Abstract
Necrosis is a pathologic form of cell death that induces an inflammatory response, and immune cell activation contributes to the development and maintenance of hypertension. Necrosis was measured in kidney, spleen and aorta of 12–13 week old male and female spontaneously hypertensive rats (SHR); male SHR had greater renal necrotic cell death than female SHR. Because male SHR have a higher blood pressure (BP) and a more pro-inflammatory T cell profile than female SHR, the current studies tested the hypothesis that greater necrotic cell death in male SHR exacerbates increases in BP and contributes to the pro-inflammatory T cell profile. Male and female SHR were randomized to receive vehicle or Necrox-5, a cell permeable inhibitor of necrosis, from 6–12 weeks of age or from 11–13 weeks of age. In both studies, Necrox-5 decreased renal necrosis and abolished the sex difference. Treatment with Necrox-5 beginning at 6 weeks of age attenuated maturation-induced increases in BP in male SHR; BP in female SHR was not altered by Necrox-5 treatment. Necrox-5 decreased pro-inflammatory renal T cells in both sexes, although sex differences were maintained. Administration of Necrox-5 for 2 weeks in SHR with established hypertension resulted in a small, but significant decrease in BP in males with no effect in females. These results suggest that greater necrotic cell death in male SHR exacerbates maturation-induced increases in BP with age contributing to sex differences in BP. Moreover, although necrosis is pro-inflammatory, it is unlikely to explain sex differences in the renal T cell profile.
Keywords: Necrox5, cell death, T cells, blood pressure, sex differences, kidney
Introduction
Hypertensive stimuli induce cell death 1–3 and vascular necrosis is a hallmark of malignant hypertension 4–6. The role of cell death in blood pressure (BP) control however, is unknown. While not extensively studied in hypertension, there is considerable interest in the sex-specific roles of cell death in injury and recovery following stroke where males have greater levels of necrotic cell death compared to females7, 8. Sex differences in cell death pathways have also been reported in cell culture studies of cortical neurons and cardiomyocytes, where cells isolated from males are more susceptible to cell death compared to cells from females9, 10. Based on well-established sex differences in hypertension, where young females have lower BP values and are less likely to be hypertensive compared to their age-matched male counterparts, sex differences in necrotic cell death could contribute to sex differences in BP control.
Cell death causes immune activation, although the induction of an immune response is dictated by how a cell dies 11–18. Regulated cell death pathways can be broadly subdivided into two categories: apoptosis and necrosis. Necrosis is a pathologic process characterized by loss of plasma membrane integrity19. Necrotic cells release their contents initiating a pro-inflammatory “danger response” 11–14, 16. In contrast, apoptosis is a controlled process20 where phagocytosis of apoptotic cells prevents the release of cellular contents to suppress immune activation 21, 22. We have previously published that there is a sex difference in the renal T cell profile in hypertension 23–25. Male spontaneously hypertensive rats (SHR) have greater pro-inflammatory renal T cell infiltration and pro-inflammatory cytokine expression compared to normotensive male Wistar Kyoto (WKY) rats and female SHR. The mechanism driving enhanced pro-inflammatory T cell activation in males is unknown.
Although T cells contribute to hypertension in both male and female experimental animals, the mechanism initiating the inflammatory response in either sex remains a fundamental question in the field. The first goal of this study was to determine the impact of sex on cell death. We found greater necrotic cell death in male SHR compared to females. Therefore additional studies were designed to test the hypothesis that greater necrotic cell death in male SHR exacerbates increases in BP and contributes to the previously published pro-inflammatory renal T cell profile.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Animals
Male and female SHR (Envigo, Inc. Indianapolis, Indiana) were used in all studies. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the Augusta University Institutional Animal Care and Use Committee. Animals were housed under conditions of constant temperature and humidity and exposed to a 12:12-h light-dark cycle. All rats were given free access to rat chow and tap water. At the end of all studies, rats were anesthetized with isoflurane and a thoracotomy was performed. Terminal blood samples were obtained via aortic puncture and tissues were harvested for flow cytometric analysis of cell death and T cells (for details on flow cytometric analysis see online Supplement).
Necrox-5 Treatment to Prevent Hypertension
To determine the role of necrosis in the development of hypertension, 6 week old male and female SHR were randomized to receive Necrox-5 (1 mg/kg; Enzo Life Sciences) or vehicle treatment (methanesulfonic acid in deionized water, pH 3, Sigma-Aldrich) via twice weekly IP injection. BP was measured weekly via tail-cuff plethysmography as previously described 26 from 6 to 12 weeks of age (n=5–6). A separate group of male and female SHR treated with Necrox-5 or vehicle beginning at 6 weeks of age were implanted with telemetry devices at 9 weeks of age, allowed 1 week to recover and BP was continuously measured during the last 3 weeks of Necrox-5 treatment from 10–13 weeks of age (n=6). Female SHR do not survive telemetry implantation prior to 9 weeks of age. Necrox-5 is a cell permeable necrosis inhibitor that selectively inhibits oxidative stress induced necrotic cell death.
Necrox-5 Treatment to Treat Hypertension
To determine whether inhibition of necrosis affects established hypertension, Necrox-5 was administered for 2 weeks in SHR beginning at 11 weeks of age. 9 week old male and female SHR were implanted with telemetry devices for the continuous measurement of BP as previously described (n=6) 27. Rats were allowed 1 week of recovery and 1 week of baseline BP recording. At 11 weeks of age rats were randomized to Necrox-5 or vehicle treatment via intraperitoneal (IP) injection 5 days a week for 2 weeks.
