Abstract
There is increasing evidence of the relevant connection and regulation between the gut and skin immune axis. In fact, oral administration of lipoteichoic acid (LTA) from Lactobacillus rhamnosus GG (LGG) prevents the development of UV-induced skin tumors in chronically exposed mice. Here we aim to evaluate whether this LTA is able to revert UV-induced immunosuppression as a mechanism involved in its anti-tumor effect and whether it has an immunotherapeutic effect against cutaneous squamous cell carcinoma. Using a mouse model of contact hypersensitivity, we demonstrate that LTA overcomes UV-induced skin immunosuppression. This effect was in part achieved by modulating the phenotype of lymph node resident dendritic cells (DCs) and the homing of skin migratory DCs. Importantly, oral LTA reduced significantly the growth stablished skin tumors once UV radiation was discontinued, demonstrating that it has a therapeutic, besides the already demonstrated preventive antitumor effect. The data presented here strongly indicates that oral administration of LTA represents a promising immunotherapeutic approach for different conditions in which the skin immune system is compromised.
Keywords: Skin diseases, Tolerance, Dendritic Cells, UV radiation, Probiotics
Graphical Abstract
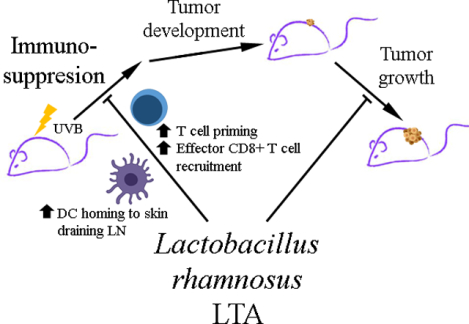
Oral administration of LTA overcomes UVB-induced immunosuppression, through increased dendritic cell homing to lymph nodes, the consequent T cell priming and, finally, CD8+ effector T cell recruitment to challenged skin. Moreover, LTA is able to reduce the growth of established UVB-induced skin tumors.
INTRODUCTION
Like other compartmentalized organs, the skin is equipped with an immune competent system that accounts for the initiation of local and systemic responses. Cutaneous immune responses may be affected by other compartmentalized immune locations such as the gut associated lymphoid tissue. In this regard, alterations of the gut microbiota as well as probiotic consumption influence the outcome of cutaneous immune responses [1]. Accordingly, gut resident immune cells sense microorganism through the recognition of molecular patterns such as lipoteichoic acid (LTA). This molecule is present in the cell wall of Gram-positive bacteria and is a highly immunogenic glycolipid. LTA exerts immunostimulatory effects by interaction with Toll-like receptors (TLR), particularly with TLR2/6, leading to the activation of NF-κB and AP-1 with subsequent transcription of pro-inflammatory cytokines and chemokines [2],[3]. Even when this is the conventional LTA-activated intracellular signaling cascade, LTA has shown to exert different immune outcomes depending on the bacterial source [3]–[6]. This could be explained by the variety of LTA structures found among bacterial species, including probiotic bacteria.
Ultraviolet (UV) B radiation is considered the most powerful inducer of skin cancer. It promotes DNA damage and subsequent mutations that in turn lead to tumor development. In the early 70ś Kripke et al. described that UVB is also capable of generating the systemic immunosuppression necessary for tumor growth [7]–[9]. UV-induced immunosuppression has been extensively studied using cutaneous hypersensitivity reactions (CHS) against different haptens, such as oxazolone and dinitrofluorobenzene [10]–[12].
We have previously demonstrated the effect of LTA from Lactobacillus rhamnosus GG (LGG) in the prophylaxis of UVB-induced squamous cell carcinoma (SCC) in chronically irradiated mice, when administered orally before UV irradiation. Mice treated with this protocol showed an increase in IFN-γ production and in the state of dendritic cells (DCs)-activation in skin draining lymph nodes (sDLN), suggesting that LTA contributes to a Th1 bias [13].
Previously, we showed that oral administration of LTA prevents the development of UV-induced SCC. To address a possible therapeutic effect, we analyzed here the role of oral administered LTA in the growth of established UV-induced SCC.
To understand a possible mechanism of the LTA anti-tumor effect, we utilized an established model of CHS to evaluate the capability of oral LTA to abrogate the immunosuppressive effect of UV and sustain skin innate and adaptive immune responses.
RESULTS AND DISCUSSION
Oral LTA overcomes UV-induced immunosuppression to oxazolone CHS reaction.
