%) | Insufficient | |
| Harms | – | No evidence | – | |
| Buprenorphine vs methadone | ||||
| Abstinence | 2 low-ROB RCTs35, 36 (N = 278) | Mixed findings. Longer abstinence with methadone in 1 RCT; no difference in 1 RCT | Insufficient | Mixed findings |
| Use | 1 low-ROB RCT35 (N = 116) | Favors Methadone. Lower use with methadone vs buprenorphine (P < 0.05) | Insufficient | |
| Retention | 2 low-ROB RCTs35, 36 (N = 278) | Mixed findings. Better retention with methadone in 1 RCT; no difference in 1 RCT | Insufficient | Mixed findings |
| Harms | 1 low-ROB RCT36 (N = 162) | Elevated LFT in 1 subject | Insufficient | |
| Disulfiram | ||||
| Abstinence | 3 RCTs37, 41, 85 (N = 296) | No difference. Continuous abstinence disulfiram vs placebo, combined RR from 3 RCTs N = 296, RR 0.96 (95% CI 0.63 to 1.45) | Low | ROB unclear overall |
| Use |
(N = 440) |
No difference. Combined RR from 4 RCTs: 0.95 (95% CI 0.64 to 1.39). The effect varied among studies, and statistical heterogeneity was highly significant (P < 0.001). | Low | Heterogeneous findings among studies |
| Retention |
1 SR4 that included 2 RCTs (N = 87): 1 unclear-ROB (N = 20),85 1 high-ROB86 (N = 67) |
Favors placebo. Treatment retention was lower with disulfiram: Meta-analysis of 7 RCTs, N = 704, RR 0.90 (95% CI 0.83 to 0.99). | Moderate | The combination of findings from all 7 studies (N = 704) was statistically homogeneous (P = 0.90) |
| Harms | 4 RCTs38–41 (N = 548) | Withdrawals due to AE ranged from 0 to 5.9%, and included elevated liver enzymes and rash. Severe AEs not otherwise reported. | Low | – |
| Naltrexone | ||||
| Abstinence |
1 unclear-ROB RCT46 (N = 416) |
No difference. 2 studies found no differences in N weeks to relapse. 1 study found no differences in abstinence (17.9% vs 17.1%, P = 0.918) | Low | Imprecision due to small number of studies; 1 study rated unclear ROB |
| Use | 1 low-ROB RCT45 (N = 80) | No difference. 1 study compared %(+) UDS at weeks 1–4; 5–8; and 9–12 and found no differences between T vs C. | Insufficient | Only 1 small RCT |
| Retention |
1 unclear-ROB RCT46 (N = 416) |
No difference. All 4 studies reported no differences in treatment retention | Low | Imprecision due small number of trials; indirectness due to behavioral co-interventions |
| Harms | 1 low RCT47 (N = 64) | No difference. In 1 trial of 64 pts., 2 in treatment arm and 11 in placebo arm experienced AE, non-significant. | Insufficient | Only 1 small RCT |
| Varenicline | ||||
| Abstinence | – | No evidence | – | |
| Use | 2 unclear-ROB RCTs51, 52 (N = 68) | No evidence. 1 study found trend toward lower use with varenicline (OR = 0.49, P = 0.08); 1 study found no difference (P = 0.84) | Insufficient | Few trials included; inconsistency of findings |
| Retention | 2 unclear-ROB RCTs51, 52 (N = 68) | No difference. 1 study reported 77% total retention with no “significant difference in time to last visit” (P = 0.1); 1 study reported 5 drop out and no differences between groups (P = 0.26) | Insufficient | Unclear risk of bias, small number of studies. |
| Harms | 1 unclear-ROB RCT51 (N = 31) | No difference. 1 trial reported no withdrawals due to AEs. | Insufficient | Unclear risk of bias, only 1 trial, few evens |
| Dopamine agonists | ||||
| Amantadine, bromocriptine, l-dopa/carbidopa, pergolide, cabergoline hydergine, and pramipexole (drugs combined in analysis) | ||||
| Abstinence | 1 SR of 11 RCTs6 (N = 731) | No difference. At 6 weeks: RR 1.12 (95% CI 0.85 to 1.47); at 4 months: RR 1.1 (95% CI 0.61 to 1.98) | Low6 | Strength of evidence was determined by the SR authors6 |
| Use | NR | NR | – | |
| Retention | 1 SR of 20 studies6 (N = 1656) | No difference. RR 1.04 (95% CI 0.94 to 1.14) | Moderate6 | |
| Harms | 1 SR of 7 studies6 (N = 252) | SAEs and withdrawals due to AE NR. | No evidence6 | |
AE, adverse event; CI, confidence interval; DSM, Diagnostic and Statistical Manual of Mental Disorders; MD, mean difference; NR, not reported; P, p value; RCT, randomized control trial; RD, risk difference; RR, relative risk; ROB, risk of bias; SAE, severe adverse event; SMD, standard mean difference; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin and norepinephrine reuptake inhibitor; SR, systematic review; UDS, urine drug screens
*The overall quality of evidence for each outcome is based on the consistency, coherence, and applicability of the body of evidence, as well as the internal validity of individual studies. The strength of evidence is classified as follows16: high, further research is very unlikely to change our confidence on the estimate of effect; moderate, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; insufficient, any estimate of effect is very uncertain
Psychopharmacotherapies
Antidepressants: Bupropion, Desipramine, Fluoxetine, Mirtazapine, Nefazodone, Paroxetine, Sertraline, Venlafaxine
Antidepressants were the most widely studied among the drug classes. We found 34 trials from two previous systematic reviews7, 17 and four subsequent trials18–21 investigating antidepressants (including bupropion) for cocaine use disorder. The more recent trials examine sertraline,20, 21 venlafaxine,18 and mirtazapine.19 Overall, there were no differences on sustained abstinence, use, retention, or harms outcomes.