Statistical Analysis
All data are expressed as mean ± SEM. Necrotic cell death in untreated male and female SHR was compared using a Student’s t-test. Renal necrotic cell death and T cells in vehicle and control treated SHR were analyzed by 2-way ANOVA. BP data within each sex were analyzed using repeated measures ANOVA with Tukey’s multiple comparisons test. Between group comparisons were made by 2-way ANOVA. For all comparisons, differences were statistically significant with p<0.05. Analyses were performed using GraphPad Prism Version 6.0 (GraphPad Software, La Jolla, CA).
Results
Male SHR have greater necrotic cell death than female SHR
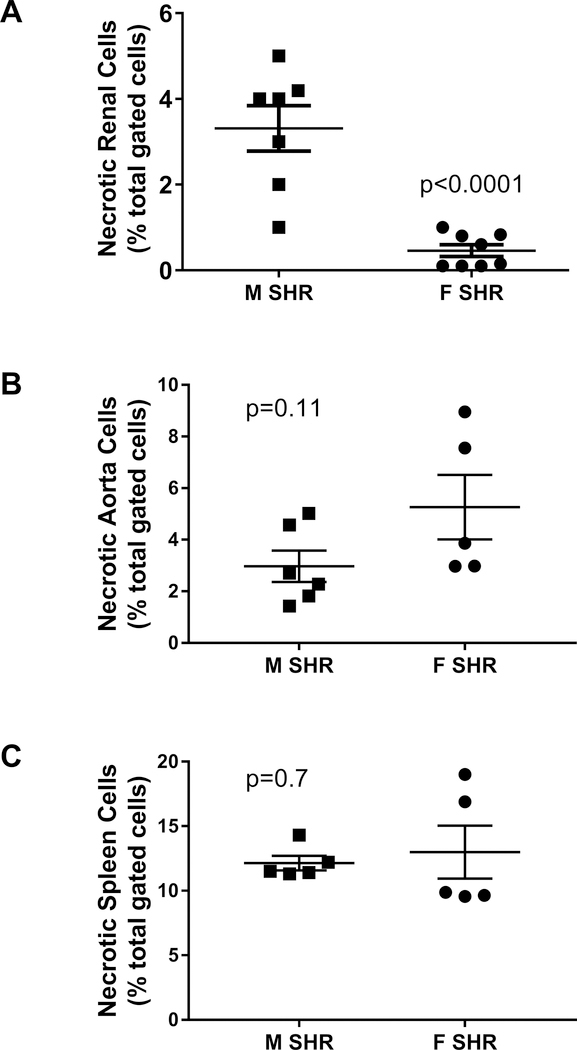
Initial studies measured necrotic cell death by flow cytometric analysis in kidneys, aorta and spleen of 13 week old male and female SHR (Figure 1). Male SHR had significantly greater renal necrotic cell death than female SHR (p<0.0001; Figure 1A). Necrotic cell death was comparable in aorta and spleen of young adult male and female SHR (p=0.11 and p=0.70, respectively; Figures 1B and 1C). To gain insight into the localization of renal cell death, kidneys were isolated from additional 13 week old male and female SHR and separated into cortex and medulla. Similar to the whole kidney, renal cortical and medullary cell death were greater in male SHR vs. female SHR (expressed as percent of total gated cells: cortex- 2.7±0.23 vs 1.2±0.13, p=0.0003; medulla- 4.3±0.22 vs 2.6±0.24, p=0.0003; n=6).
Figure 1:
Necrotic cell death measured by flow cytometric analysis in whole kidney (panel A), thoracic aorta with perivascular adipose tissue intact (panel B), and spleen (panel C) of 13 week old male (M) and female (F) spontaneously hypertensive rats (SHR). Data were compared via Student’s t-test; n=5–8.
Renal necrotic cell death was next measured in 6 week old SHR prior to the development of established hypertension and in 15 week old SHR with established hypertension. Renal necrosis was comparable between 6 week old male and female SHR (expressed as percent of total gated kidney cells: 2.4±0.2 and 1.8±0.1, respectively; n=6) and increased with age only in males (expressed as percent of total gated kidney cells: 5.4±0.2 and 1.7±0.3, respectively; effect of sex: p<0.0001; effect of age: p<0.0001; interaction: p<0.0001).
Inhibition of necrosis attenuates the development of hypertension in male SHR
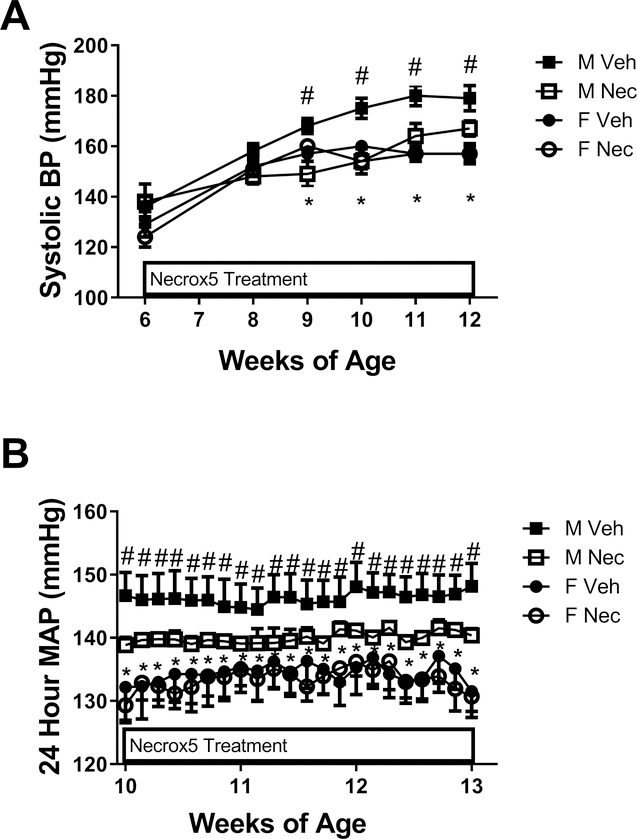
To determine the contribution of necrotic cell death to the development of hypertension in male and female SHR, rats were treated with Necrox-5 or vehicle beginning at 6 weeks of age prior to the development of sex differences in renal necrosis and BP was measured (Figure 2). Systolic BP was greater in male SHR compared to female SHR at baseline, and BP remained greater in males throughout the treatment period (p<0.05). Treatment with Necrox-5 attenuated the age-dependent increase in BP in male SHR when measured by tail-cuff (final systolic BP: 168±3 vs. 180±4 mmHg, respectively; p=0.078; Figure 2A) with no effect in females (final systolic BP: 157±3 vs. 158±4 mmHg; NS). A separate set of rats were randomized to vehicle or Necrox-5 treatment at 6 weeks of age then instrumented with telemetry devices for the continuous measurement of mean arterial pressure (MAP) beginning at 10 weeks of age. MAP was greater in males compared to females at both the beginning and the end of the treatment period (p<0.05). Consistent with the tail-cuff data, MAP was significantly lower in Necrox-5-treated male SHR compared to vehicle-treated rats at the end of the treatment period (final MAP: 149±2 vs. 140±1 mmHg, respectively; p=0.036; Figure 2B). MAP was not altered by Necrox5 in females (final MAP: 132±3 vs. 131±3 mmHg; NS).