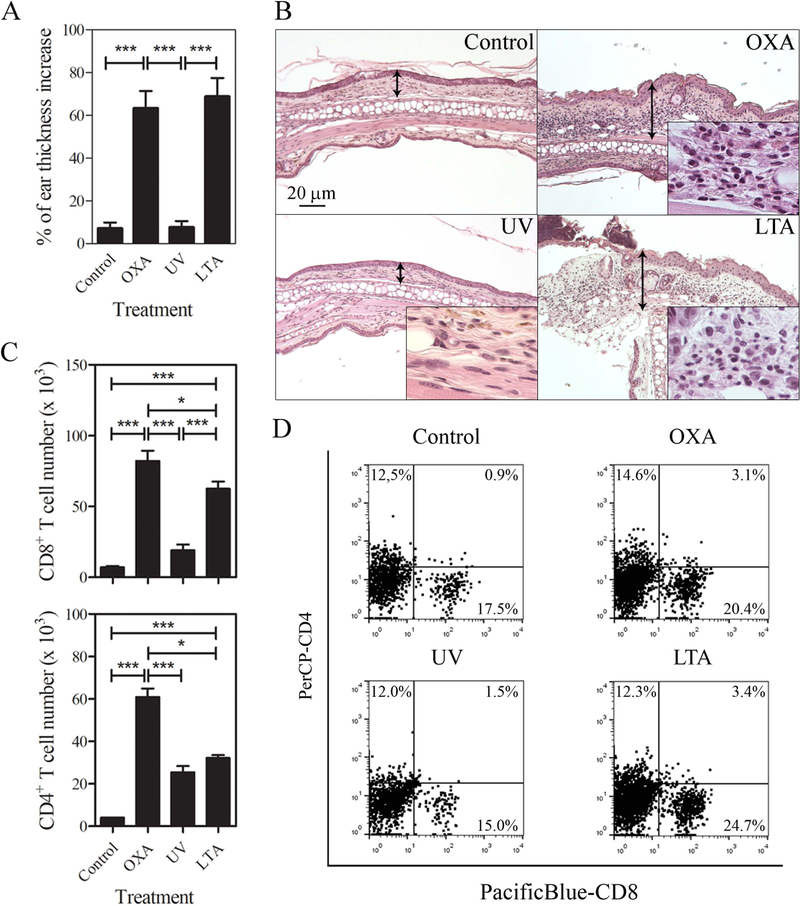
The capacity of oral LTA to revert UV-induced immunosuppression was evaluated in an experimental model of CHS to the skin sensitizer OXA. As expected, mice that were sensitized and elicited with OXA developed a potent CHS reaction compared to untreated mice or mice that were UV irradiated prior to OXA sensitization. Importantly, mice that were treated with oral LTA before UV radiation and exposed to OXA showed a significant increase in ear thickness compared to the OXA treated and UV-irradiated control group (Fig 1A and suppl. 1A). The previous results were further confirmed by histological analysis of the CHS effector site. Mice ears were dissected 48h following elicitation and the intensity and characteristics of the cellular infiltrate was analyzed. The ear sections showed intense mononuclear cell infiltrate, epidermal hyperplasia in OXA induced CHS, which was abrogated by UV radiation (Fig 1B). Importantly, the CHS response was completely restored by oral administration of LTA (Fig 1B). The characteristics and quantification of the skin cellular infiltrate analyzed by flow cytometry (FACS), showed a high number of CD8+ T cells in mice treated with OXA in the absence of UV-radiation. The number of CD8+ T cells was significantly reduced in UV irradiated mice, and it was increased by LTA administration to a similar extent shown in non-irradiated and OXA treated mice (Fig 1C, D and suppl. 1B and D). The number of effector CD4+ T cells was also reduced by UV radiation, but LTA was unable to restore their number during the effector phase of CHS (Fig 1C and D).
Figure 1.
Oral LTA overcomes UV-induced immunosuppression to oxazolone CHS reaction. (A) Quantitative comparison of the percent of ear thickness increase in BL6 mice skin sensitized and elicited with oxazolone 5 d later. The bar diagram depicts ear thickness increase 24 h following elicitation phase of the CHS. Groups compared include non-irradiated, and vehicle challenged (Control), non-irradiated and challenged with oxazolone (OXA), UV-irradiated and challenged with oxazolone (UV) or oral LTA-treated, UV-irradiated and challenged with oxazolone (LTA). Results are expressed as means±1SE of five mice per experimental group in one of two independent experiments. (B) Histological images of one representative skin section of mice ears shown in A. H&E X100, insets show the characteristics of the leukocyte infiltrate at higher magnification (X 500). (C) Quantification by flow cytometry using CountBright™ fluorescent beads of infiltrating CD4+ (FITC-CD3 and PerCP-CD4 Ab) and CD8+ (PacificBlue-CD8b Ab) T cell in mice ears. Results are expressed as means±1SE of 3 samples composed of 4 pooled ears each in one of two independent experiments (D) One representative flow cytometry dot-plot from each experimental group of PE-CD45 pre-gated CD4+ and CD8+ T cells quantified in C.
*p <0.05, **p <0.01, ***p <0.001 (One-way ANOVA and Tukey post hoc test).
Together these data demonstrate that oral administration of LTA overcomes the immunosuppressive effect of UV in the skin that precludes the development of the efficient cellular immune response accounting for CHS reactions.
Given the novel capacity of LTA to revert UV-induced immunosuppression, we further investigated whether oral LTA had an effect in the skin inflammatory response during sensitization phase of the CHS and in the priming of effector T cells in sDLN.
