Abstract
Objective
Accumulating evidence indicates that regulatory T cells (Tregs) may be involved in the pathogenesis of ankylosing spondylitis (AS). As different markers have been used to identify Tregs, some studies on the proportions of Tregs in AS patients have generated considerable controversy. To clarify the status of Tregs in such patients, we determine the proportion changes of peripheral Tregs during development of the disease, with different cellular markers.
Methods
We systematically searched Embase, PubMed, Cochrane, Web of Knowledge, FDA.gov, and Clinical Trials.gov for the studies reporting the proportion of Tregs in AS patients. Using the PRISMA guidelines, we performed a random-effects meta-analysis of the frequencies of peripheral Tregs defined in different ways. Inconsistency was evaluated using the I-squared index (I2), and publication bias was assessed by examining funnel plot asymmetry using the Begger and Egger tests.
Results
A total 29 studies involving 1732 participants were included in the meta-analysis. Their conclusions of using the diversity of Tregs surface markers were inconsistent with each other. No significant difference in the proportions of Tregs was evident regardless of the definitions used [−0.709, (−1.455, 0.037, p = 0.063), I2 = 97.3%]. Six studies used “single CD25-positive” cells as Tregs, which revealed a significant increase in AS patients compared with healthy blood donors [0.736, (0.138, 1.334), p = 0.016, I2 = 80.7%]. Notably, the proportions of “CD4+CD25+FOXP3+,” “CD4+CD25highCD127low/−,” or “CD4+CD25+CD127low” T cells were lower in AS patients [−2.856, (−4.645, −1.066), p = 0.002; −1.812, (−2.648, −0.977), p < 0.001; −1.12, (−1.605, −0.635), p < 0.001]. Tregs defined as “CD25high,” “CD25bright,” “CD25bright/highCD127low/−,” “CD4+FOXP3+,” “CD4+CD25highFOXP3+,” and “CD4+CD25+CD127−” did not differ in proportion between AS patients and healthy blood donors.
Conclusions
The levels of Tregs varied based on the cellular identification markers used. The proportions of CD4+CD25+FOXP3+Tregs, CD4+CD25highCD127low/−, or CD4+CD25+CD127low in blood of AS patients were significantly decreased as compared with those in healthy blood donors, and our findings lend support to the idea that the Treg status of AS patients is important. And we recommend the above as the best definition of Tregs when evaluating the status of such patients.
1. Introduction
Ankylosing spondylitis (AS) is a common inflammatory rheumatic disease that affects the axial skeleton, causing characteristic inflammatory back pain, asymmetrical peripheral oligoarthritis, enthesitis, and specific organ involvement such as anterior uveitis, psoriasis, and chronic inflammatory bowel disease, which can lead to structural and functional impairments and a decrease in quality of life [1]. To date, the disease etiology remains unclear. Reduced proportion and deficient function of CD4+ regulatory T cells (Tregs, with immune modulation and suppression) have been implicated in the pathogenesis of different immune-mediated rheumatic diseases [2–4]. In the case of AS, few studies have been carried out to analyze the levels of Tregs in the peripheral blood of patients; however, low percentages [5–8] or functional impairment of Tregs [9, 10] has been reported in the peripheral blood (PB) of patients with AS, suggesting an imbalance between Tregs and the adaptive immune response. Moreover, AS patients treated with anti-TNF therapy showed similar levels of Treg cells to those observed in healthy subjects [11]. These data suggest a possible role of Tregs in AS.
However, initial studies of Treg status in PB of patients with AS are controversial. One reason for the inconsistencies may be the multiple phenotypes of Tregs, which have been identified using different markers [12]. Tregs were first described as a peripheral CD4+ subset expressing interleukin- (IL-) 2 receptor alpha chains (CD25) [13]. As early as 2004, Cao et al. [14] used CD4+CD25bright to identify peripheral Tregs in peripheral blood of AS patients. However, CD25 was expressed not only on Tregs but also on activated cells lacking regulatory functions, although the CD4+ T cell subset expressed the highest levels of CD25 (CD4+, CD25high) and exhibited in vitro immunosuppressive features [15]. Forkhead box protein P3 (FOXP3), a transcription factor expressed at high levels in authentic Tregs, plays a key role in Treg development and is thought to be one of the most specific Treg cell markers [16]. Since 2008, scholars have been studying the proportion and function of peripheral FOXP3+Tregs of AS patients [9, 17]. However, the marker cannot be used to sort live cells, as the protein is intracellular. In addition, CD127, the alpha chain of the IL-7 receptor, was reported to be upregulated on human T cells after activation and downregulated on Tregs [18]. Thus, costaining for CD127 and CD25 has been proposed to efficiently discriminate between Tregs and activated T cells [19]. The study of CD4+CD25+CD127−Tregs in AS patients began in 2011. Zhao et al. used CD25+CD127− to define peripheral Tregs in new-onset AS patients firstly [6]. Furthermore, CD8+CD122+ T cell is a newly discovered natural immune regulatory T cells with immune negative regulation function [20], which may be involved in the pathogenesis and disease progression of AS [21]. The available data on the proportions and phenotypes of Tregs of AS patients are contradictory; some studies using the same or different markers to analyze peripheral Tregs of AS patients have obtained different or even opposite results [22–25].
To better understand Treg malfunctions in patients with AS, we meta-analyzed reports documenting the proportion of peripheral Treg cells among CD4+ T cells in the PB of patients with AS, as well as healthy blood donors in this study.
2. Methods
2.1. Data Sources and Searches
This meta-analysis was consistent with that of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement, and it had been registered at the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42019120790). We searched for relevant studies published between January 1, 1950, and November 1, 2018, using PubMed, Embase, Cochrane, Web of Knowledge, Clinical Trials.gov, and FDA.gov, with no restrictions in terms of the primary outcome or publication language. We used the MeSH terms “Spondylitis, Ankylosing” and “T−lymphocytes, regulatory” and their combination. All potentially eligible studies were considered except for reviews and murine experiments. Key articles listed in the references were retrieved manually.
2.2. Study Selection and Data Extraction
The inclusion criteria were evaluation of the proportion of Tregs among CD4+ T cells of AS patients using the 1984 Modified New York AS Criteria [26], available as a full text article, and information on the number of patients and healthy blood donors. Two investigators independently selected and identified relevant publications, and a third investigator resolved any disagreements. The evidence levels of the studies were assessed based on the 2011 guidelines of the Oxford Centre for Evidence-Based Medicine. Quality assessment was done with the Newcastle-Ottawa Quality Assessment Scale, which can be used to assess the quality of nonrandomized studies.
We recorded patient baseline characteristics and their country of origin, the year of publication, the number of patients and healthy blood donors, the definition of Tregs used (including CD4+CD25+, CD4+CD25bright, CD4+CD25high, CD25low/−FOXP3+, FOXP3+, CD25+FOXP3+, CD25highFOXP3+, CD25+CD127−, CD25bright/highCD127low/−, and CD25highCD127low/−FOXP3+), and the mean (or median) and standard deviation (SD) of the proportion of Tregs among CD4+ T cells. Data on the proportion of Tregs in patients with HLA-B27(+) and HLA-B27(−) were also extracted.