Figure 2:
Blood pressure (BP) in male (M) and female (F) spontaneously hypertensive rats (SHR) treated with vehicle or Necrox-5 beginning at 6 weeks of age. Systolic blood pressure (BP) was measured by tail-cuff in M and F SHR treated with vehicle or Necrox-5 from 6 to 12 weeks of age (panel A; n=5–6). Mean arterial pressure (MAP) was measured in a separate set of M and F SHR treated with Necrox-5 or vehicle beginning at 6 weeks of age and implanted with telemetry devices at 9 weeks of age (panel B; n=6). Rats were allowed 1 week to recover and MAP was measured from 10–13 weeks of age. Data within each sex were analyzed using repeated measures ANOVA with Tukey’s multiple comparisons test and between group comparisons were made by 2-way ANOVA. # indicates p<0.05 vs. male Necrox-5 (treatment effect); * indicates p<0.05 vs. male SHR (sex effect).
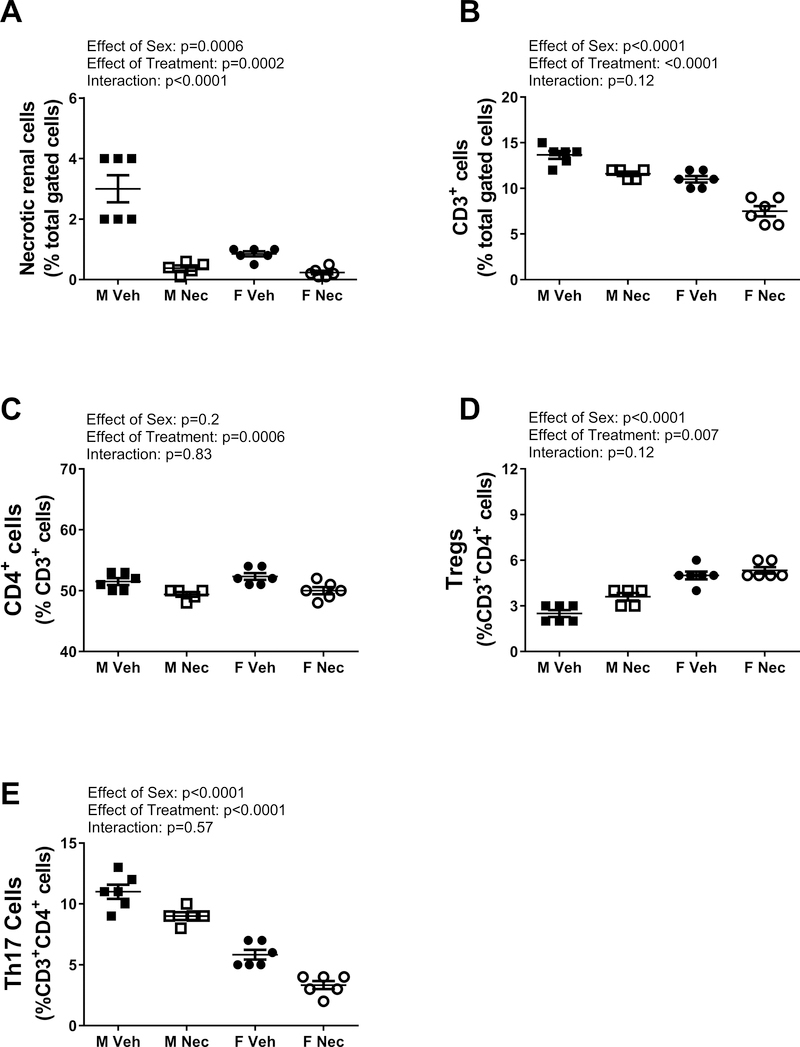
At the end of the treatment period, kidneys were collected to confirm the effectiveness of Necrox-5 to lower necrosis. Treatment with Necrox-5 for 6 weeks significantly decreased renal necrosis in both sexes (effect of treatment: p=0.0002; Figure 3A), although the effect was greater in males abolishing the sex difference (effect of sex: p= 0.0006; interaction: p<0.0001). To assess the selectivity of chronic Necrox-5 treatment for necrosis, renal apoptosis was also measured in vehicle and Necrox-5-treatred male and female SHR at the end of the treatment period. Renal apoptosis was reduced by Necrox-5 in both sexes (expressed as percent of total gated kidney cells: male vehicle: 4±0.3 vs. male Necrox-5: 3.2±0.2; female vehicle: 5.2±0.7 vs. female Necrox-5: 2.7±0.4; effect of treatment: p=0.003; n=5–6), and the decrease was comparable between the sexes (effect of sex: p=0.52; interaction: P=0.09).