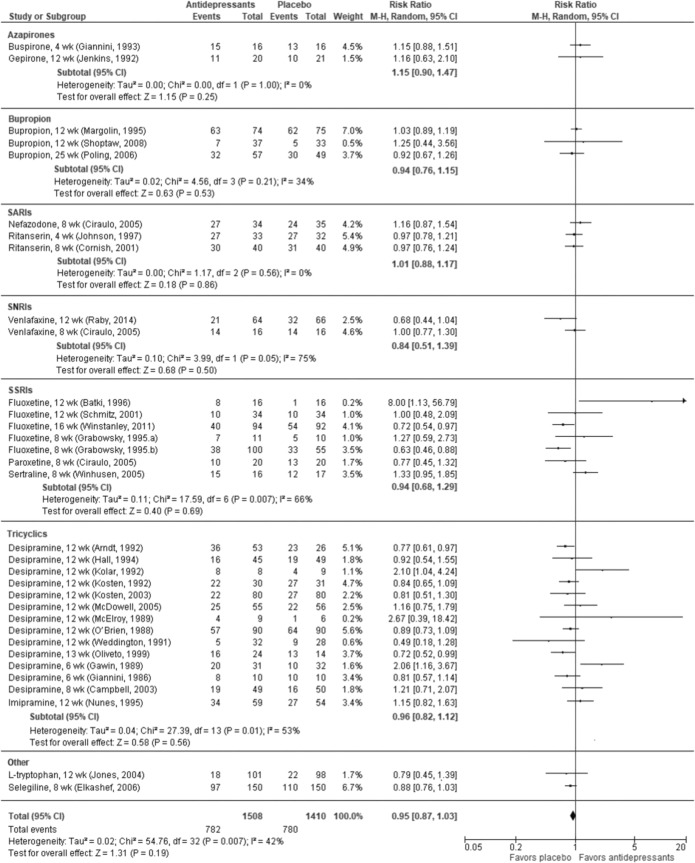
In a meta-analysis combining 10 RCTs7, 17, 18 across all antidepressants, abstinence occurred more frequently in the antidepressant groups than placebo (RR 1.27, 95% CI 0.99 to 1.63), but the difference did not reach statistical significance (P = 0.06; Online Appendix Figure 2). We found moderate SOE of no difference on cocaine use between antidepressants as a class and placebo. Findings were consistent across four RCTs reported in a systematic review17 (RR 1.05, 95% CI 0.91 to 1.21) and two additional RCTs.18, 19 We found high SOE that antidepressants as a class are no better than placebo for study retention (33 RCTs; RR 0.95, 95% CI 0.87 to 1.03; Fig. 2). There were no differences in dropouts due to AEs (moderate SOE) or SAEs (low SOE).
Figure 2.
Treatment retention in RCTs of antidepressants vs placebo for cocaine use disorder.
Selective Serotonin Reuptake Inhibitors: Fluoxetine, Paroxetine, and Sertraline
We found two low risk of bias (ROB) RCTs20, 21 and seven RCTs in the SR of antidepressants17 that provide moderate SOE that selective serotonin reuptake inhibitors (SSRIs) do not improve study retention (N = 527; RR 0.94, 95% CI 0.68 to 1.29; Fig. 2). However, three RCTs (N = 251) in the SR17 provide low-strength evidence of a higher risk of dropout due to AEs with SSRIs compared with placebo (RR 3.55, 95% CI 1.11 to 11.34).
Relapse Prevention: Sertraline
We found two trials (N = 116) examining sertraline for relapse prevention among subjects who were cocaine-abstinent at baseline.20, 21 These RCTs provided a 2-week residential treatment program during which subjects were required to achieve abstinence in order to continue treatment in a 10-week outpatient program. Patients treated with sertraline were less likely to experience lapse (first cocaine positive or missing UDS samples [combined RR 0.80, 95% CI 0.63 to 1.02]) and relapse (two consecutive cocaine positive or missing UDS [combined RR 0.75, 95% CI 0.58 to 0.98; Online Appendix Figure 3]), although only the latter finding was statistically significant. Retention was also higher versus placebo (combined RR 1.43, 95% CI 0.94 to 2.15; Online Appendix Figure 4), though the difference was not statistically significant (P = 0.09).
Bupropion
There were three trials reported in two existing SRs7, 17 that examined bupropion for cocaine use disorder. There was low SOE that bupropion improved abstinence versus placebo (2 RCTs; combined RR 1.63, 95% CI 1.03 to 2.59).7 Bupropion had no effect on cocaine use (low SOE) or retention (3 RCTs, combined RR 0.94, 95% CI 0.76 to 1.15; moderate SOE; Fig. 2). We found insufficient evidence related to harms.
Antipsychotics: Aripiprazole, Haloperidol, Lamotrigine, Olanzapine, Quetiapine, Risperidone, Reserpine
Fourteen RCTs (N = 719) in an existing SR8 and one additional RCT22 examined antipsychotics as a class for the treatment of cocaine use disorder. The additional RCT22 of recently abstinent subjects found no difference between 15 mg of aripiprazole and placebo for any outcome of interest. Overall, we found low SOE that antipsychotics did not improve abstinence8 or reduce cocaine use,8, 22 and insufficient evidence for lapse and relapse in participants abstinent at baseline.22 We found moderate SOE that antipsychotics improve study retention compared with placebo based on findings from eight RCTs in the SR (RR 0.75, 95% CI 0.57 to 0.97),8 and the additional RCT.22 We found insufficient evidence to form conclusions on harms.