2.3. Statistical Analysis
For continuous outcomes (the proportions of Tregs among CD4+/CD8+ T cells of patients with AS and healthy blood donors), we calculated standardized mean differences (SMDs) and compared these values by using a random-effects model (REM) (the DerSimonian and Laird method) [27]. When Treg percentages were reported as medians with interquartile ranges (IQRs), we calculated means and SD (SD = IQR/1.35). The Cochrane chi-squared test was used to explore between-study heterogeneity. As heterogeneity was high (I2 > 75%), we drew forest plots and performed subgroup analyses to explore the possible effects of study characteristics on outcomes. Publication bias was assessed by examining funnel plot asymmetry using the Begger and Egger tests (p ≥ 0.05). A preplanned sensitivity analysis was performed by omitting each study individually and calculating the remaining pooled effect. All statistical analyses were conducted using Stata software (ver. 12.0).
3. Results
3.1. Study Characteristics
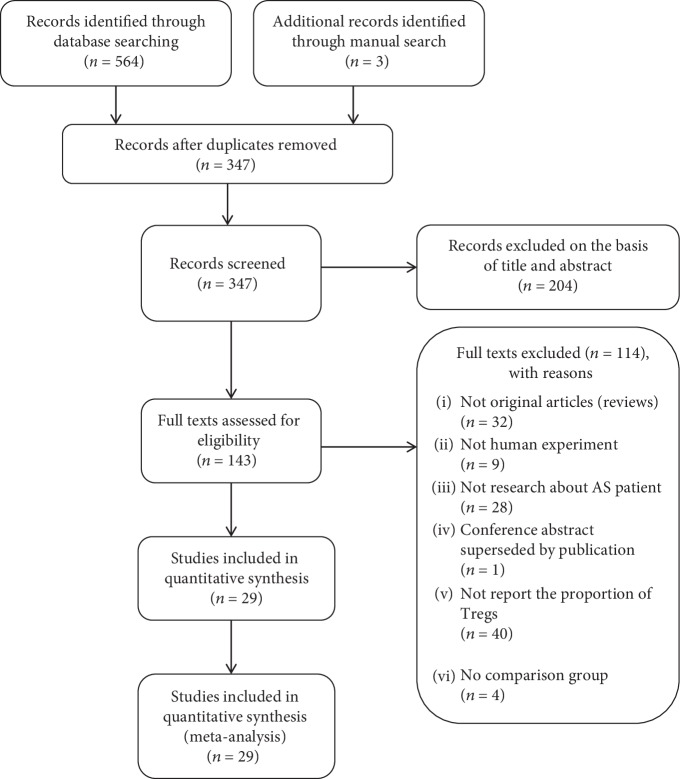
We identified 564 studies. And 29 of them (with data on 980 patients and 752 healthy blood donors) were included in the analysis (Figure 1), and all of them used a reliable flow cytometric analysis to detect the proportions of peripheral Tregs. The details are shown in Table 1. The average age of the AS patients was between 24.8 and 52.13 years, the proportion of males ranged from 0 to 100%, the average disease duration was from 1.6 to 13.3 years, the average erythrocyte sedimentation rate (ESR) was from 15.2 to 57.3 mm/hour, the average C-reactive protein (CRP) was from 6.63 to 77.1 mg/l, and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) [28] from was 1.19 to 51.94. Patients were treated with glucocorticoids, NSAIDs, DMARDs, immunosuppressants including cyclophosphamide (CTX) and cyclosporine, and biological agents. All healthy blood donors were age and sex matched, healthy, and without any autoimmune disease. All studies were poor-quality case-control studies or case series; thus, they were all of evidence level 4. We regarded all studies as case-control studies and scored them using the Newcastle-Ottawa Quality Assessment Scale (NOQAS); all studies had a score of 3–5.
Figure 1.
The study selection process.
Table 1.
Characteristics of the individual studies included in the meta-analysis.
| Author (ref.) | Publish year | Country | ELa | Q b | Case numbers | Tregs' definition | % of Tregs among CD4+ T cells [mean (or median) ± SD] | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AS | HC | AS | HD | p | ||||||
| Duojia Cao et al. [14] | 2004 | Sweden | 4 | 6 | 10 | 29 | CD25brightCD4+ | 1.31 ± 0.68 | 1.23 ± 0.64 | ns |
| Jau-Ling Suen et al. [17] | 2008 | Taiwan, China | 4 | 6 | 23 | 36 | CD4+CD25highFOXP3+ | 0.97 ± 0.33 | 0.86 ± 0.39 | ns |
| Éric Toussirot et al. [32] | 2009 | France | 4 | 6 | 32 | 15 | CD4+CD25+FOXP3+ | 8.2 ± 0.61 | 7.94 ± 1.04 | ns |
| Frauke Forger et al. [29] | 2009 | Swiss | 4 | 7 | 15 | 18 | CD4+CD25high | 2.22 ± 1.47 | 2.12 ± 1.42 | <0.01 |
| Francesco Ciccia et al. [22] | 2010 | Italy | 4 | 8 | 18 | 15 | CD4+CD25high | 1.08 ± 0.4 | 0.25 ± 0.12 | <0.05 |
| Christian Dejaco et al. [23] | 2010 | Austria | 4 | 5 | 22 | 17 | CD4+CD25high | 13.54 ± 16.55 | 3.08 ± 2.48 | ns |
| Heiner Appel et al. [33] | 2011 | Germany | 4 | 6 | 19 | 20 | CD4+FOXP3+ | 5.55 ± 2.54 | 5.18 ± 1.99 | ns |
| Ming-Han Chen et al. [30] | 2011 | Taiwan, China | 4 | 7 | 23 | 25 | CD4+CD25high+ | 2.18 ± 0.11 | 2.16 ± 0.1 | ns |
| Yanfeng Wu et al. [5] | 2011 | China | 4 | 8 | 51 | 49 | CD4+CD25+FOXP3+ | 1.23 ± 0.13 | 2.56 ± 0.16 | <0.001 |
| S-S Zhao et al. [6] | 2011 | China | 4 | 8 | 14 | 18 | CD4+CD25highCD127low/− | 0.57 ± 0.29 | 1.65 ± 0.75 | <0.001 |
| Katayoon Bidad et al. [46] | 2012 | Iran | 4 | 7 | 18 | 18 | CD4+FOXP3+ RORγt−Tbet− | 9.7 ± 1.2 | 16.1 ± 3 | 0.048 |
| Leonardo Limon-Camacho et al. [11] | 2012 | Mexico | 4 | 5 | 39 | 25 | CD4+FOXP3+ | 7.3 ± 1.3 | 5.3 ± 1.7 | 0.01 |
| Yong Gao et al. [34] | 2012 | China | 4 | 8 | 40 | 37 | CD4+CD25+FOXP3+ | 3.77 ± 0.81 | 4.69 ± 1.23 | <0.05 |