Figure 3:
Necrotic cell death and T cells measured by flow cytometric analysis in whole kidney from male (M) and female (F) spontaneously hypertensive rats (SHR) treated with vehicle or Necrox-5 from 6 to 12 weeks of age. Necrotic cells (Panel A) and CD3+ T cells (panel B) are expressed as % total gated kidney cells; CD4+ T cells (panel C) are expressed as % CD3+ T cells; Tregs (panel D) and Th17 cells (panel E) are expressed as % CD3+CD4+ cells. Data were compared using a 2-way ANOVA; n=5–6.
Rats were placed in metabolic cages at the end of the treatment period to determine the impact of Necrox-5 on 24 hour food intake, water intake, and urine output. There were no effects of either sex or Necrox-5 treatment on food intake, water intake, or urine output (online Supplement Table S1). Males randomized to Necrox-5 treatment were smaller than vehicle control males at baseline, although final body weight in males, body weight in females and kidney weights were not altered by Necrox-5. However, males had greater body and kidney weights than females. Urinary protein and creatinine excretion, as well as plasma creatinine and GFR were also measured to assess renal function. There was no effect of Necrox-5 on any parameter of renal health, although males had greater protein and creatinine excretion than females (online Supplement Table S2).
Necrotic cell death is pro-inflammatory 13–16, therefore we also assessed the contribution of necrosis to the renal T cell profile (Figure 3). Treatment with Necrox-5 resulted in a significant decrease in total CD3+ renal T cells in both sexes (effect of treatment: p<0.0001; interaction: p=0.12; Figure 3B) as well as a decrease in CD4+ T cells (effect of treatment: p=0.0006; effect of sex: p=0.20; interaction: p=0.83; Figure 3C). Necrox-5 also significantly increased renal Tregs (effect of treatment: p=0.0067; interaction: p=0.12; Figure 3D) and decreased renal Th17 cells in both sexes (effect of treatment: p<0.0001; interaction: p=0.57; Figure 3E). Sex differences in CD3+ T cells (effect of sex: p<0.0001), Th17 cells (effect of sex: p<0.0001) and Tregs (effect of sex: p<0.0001) were not altered by chronic Necrox-5 treatment.
Inhibition of necrosis in established hypertension deceases blood pressure in adult male SHR
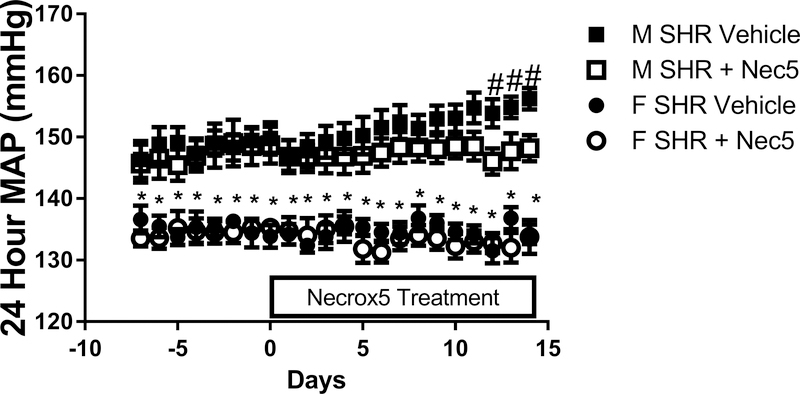
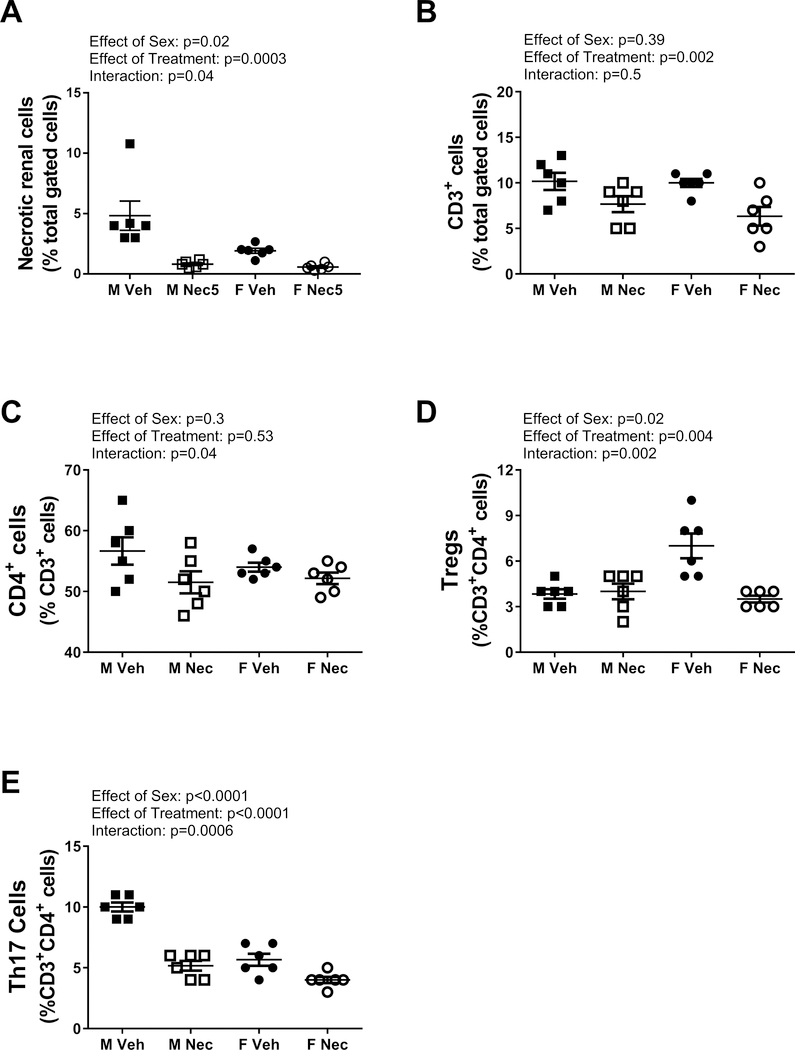
To determine the contribution of necrotic cell death to the maintenance of established hypertension in adult male and female SHR, rats were treated with Necrox-5 or vehicle from 11 to 13 weeks of age and BP was measured by telemetry (Figure 4). BP was greater in male SHR than female SHR at baseline and throughout the treatment period (p<0.001). BP increased during the course of the experiment in vehicle-treated control male SHR, and this effect was attenuated in Necrox-5-treated male SHR. As a result, at the end of the 2 week treatment period MAP was significantly less in the Necrox-5-treated male SHR vs. vehicle-treated males (final MAP: 156±2 vs. 148±2 mmHg, respectively; p=0.016). There was no effect of Necrox-5 on BP in young adult female SHR (final MAP: 134±2 vs. 133±3 mmHg; NS). At the end of the treatment period, we again confirmed that treatment with Necrox-5 decreased necrosis (effect of treatment: p=0.0003; figure 5A), and the effect was again greater in males compared to females abolishing the sex difference in renal necrosis (effect of sex: p= 0.02; interaction: p=0.04).