Oral LTA restores skin inflammation during CHS sensitization by activating DCs and priming T cell
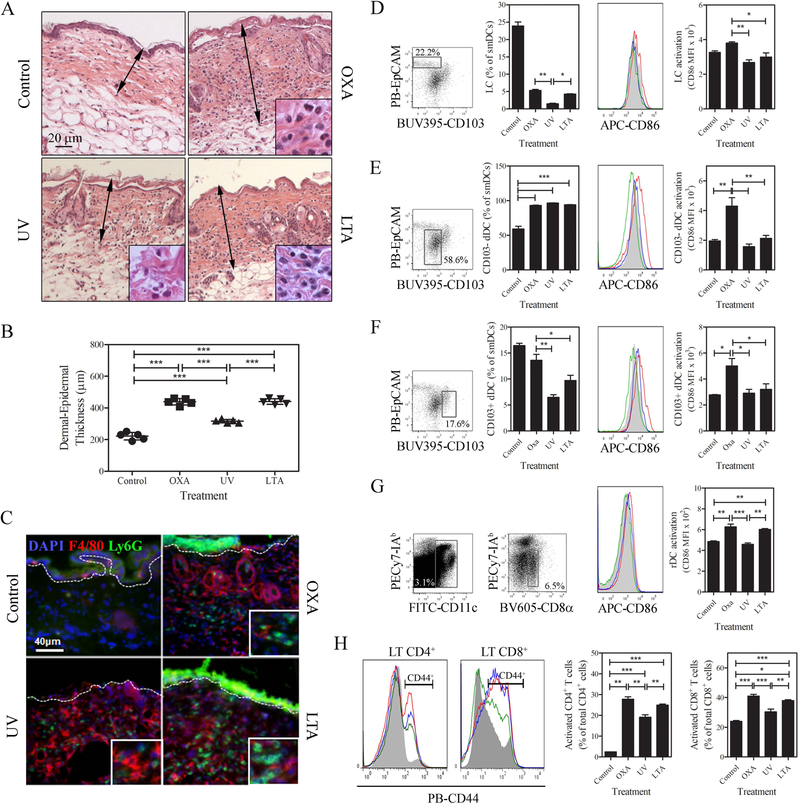
The role of LTA to modify the skin inflammatory response to OXA during CHS sensitization was analyzed in the abdominal skin of mice UV-radiated or not and, treated or not with oral LTA. The intensity and characterization of the cutaneous cellular infiltrate was analyzed by H&E and immunofluorescence respectively. Mice treated with OXA in the absence of UV-radiation showed an increase in epidermal-dermal thickness (Fig 2A). Irradiated mice sensitized with OXA showed a significant increase of skin thickness, but this increase was significantly lower than that observed in mice treated with OXA in the absence of UV- radiation (Fig. 2A and B). Importantly, the epidermal-dermal thickness in LTA treated mice was similar to that observed in non-irradiated mice treated with OXA (Fig 2A and 2B) andcorrelated with intense inflammatory infiltrate composed by Ly6G+ polymorphonuclear cells (PMN) and F4/80+ macrophages (Fig 2C). These results indicate that oral LTA increases the skin inflammatory response during the sensitization phase of CHS.
Figure 2.
Oral LTA restores skin inflammation during the CHS sensitization phase through activation of DCs and T cell priming in sDLN. (A) Histological images of the sensitization site (abdominal skin) dissected 48 h after sensitization with OXA of BL6 mice previously treated with oral LTA, UV or oral LTA and UV. The sections from the skin of mice treated with OXA show significant thickness increase of the epidermis and dermis (black arrows) and intense inflammatory infiltrate compared to vehicle treated control mice (H&E, X 100). Insets show the characteristics of leukocyte infiltrate at higher magnification (X 500). (B) Comparison of skin thickness of mouse treated as described in A. Mean ± SD of epidermal-dermal thickness (n=5) measured in skin sections stained with H&E. (C) Immunofluorescence images of sections of mouse skin treated as described in A. Polymorphonuclear cells and macrophages are identified by their expression of Ly6G (green) (Alexa488-Ly6G Ab) and F4/80 (red) (Alexa647-F4/80 Ab) molecules respectively. Cell nuclei were stained with DAPI. IF (X200, insets X500). The dashed white line indicates the epidermal-dermal junction. (D-F) Quantification of smiDC and CD86 (APC-CD86 Ab) expression in sDLN 48 h after OXA sensitization. Histograms represent control (full gray), OXA (red), UV (green) and LTA treated group (blue), (D) Langerhans cells, (E) CD103− dermal DCs, (F) CD103+ dermal DCs, (G) resident DCs (BV605-CD8α Ab) CD86 expression at the same time point in sDLN. Cell abundance results are expressed as mean percentages of total smiDC ± SD and CD86 expression results are expressed as mean fluorescent intensity ± SD (n=3). (H) CD4+CD44+ (PECy7-CD4 and PacificBlue or PB-CD44 Ab) and CD8+CD44+ (FITC-CD8b and PacificBlue or PB -CD44 Ab) T cells (Alexa 555-CD3 Ab) in sDLN 4 days after OXA sensitization in the control (full gray), OXA (red), UV (green) and LTA treated group (blue). Bar diagrams are means ± SD (n=4 sDLN / group). *p <0.05, **p <0.01, ***p <0.001 (One-way ANOVA and Tukey post hoc test). All the data correspond to one of two independent experiments.