Psychostimulants: Dexamphetamine, Lisdexamfetamine, Mazindol, Methamphetamine, Methylphenidate, Mixed Amphetamine Salts, Modafinil, Selegiline
A SR of 14 RCTs examined psychostimulants for treatment of cocaine use disorder. These trials reported low-strength evidence that psychostimulants improved abstinence versus placebo (RR 1.36, 95% CI 1.05 to 1.77).7 Although one study of bupropion (which we classified as an antidepressant) was included in the combined estimate, its removal does not change the conclusion.23 There were no significant differences between groups for cocaine use during the trial period (SOE low), study retention (moderate SOE), or harms (moderate SOE).
Cognitive Enhancing Drugs: Atomoxetine, Memantine
We found two small RCTs, including one examining memantine (low ROB) and the other examining atomoxetine (unclear ROB), that provide insufficient evidence to draw conclusions about the effects of cognitive enhancing drugs on any outcome of interest.24, 25
Anxiolytics: Buspirone
We identified only one small (N = 62), multi-site, high-ROB RCT that compared 60 mg of buspirone to placebo, along with CM and once weekly optional individual or group psychosocial treatment and relapse prevention.26 This provides insufficient evidence for the use of buspirone for cocaine use disorder.
Other Pharmacotherapies
Anticonvulsants and Muscle Relaxants: Baclofen, Carbamazepine, Gabapentin, Lamotrigine, Phenytoin, Tiagabine, Topiramate, Vigabatrin
We identified 20 RCTs in a prior SR,5 and three additional RCTs27–29 examining the effectiveness of anticonvulsants and muscle relaxants. The SR examined anticonvulsants and found moderate SOE that anticonvulsants as a class are no different than placebo for retention (17 RCTs, combined RR 0.95, 95% CI 0.86 to 1.05) or cocaine use (9 RCTs, RR 0.92, 95% CI 0.84 to 1.02). We identified one additional RCT (N = 60) that found improved abstinence and a reduction in cocaine use associated with topiramate, and no difference in retention.29 Two additional RCTs27, 28 compared baclofen (60 mg and 20 mg) to placebo. Neither study reported differences between groups on any of the outcomes of interest. Across all anticonvulsants and muscle relaxants as a class, there is insufficient evidence to form conclusions about the effects on abstinence, moderate SOE of no difference from placebo on cocaine use and study retention, and insufficient evidence on harms.
Topiramate
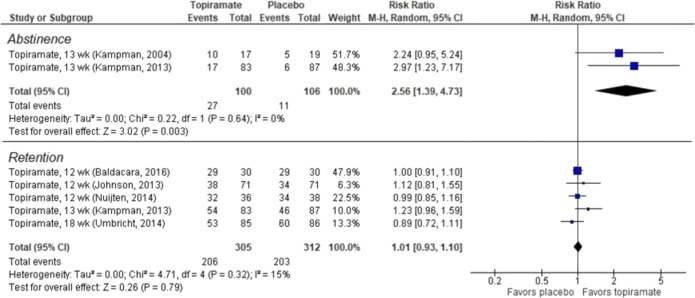
Five RCTs—four30–34 from an existing SR5 and one additional RCT29—examined topiramate for cocaine use disorder. We found low SOE favoring topiramate over placebo for abstinence (2 RCTs, combined RR 2.56, 95% CI, 1.39 to 4.73; Fig. 3) and moderate SOE that topiramate was no different from placebo for study retention (5 RCTs, combined RR 1.01, 95% CI 0.93 to 1.10; Fig. 3). There was insufficient evidence to form conclusions on AEs.
Figure 3.
Abstinence and retention in RCTs of topiramate vs placebo for cocaine use disorder.
Medications FDA-Approved for Other Substance Use Disorders: Acamprosate, Buprenorphine, Buprenorphine and Naloxone, Disulfiram, Methadone, Naltrexone, Varenicline
One SR4 and 18 RCTs35–52 examined FDA-approved pharmacotherapies for other substance use disorders. We found low SOE from six trials43–48 that naltrexone was no different than placebo for abstinence or retention. There was insufficient evidence on use reduction and AEs. For studies of acamprosate,50 varenicline,51, 52 buprenorphine plus naloxone,49 and methadone compared directly with buprenorphine,35, 36 there was insufficient evidence to form conclusions on the outcomes of interest.
Disulfiram
Disulfiram for the treatment of cocaine use disorder was examined in a previous SR4 of seven RCTs and in five more recently published RCTs.37–41 There was low SOE that disulfiram does not increase abstinence (3 RCTs, combined RR 0.96, 95% CI 0.63 to 1.45; Figure 7) or increase harms versus placebo. We found moderate SOE that disulfiram worsened rates of retention versus placebo (7 RCTs, combined RR 0.90, 95% CI 0.83 to 0.99; Online Appendix Figure 5). The effects of disulfiram on cocaine use were significantly heterogeneous (I2 = 97%, P < 0.00001) in a meta-analysis of four RCTs (Online Appendix Figure 5), and the evidence was therefore insufficient for drawing conclusions.
Dopamine Agonists: Amantadine, Bromocriptine, Cabergoline Hydergine, l-Dopa/Carbidopa, Pergolide, Pramipexole
A 2015 SR of 24 trials found no difference between dopamine agonists and placebo on retention (moderate SOE), abstinence (low SOE), and a lack of evidence on AEs.6 We identified no additional trials of examining dopamine agonists for cocaine use disorder.
Other Pharmacotherapies
Nineteen trials53–71 examined the effects of other drugs or drug combinations for cocaine use disorder (Table 2). Although there is insufficient evidence to form conclusions due to limited power, positive findings on abstinence and use reduction were reported in studies of doxazosin,59 ondansetron,66 propranolol,70 and topiramate combined with mixed amphetamine salts.64 There were no positive or negative findings on the outcomes of interest for any of the other drugs or drug combinations.