Figure 4:
24 hour mean arterial pressure (MAP) measured by telemetry in male (M) and female (F) spontaneously hypertensive rats (SHR) treated with vehicle or Necrox-5 from 11 to 13 weeks of age (panel A). Data within each sex were analyzed using repeated measures ANOVA with Tukey’s multiple comparisons test and between group comparisons were made by 2-way ANOVA; n=6. # indicates p<0.05 vs. male Necrox-5 (treatment effect); * indicates p<0.05 vs. male SHR (sex effect).
Figure 5:
Necrotic cell death and T cells measured by flow cytometric analysis in whole kidney from male (M) and female (F) spontaneously hypertensive rats (SHR) treated with vehicle or Necrox-5 from 11 to 13 weeks of age (panel A). Necrotic cells (Panel A) and CD3+ T cells (panel B) are expressed as % total gated kidney cells; CD4+ T cells (panel C) are expressed as % CD3+ T cells; Tregs (panel D) and Th17 cells (panel E) are expressed as % CD3+CD4+ cells. Data were compared using a 2-way ANOVA; n=6.
The renal T cell profile was assessed following 2 weeks of Necrox-5 treatment (Figure 5). Necrox-5 resulted in a significant decrease in total CD3+ renal T cells in both sexes (effect of treatment: p=0.002; effect of sex: p=0.39; interaction: p=0.50; Figure 5B) and a decrease in renal CD4+ T cells only in male SHR (effect of treatment: p=0.53; effect of sex: p=0.3; interaction: p=0.04; Figure 5C). Necrox-5 significantly decreased pro-inflammatory Th17 cells, and the decrease was greater in males compared to females abolishing the sex difference (effect of treatment: p<0.0001; effect of sex: p<0.0001; interaction: P=0.0006; Figure 5D). In contrast, anti-inflammatory Tregs were decreased with 2 weeks of Necrox-5 only in female SHR, also abolishing the sex difference (effect of treatment: p=0.004; effect of sex: p=0.018; interaction: p=0.002; Figure 5E).
Discussion
The main finding from this study is that greater necrotic cell death contributes to the onset and maintenance of hypertension in male SHR. These results support our hypothesis that cell death is an early event that occurs in response to hypertensive stimuli that leads to further, sustained increases in BP. Moreover, males appear to be more susceptible to necrotic cell death than females and this may be an important mechanism contributing to established sex differences in BP in young adult SHR.
It is well established that there is a sex difference in BP in young adult SHR, with males having a higher BP compared to age-matched females28, 29. The mechanism(s) mediating sex differences in BP have been highly investigated and many pathways have been implicated underscoring the complex nature of BP control. In the current study, we have identified cell death as an additional factor that contributes to BP control in a sex-specific manner. Indeed, sex differences in cell death may represent a unifying mechanism underlying sex differences in many hypertensive stimuli including angiotensin II and oxidative stress 30–32. Studies in the 1960’s noted that malignant hypertension was associated with the development of necrotic lesions in the vasculature3, 6, and later autopsy studies in humans found that the necrotic lesions were most common in the kidneys and gastrointestinal tract4. However, little has been done to examine cell death in essential hypertension. In the current study we found modest amounts of necrotic cell death in the kidney, aorta, and spleen of young adult SHR, with a sex difference only noted in the kidney of adult SHR with hypertension. Furthermore, systemic administration of an inhibitor of necrosis lowered BP only in males, making it tempting to speculate that renal necrosis specifically contributes to increases in BP in male SHR. Indeed, correlation analyses performed between MAP and levels of renal necrosis showed a positive significant correlation between MAP and renal necrosis, and this positive correlation was most evident in the males (Supplementary figure S3). The decrease in BP in male SHR with Necrox-5 was not accompanied by a decrease in indices of renal injury, although this is not surprising as young adult SHR are not a model of renal injury29, 33, 34.