The skin inflammatory response following OXA sensitization supports the T cell stimulatory function of skin dendritic cells (sDC) that initiate the adaptive immunity accounting for the development of efficient CHS. Conversely, UV-radiation impairs the development of CHS, an effect that is reversed by oral administration of LTA.
Therefore, we investigated whether LTA influenced the numbers and phenotype of migratory skin migratory DCs (smiDC) and LN resident DCs, in the DLN of OXA sensitized skin in the presence or not of UV-radiation. Skin migratory DCs homing in DLN were identified by the expression of CD11c and Ia2 high molecules (suppl. Fig 2A). Three subsets of smiDC were further analyzed i) EpCam+ CD24high CD103− Langerhans cells (LCs), ii) CD103−EpCam−CD24int conventional dermal DCs (dDC), and iii) CD103+EpCam−CD24high (CD103+dDC), (Fig 3D–G and suppl. Fig 2B and C). The LN resident DCs were identified by the phenotype CD11chigh CD8α+CD11b−. The maturation stage of different DC populations was assessed by the expression of surface CD86. As previously described, following skin UV radiation the percentage of LCs homing in sDLN was significantly reduced [14]–[16], and their migration was re-established in mice treated with LTA. However, in both experimental variables, the expression of CD86 remained low in those cells (Fig 2D). The role of LC in the CHS reaction is still a matter of debate, but it has been proposed that LC are, indeed, required for the CHS reaction [17]. However, Noordegraaf et al have shown that the presence of LC is critical when animals are sensitized and challenged with low doses of OXA (0.5% and 0.25% v/v respectively) while the CHS reaction is partially reduced in the absence of LC when animals are sensitized and challenged with high doses of OXA (2% and 0.5% v/v respectively)[18]. Conversely, UV irradiation did not affect the percentage of conventional dDC in sDLN but slightly reduced CD86 expression on this cell population. LTA treatment did not modulate the migratory capacity of conventional dDC or their expression of CD86 (Fig 2E). The percentage of CD103+dDC and the expression of CD86 were decreased in the sDLN of UV irradiated mice and LTA was unable to revert these UV effects (Fig 2F). Of note, the limitations of analyzing cell population percentages in sDLN after sensitization have to be taken into account, since the migration of dDC appears to be differentially modulated by OXA sensitization. Lymph node resident DCs, showed a slight but significant reduction in CD86 expression after UV radiation. Surprisingly, oral administration of LTA increased the expression of CD86 to similar levels observed in OXA sensitized non-irradiated mice (Figure 2G). The fact that LN resident DCs cells are involved in Ag cross-presentation in the CHS reaction [19], this result suggests that the stimulatory effects of LTA in CHS, and its capacity to revert UV-induced immunosuppression could be in part mediated by promoting the immune function of LN resident DCs.
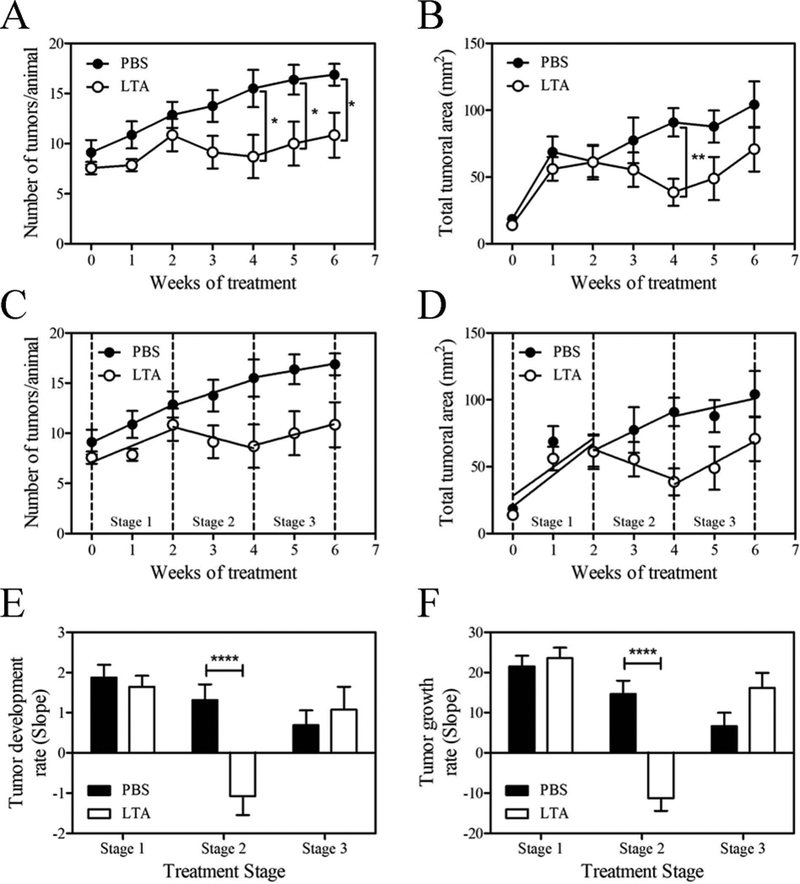
Figure 3.