Pharmacotherapies for Comorbid Opioid Use Disorder
Data from 6 SRs4, 5, 7, 8, 17, 72 and 14 additional RCTs22, 35–41, 49, 51, 54, 61, 62, 69 contribute to the evidence on pharmacotherapy for the treatment of cocaine use disorder in adults with comorbid opioid use disorder. Table 5 summarizes the findings of pharmacotherapies studied in patients with comorbid opioid use disorder, and additional details are provided in an online data supplement (Online Appendix Table 7).
We found low SOE that antidepressants are more effective than placebo for cocaine abstinence,7, 17, 72 and that psychostimulants are more effective than placebo for reducing cocaine use in patients with comorbid opioid use disorder.8, 22 However, we also found moderate SOE that both retention and dropouts due to AEs were higher in subjects receiving antidepressants versus placebo, and moderate SOE of poorer retention associated with disulfiram. There was no difference between placebo and antipsychotics or psychostimulants on retention (low SOE). All other medication/outcomes were insufficient to form conclusions.
DISCUSSION
In this review, we identified seven SRs and 48 RCTs examining a variety of pharmacotherapies for cocaine use disorder. We found no strong evidence that any drug class was effective in increasing abstinence, reducing use, or improving retention rates for cocaine use disorder. However, we found low SOE that bupropion, psychostimulants, and topiramate may improve abstinence, and low SOE that sertraline may reduce relapse rates in abstinent patients. There was moderate SOE that antipsychotics may improve treatment retention. We also found moderate SOE that disulfiram may actually worsen treatment retention, and low SOE that SSRIs were associated with higher dropouts due to AEs (Table 4).
To our knowledge, this is the first report to summarize multiple classes of medications used in treatment of cocaine use disorder, which continues to be a global public health problem with increasing morbidity and mortality.73 One motivation for this review was to find potentially promising treatments and targets for future research for a devastating condition that has been historically difficult to treat with pharmacotherapy. Indeed, we did identify several promising treatments that may be good areas in which to prioritize future research (Table 3). Post hoc analyses in RCTs of bupropion suggest that it may be effective for patients with comorbid depression and in conjunction with CM. We also found that psychostimulants—which serve as a form of agonist replacement therapy—may improve abstinence outcomes. Finally, we found that topiramate, thought to work via GABAergic pathways to regulate dopamine release, was potentially effective for abstinence and warrants continued exploration.74
Our review complements and extends the findings of prior SRs by examining and summarizing data across all drug classes. We defined abstinence as 2 or more weeks of negative UDS—which meant excluding studies using other measures of abstinence. We summarized retention as an outcome, recognizing that improving retention in treatment increases the chances for successful recovery of stimulant use disorders; therefore, we did not consider study retention in our SOE assessment. We were limited in our ability to compare and meta-analyze results across studies because many studies did not report these data, or used different measures, and future research should look to standardize outcome reporting such as 3 or more-week abstinence to compare efficacy across trials and drug classes. It is also possible that the lack of significant findings was due to insufficient power to detect differences.
A lack of engagement in treatment on the part of some study participants who are actively using stimulants may affect the efficacy of pharmacotherapies; retention rates varied widely across studies (24–97%), and overall low rates of retention may have affected the assessment of treatment effectiveness in the majority of studies (attrition was greater than 20% in more than a third of the trials reporting retention rates). Unfortunately, pharmacotherapy alone (aside from antipsychotics) does not appear to be effective in improving treatment retention rates. Two areas of promise that are notable include those in which patients have already demonstrated engagement in treatment, or may have another rationale for ongoing engagement, as is the case for some patients with comorbid opiate use disorder. Indeed, we found low SOE that antidepressants and psychostimulants improved cocaine use outcomes in patients with comorbid opioid use disorder (Table 5). Given that the prevalence of cocaine use among heroin users is between 30 and 80%,75 and concurrent opioid use increases risk of death due to cocaine,76 further investigation on treatments for comorbid opioid use disorder is warranted.
Furthermore, more trials of medications that integrate evidence-based psychosocial and behavioral interventions are necessary to move the field forward. Given the largely disappointing pharmacotherapy results, these interventions (e.g., CM, CBT), alone or in combination, continue to be mainstays of treatment and management of stimulant use disorders.77, 78 A systematic review by Minozzi et al. found that any psychosocial treatment likely reduces dropout rates and may increase the period of abstinence (most of the studies reviewed included CM in addition to treatment as usual).78 The combination of pharmacotherapy with CM is an important area for future research as we do not know how medication may enhance the effectiveness of these interventions.79, 80 When we compared studies with a CM co-intervention to those without, we found that pharmacotherapeutic effects were similar in both.81
Our SR has several limitations. Our scope was broad, and we relied on existing SRs when available. We sought to minimize the disadvantages of using existing SRs by only including those that met key quality criteria; conducting updated searches to identify more recent trials; and combining data in meta-analysis from trials in previous SRs with newer trials from our search. Our definition of abstinence (3 or more weeks) served as a proxy for sustained abstinence, and the effects of treatment on long-term abstinence cannot be directly interpolated. Our search was limited to English language studies; however, the likelihood is low that the exclusion of non-English language studies would alter conclusions.82
CONCLUSIONS
We found no strong or consistent evidence that any drug class was effective in increasing abstinence, reducing use, or improving treatment retention for people with cocaine use disorder. There are several promising classes deserving of further research including psychostimulants, bupropion, topiramate, and treatment of patients with comorbid opioid use disorder.