Inhibition of necrosis did not completely prevent increases in BP with maturation in males or abolish the sex difference in BP, although our findings support the hypothesis that necrotic cell death is a novel pathway contributing to the development of hypertension in male SHR. Consistent with the current findings, treatment of male SHR with chloroquine to inhibit endosomal TLR signaling by circulating mitochondrial DNA attenuates age-related increases in systolic BP with no effect on systolic BP in established hypertension35. Taken together, these data lend support to the notion that cell death may be a central pathway involved in the development of hypertension in male SHR. We propose that necrotic cell death and the subsequent release of danger associated molecular patterns, including mitochondrial DNA, is an early event that contributes to increases of BP and the development of hypertension in male SHR. In contrast, inhibition of necrosis in established hypertension had a much smaller impact on BP, suggesting that inhibiting necrosis once hypertension has developed is of little utility. This finding may not be surprising if cell death is an early initiating event that underlies the activation of several additional pathways known to control BP culminating in the development of hypertension. As a result, preventing this cascade of events is much more impactful than attempting to reverse them.
Greater necrosis in male SHR is consistent with cell culture studies showing that isolated cells from males are more prone to necrotic cell death compared to cells from females, in part because cells from females show a better survival in response to a variety of stressors36–38. The reason for the sex difference in renal necrosis is unknown, although it may be related to sex differences in susceptibility of cells to cell death and/or cell death machinery. Males have been reported to have higher levels of RIP1, a kinase involved in necroptosis, and less caspase 3, a protein controlling apoptosis, compared to females following brain injury39. Estrogens have been implicated in conferring protection against cell death via activation of Fas/FasL in bone40 and inhibition of PARP1 in kidneys41. Alternatively, studies have identified intrinsic peptides that confer protection against cell death, including renalase. Renalase protects against renal tubular and myocardial cell death and, of relevance to the current study, renalase levels are reduced in experimental models of hypertension42, 43. Future studies will be designed to identify the cells that are most susceptible cell death in hypertension and assess potential sex differences on cell death machinery in SHR.
Necrosis is associated with the loss of the plasma membrane integrity which initiates an inflammatory response and promotes immune cell infiltration17, 18. We examined splenic, aortic, and renal cell death in the current study. The spleen is the largest lymphoid organ in the body and plays a fundamental role in the maintenance of immune homeostasis which is now known to contribute to the development of hypertension, and vascular inflammation and dysfunction contributes to the development of hypertension44–46. However, we observed a sex difference in necrotic cell death only in the kidney of adult SHR. Moreover, the absence of a sex difference in renal necrosis in young, pre-hypertensive SHR makes it tempting to speculate that renal necrosis in particular is important in BP control in SHR. Recent studies have established that increases in renal perfusion pressure are required for renal T cell infiltration in male Dahl rats47. Increases in renal perfusion pressure can result in baurotrauma and initiate cell death, leading to immune cell infiltration/activation and increases in BP. These studies would suggest that renal cell death may be central to the development of hypertension; future studies are planned to directly address this hypothesis.
Renal T cell infiltration has been shown to increase BP and we previously published that there is a sex difference in renal T cells in SHR where males have more pro-inflammatory Th17 cells, while females have more anti-inflammatory Tregs. The mechanism mediating sex differences in the renal T cell profile is unknown. Therefore additional studies measured the renal T cell profile following Necrox-5 treatment since greater necrotic cell death in male SHR is associated with a more pro-inflammatory renal T cell profile in the current study. Both Necrox-5 treatment protocols decreased total renal T cells and pro-inflammatory Th17 cells in both sexes. Despite male SHR having greater necrosis, inhibition of necrosis did not have a greater impact on the renal T cell profile in males and sex differences in renal Th17 cells were maintained following chronic Necrox-5 treatment. Therefore, while necrotic cell death contributes to the increase in pro-inflammatory T cells in both sexes, this does not mediate the sex difference in the renal T cell profile. Interestingly, the acute (2 week) treatment with Necrox-5 abolished the sex difference in Th17 cells with minimal impact on BP in males and no change in BP in the female. This suggests that in established hypertension, renal necrosis contributes more to the renal pro-inflammatory T cell profile than it does to the control of BP. Although the 2 week treatment period did not induce large changes in resting BP, physiological responses to any challenge that activates the immune system may be altered.
Tregs are anti-inflammatory and anti-hypertensive, and we previously reported that female SHR have a BP-dependent increase in Tregs that is not observed in males24. In the current study, the sex difference in Tregs was maintained with chronic inhibition of necrosis. In contrast, 2 weeks of Necrox-5 treatment abolished the sex difference by decreasing Tregs preferentially in female SHR. Tregs have been shown to confer protection against cell death and tissue injury as well as promote tissue repair and proliferation 48, therefore it is possible that greater Tregs in females attenuates cell death relative to males. Moreover, females tend to be more resilient to tissue injury than males and depletion of Tregs is associated with more severe injury in females compared to their male counterparts, further supporting a key role for Tregs in the female 48. The finding that 2 weeks of Necrox-5 treatment in young adult female SHR decreased Tregs with no effect on BP leads us to speculate the female SHR respond to cell death by upregulating Tregs to limit injury. Since Necrox-5 lowered necrosis in females as well as males, the need for Tregs was lessened and renal Tregs were decreased. Future studies will test this hypothesis.
It should be noted that the 2 weeks of treatment with Necrox-5 in the established hypertension experiment was relatively short compared to the 6 week treatment protocol. It is possible that longer treatment times could have resulted in a more pronounced effect on BP. Apoptosis was also measured in rats chronically treated with Necrox-5 to assess the selectivity of the drug for necrotic cell death since in the heart and neutrophils reducing necrosis increases apoptosis49, 50. However, Necrox-5 resulted in a significant decrease in apoptosis as well as necrosis in both male and female SHR. While it is possible that the BP-lowering effect of Necrox-5 may also be related to the decrease in apoptosis, apoptosis is largely thought to be a protective form of cell death51, 52 therefore it is unlikely that decreases in apoptosis in males would decrease BP. In addition, BP in females was unaffected by Necrox-5 despite females tending to have greater decreases in apoptosis. However, future studies will be designed to specifically test the contribution of apoptotic cell death to BP control.