Oral LTA reduces UV-induced tumor development once UV radiation is suspended.
(A) The number of tumors and (B) total tumor areas per SKH:1 mouse during the 6 weeks of treatment, for the PBS and LTA treated group. The results are expressed as mean tumor number or total tumor area ± SD. (C) Time-course analysis in three stages for tumor development and (D) tumor growth: Stage 1 absence of effect, Stage 2 anti-tumor effect, Stage 3 effect loss after discontinued LTA treatment. For each stage a linear regression was performed, and the slopes were compared between experimental groups (E, F). The results are expressed as the slope ± SD. *p <0.05, **p <0.01, ***p <0.001; (unpaired Student t-test). The data correspond to one of two independent experiments (n=8).
The role of oral LTA in modulating T cell priming in the context of CHS was assessed by quantification of CD4+CD44+ and CD8+CD44+ T cells in the sDLN 96 h after sensitization by FACS. As expected, UV radiation significantly reduced the percentage of CD4+CD44+ and CD8+CD44+ T cells and oral LTA administration was able to recover the percentage of CD4+CD44+ and CD8+CD44+ T cells to similar levels observed in mice that were sensitized with OXA in the absence of UV radiation (Fig 2H).
Taken together, we can conclude that oral administration of LTA affects skin innate and adaptive immune responses accounting for the development of CHS, overcoming the immunosuppressive effect of UV radiation.
Oral LTA reduces UV-induced tumor growth once UV radiation is suspended
To address the clinical relevance of our results, we used a chronic UV-induced SCC mice model. Chronic exposure to UV radiation produces cutaneous inflammation and promotes T cell priming against transformed epidermal cells. However, as T cells are primed under the immunosuppressive effects of UV radiation, the immune system fails to reject the tumor [20].
In a previous work we demonstrated the ability of oral LTA to prevent the development of UV-induced SSC by treating the animals from the beginning of a chronic irradiation schedule. Here, we analyzed the potential therapeutic effect of oral LTA in SSC once tumors were already stablished and UV-irradiation suspended. For this purpose, mice were chronically irradiated as previously described [13]. Once all mice had at least 5 tumors of 1 mm diameter, we interrupted UV irradiation and began with LTA oral administration.
Four weeks after LTA treatment mice showed lower number of smaller sized tumors (supplementary Fig 2D). The LTA ability to decrease the number of tumors was sustained throughout the end of the experiment (week 6) (Fig 3A). However, the effect of LTA to reduce the total tumor area was lost after the suspension of the LTA treatment (Fig 3B).
We observed that the kinetics in TN and total tumor area presents three stages: i) from the beginning of the treatment to week 2, where LTA had no effect, ii) from week 2 to 4, where LTA produced its antitumor effect and iii) when the treatment was suspended, and the effect of LTA was lost. Therefore, we analyzed the kinetics of tumor growth during these 3 stages. By using linear regression analysis (Fig 3C and D) we observed a highly significant effect of LTA on tumor development and growth during the stage 2, with no significant differences in stages 1 and 3 (Fig 3E and F). In this context, with tumor cells as the only challenge present in the skin, LTA was able to induce the observed antitumor effect. However, it took two weeks of treatment before an effect could be observed. This effect persisted during the administration of LTA but was lost after LTA-treatment suspension.
These results show that oral LTA can modulate skin immune responses against complex stimuli, like those produced during tumor development, and that the stimulatory effect of LTA needs to be persistent to maintain the activity of the immune response. Moreover, we here demonstrated that LTA acts not only as a prophylactic agent, but also as a potential therapeutic strategy for SCC treatment.
CONCLUDING REMARKS
In the present work we demonstrate that oral LTA can overcome the immunosuppressive effect of UV radiation by modulating the skin immune system. Particularly, skin DCs activation and T cell priming are involved in this effect. Moreover, LTA prove to be useful not only as a prophylactic agent but also as a therapeutic strategy for SCC treatment. Altogether, this article contributes to deepen the knowledge on the complex interactions between the host and derived probiotic molecules, which have been called “probiotaceuticals” [21], and proposes LTA from Lactobacillus rhamnosus GG as a promising therapeutic tool for diverse skin conditions where the local immune response is being compromised.
MATERIALS AND METHODS
Lipoteichoic acid purification
LTA was isolated as previously described [22]. LTA preparation was tested for purity by Western blot, as described previously by Weill [13].
Animal models, general procedures and LTA administration
Female C57BL/6 (The Jackson Laboratory) and Crl:SKH-1-hrBR hairless mice (Charles River Laboratories) were used at 8–12 weeks of age. They were housed in quarters with a 12h light–12h dark cycle and maintained with water and food ad libitum. Twenty-four to 48h before radiation procedure, hair from the back of C57BL/6 mice was removed using electric clipper and commercial depilatory cream. All radiations were performed as previously described [23]. LTA oral administration was performed as previously described [13]. All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee or, the guidelines established by the Consejo Nacional de Investigaciones Científicas y Técnicas (Argentina) and were approved by the Review Board of Ethics of the Instituto de Estudios de la Inmunidad Humoral.