Electronic Supplementary Material
(DOCX 756 kb)
Acknowledgments
The authors wish to thank Robin Paynter for developing the search strategy and running electronic searches.
Funders
This research was funded by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Quality Enhancement Research Initiative. Dr. Chan’s time was supported by grant number K12HS022981 from the Agency for Healthcare Research and Quality.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Disclaimer
The findings and conclusions in this document are those of the authors who are responsible for its contents; the findings and conclusions do not necessarily represent the views of the Department of Veterans Affairs or the US government.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lipari RN, Van Horn SL. Trends in substance use disorders among adults aged 18 or older. The CBHSQ Report. Rockville: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2017. [PubMed] [Google Scholar]
- 2.Goldstein RA, DesLauriers C, Burda A, Johnson-Arbor K. Cocaine: history, social implications, and toxicity: a review. Semin Diagn Pathol. 2009;26(1):10–7. doi: 10.1053/j.semdp.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Riezzo I, Fiore C, De Carlo D, Pascale N, Neri M, Turillazzi E, et al. Side effects of cocaine abuse: multiorgan toxicity and pathological consequences. Curr Med Chem. 2012;19(33):5624–46. doi: 10.2174/092986712803988893. [DOI] [PubMed] [Google Scholar]
- 4.Pani PP, Trogu E, Vacca R, Amato L, Vecchi S, Davoli M. Disulfiram for the treatment of cocaine dependence. Cochrane Database Syst Rev 2010(1):CD007024. doi: 10.1002/14651858.CD007024.pub2. [DOI] [PubMed]
- 5.Minozzi S, Cinquini M, Amato L, Davoli M, Farrell MF, Pani PP, et al. Anticonvulsants for cocaine dependence. Cochrane Database Syst Rev 2015(4):CD006754. doi: 10.1002/14651858.CD006754.pub4. [DOI] [PMC free article] [PubMed]
- 6.Minozzi S, Amato L, Pani PP, Solimini R, Vecchi S, De Crescenzo F, et al. Dopamine agonists for the treatment of cocaine dependence. Cochrane Database Syst Rev 2015(5):CD003352. doi: 10.1002/14651858.CD003352.pub4. [DOI] [PMC free article] [PubMed]
- 7.Castells X, Cunill R, Perez-Mana C, Vidal X, Capella D. Psychostimulant drugs for cocaine dependence. Cochrane Database Syst Rev 2016;9:CD007380. doi: 10.1002/14651858.CD007380.pub4. [DOI] [PMC free article] [PubMed]
- 8.Indave BI, Minozzi S, Pani PP, Amato L. Antipsychotic medications for cocaine dependence. Cochrane Database Syst Rev 2016;3:CD006306. doi: 10.1002/14651858.CD006306.pub3. [DOI] [PMC free article] [PubMed]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(6):e1000097. doi: 10.1371/journal.pmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011 [cited 2017 March 24]. Available from: http://handbook.cochrane.org/.
- 11.Kiluk BD, Carroll KM, Duhig A, Falk DE, Kampman K, Lai S, et al. Measures of outcome for stimulant trials: ACTTION recommendations and research agenda. Drug Alcohol Depend. 2016;158:1–7. doi: 10.1016/j.drugalcdep.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkman N, Lohr K, Ansari M, McDonagh M, Balk E, Whitlock E, et al. Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. Rockville: Agency for Healthcare Research and Quality; Methods Guide for Comparative Effectiveness Reviews (AHRQ Publication No. 13(14)-EHC130-EF); 2013. [PubMed] [Google Scholar]
- 17.Pani PP, Trogu E, Vecchi S, Amato L. Antidepressants for cocaine dependence and problematic cocaine use. Cochrane Database Syst Rev. 2011(12):CD002950. doi: 10.1002/14651858.CD002950.pub3. [DOI] [PMC free article] [PubMed]
- 18.Raby WN, Rubin EA, Garawi F, Cheng W, Mason E, Sanfilippo L, et al. A randomized, double-blind, placebo-controlled trial of venlafaxine for the treatment of depressed cocaine-dependent patients. Am J Addict. 2014;23(1):68–75. doi: 10.1111/j.1521-0391.2013.12065.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afshar M, Knapp CM, Sarid-Segal O, Devine E, Colaneri LS, Tozier L, et al. The efficacy of mirtazapine in the treatment of cocaine dependence with comorbid depression. Am J Drug Alcohol Abuse. 2012;38(2):181–6. doi: 10.3109/00952990.2011.644002.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancino MJ, McGaugh J, Chopra MP, Guise JB, Cargile C, Williams DK, et al. Clinical efficacy of sertraline alone and augmented with gabapentin in recently abstinent cocaine-dependent patients with depressive symptoms. J Clin Psychopharmacol. 2014;34(2):234–9. doi: 10.1097/JCP.0000000000000062.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveto A, Poling J, Mancino MJ, Williams DK, Thostenson J, Pruzinsky R, et al. Sertraline delays relapse in recently abstinent cocaine-dependent patients with depressive symptoms. Addiction. 2012;107(1):131–41. doi: 10.1111/j.1360-0443.2011.03552.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran LM, Phillips KA, Kowalczyk WJ, Ghitza UE, Agage DA, Epstein DH, et al. Aripiprazole for cocaine abstinence: a randomized-controlled trial with ecological momentary assessment. Behav Pharmacol. 2017;28(1):63–73. doi: 10.1097/FBP.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63(2):219–28. doi: 10.1001/archpsyc.63.2.219.. [DOI] [PubMed] [Google Scholar]