Perspectives
Despite all of the currently available anti-hypertensive medications on the market, control of BP in patients with essential hypertension remains sub-optimal. In this study we identified a novel role for necrosis in mediating age-related increases in BP in male SHR. Further studies are needed to unveil the molecular mechanisms underlying the sex difference observed in cell death profile in order to provide an additional target for a better control of BP in the hypertensive population.
Supplementary Material
Novelty and Significance.
1). What Is New
Although it has been known for decades that hypertensive stimuli can induce barotrauma and localized cell death, the impact of cell death on the development of hypertension has not been examined. The current studies establish a role for necrotic cell death in the development of hypertension in male, but not female, hypertensive rats.
2). What Is Relevant?
The mechanisms that drive the development of hypertension in either sex remain unknown. Greater understanding of the initiating events that increase blood pressure can lead to the development of more effective therapeutics in both men and women with hypertension. The current study identifies a novel role for necrosis in the development of hypertension in males.
3). Summary
The main finding from this study is that greater necrotic cell death contributes to the onset and maintenance of hypertension in male SHR. Moreover, males appear to be more susceptible to necrotic cell death than females and this may be an important mechanism contributing to established sex differences in BP in young adult SHR.
Acknowledgments
We would like to acknowledge the excellent technical assistance of Katherine Hatcher for telemetry implantation surgeries.
Source(s) of Funding: The work was funded by the National Institute of Health (R01HL127091 and P01HL134604 to J.C.S.) and the American Heart Association (17EIA33410565 to J.C.S).
Footnotes
Conflict(s) of Interest/Disclosure(s)
None
References
- 1.Sasaki Y, Ikeda Y, Iwabayashi M, Akasaki Y, Ohishi M. The impact of autophagy on cardiovascular senescence and diseases. International heart journal. 2017;58:666–673 [DOI] [PubMed] [Google Scholar]
- 2.Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: Cell survival and cell death. International journal of cell biology. 2010;2010:214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner DL. Arteriolar necrosis and the prenecrotic phase of experimental hypertension. Quarterly journal of experimental physiology and cognate medical sciences. 1963;48:156–163 [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson F Hypertensive arteriolar necrosis revisited. Blood pressure. 1997;6:71–77 [DOI] [PubMed] [Google Scholar]
- 5.Kerenyi T, Jellinek H, Huttner I, Goracz G, Konyar E. Fibrinoid necrosis of the vascular wall in experimental malignant hypertension. Acta morphologica Academiae Scientiarum Hungaricae. 1966;14:175–182 [PubMed] [Google Scholar]
- 6.Schaffenburg C, Goldblatt H. Pathogenesis of arteriolar necrosis of malignant hypertension. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.). 1957;96:421–423 [DOI] [PubMed] [Google Scholar]
- 7.Spychala MS, Honarpisheh P, McCullough LD. Sex differences in neuroinflammation and neuroprotection in ischemic stroke. Journal of neuroscience research. 2017;95:462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rafikov R, Nair V, Sinari S, Babu H, Sullivan JC, Yuan JX, Desai AA, Rafikova O. Gender difference in damage-mediated signaling contributes to pulmonary arterial hypertension. Antioxidants & redox signaling. 2019. March 20. doi: 10.1089/ars.2018.7664. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairbanks SL, Young JM, Nelson JW, Davis CM, Koerner IP, Alkayed NJ. Mechanism of the sex difference in neuronal ischemic cell death. Neuroscience. 2012;219:183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, He Q, Sun Y, Dai X, Yang XP. Female adult mouse cardiomyocytes are protected against oxidative stress. Hypertension (Dallas, Tex. : 1979). 2010;55:1172–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez Fernandez D, Lamkanfi M. Inflammatory caspases: Key regulators of inflammation and cell death. Biological chemistry. 2015;396:193–203 [DOI] [PubMed] [Google Scholar]
- 12.Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell death and differentiation. 2018;25:486–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidovich P, Kearney CJ, Martin SJ. Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biological chemistry. 2014;395:1163–1171 [DOI] [PubMed] [Google Scholar]
- 14.Linkermann A, Stockwell BR, Krautwald S, Anders HJ. Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nature reviews. Immunology. 2014;14:759–767 [DOI] [PubMed] [Google Scholar]
- 15.Tonnus W, Linkermann A. The in vivo evidence for regulated necrosis. Immunological reviews. 2017;277:128–149 [DOI] [PubMed] [Google Scholar]
- 16.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein hmgb1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195 [DOI] [PubMed] [Google Scholar]
- 17.Nagata S, Tanaka M. Programmed cell death and the immune system. Nature reviews. Immunology. 2017;17:333–340 [DOI] [PubMed] [Google Scholar]
- 18.Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nature reviews. Immunology. 2017;17:262–275 [DOI] [PubMed] [Google Scholar]
- 19.Golstein P, Kroemer G. Cell death by necrosis: Towards a molecular definition. Trends in biochemical sciences. 2007;32:37–43 [DOI] [PubMed] [Google Scholar]
- 20.Ortiz A, Justo P, Catalan MP, Sanz AB, Lorz C, Egido J. Apoptotic cell death in renal injury: The rationale for intervention. Current drug targets. Immune, endocrine and metabolic disorders. 2002;2:181–192 [PubMed] [Google Scholar]