| Li Xueyi et al. [7] | 2013 | China | 4 | 6 | 222 | 68 | CD4+CD25+FOXP3+ | 2.14 ± 0.44 | 4.99 ± 0.49 | <0.001 |
| Lingying Ye et al. [62] | 2013 | China | 4 | 6 | 21 | 22 | CD4+CD45RO+FOXP3high | 0.48 ± 0.07 | 0.73 ± 0.07 | <0.05 |
| Wei Ji et al. [39] | 2014 | China | 4 | 7 | 20 | 20 | CD4+CD25+CD127low | 40.1 ± 17.5 | 58.6 ± 10.2 | <0.05 |
| Zhang Xin et al. [8] | 2014 | China | 4 | 5 | 10 | 10 | CD4+CD25+FOXP3+ | 2.72 ± 0.26 | 5.17 ± 0.31 | <0.001 |
| Hsien-Tzung Liao et al. [31] | 2015 | Taiwan, China | 4 | 8 | 69 | 30 | CD4+CD25+FOXP3+ | 1.73 ± 1.08 | 1.51 ± 0.48 | <0.001 |
| Yuxing Shan et al. [24] | 2015 | China | 4 | 6 | 20 | 10 | CD4+FOXP3+CXCR5+ | 5.57 ± 1.28 | 3.08 ± 0.59 | <0.0001 |
| Chenggong Wang et al. [35] | 2015 | China | 4 | 6 | 45 | 20 | CD4+CD25+FOXP3+ | 1.81 ± 0.81 | 1.23 ± 0.52 | ns |
| Elliott TJ Dunn et al. [36] | 2016 | New Zealand | 4 | 7 | 6 | 10 | CD4+FOXP3+CD25high | 1.43 ± 0.37 | 0.43 ± 0.15 | ns |
| Huifang Guo et al. [9] | 2016 | China | 4 | 8 | 39 | 17 | CD4+CD25highFOXP3+ | 5.62 ± 0.4 | 5.89 ± 0.2 | ns |
| Zhongliang Duan et al. [40] | 2017 | China | 4 | 7 | 21 | 16 | CD4+CD25+CD127low | 2.7 ± 0.8 | 3.47 ± 0.83 | 0.03 |
| Zofia Gula et al. [37] | 2017 | Poland | 4 | 7 | 48 | 23 | CD4+FOXP3+ | 28.83 ± 11.71 | 34.39 ± 20.65 | ns |
| Dan Xu et al. [25] | 2017 | China | 4 | 7 | 17 | 93 | CD4+CD25+FOXP3+ | 22.58 ± 12.8 | 35.57 ± 6.48 | <0.01 |
| Mingfei Wang et al. [10] | 2018 | China | 4 | 7 | 26 | 26 | CD4+CD25+ FOXP3+CD127− | 6.32 ± 1.5 | 5.44 ± 1.02 | <0.05 |
| Mohammad Javad Fattahietal [38]. | 2018 | Iran | 4 | 7 | 30 | 15 | CD4+CD25+FOXP3+ | 2.7 ± 0.23 | 3.3 ± 0.47 | 0.45 |
| Renfang Han et al. [21] | 2018 | China | 4 | 6 | 40 | 40 | CD8+CD122+ | 10.72 ± 6.32 | 1.21 ± 0.82 | <0.05 |
| Sonja Dulic et al. [41] | 2018 | Hungary | 4 | 8 | 22 | 10 | CD4+CD25+CD127– | 5.708 ± 2.05 | 5.715 ± 0.79 | ns |
AS: ankylosing spondylitis; HD: healthy donors. aEvidence level (EL) of each study was based on Oxford Centre for Evidence-Based Medicine 2011. bQuality (Q) of each study was based on the Newcastle-Ottawa Quality.
3.2. The Proportion of Peripheral Tregs of AS Patients
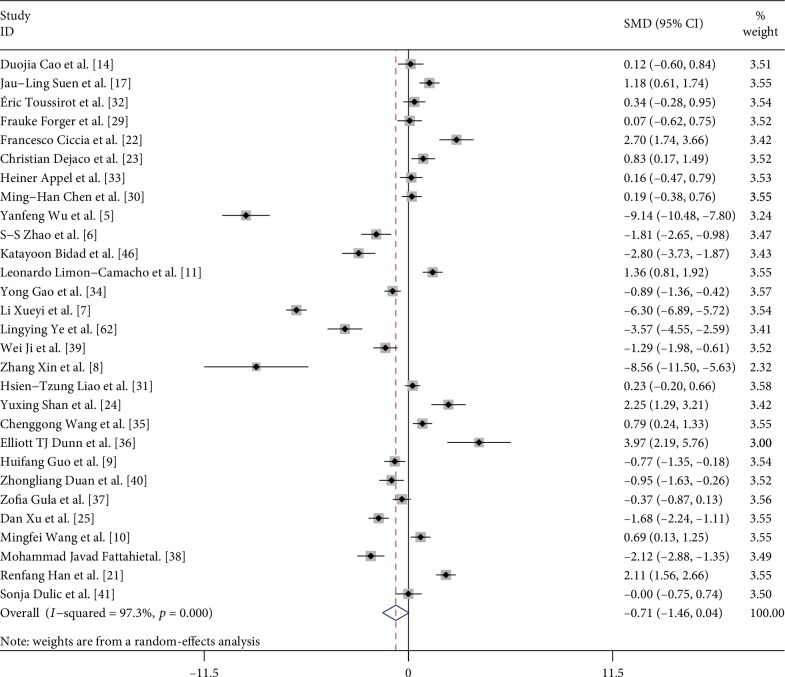
First, we performed a meta-analysis of the proportion of Tregs between AS patients and control subjects in all studies, neglecting the definition methods of Tregs (Figure 2). Unexpectedly, there was no significant difference in the proportion of Tregs in PB between AS patients and healthy blood donors in all studies [−0.709, (−1.455, 0.037, p = 0.063)]. Besides, there was statistically significant heterogeneity between studies (I2 = 97.3%). In this analysis, there was no publication bias on Egger test (p = 0.227).
Figure 2.
Forest plot of the overall meta-analysis of regulatory T cell (Treg) proportions in peripheral blood (PB), regardless of the Treg definitions used, between ankylosing spondylitis (AS) patients and healthy blood donors (HD).
We hypothesized that the cause of unexpected results may be the different definition methods of Tregs. Thus, we performed a subgroup analysis based on the Treg definitions to explore the potential sources of heterogeneity. First, we analyzed studies that identified Tregs only as “CD25-positive” in CD4+ T cell subpopulations. A pooled analysis of all 6 trials [14, 22, 23, 29–31] revealed a significant increase in the proportion of Tregs in AS patients compared with healthy blood donors [0.736, (0.138, 1.334), p = 0.016] with statistically significant between-study heterogeneity (I2 = 80.7%, p < 0.001) and no significant between-study publication bias detected by the Egger test (t = 0.72, p = 0.513). In detail, we found a significant increase in the proportion of Tregs between AS patients and healthy blood donors when Tregs were defined as “CD4+CD25+” cells [0.846, (0.401, 1.291), p < 0.001] [31]. However, the proportion of Tregs defined as “CD4+CD25high” cells [0.892, (−0.078, 1.862), p = 0.071] [22, 23, 29, 30] and as “CD4+CD25bright” cells [0.123, (−0.596, 0.842), p = 0.737] [14] did not differ significantly between patients and healthy blood donors (Table 2).
Table 2.