- 24.Bisaga A, Aharonovich E, Cheng WY, Levin FR, Mariani JJ, Raby WN, et al. A placebo-controlled trial of memantine for cocaine dependence with high-value voucher incentives during a pre-randomization lead-in period. Drug Alcohol Depend. 2010;111(1–2):97–104. doi: 10.1016/j.drugalcdep.2010.04.006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh SL, Middleton LS, Wong CJ, Nuzzo PA, Campbell CL, Rush CR, et al. Atomoxetine does not alter cocaine use in cocaine dependent individuals: double blind randomized trial. Drug Alcohol Depend. 2013;130(1–3):150–7. doi: 10.1016/j.drugalcdep.2012.10.024.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winhusen TM, Kropp F, Lindblad R, Douaihy A, Haynes L, Hodgkins C, et al. Multisite, randomized, double-blind, placebo-controlled pilot clinical trial to evaluate the efficacy of buspirone as a relapse-prevention treatment for cocaine dependence. J Clin Psychiatry. 2014;75(7):757–64. doi: 10.4088/JCP.13m08862.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh Y-CM, Kintaudi PC, Charuvastra VC, et al. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003;64(12):1440–8. doi: 10.4088/JCP.v64n1207. [DOI] [PubMed] [Google Scholar]
- 28.Kahn R, Biswas K, Childress A-R, Shoptaw S, Fudala PJ, Gorgon L, et al. Multi-center trial of baclofen for abstinence initiation in severe cocaine-dependent individuals. Drug Alcohol Depend. 2009;103(1–2):59–64. doi: 10.1016/j.drugalcdep.2009.03.011.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldacara L, Cogo-Moreira H, Parreira BL, Diniz TA, Milhomem JJ, Fernandes CC, et al. Efficacy of topiramate in the treatment of crack cocaine dependence: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2016;77(3):398–406. doi: 10.4088/JCP.14m09377. [DOI] [PubMed] [Google Scholar]
- 30.Johnson BA, Ait-Daoud N, Wang X-Q, Penberthy JK, Javors MA, Seneviratne C, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338–46. doi: 10.1001/jamapsychiatry.2013.2295. [DOI] [PubMed] [Google Scholar]
- 31.Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75(3):233–40. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Kampman KM, Pettinati HM, Lynch KG, Spratt K, Wierzbicki MR, O’Brien CP. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133(1):94–9. doi: 10.1016/j.drugalcdep.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nuijten M, Blanken P, van den Brink W, Hendriks V. Treatment of crack-cocaine dependence with topiramate: a randomized controlled feasibility trial in The Netherlands. Drug Alcohol Depend. 2014;138:177–84. doi: 10.1016/j.drugalcdep.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Umbricht A, DeFulio A, Winstanley EL, Tompkins DA, Peirce J, Mintzer MZ, et al. Topiramate for cocaine dependence during methadone maintenance treatment: a randomized controlled trial. Drug Alcohol Depend. 2014;140:92–100. doi: 10.1016/j.drugalcdep.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54(8):713–20. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- 36.Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, Kosten TR. Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. Am J Psychiatry. 2005;162(2):340–9. doi: 10.1176/appi.ajp.162.2.340. [DOI] [PubMed] [Google Scholar]
- 37.Carroll KM, Nich C, Petry NM, Eagan DA, Shi JM, Ball SA. A randomized factorial trial of disulfiram and contingency management to enhance cognitive behavioral therapy for cocaine dependence. Drug Alcohol Depend. 2016;160:135–42. doi: 10.1016/j.drugalcdep.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carroll KM, Nich C, Shi JM, Eagan D, Ball SA. Efficacy of disulfiram and Twelve Step Facilitation in cocaine-dependent individuals maintained on methadone: a randomized placebo-controlled trial. Drug Alcohol Depend. 2012;126(1–2):224–31. doi: 10.1016/j.drugalcdep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, et al. Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine beta-hydroxylase. Biol Psychiatry. 2013;73(3):219–24. doi: 10.1016/j.biopsych.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveto A, Poling J, Mancino MJ, Feldman Z, Cubells JF, Pruzinsky R, et al. Randomized, double blind, placebo-controlled trial of disulfiram for the treatment of cocaine dependence in methadone-stabilized patients. Drug Alcohol Depend. 2011;113(2–3):184–91. doi: 10.1016/j.drugalcdep.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schottenfeld RS, Chawarski MC, Cubells JF, George TP, Lappalainen J, Kosten TR. Randomized clinical trial of disulfiram for cocaine dependence or abuse during buprenorphine treatment. Drug Alcohol Depend. 2014;136:36–42. doi: 10.1016/j.drugalcdep.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Shorter D, Nielsen DA, Huang W, Harding MJ, Hamon SC, Kosten TR. Pharmacogenetic randomized trial for cocaine abuse: disulfiram and alpha1A-adrenoceptor gene variation. Eur Neuropsychopharmacol. 2013;23(11):1401–7. doi: 10.1016/j.euroneuro.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pettinati HM, Kampman KM, Lynch KG, Dundon WD, Mahoney EM, Wierzbicki MR, et al. A pilot trial of injectable, extended-release naltrexone for the treatment of co-occurring cocaine and alcohol dependence. Am J Addict. 2014;23(6):591–7. doi: 10.1111/j.1521-0391.2014.12146.x. [DOI] [PubMed] [Google Scholar]