- 21.Getts DR, McCarthy DP, Miller SD. Exploiting apoptosis for therapeutic tolerance induction. Journal of immunology (Baltimore, Md. : 1950). 2013;191:5341–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luan YY, Yin CF, Qin QH, Dong N, Zhu XM, Sheng ZY, Zhang QH, Yao YM. Effect of regulatory t cells on promoting apoptosis of t lymphocyte and its regulatory mechanism in sepsis. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2015;35:969–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brinson KN, Elmarakby AA, Tipton AJ, Crislip GR, Yamamoto T, Baban B, Sullivan JC. Female shr have greater blood pressure sensitivity and renal t cell infiltration following chronic nos inhibition than males. American journal of physiology. Regulatory, integrative and comparative physiology. 2013;305:R701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory t lymphocyte infiltration than males. American journal of physiology. Regulatory, integrative and comparative physiology. 2012;303:R359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory t cells in response to elevations in blood pressure. Hypertension (Dallas, Tex. : 1979). 2014;64:557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollock DM, Polakowski JS, Opgenorth TJ, Pollock JS. Role of endothelin eta receptors in the hypertension produced by 4-day l-nitroarginine methyl ester and cyclosporine treatment. European journal of pharmacology. 1998;346:43–50 [DOI] [PubMed] [Google Scholar]
- 27.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol. 2001;281:F144–150 [DOI] [PubMed] [Google Scholar]
- 28.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;293:R1573–1579 [DOI] [PubMed] [Google Scholar]
- 29.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1–7) receptor antagonism equalizes angiotensin ii-induced hypertension in male and female spontaneously hypertensive rats. Hypertension (Dallas, Tex. : 1979). 2010;56:658–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clinical and experimental pharmacology & physiology. 2007;34:938–945 [DOI] [PubMed] [Google Scholar]
- 31.Bhatia K, Elmarakby AA, El-Remessy AB, Sullivan JC. Oxidative stress contributes to sex differences in angiotensin ii-mediated hypertension in spontaneously hypertensive rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2012;302:R274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan JC. Sex and the renin-angiotensin system: Inequality between the sexes in response to ras stimulation and inhibition. American journal of physiology. Regulatory, integrative and comparative physiology. 2008;294:R1220–1226 [DOI] [PubMed] [Google Scholar]
- 33.Griffin KA. Hypertensive kidney injury and the progression of chronic kidney disease. Hypertension (Dallas, Tex. : 1979). 2017;70:687–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braun MC, Herring SM, Gokul N, Monita M, Bell R, Hicks MJ, Wenderfer SE, Doris PA. Hypertensive renal disease: Susceptibility and resistance in inbred hypertensive rat lines. Journal of hypertension. 2013;31:2050–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy CG, Wenceslau CF, Goulopoulou S, Baban B, Matsumoto T, Webb RC. Chloroquine suppresses the development of hypertension in spontaneously hypertensive rats. American journal of hypertension. 2017;30:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. The Journal of biological chemistry. 2004;279:38563–38570 [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Li PP, Feng X, Barker JL, Smith SV, Rubinow DR. Sex-related differences in neuronal cell survival and signaling in rats. Neuroscience letters. 2003;337:65–68 [DOI] [PubMed] [Google Scholar]
- 38.Giampietri C, Petrungaro S, Filippini A, Ziparo E. Sex-related differences in death control of somatic cells. Journal of cellular and molecular medicine. 2013;17:550–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Y, Liu W, Koch LG, Britton SL, Keep RF, Xi G, Hua Y. Susceptibility to intracerebral hemorrhage-induced brain injury segregates with low aerobic capacity in rats. Neurobiology of disease. 2013;49:22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura T, Imai Y, Matsumoto T, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of fas ligand in osteoclasts. Cell. 2007;130:811–823 [DOI] [PubMed] [Google Scholar]
- 41.Jog NR, Caricchio R. Differential regulation of cell death programs in males and females by poly (adp-ribose) polymerase-1 and 17beta estradiol. Cell death & disease. 2013;4:e758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Xie Z, Lin M, Huang R, Liang Z, Huang W, Jiang W. Renalase protects the cardiomyocytes of sprague-dawley rats against ischemia and reperfusion injury by reducing myocardial cell necrosis and apoptosis. Kidney & blood pressure research. 2015;40:215–222 [DOI] [PubMed] [Google Scholar]
- 43.Fedchenko V, Globa A, Buneeva O, Medvedev A. Renalase mrna levels in the brain, heart, and kidneys of spontaneously hypertensive rats with moderate and high hypertension. Medical science monitor basic research. 2013;19:267–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. The Journal of experimental medicine. 2018;215:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikolajczyk TP, Nosalski R, Szczepaniak P, Budzyn K, Osmenda G, Skiba D, Sagan A, Wu J, Vinh A, Marvar PJ, Guzik B, Podolec J, Drummond G, Lob HE, Harrison DG, Guzik TJ. Role of chemokine rantes in the regulation of perivascular inflammation, t-cell accumulation, and vascular dysfunction in hypertension. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30:1987–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nosalski R, Guzik TJ. Perivascular adipose tissue inflammation in vascular disease. British journal of pharmacology. 2017;174:3496–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW Jr. Increased perfusion pressure drives renal t-cell infiltration in the dahl salt-sensitive rat. Hypertension (Dallas, Tex. : 1979). 2017;70:543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang C, Li L, Feng K, Fan D, Xue W, Lu J. ‘Repair’ treg cells in tissue injury. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;43:2155–2169 [DOI] [PubMed] [Google Scholar]
- 49.Zhe-Wei S, Li-Sha G, Yue-Chun L. The role of necroptosis in cardiovascular disease. Frontiers in pharmacology. 2018;9:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jie H, He Y, Huang X, Zhou Q, Han Y, Li X, Bai Y, Sun E. Necrostatin-1 enhances the resolution of inflammation by specifically inducing neutrophil apoptosis. Oncotarget. 2016;7:19367–19381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szondy Z, Sarang Z, Kiss B, Garabuczi E, Koroskenyi K. Anti-inflammatory mechanisms triggered by apoptotic cells during their clearance. Frontiers in immunology. 2017;8:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin SJ, Henry CM, Cullen SP. A perspective on mammalian caspases as positive and negative regulators of inflammation. Molecular cell. 2012;46:387–397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.