Subgroup analysis based on different definitions of Tregs in PB of patients with AS.
| Definition of Tregs | Number of studies | Test of association | Test of heterogeneity | Egger's test | ||||
|---|---|---|---|---|---|---|---|---|
| SMD | 95% CI | p value | I 2 | p value | t | p value | ||
| Single CD25-positive | 6 | 0.736 | (0.138, 1.334) | 0.016 | 80.7% | <0.001 | 0.72 | 0.513 |
| CD4+CD25+ | 1 | 0.846 | (0.401, 1.291) | <0.001 | – | – | – | – |
| CD4+CD25high | 4 | 0.892 | (−0.078, 1.862) | 0.071 | 87% | <0.001 | 2.74 | 0.112 |
| CD4+CD25bright | 1 | 0.123 | (−0.596, 0.842) | 0.737 | – | – | – | – |
| Associated with FOXP3-positive | 18 | −1.004 | (−1.966, −0.042) | 0.041 | 97.9% | <0.001 | 0.97 | 0.795 |
| CD4+FOXP3+ | 3 | 0.383 | (−0.663, 1.429) | 0.473 | 90.4% | <0.001 | −11.62 | 0.143 |
| CD4+CD25+FOXP3+ | 9 | −2.856 | (−4.645, −1.066) | 0.002 | 98.6% | <0.001 | 6.06 | 0.42 |
| CD4+CD25highFOXP3+ | 3 | 0.868 | (−0.756, 2.492) | 0.295 | 92.6% | <0.001 | 2.91 | 0.862 |
| CD4+CD25low/−FOPX3+ | 3 | 0.683 | (0.161, 1.206) | 0.01 | 68.4% | 0.042 | 9.58 | 0.783 |
| Associated with CD127-negative | 4 | −1.003 | (−1.713, −0.294) | 0.006 | 73.1% | 0.011 | −0.37 | 0.747 |
| CD4+CD25highCD127low/− | 1 | −1.812 | (−2.648, −0.977) | <0.001 | – | – | – | – |
| CD4+CD25+CD127low | 2 | −1.12 | (−1.605, −0.635) | <0.001 | 0.0% | 0.486 | – | – |
| CD4+CD25+CD127− | 1 | −0.004 | (−0.751, 0.744) | 0.992 | – | – | – | – |
PB: peripheral blood; AS: ankylosing spondylitis; SMD: standard mean difference; CI: confidence interval; I2: I-squared index. Magnitude of Cohen's d effect size (SMD): 0.2–0.5, small effect; 0.5–0.8, medium effect; and ≥0.8, large effect.
Then, we analyzed studies in which Tregs were defined as “FOXP3+” cells. A pooled analysis of all 18 trials [5, 7, 8, 11, 17, 25, 31–38] revealed a significant decrease in the proportion of such Tregs between AS patients and healthy blood donors [−1.004, (−1.966, −0.042), p = 0.041]. Statistically significant heterogeneity was evident among the studies (I2 = 97.9%, p < 0.001). The Egger test detected no publication bias (t = 0.97, p = 0.795). Among the studies, 9 [5, 7, 8, 25, 31, 32, 34, 35, 38] used “CD4+CD25+FOXP3+” to define Tregs, which showed that the proportion of Tregs in AS patients appeared to be lower than in healthy blood donors [−2.856, (−4.645, −1.066), p = 0.002]. However, pooling of these data with those of other studies [17, 31] identifying Tregs as “CD4+CD25low/−FOPX3+” cells revealed a higher proportion of Tregs in patients than in healthy blood donors [0.683, (0.161, 1.206), p = 0.01]. Tregs were identified as simply “FOXP3+” cells [11, 33, 37]; and “CD25highFOXP3+” cells [9, 17, 36] [0.383, (−0.663, 1.429), p = 0.473; 0.868, (−0.756, 2.492), p = 0.295] were not shown to be significantly different between patients and healthy blood donors (Table 2).
Finally, the other four groups [6, 39–41] that used “CD127-negative” in CD4+ T cell subgroups to define Tregs showed that such cell numbers decreased in AS patients [−1.003, (−1.713, −0.294), p = 0.006] with statistical heterogeneity (I2 = 73.1%, p = 0.011) and no publication bias (t = −0.37, p = 0.747). More specifically, pooling the data of studies in which Tregs were identified as “CD4+CD25highCD127low/−” cells [6] and “CD4+CD25+CD127low” cells [39, 40] revealed a significant decrease between AS patients and healthy blood donors [−1.812, (−2.648, −0.977), p < 0.001; −1.12, (−1.605, −0.635), p < 0.001], but no significant difference was observed when Tregs were defined as “CD4+CD25+CD127−” cells [−0.004, (−0.751, 0.744), p = 0.992] [41] (Table 2).
Due to the heterogeneity in the meta-analysis, the random-effects model was applied in preparing forest plots. We hypothesized that the significant heterogeneity might have been caused by differences in the experimental methods, and clinical type and severity of disease among the different studies.
3.3. Disease Activity and the Proportion of Tregs in PB
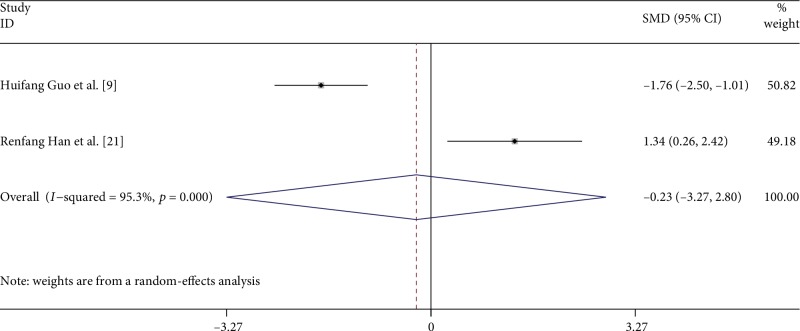
To further assess the effect of disease activity, we analyzed 2 studies [9, 21] that reported the proportion of Tregs in active and stable AS patients regardless of the Tregs definitions used (Figure 3). All of these 2 studies used the Ankylosing Spondylitis Disease Activity Score (ASDAS) [42–44] to evaluate the disease activity. Guo H. et al. [9] found no significant differences in the percentages of Tregs among patients with active AS and patients with stable AS, but Han R. et al. [21] showed a significant increase. We found no difference in the proportion of Tregs in patients with active compared with stable AS [−0.234, (−3.267, 2.799), p = 0.880]. The heterogeneity, as assessed by the I2 statistic, was 95.3% (p < 0.0001).
Figure 3.
Forest plot of the overall meta-analysis of regulatory T cell (Treg) proportions in peripheral blood (PB), regardless of the Treg definition used, in patients with active and stable AS.
4. Discussion
It is now widely accepted that Treg cells play a key role in the maintenance of immune tolerance and homeostasis [3, 45]. However, the role of Tregs in peripheral immune tolerance in patients with AS has not been fully elucidated in previous studies [7, 21, 31]. During the process, the markers used in the identification of Tregs are inconsistent by flow cytometry in previous studies; therefore, the proportion of peripheral Treg of AS patients has always been reported controversially [5, 24, 29, 46]. Our overall meta-analysis found no significant difference in Treg proportions between patients and healthy blood donors, although significant between-study heterogeneity was evident. We considered that the primary reasons for such unexpected results were due to inconsistent definitions of Tregs based on diverse markers used; thus, we subanalyzed the Treg data by the markers used for Treg identification, including CD25, FOXP3, and CD127.