- 44.Schmitz JM, Lindsay JA, Green CE, Herin DV, Stotts AL, Moeller FG. High-dose naltrexone therapy for cocaine-alcohol dependence. Am J Addict. 2009;18(5):356–62. doi: 10.3109/10550490903077929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitz JM, Stotts AL, Sayre SL, DeLaune KA, Grabowski J. Treatment of cocaine-alcohol dependence with naltrexone and relapse prevention therapy. Am J Addict. 2004;13(4):333–41. doi: 10.1080/10550490490480982. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167–80. doi: 10.1016/S0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 47.Hersh D, Van Kirk JR, Kranzler HR. Naltrexone treatment of comorbid alcohol and cocaine use disorders. Psychopharmacology. 1998;139(1–2):44–52. doi: 10.1007/s002130050688. [DOI] [PubMed] [Google Scholar]
- 48.Pettinati HM, Kampman KM, Lynch KG, Suh JJ, Dackis CA, Oslin DW, et al. Gender differences with high-dose naltrexone in patients with co-occurring cocaine and alcohol dependence. J Subst Abus Treat. 2008;34(4):378–90. doi: 10.1016/j.jsat.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ling W, Hillhouse MP, Saxon AJ, Mooney LJ, Thomas CM, Ang A, et al. Buprenorphine + naloxone plus naltrexone for the treatment of cocaine dependence: the Cocaine Use Reduction with Buprenorphine (CURB) study. Addiction. 2016;111(8):1416–27. doi: 10.1111/add.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kampman KM, Dackis C, Pettinati HM, Lynch KG, Sparkman T, O’Brien CP. A double-blind, placebo-controlled pilot trial of acamprosate for the treatment of cocaine dependence. Addict Behav. 2011;36(3):217–21. doi: 10.1016/j.addbeh.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M. The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: a pilot study. Am J Addict. 2010;19(5):401–8. doi: 10.1111/j.1521-0391.2010.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plebani JG, Lynch KG, Yu Q, Pettinati HM, O’Brien CP, Kampman KM. Results of an initial clinical trial of varenicline for the treatment of cocaine dependence. Drug Alcohol Depend. 2012;121(1–2):163–6. doi: 10.1016/j.drugalcdep.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malcolm R, LaRowe S, Cochran K, Moak D, Herron J, Brady K, et al. A controlled trial of amlodipine for cocaine dependence: a negative report. J Subst Abus Treat. 2005;28(2):197–204. doi: 10.1016/j.jsat.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Sofuoglu M, Poling J, Babuscio T, Gonsai K, Severino K, Nich C, et al. Carvedilol does not reduce cocaine use in methadone-maintained cocaine users. J Subst Abus Treat. 2017;73:63–9. doi: 10.1016/j.jsat.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reid MS, Angrist B, Baker S, Woo C, Schwartz M, Montgomery A, et al. A placebo-controlled screening trial of celecoxib for the treatment of cocaine dependence. Addiction. 2005;100(Suppl 1):32–42. doi: 10.1111/j.1360-0443.2005.00989.x. [DOI] [PubMed] [Google Scholar]
- 56.Licata SC, Penetar DM, Ravichandran C, Rodolico J, Palmer C, Berko J, et al. Effects of daily treatment with citicoline: a double-blind, placebo-controlled study in cocaine-dependent volunteers. J Addict Med. 2011;5(1):57–64. doi: 10.1097/ADM.0b013e3181d80c93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kennedy AP, Gross RE, Whitfield N, Drexler KPG, Kilts CD. A controlled trial of the adjunct use of D-cycloserine to facilitate cognitive behavioral therapy outcomes in a cocaine-dependent population. Addict Behav. 2012;37(8):900–7. doi: 10.1016/j.addbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shoptaw S, Majewska MD, Wilkins J, Twitchell G, Yang X, Ling W. Participants receiving dehydroepiandrosterone during treatment for cocaine dependence show high rates of cocaine use in a placebo-controlled pilot study. Exp Clin Psychopharmacol. 2004;12(2):126–35. doi: 10.1037/1064-1297.12.2.126. [DOI] [PubMed] [Google Scholar]
- 59.Shorter D, Lindsay JA, Kosten TR. The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: A pilot study. Drug Alcohol Depend. 2013;131(1–2):66–70. doi: 10.1016/j.drugalcdep.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sofuoglu M, Carroll KM. Effects of galantamine on cocaine use in chronic cocaine users. Am J Addict. 2011;20(3):302–3. doi: 10.1111/j.1521-0391.2011.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Margolin A, Kantak K, Copenhaver M, Avants SK. A preliminary, controlled investigation of magnesium L-aspartate hydrochloride for illicit cocaine and opiate use in methadone-maintained patients. J Addict Dis. 2003;22(2):49–61. doi: 10.1300/J069v22n02_04. [DOI] [PubMed] [Google Scholar]
- 62.Reid MS, Angrist B, Baker SA, O’Leary S, Stone J, Schwartz M, et al. A placebo controlled, double-blind study of mecamylamine treatment for cocaine dependence in patients enrolled in an opiate replacement program. Subst Abus. 2005;26(2):5–14. doi: 10.1300/J465v26n02_02. [DOI] [PubMed] [Google Scholar]
- 63.Kablinger AS, Lindner MA, Casso S, Hefti F, DeMuth G, Fox BS, et al. Effects of the combination of metyrapone and oxazepam on cocaine craving and cocaine taking: a double-blind, randomized, placebo-controlled pilot study. J Psychopharmacol. 2012;26(7):973–81. doi: 10.1177/0269881111430745. [DOI] [PubMed] [Google Scholar]