In order to obtain single cell suspensions to be analyzed by flow cytometry, organs were cut into small pieces, treated with 1.5mg/ml collagenase type IV and 40 μg/ml DNAse I (Sigma-Aldrich, St Louis, MO) for 90 minutes and filtered through 40 μm cell mesh.
Prevention of the skin UV tolerogenic effect by oral administration of LTA
C57/BL6 mice (n=5/experimental group) received oral LTA or PBS (control) by gavage every other day during the complete experimental procedure. Twenty-four hours after the 8th dose, the ear skin was protected, and mice were exposed to one application of 150 mJ/cm2 of UVB in their shaved dorsal skin for three consecutive days, using an 8 watt 302 nm lamp (range 280–350 nm, Ultraviolet Products, Upland, CA, U.S.A.). This dose correspond to 1.5 minimal erythemal dose and its proven to induce immunosuppression in similar irradiation schedules [17],[24]. Twenty-four h after the last radiation dose, animals were sensitized with 150 μl of 3% w/v oxazolone (OXA) (Sigma-Aldrich) in ethanol (vehicle) on their shaved, non-irradiated, lower abdomen. Six days later, the dorsal and ventral sides of the right ears were challenged with 1% w/v OXA, or vehicle (control). Ear thickness was evaluated using a quick mini digital micrometer (Mitutoyo, Buenos Aires, Argentina) up to 72h. Percentage of ear thickness increase was calculated the formula 100 × [(thickness of challenged ear − thickness of unchallenged ear)/thickness of unchallenged ear].
For histologic analysis, ears were dissected and skin samples were paraffin embedded, sectioned, stained with H&E and analyzed by light microscopy using an Axiostar plus microscope (Zeiss, Oberkochen, Germany). Characterization and quantification of the cellular infiltrate in effector site by flow cytometry, was performed in cell suspensions of 4 dissected pooled ears from 2 mice per experimental condition 48h after CHS elicitation, in two independent experiments. After Fc blocking, cells were stained with Zombie Aqua Fixable Dye (Biolegend, San Diego, CA) for cell viability and labeled with FITC-CD3 (only in the second experiment), PE-CD45, PacificBlue-CD8b and APC-CD4 (BD Bioscience, San Jose, CA) monoclonal antibodies. The gating strategy used is shown in supplementary Figure 1C. Absolute cell numbers were calculated using CountBright™ (Molecular Probes, Eugene, OR) according to manufacturer protocols. The results were expressed as cell number per ear. Data was acquired on a LSR Fortessa cytometer (BD Bioscence) and analyzed with FlowJo® v9 software.
The analysis of the inflammatory infiltrate during CHS priming was analyzed in mouse abdominal skin dissected 48h after sensitization. Two abdominal skin samples per mouse were obtained dissected, embedded in paraffin and serial sections, were stained with H&E. For characterization of leukocyte subsets, skin samples were snap-frozen cryostat-sectioned, blocked with 5% goat serum (Sigma-Aldrich) and stained with 1:100 Alexa 555-CD3 Ab, Alexa488-Ly6G Ab and Alexa647-F4/80 Ab (BD Bioscience). Nuclei were counterstained with DAPI (Molecular Probes). Finally, samples were fixed in 4% buffered neutral formalin and mounted. Tissue sections were analyzed using an AxioStar Plus microscope (Zeiss) equipped with epifluorescence and a digital camera (AxioCam MRc; Zeiss).
Analysis of dendritic cell subsets was performed by flow cytometry in cell suspensions of inguinal skin draining lymph nodes obtained 48h after sensitization. For flow cytometry analysis, cells were incubated with Fc receptor CD16/32 Ab (BD Bioscience) and labeled with BUV395-CD103, PE-CD24a, FITC-CD11c, PerCPCy5.5-CD11b, APC-CD86, BV605-CD8α, PECy7-IAb (BD Bioscience) and PacificBlue-EpCAM (PB-EpCam, Miltenyi, Bergisch Gladbach, Germany).
Characterization of T cell populations and their activation state was performed in cell suspensions of sDLN obtained 4 d after sensitization and labeled with PECy7-CD4, FITC-CD8b, PacificBlue-CD44 (PB-CD44, BD Bioscience).
We have adhered to the Guidelines for the use of flow cytometry and cell sorting in immunological studies.
LTA treatment in established UV-induced tumors
Crl:SKH-1-hrBR hairless mice were irradiated three times a week with 75 mJ/cm2, as previously described [13]. The radiation schedule was suspended once every mouse had developed at least 5 tumors of 1 mm diameter, after approximately 4 months of irradiation. At that time, mice were separated in two groups and orally administered with LTA or PBS, three times each week for a period of 4 weeks. Thereafter oral treatment was suspended, and mice were euthanized 2 weeks later. Mice were photographed weekly starting the date of the first oral dose until the end of the experiment. The photographs were analyzed with ImageJ software (National Institutes of Health), to determine the TN and total tumor area.