Currently, researches on Tregs mainly focus on CD4+Tregs. Expression of CD25 (α chain of family IL-2R) correlates positively with Treg functionality [47]. The Treg-suppressive capacity is restricted to the CD4+ T cells that express the highest levels of CD25 [48]. We found out that AS patients had a higher proportion of Tregs termed “single CD25-positive” than had healthy blood donors. However, when Tregs were defined as “CD4+CD25high” or “CD4+CD25bright,” no significant differences were found between AS patients and healthy blood donors. And other activated CD4+ T cells also express CD25 [45, 48], indicating that use of the surface marker CD25 alone is inadequate. In 2008, Han G. et al. [49] found out that CD25high cells that included a large proportion of FOXP3− cells could not be classified as Tregs. The expressions of the transcription factor FOXP3 or other markers are considered more specific for the identification of Tregs than CD25 [50].
FOXP3 is a pivotal regulator of Treg fictional gene expression, being required for both Treg generation and survival [51]. The mutations of the FOXP3 gene disturb the function of Tregs, therefore resulting in the development of various autoimmune diseases [52]. Decreased FOXP3 expression causes an immune disease by subverting the suppressive function of Treg cells and converting Treg cells into effector cells [53]. However, when Tregs were defined as “FOXP3-positive” cells, the proportions of such cells did not differ between AS patients and healthy blood donors because the definitions of Tregs were complicated by the addition of CD25 status, giving “CD25-negative and FOXP3-positive” and “CD25 and FOXP3 double positive.” We also found that AS patients had a higher proportion of Tregs termed “CD4+CD25low/−FOXP3+” than had healthy blood donors. This phenomenon may be explained by the findings that the CD4+CD25low/−FOPX3+ cells were dysfunctional Tregs [54, 55] and may even be previously activated conventional T cells [56].
However, the detection of FOXP3 requires cell permeabilization, thereby preventing isolation of viable Tregs. Subsequently, the extracellular marker CD127 was established for the identification of Tregs [57–59]. Some scholars believe that CD4+CD25+CD127low/− is the best surface marker of natural Tregs and alive Tregs, which not only can avoid interference of other activated T cells, but can also be used to conduct preliminary functional studies [19]. We found that the ratio of “CD127-negative” in peripheral blood of patients with AS was significantly lower than that of the control group, further suggesting that CD127 combined with other markers could indeed be used to label Tregs.
CD8+Tregs are similar to CD4+Treg and also have immunomodulatory effects. However, due to the lack of specific surface markers, few studies have been conducted on CD8+Treg [60, 61]. In 2015, Churland G. et al. [61] have found that the proportion of CD8+Tregs in the peripheral blood of healthy people is less than one-tenth of that of CD4+Tregs, which makes the study of CD8+Tregs more difficult. In this study, we found that only one study [21] reported the expression of CD8+Treg in peripheral blood of patients with AS, and a comprehensive analysis showed that there was a higher proportion of CD8+Treg in AS group than in the healthy control group. The specific marker, expression, and function of CD8+Treg need further study.
Some studies have used other markers to indicate different subsets of Tregs [24, 46, 62]. Human FOXP3+ cells have been subdivided into three functionally and phenotypically distinct subsets [63]: naïve Tregs (FOXP3+CD45RA+), short-lived and highly suppressive activated Tregs (FOXP3highCD45RA−), and non-Tregs (FOXP3low CD45RA−). Although human naturally occurring Tregs may express either CD45RA or CD45RO, the majority of natural Tregs in adults are CD45RO+, which increases significantly with age [64, 65]. Ye L. et al. [62] found that in AS patients, the frequencies of effector Tregs (CD4+FOXP3highCD45RO+) and naïve Tregs (CD4+FOXP3lowCD45RO−) were decreased. T-bet is an immune cell-specific member of the T-box family of transcription factors, which is required for the functional fitness of pTregs (also known as induced or adaptive Tregs [66–68]). Only Bidad K. [46] observed that FOXP3+CD4+RORγt−Tbet− Tregs in AS patients were significantly lower than in healthy blood donors. A specialized subset of Tregs that are characterized by a high expression level of CXC chemokine receptor 5 (CXCR5), T follicular regulatory (Tfr) cells are important for the control of humoral immune responses [69, 70]. To date, it is still challenging to value the real status of above Treg subsets in patients with AS.
Further, the controversial status of Tregs in PB of AS patients might also be related to the different disease status, such as different treatment, disease activity, or markers of inflammation. It appears that the effects of corticosteroids (CS) on Treg numbers in patients with autoimmune diseases are disease-specific [71]. Treg cell numbers increased in CS-treated patients with SLE [72, 73] but decreased in CS-treated patients with psoriasis [74] and was not clearly defined in multiple sclerosis patients [75, 76]. Some studies found that disease-modifying antirheumatic drugs (DMARDs) can normalize the distribution of Tregs in RA patients [77–79]. Long-term anti-TNF therapy may increase Tregs in AS and other autoimmune diseases [41, 62, 80]. However, studies about CS and DMARDs on peripheral blood Tregs in AS patients are still lacking. In addition to drugs, disease activity also affects the proportion of peripheral blood Tregs [81, 82]. But our subgroup analysis found no difference in the proportion of Tregs in patients with active compared with stable AS. However, this conclusion needs to be confirmed by more studies on the proportion of Tregs and the activity of AS. One study also observed that the highest correlation coefficient was between CD4+CD25+FoxP3+Tregs and CRP or ESR [31]. But the true relationship between Tregs and inflammatory markers needs further studies.
Our meta-analysis had several limitations. Firstly, severity of the disease and clinical subtypes in AS patients were not consistent across studies. Moreover, although we did a subgroup analysis of disease activity, the results are questionable due to the small number of studies included. Second, we did not consider disease duration or treatment, as both the drugs used and disease staging were inconsistent; however, these factors might affect the proportion of Tregs in PB. Thirdly, there were differences in experimental methods between studies. A flow cytometric expert must run through all experiments: Some of the flow cytometric assays in the papers used here might even be disqualified. Meanwhile, the definition of Tregs in some studies also included CD127low or CD25high rather than completely the same definition makers. Moreover, Tregs are usually evaluated in PB, in which tissue Treg status may fluctuate.
5. Conclusion
Our study suggests that the reported variations of Treg status among AS patients are due to using inconsistent definitions or markers for Tregs. We found the best definition of Tregs as CD4+CD25+FOXP3+ or CD4+CD25high/+CD127low/−. Further studies are needed to validate our results in independent cohorts of patients with larger sample sizes using the above definitions of Tregs as accurate and standard definition of Tregs. Our findings lend support to the idea that the Treg status of AS patients is important.
Acknowledgments
We wish to acknowledge funding in support of XFL and CHW by the National Science Foundation of China (81871295 and 81471618).
Disclosure
National Science Foundation of China had no involvement in the study design; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
NLL, SXZ, and XFL participated in the study design. NLL, JW, and JQZ participated in the data collection and interpreted the data. NLL and SXZ performed the statistical analysis under the supervision of CH W and XFL. NLL and SXZ wrote the draft manuscript, and CHW and CG helped revise the manuscript. All authors read and approved the final manuscript. Na-Lin Lai and Sheng-Xiao Zhang contributed to the work equally and should be regarded as co-first authors.