- 64.Mariani JJ, Pavlicova M, Bisaga A, Nunes EV, Brooks DJ, Levin FR. Extended-release mixed amphetamine salts and topiramate for cocaine dependence: a randomized controlled trial. Biol Psychiatry. 2012;72(11):950–6. doi: 10.1016/j.biopsych.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.LaRowe SD, Kalivas PW, Nicholas JS, Randall PK, Mardikian PN, Malcolm RJ. A double-blind placebo-controlled trial of N-acetylcysteine in the treatment of cocaine dependence. Am J Addict. 2013;22(5):443–52. doi: 10.1111/j.1521-0391.2013.12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson BA, Roache JD, Ait-Daoud N, Javors MA, Harrison JM, Elkashef A, et al. A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of cocaine dependence. Drug Alcohol Depend. 2006;84(3):256–63. doi: 10.1016/j.drugalcdep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 67.Schmitz JM, Green CE, Hasan KM, Vincent J, Suchting R, Weaver MF, et al. PPAR-gamma agonist pioglitazone modifies craving intensity and brain white matter integrity in patients with primary cocaine use disorder: a double-blind randomized controlled pilot trial. Addiction. 2017;112(10):1861–8. doi: 10.1111/add.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kampman K, Majewska MD, Tourian K, Dackis C, Cornish J, Poole S, et al. A pilot trial of piracetam and ginkgo biloba for the treatment of cocaine dependence. Addict Behav. 2003;28(3):437–48. doi: 10.1016/S0306-4603(02)00226-5. [DOI] [PubMed] [Google Scholar]
- 69.Sofuoglu M, Poling J, Gonzalez G, Gonsai K, Oliveto A, Kosten TR. Progesterone effects on cocaine use in male cocaine users maintained on methadone: a randomized, double-blind, pilot study. Exp Clin Psychopharmacol. 2007;15(5):453–60. doi: 10.1037/1064-1297.15.5.453. [DOI] [PubMed] [Google Scholar]
- 70.Kampman KM, Volpicelli JR, Mulvaney F, Alterman AI, Cornish J, Gariti P, et al. Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend. 2001;63(1):69–78. doi: 10.1016/S0376-8716(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 71.Gilgun-Sherki Y, Eliaz RE, McCann DJ, Loupe PS, Eyal E, Blatt K, et al. Placebo-controlled evaluation of a bioengineered, cocaine-metabolizing fusion protein, TV-1380 (AlbuBChE), in the treatment of cocaine dependence. Drug Alcohol Depend. 2016;166:13–20. doi: 10.1016/j.drugalcdep.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 72.Castells X, Kosten TR, Capella D, Vidal X, Colom J, Casas M. Efficacy of opiate maintenance therapy and adjunctive interventions for opioid dependence with comorbid cocaine use disorders: A systematic review and meta-analysis of controlled clinical trials. Am J Drug Alcohol Abuse. 2009;35(5):339–49. doi: 10.1080/00952990903108215. [DOI] [PubMed] [Google Scholar]
- 73.Ahmad FB, Rossen LM, Spencer MR, Warner M, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. 2018. Designed by LM Rossen, A Lipphardt, FB Ahmad, JM Keralis, and Y Chong: National Center for Health Statistics. Available from https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm.
- 74.Singh M, Keer D, Klimas J, Wood E, Werb D. Topiramate for cocaine dependence: a systematic review and meta-analysis of randomized controlled trials. Addiction. 2016;111(8):1337–46. doi: 10.1111/add.13328. [DOI] [PubMed] [Google Scholar]
- 75.McCall Jones C, Baldwin GT, Compton WM. Recent Increases in Cocaine-Related Overdose Deaths and the Role of Opioids. Am J Public Health. 2017;107(3):430–2. doi: 10.2105/ajph.2016.303627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leeman RF, Sun Q, Bogart D, Beseler CL, Sofuoglu M. Comparisons of Cocaine-Only, Opioid-Only, and Users of Both Substances in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Subst Use Misuse. 2016;51(5):553–64. doi: 10.3109/10826084.2015.1122063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phillips KA, Epstein DH, Preston KL. Psychostimulant addiction treatment. Neuropharmacology. 2014;87:150–60. doi: 10.1016/j.neuropharm.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minozzi S, Saulle R, De Crescenzo F, Amato L. Psychosocial interventions for psychostimulant misuse. Cochrane Database Syst Rev. 2016;9:CD011866. doi: 10.1002/14651858.CD011866.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klimas J, Wood E, Werb D. How can we investigate the role of topiramate in the treatment of cocaine use disorder more thoroughly? Addiction. 2017;112(1):182–3. doi: 10.1111/add.13618.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Darke S, Farrell M. Commentary on Singh et al. (2016): Still searching for the answer. Addiction. 2016;111(8):1347. doi: 10.1111/add.13369.. [DOI] [PubMed] [Google Scholar]
- 81.Chan B, Kondo K, Ayers C, Freeman M, Montgomery J, Paynter R, et al. Pharmacotherapy for Stimulant Use Disorders: A Systematic Review of the Evidence. VA ESP Project 05–225; 2018. [PubMed]
- 82.Moher D, Pham B, Lawson ML, Klassen TP. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technol Assess. 2003;7(41):1–90. doi: 10.3310/hta7410. [DOI] [PubMed] [Google Scholar]
- 83.Brodie JD, Case BG, Figueroa E, Dewey SL, Robinson JA, Wanderling JA, et al. Randomized, double-blind, placebo-controlled trial of vigabatrin for the treatment of cocaine dependence in Mexican parolees. Am J Psychiatry. 2009;166(11):1269–77. doi: 10.1176/appi.ajp.2009.08121811. [DOI] [PubMed] [Google Scholar]
- 84.Somoza EC, Winship D, Gorodetzky CW, Lewis D, Ciraulo DA, Galloway GP, et al. A multisite, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of vigabatrin for treating cocaine dependence. JAMA Psychiatry. 2013;70(6):630–7. doi: 10.1001/jamapsychiatry.2013.872. [DOI] [PubMed] [Google Scholar]
- 85.George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biol Psychiatry. 2000;47(12):1080–6. doi: 10.1016/S0006-3223(99)00310-8. [DOI] [PubMed] [Google Scholar]
- 86.Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, et al. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95(2):219–28. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 756 kb)