Statistical analysis
Results are expressed as means ± 1standard error. Comparisons between two means were obtained by Student test and Mann-Whitney post hoc test. More than two means were compared by one-way ANOVA and Tukey post hoc test was performed. Graphical and statistical analyses were performed with GraphPad Prism 5.0 (GraphPad Software) and GraphPad Instat 2.0 (GraphPad Software), respectively. Differences with p<0.05 between values were considered significantly different (*p<0.05; **p<0.01, ***p<0.001 y ****p<0.0001; ns: non-significant differences).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Universidad de Buenos Aires (UBACyT 2013–2016, to D.H.G.M.) the Agencia Nacional de Promoción Científica y Tecnológica (PICT-2012–0264, to D.H.G.M.), and a Fulbright Fellowship (to A.D.F.), and NIH grants R01AR071277 and R01AR078249 (to A.T.L.).
Valeria Campo and Eliana Cela contributed to the experimental work included in this manuscript. Bill Shufesky performed cryosections IF staining and image acquisition, while Olga Tkacheva contributed with technical support in the CHS experiments and flow cytometry. Adrian Morelli contributed intellectually to experimental planning of CHS. Juliana Leoni contributed in the article correction. Mariela Laura Paz helped in the design of the CHS model and the tumor development experiments as well as in the writing of this manuscript. Adriana Larregina provided intellectual input, helped programing in vivo experiments of CHS, performed acquisition by flow cytometry, and H&E and IF analysis of skin sections and in the manuscript correction. Daniel Gonzalez Maglio provided intellectual input, helped in the design of all the experiments, performed the figures of the manuscript and helped in the writing of this manuscript. Adrian Friedrich performed the research, designed and analyzed the experiments and wrote the manuscript.
The authors would also like to thank Carolina Mourelle and Angélica Miranda for their technical support, and advice in relation to mouse care and management. The authors would also like to thank Placida Baz and Ariel Billordo for their technical support in flow cytometry.
ABBREVIATIONS
- LTA
lipoteichoic acid
- LGG
Lactobacillus rhamnosus GG
- DCs
dendritic cells
- CHS
contact hypersensitivity
- SCC
squamous cell carcinoma
- OXA
oxazolone
- TN
tumor number
- Sdln
skin draining lymph node
- smiDC
skin migratory dendritic cells
- LC
Langerhans’ cells
- dDC
dermal dendritic cells
- MHCII
Major Histocompatibility Complex Class II
Footnotes
CONFLICT OF INTEREST
The authors declare no commercial or financial conflict of interest.
REFERENCES
- 1.Friedrich A, Paz M, Leoni J, González Maglio D. Message in a Bottle: Dialog between Intestine and Skin Modulated by Probiotics. Int. J. Mol. Sci 2017; 18:1067 Available at: http://www.ncbi.nlm.nih.gov/pubmed/28598354 [Accessed November 30, 2017].DOI: 10.3390/ijms18061067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckley JM, Wang JH, Redmond HP. Cellular reprogramming by gram-positive bacterial components: a review. J. Leukoc. Biol 2006; 80:731–741. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16885502 [Accessed May 3, 2018].DOI: 10.1189/jlb.0506312. [DOI] [PubMed] [Google Scholar]
- 3.Ryu YH, Baik JE, Yang JS, Kang SS, Im J, Yun CH, Kim DW, et al. Differential immunostimulatory effects of Gram-positive bacteria due to their lipoteichoic acids. Int. Immunopharmacol 2009; 9:127–133. Available at: 10.1016/j.intimp.2008.10.014.DOI: 10.1016/j.intimp.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Kim HG, Kim N-R, Gim MG, Lee JM, Lee SY, Ko MY, Kim JY, et al. Lipoteichoic acid isolated from Lactobacillus plantarum inhibits lipopolysaccharide-induced TNF-alpha production in THP-1 cells and endotoxin shock in mice. J. Immunol 2008; 180:2553–61. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18250466 [Accessed December 1, 2017]. [DOI] [PubMed] [Google Scholar]
- 5.Molina MA, Díaz AM, Hesse C, Ginter W, Gentilini MV, Nuñez GG, Canellada AM, et al. Immunostimulatory Effects Triggered by Enterococcus faecalis CECT7121 Probiotic Strain Involve Activation of Dendritic Cells and Interferon-Gamma Production Bayry J, ed. PLoS One. 2015; 10:e0127262 Available at: http://www.ncbi.nlm.nih.gov/pubmed/25978357 [Accessed February 6, 2018].DOI: 10.1371/journal.pone.0127262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, Yoshikai Y. Lipoteichoic Acids from Lactobacillus Strains Elicit Strong Tumor Necrosis Factor Alpha-Inducing Activities in Macrophages through Toll-Like Receptor 2. Clin. Diagn. Lab. Immunol 2003; 10:259–266. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC150522/%5Cnhttp://www.ncbi.nlm.nih.gov/pmc/articles/PMC150522/pdf/0008.pdf.DOI: 10.1128/CDLI.10.2.259-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher MS, Kripke ML. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc. Natl. Acad. Sci. U. S. A 1977; 74:1688–92. Available at: http://www.ncbi.nlm.nih.gov/pubmed/300876 [Accessed February 3, 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J. Natl. Cancer Inst 1974; 53:1333–6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/4139281 [Accessed May 5, 2017]. [DOI] [PubMed] [Google Scholar]