Supplementary Materials
Supplementary Table 1: background of AS patients in each study. Supplementary Figure 1: forest plot of the overall meta-analysis of regulatory T cell (Treg) proportions in peripheral blood (PB), identified as single CD25-positive, between ankylosing spondylitis (AS) patients and healthy blood donors (HD). Supplementary Figure 2: forest plot of the overall meta-analysis of regulatory T cell (Treg) proportions in peripheral blood (PB), identified with FOXP3-positive, between ankylosing spondylitis (AS) patients and healthy blood donors (HD). Supplementary Figure 3: forest plot of the overall meta-analysis of regulatory T cell (Treg) proportions in peripheral blood (PB), identified with CD127-negative, between ankylosing spondylitis (AS) patients and healthy blood donors (HD).
References
- 1.Braun J., Sieper J. Ankylosing spondylitis. The Lancet. 2007;369(9570):1379–1390. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 2.Dejaco C., Duftner C., Grubeck-Loebenstein B., Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117(3):289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liston A., Gray D. H. Homeostatic control of regulatory T cell diversity. Nature Reviews Immunology. 2014;14(3):154–165. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 4.Gizinski A. M., Fox D. A. T cell subsets and their role in the pathogenesis of rheumatic disease. Current Opinion in Rheumatology. 2014;26(2):204–210. doi: 10.1097/bor.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y., Ren M., Yang R., et al. Reduced immunomodulation potential of bone marrow-derived mesenchymal stem cells induced CCR4+CCR6+Th/Treg cell subset imbalance in ankylosing spondylitis. Arthritis Research & Therapy. 2011;13(1, article R29) doi: 10.1186/ar3257. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Zhao S. S., Hu J. W., Wang J., Lou X.-J., Zhou L.-L. Inverse correlation between CD4+CD25highCD127low/− regulatory T-cells and serum immunoglobulin A in patients with new-onset ankylosing spondylitis. The Journal of International Medical Research. 2011;39(5):1968–1974. doi: 10.1177/147323001103900543. [DOI] [PubMed] [Google Scholar]
- 7.Xueyi L., Lina C., Zhenbiao W., Qing H., Qiang L., Zhu P. Levels of circulating Th17 cells and regulatory T cells in ankylosing spondylitis patients with an inadequate response to anti-TNF-α therapy. Journal of Clinical Immunology. 2013;33(1):151–161. doi: 10.1007/s10875-012-9774-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X., Wang P., Wu Y. F., et al. Allogeneic blood transfusion alleviates hip joint pain induced by ankylosing spondylitis. Chinese Journal of Tissue Engineering Research. 2014;18(9):1465–1470. doi: 10.3969/j.issn.2095-4344.2014.09.026. [DOI] [Google Scholar]
- 9.Guo H., Zheng M., Zhang K., et al. Functional defects in CD4+ CD25high FoxP3+ regulatory cells in ankylosing spondylitis. Scientific Reports. 2016;6(1) doi: 10.1038/srep37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M., Liu C., Bond A., et al. Dysfunction of regulatory T cells in patients with ankylosing spondylitis is associated with a loss of Tim-3. International Immunopharmacology. 2018;59:53–60. doi: 10.1016/j.intimp.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Limón-Camacho L., Vargas-Rojas M. I., Vázquez-Mellado J., et al. In vivo peripheral blood proinflammatory T cells in patients with ankylosing spondylitis. The Journal of Rheumatology. 2012;39(4):830–835. doi: 10.3899/jrheum.110862. [DOI] [PubMed] [Google Scholar]
- 12.Bonelli M., Smolen J. S., Scheinecker C. Treg and lupus. Annals of the Rheumatic Diseases. 2010;69(Supplement 1):i65–i66. doi: 10.1136/ard.2009.117135. [DOI] [PubMed] [Google Scholar]
- 13.Arenas-Ramirez N., Woytschak J., Boyman O. Interleukin-2: biology, design and application. Trends in Immunology. 2015;36(12):763–777. doi: 10.1016/j.it.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Cao D., van Vollenhoven R., Klareskog L., Trollmo C., Malmström V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Research & Therapy. 2004;6(4):R335–R346. doi: 10.1186/ar1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Létourneau S., Krieg C., Pantaleo G., Boyman O. IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. The Journal of Allergy and Clinical Immunology. 2009;123(4):758–762. doi: 10.1016/j.jaci.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. J Immunol, 2017, 198(3): 981-985. [DOI] [PubMed] [Google Scholar]
- 17.Suen J. L., Li H. T., Jong Y. J., Chiang B.-L., Yen J.-H. Altered homeostasis of CD4+ FoxP3+ regulatory T-cell subpopulations in systemic lupus erythematosus. Immunology. 2009;127(2):196–205. doi: 10.1111/j.1365-2567.2008.02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banham A. H. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3+ regulatory T cells. Trends in Immunology. 2006;27(12):541–544. doi: 10.1016/j.it.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Sun J., Tang D. N., Fu T., Sharma P. Identification of human regulatory T cells in the setting of T-cell activation and anti–CTLA-4 immunotherapy on the basis of expression of latency-associated peptide. Cancer Discovery. 2012;2(2):122–130. doi: 10.1158/2159-8290.CD-11-0236. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y. H., Ishida Y., Rifa'i M., Shi Z., Isobe K., Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. The Journal of Immunology. 2008;180(2):825–832. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- 21.Han R., Yang X., Chen M., et al. Changes and clinical significance of CD8+CD122+ T cells in the peripheral blood of patients with ankylosing spondylitis. Clinical Rheumatology. 2018;37(3):639–646. doi: 10.1007/s10067-017-3887-z. [DOI] [PubMed] [Google Scholar]
- 22.Ciccia F., Accardo-Palumbo A., Giardina A., et al. Expansion of intestinal CD4+CD25(high) Treg cells in patients with ankylosing spondylitis: a putative role for interleukin-10 in preventing intestinal Th17 response. Arthritis and Rheumatism. 2010;62(12):3625–3634. doi: 10.1002/art.27699. [DOI] [PubMed] [Google Scholar]
- 23.Dejaco C., Duftner C., Klauser A., Schirmer M. Altered T-cell subtypes in spondyloarthritis, rheumatoid arthritis and polymyalgia rheumatica. Rheumatology International. 2010;30(3):297–303. doi: 10.1007/s00296-009-0949-9. [DOI] [PubMed] [Google Scholar]
- 24.Shan Y., Qi C., Zhao J., et al. Higher frequency of peripheral blood follicular regulatory T cells in patients with new onset ankylosing spondylitis. Clinical and Experimental Pharmacology & Physiology. 2015;42(2):154–161. doi: 10.1111/1440-1681.12330. [DOI] [PubMed] [Google Scholar]
- 25.Xu D., Fan J., Chen Q., Qin K., Li X., Gao C. Low dose IL-2 therapy can recovery Th17/Treg cell balance in patients with ankylosing spondylitis. Annals of the Rheumatic Diseases. 2017;76:p. 63. doi: 10.1136/annrheumdis-2017-eular.2564. [DOI] [Google Scholar]
- 26.van der Linden S., Valkenburg H. A., Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis and Rheumatism. 1984;27(4):361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemporary Clinical Trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Garrett S., Jenkinson T., Kennedy L. G., Whitelock H., Gaisford P., Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. The Journal of Rheumatology. 1994;21(12):2286–2291. [PubMed] [Google Scholar]