- 9.Kripke ML, Fisher MS. Immunologic parameters of ultraviolet carcinogenesis. J. Natl. Cancer Inst 1976; 57:211–5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1003502 [Accessed May 5, 2017]. [DOI] [PubMed] [Google Scholar]
- 10.Byrne SN, Halliday GM. B Cells Activated in Lymph Nodes in Response to Ultraviolet Irradiation or by Interleukin-10 Inhibit Dendritic Cell Induction of Immunity. J. Invest. Dermatol 2005; 124:570–578. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15737198 [Accessed December 19, 2017].DOI: 10.1111/j.0022-202X.2005.23615.x. [DOI] [PubMed] [Google Scholar]
- 11.Moodycliffe AM, Kimber I, Norval M. Role of tumour necrosis factor-alpha in ultraviolet B light-induced dendritic cell migration and suppression of contact hypersensitivity. Immunology. 1994; 81:79–84. [PMC free article] [PubMed] [Google Scholar]
- 12.Lappin MB, Kimber I, Dearman RJ, Norval M. Exposure of UVB-sensitive mice to immunosuppressive doses of UVB in vivo fails to affect the accessory function or the phenotype of draining lymph node dendritic cells. Exp. Dermatol 1996; 5:286–94. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8981028 [Accessed May 3, 2018]. [DOI] [PubMed] [Google Scholar]
- 13.Weill FS, Cela EM, Paz ML, Ferrari A, Leoni J, González Maglio DH. Lipoteichoic acid from Lactobacillus rhamnosus GG as an oral photoprotective agent against UV-induced carcinogenesis. Br. J. Nutr 2013; 109:457–66. Available at: http://journals.cambridge.org/abstract_S0007114512001225.DOI: 10.1017/S0007114512001225. [DOI] [PubMed] [Google Scholar]
- 14.Kurimoto I, Streilein JW. Deleterious effects of cis-urocanic acid and UVB radiation on Langerhans cells and on induction of contact hypersensitivity are mediated by tumor necrosis factor-alpha. J. Invest. Dermatol 1992; 99:69S–70S. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1431236 [Accessed January 2, 2018]. [DOI] [PubMed] [Google Scholar]
- 15.Simon JC, Tigelaar RE, Bergstresser PR, Edelbaum D, Cruz PD. Ultraviolet B radiation converts Langerhans cells from immunogenic to tolerogenic antigen-presenting cells. Induction of specific clonal anergy in CD4+ T helper 1 cells. J. Immunol 1991; 146:485–91. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1670944 [Accessed December 20, 2017]. [PubMed] [Google Scholar]
- 16.Aberer W, Schuler G, Stingl G, Hönigsmann H, Wolff K. Ultraviolet light depletes surface markers of Langerhans cells. J. Invest. Dermatol 1981; 76:202–10. Available at: http://www.ncbi.nlm.nih.gov/pubmed/6453905 [Accessed December 20, 2017]. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz A, Noordegraaf M, Maeda A, Torii K, Clausen BE, Schwarz T. Langerhans Cells Are Required for UVR-Induced Immunosuppression. J. Invest. Dermatol 2010; 130:1419–1427. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20090769 [Accessed December 20, 2017].DOI: 10.1038/jid.2009.429. [DOI] [PubMed] [Google Scholar]
- 18.Noordegraaf M, Flacher V, Stoitzner P, Clausen BE. Functional redundancy of langerhans cells and langerin+ dermal dendritic cells in contact hypersensitivity. J. Invest. Dermatol 2010; 130:2752–2759.DOI: 10.1038/jid.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, et al. Migratory Dendritic Cells Transfer Antigen to a Lymph Node-Resident Dendritic Cell Population for Efficient CTL Priming. Immunity. 2006; 25:153–162. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16860764 [Accessed November 27, 2017].DOI: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz T Photoimmunosuppression. Photodermatol. Photoimmunol. Photomed 2002; 18:141–5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12207678 [Accessed October 23, 2018]. [DOI] [PubMed] [Google Scholar]
- 21.Howarth GS. Probiotic-Derived Factors: Probiotaceuticals? J. Nutr 2010; 140:229–230. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20018806 [Accessed February 15, 2017].DOI: 10.3945/jn.109.118844. [DOI] [PubMed] [Google Scholar]
- 22.Morath S, von Aulock S, Hartung T. Structure/function relationships of lipoteichoic acids. J. Endotoxin Res. 2005; 11:348–356. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16303090.DOI: 10.1179/096805105X67328. [DOI] [PubMed] [Google Scholar]
- 23.Cela EM, Friedrich A, Paz ML, Vanzulli SI, Leoni J, Gonzalez Maglio DH. Time-course study of different innate immune mediators produced by UV-irradiated skin: Comparative effects of short and daily versus a single harmful UV exposure. Immunology. 2015; 145:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guéniche A, Philippe D, Bastien P, Blum S, Buyukpamukcu E, Castiel-Higounenc I. Probiotics for photoprotection. Dermatoendocrinol. 2009; 1:275–279.DOI: 10.4161/derm.1.5.9849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.