- 29.Forger F., Villiger P. M., Ostensen M. Pregnancy in patients with ankylosing spondylitis: do regulatory T cells play a role? Arthritis and Rheumatism. 2009;61(2):279–283. doi: 10.1002/art.24161. [DOI] [PubMed] [Google Scholar]
- 30.Chen M. H., Chen W. S., Lee H. T., Tsai C.-Y., Chou C.-T. Inverse correlation of programmed death 1 (PD-1) expression in T cells to the spinal radiologic changes in Taiwanese patients with ankylosing spondylitis. Clinical Rheumatology. 2011;30(9):1181–1187. doi: 10.1007/s10067-011-1721-6. [DOI] [PubMed] [Google Scholar]
- 31.Liao H. T., Lin Y. F., Tsai C. Y., Chou C.-T. Regulatory T cells in ankylosing spondylitis and the response after adalimumab treatment. Joint, Bone, Spine. 2015;82(6):423–427. doi: 10.1016/j.jbspin.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Toussirot É., Saas P., Deschamps M., et al. Increased production of soluble CTLA-4 in patients with spondylarthropathies correlates with disease activity. Arthritis Research & Therapy. 2009;11(4, article R101) doi: 10.1186/ar2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appel H., Wu P., Scheer R., et al. Synovial and peripheral blood CD4+FoxP3+ T cells in spondyloarthritis. The Journal of Rheumatology. 2011;38(11):2445–2451. doi: 10.3899/jrheum.110377. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y., Song Y., Fan Y. X., et al. The alteration of Thl7 cells and CD4+CD25+FoxP3+ regulatory T cells in patients with ankylosing spondylitis. Chinese Journal of Microbiology and Immunology. 2012;32(4):318–322. doi: 10.3760/cma.j.issn.0254-5101.2012.04.006. [DOI] [Google Scholar]
- 35.Wang C., Liao Q., Hu Y., Zhong D. T lymphocyte subset imbalances in patients contribute to ankylosing spondylitis. Experimental and Therapeutic Medicine. 2015;9(1):250–256. doi: 10.3892/etm.2014.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunn E. T., Taylor E. S., Stebbings S., Schultz M., Butt A. G., Kemp R. A. Distinct immune signatures in the colon of Crohn's disease and ankylosing spondylitis patients in the absence of inflammation. Immunology and Cell Biology. 2016;94(5):421–429. doi: 10.1038/icb.2015.112. [DOI] [PubMed] [Google Scholar]
- 37.Gula Z., Stec M., Rutkowska-Zapala M., et al. The absolute number of circulating nonclassical (CD14+CD16++) monocytes negatively correlates with DAS28 and swollen joint count in patients with peripheral spondyloarthritis. Polish Archives of Internal Medicine. 2017;127(12):846–853. doi: 10.20452/pamw.4142. [DOI] [PubMed] [Google Scholar]
- 38.Fattahi M. J., Ahmadi H., Jafarnezhad-Ansariha F., et al. Oral administration effects of β-D-mannuronic acid (M2000) on Th17 and regulatory T cells in patients with ankylosing spondylitis. Biomedicine & Pharmacotherapy. 2018;100:495–500. doi: 10.1016/j.biopha.2018.02.059. [DOI] [PubMed] [Google Scholar]
- 39.Ji W., Li H., Gao F., Chen Y., Zhong L., Wang D. Effects of tripterygium glycosides on interleukin-17 and CD4+CD25+CD127low regulatory T-cell expression in the peripheral blood of patients with ankylosing spondylitis. Biomedical Reports. 2014;2(4):517–520. doi: 10.3892/br.2014.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan Z., Gui Y., Li C., et al. The immune dysfunction in ankylosing spondylitis patients. Bioscience Trends. 2017;11(1):69–76. doi: 10.5582/bst.2016.01171. [DOI] [PubMed] [Google Scholar]
- 41.Dulic S., Vásárhelyi Z., Bajnok A., et al. Analysis of the T-cell subset composition in ankylosing spondylitis patients with long-standing anti-TNF therapy. Annals of the Rheumatic Diseases. 2017;76:p. 780. doi: 10.1136/annrheumdis-2017-eular.6335. [DOI] [Google Scholar]
- 42.Lukas C., Landewe R., Sieper J., et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Annals of the Rheumatic Diseases. 2009;68(1):18–24. doi: 10.1136/ard.2008.094870. [DOI] [PubMed] [Google Scholar]
- 43.van der Heijde D., Lie E., Kvien T. K., et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Annals of the Rheumatic Diseases. 2009;68(12):1811–1818. doi: 10.1136/ard.2008.100826. [DOI] [PubMed] [Google Scholar]
- 44.Machado P., Landewe R., Lie E., et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Annals of the Rheumatic Diseases. 2011;70(1):47–53. doi: 10.1136/ard.2010.138594. [DOI] [PubMed] [Google Scholar]
- 45.Sakaguchi S. Regulatory T cells: history and perspective. Methods in Molecular Biology. 2011;707:3–17. doi: 10.1007/978-1-61737-979-6_1. [DOI] [PubMed] [Google Scholar]
- 46.Bidad K., Salehi E., Jamshidi A., et al. Effect of all-transretinoic acid on Th17 and T regulatory cell subsets in patients with ankylosing spondylitis. The Journal of Rheumatology. 2013;40(4):476–483. doi: 10.3899/jrheum.121100. [DOI] [PubMed] [Google Scholar]
- 47.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. The Journal of Immunology. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 48.Baecher-Allan C., Brown J. A., Freeman G. J., Hafler D. A. CD4+CD25high regulatory cells in human peripheral blood. The Journal of Immunology. 2001;167(3):1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 49.Han G. M., O'Neil-Andersen N. J., Zurier R. B., Lawrence D. A. CD4+CD25high T cell numbers are enriched in the peripheral blood of patients with rheumatoid arthritis. Cellular Immunology. 2008;253(1-2):92–101. doi: 10.1016/j.cellimm.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dejaco C., Duftner C., Schirmer M. Are regulatory T-cells linked with aging? Experimental Gerontology. 2006;41(4):339–345. doi: 10.1016/j.exger.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Lu L., Barbi J., Pan F. The regulation of immune tolerance by FOXP3. Nature Reviews Immunology. 2017;17(11):703–717. doi: 10.1038/nri.2017.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noack M., Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmunity Reviews. 2014;13(6):668–677. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Wan Y. Y., Flavell R. A. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445(7129):766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 54.Nadkarni S., Mauri C., Ehrenstein M. R. Anti-TNF-α therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-β. The Journal of Experimental Medicine. 2007;204(1):33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonelli M., Savitskaya A., Steiner C. W., Rath E., Smolen J. S., Scheinecker C. Phenotypic and functional analysis of CD4+CD25−Foxp3+ T cells in patients with systemic lupus erythematosus. The Journal of Immunology. 2009;182(3):1689–1695. doi: 10.4049/jimmunol.182.3.1689. [DOI] [PubMed] [Google Scholar]
- 56.Yang H. X., Zhang W., Zhao L. D., et al. Are CD4+CD25−Foxp3+ cells in untreated new-onset lupus patients regulatory T cells? Arthritis Research & Therapy. 2009;11(5, article R153) doi: 10.1186/ar2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu W., Putnam A. L., Xu-Yu Z., et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. Journal of Experimental Medicine. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hartigan-O'Connor D. J., Poon C., Sinclair E., McCune J. M. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. Journal of Immunological Methods. 2007;319(1-2):41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Ziegler S. F. FOXP3: of mice and men. Annual Review of Immunology. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 60.Xystrakis E., Dejean A. S., Bernard I., et al. Identification of a novel natural regulatory CD8 T-cell subset and analysis of its mechanism of regulation. Blood. 2004;104(10):3294–3301. doi: 10.1182/blood-2004-03-1214. [DOI] [PubMed] [Google Scholar]
- 61.Churlaud G., Pitoiset F., Jebbawi F., et al. Human and mouse CD8+CD25+FOXP3+ regulatory T cells at steady state and during interleukin-2 therapy. Frontiers in Immunology. 2015;6:p. 171. doi: 10.3389/fimmu.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye L., Zhang L., Goodall J., Gaston H., Xu H. Altered frequencies of regulatory T-cell subsets in ankylosing spondylitis and rheumatoid arthritis patients and their response to anti-TNF therapy. Rheumatology. 2013;52:135–136. [Google Scholar]
- 63.Miyara M., Yoshioka Y., Kitoh A., et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 64.Taams L. S., Smith J., Rustin M. H., Salmon M., Poulter L. W., Akbar A. N. Human anergic/suppressive CD4+CD25+ T cells: a highly differentiated and apoptosis-prone population. European Journal of Immunology. 2001;31(4):1122–1131. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 65.Booth N. J., McQuaid A. J., Sobande T., et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. The Journal of Immunology. 2010;184(8):4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 66.Lazarevic V., Glimcher L. H., Lord G. M. T-bet: a bridge between innate and adaptive immunity. Nature Reviews Immunology. 2013;13(11):777–789. doi: 10.1038/nri3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koch M. A., Tucker-Heard G., Perdue N. R., Killebrew J. R., Urdahl K. B., Campbell D. J. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nature Immunology. 2009;10(6):595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sprouse M. L., Scavuzzo M. A., Blum S., et al. High self-reactivity drives T-bet and potentiates Treg function in tissue-specific autoimmunity. JCI Insight. 2018;3(2) doi: 10.1172/jci.insight.97322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chung Y., Tanaka S., Chu F., et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nature Medicine. 2011;17(8):983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wollenberg I., Agua-Doce A., Hernández A., et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. The Journal of Immunology. 2011;187(9):4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 71.Cari L., De Rosa F., Nocentini G., Riccardi C. Context-dependent effect of glucocorticoids on the proliferation, differentiation, and apoptosis of regulatory T cells: a review of the empirical evidence and clinical applications. International Journal of Molecular Sciences. 2019;20(5) doi: 10.3390/ijms20051142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Azab N. A., Bassyouni I. H., Emad Y., El-Wahab G. A. A., Hamdy G., Mashahit M. A. CD4+CD25+ regulatory T cells (TREG) in systemic lupus erythematosus (SLE) patients: the possible influence of treatment with corticosteroids. Clinical Immunology. 2008;127(2):151–157. doi: 10.1016/j.clim.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 73.Mathian A., Jouenne R., Chader D., et al. Regulatory T cell responses to high-dose methylprednisolone in active systemic lupus erythematosus. PLoS One. 2015;10(12, article e0143689) doi: 10.1371/journal.pone.0143689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keijsers R. R. M. C., Joosten I., Hendriks A. G. M., Koenen H. J. P. M., van Erp P. E. J., van de Kerkhof P. C. M. Balance of Treg versus T-effector cells during systemic treatment with adalimumab and topical treatment with calcipotriol-betamethasone dipropionate ointment. Experimental Dermatology. 2015;24(1):65–67. doi: 10.1111/exd.12575. [DOI] [PubMed] [Google Scholar]
- 75.Braitch M., Harikrishnan S., Robins R. A., et al. Glucocorticoids increase CD4+CD25high cell percentage and Foxp3 expression in patients with multiple sclerosis. Acta Neurologica Scandinavica. 2009;119(4):239–245. doi: 10.1111/j.1600-0404.2008.01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muls N. G., Dang H. A., Sindic C. J. M., van Pesch V. Regulation of Treg-associated CD39 in multiple sclerosis and effects of corticotherapy during relapse. Multiple Sclerosis. 2015;21(12):1533–1545. doi: 10.1177/1352458514567215. [DOI] [PubMed] [Google Scholar]
- 77.Guggino G., Giardina A., Ferrante A., et al. The in vitro addition of methotrexate and/or methylprednisolone determines peripheral reduction in Th17 and expansion of conventional Treg and of IL-10 producing Th17 lymphocytes in patients with early rheumatoid arthritis. Rheumatology International. 2015;35(1):171–175. doi: 10.1007/s00296-014-3030-2. [DOI] [PubMed] [Google Scholar]
- 78.Yu X., Wang C., Luo J., Zhao X., Wang L., Li X. Combination with methotrexate and cyclophosphamide attenuated maturation of dendritic cells: inducing Treg skewing and Th17 suppression in vivo. Clinical and Developmental Immunology. 2013;2013:12. doi: 10.1155/2013/238035.238035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lina C., Conghua W., Nan L., Ping Z. Combined treatment of etanercept and MTX reverses Th1/Th2, Th17/Treg imbalance in patients with rheumatoid arthritis. Journal of Clinical Immunology. 2011;31(4):596–605. doi: 10.1007/s10875-011-9542-6. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen D. X., Ehrenstein M. R. Anti-TNF drives regulatory T cell expansion by paradoxically promoting membrane TNF-TNF-RII binding in rheumatoid arthritis. The Journal of Experimental Medicine. 2016;213(7):1241–1253. doi: 10.1084/jem.20151255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Valencia X., Yarboro C., Illei G., Lipsky P. E. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. The Journal of Immunology. 2007;178(4):2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 82.Zhang S. X., Ma X. W., Li Y. F., et al. The proportion of regulatory T cells in patients with systemic lupus erythematosus: a meta-analysis. Journal of Immunology Research. 2018;2018:11. doi: 10.1155/2018/7103219.7103219 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: background of AS patients in each study. Supplementary Figure 1: forest plot of the overall meta-analysis of regulatory T cell (Treg) proportions in peripheral blood (PB), identified as single CD25-positive, between ankylosing spondylitis (AS) patients and healthy blood donors (HD). Supplementary Figure 2: forest plot of the overall meta-analysis of regulatory T cell (Treg) proportions in peripheral blood (PB), identified with FOXP3-positive, between ankylosing spondylitis (AS) patients and healthy blood donors (HD). Supplementary Figure 3: forest plot of the overall meta-analysis of regulatory T cell (Treg) proportions in peripheral blood (PB), identified with CD127-negative, between ankylosing spondylitis (AS) patients and healthy blood donors (HD).