Abstract
Nasopharyngeal adenocarcinomas are rare tumours, and include neoplasms arising from the nasopharyngeal surface epithelium as well as those of minor salivary gland origin, each of which is distinct from the other. The former encompasses nasopharyngeal papillary adenocarcinoma (NPAC), also known as low grade NPAC and thyroid-like NPAC, an extremely unusual malignancy bearing histomorphological similarity to papillary thyroid carcinoma, and displaying indolent clinical behaviour. We report the case of a 41-year-old lady who developed NPAC as a second malignancy five-and-a-half years after being diagnosed and treated for a diffuse astrocytoma in the frontal lobe. In addition, we discuss the differential diagnosis, as well as raise certain pathogenetic considerations with regard to this unique neoplasm.
Keywords: Nasopharyngeal carcinoma, Adenocarcinoma, Papillary carcinoma, Radiotherapy, Glioma
Introduction
The nasopharynx is host to a wide range of malignancies, including epithelial, mesenchymal and hematolymphoid neoplasms. Among these, the majority are carcinomas, including keratinizing and non-keratinizing types [1]. Nasopharyngeal adenocarcinomas are rare tumors, accounting for less than 0.5% of nasopharyngeal malignancies [2–4]. They include nasopharyngeal papillary adenocarcinoma (NPAC), also known as low grade NPAC and thyroid-like NPAC, which originates from the nasopharyngeal surface epithelium, as well as adenocarcinomas of salivary gland origin. NPAC is a low grade malignancy bearing histomorphological similarity to papillary thyroid carcinoma (PTC), of which 41 cases have been reported in English literature till date. Most reported cases are from East Asian countries, with only one case from the Indian subcontinent [5]. We report the first case of this unusual tumor which occurred in a patient with previous history of a brain tumor, for which she received radiation therapy.
Case Report
This 41-year-old lady presented to the Otorhinolaryngology OPD with complaints of epistaxis, and fullness of the left ear for 1 month. The patient had previous history of a frontal diffuse astrocytoma, WHO grade II, for which she had been operated five and a half years ago, following which she had received external beam radiation therapy: 3D conformal radiotherapy was administered using 6 MV photon beam energy to left lateral and right anterior oblique fields in Linac CL 2300, which was planned with multileaf collimators. A total dose of 56 Gy was delivered in 28 fractions over 7 weeks. The tumor was extending to the left basifrontal region, suggesting that the upper nasopharyngeal mucosa would be exposed to radiation. Following this, the patient was on regular follow up with Neurosurgery, when she developed the present complaints.
On examination, a reddish, vascular growth was seen on the left side of posterior wall of nasopharynx protruding into the left choana (Fig. 1a, b). Cervical lymph nodes were not palpable. Non-contrast CT scan of nose and paranasal sinuses showed a lobulated heterogeneous mass in the nasopharynx. Contrast enhanced MRI showed a large, contrast-enhancing soft tissue lesion approximately 2.7 cm × 1.7 cm × 0.9 cm in size, involving the nasopharynx with minimal extension into the left parapharyngeal space (Fig. 1c–e). There was no extension into the sphenoid sinus, clivus, or intracranially. A biopsy was taken from the mass.
Fig. 1.
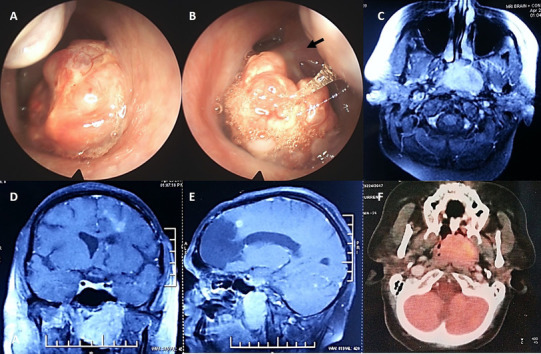
Clinical and Endoscopic images of right (a) and left nasal cavities (b) showing a large reddish, vascular mass almost filling bilateral choanae, attached to the left supero-lateral wall of nasopharynx with a stalk. T1-weighted contrast enhanced MRI of nose and brain revealed a large enhancing soft tissue lesion involving nasopharynx with minimal extension into left parapharyngeal space; encephalomalacia is noted in left frontal lobe (c, d, e). Axial PET CT revealed a FDG avid tumor completely occupying the nasopharynx with no abnormal uptake elsewhere (f)
Histological examination revealed an invasive carcinoma with papillary and glandular architecture (Fig. 2a). Areas of transition between the overlying respiratory epithelium and the tumor were present. Some of the papillae had hyalinised fibrovascular cores (Fig. 2b). Tumor cells lining the papillae were cuboidal with scant to moderate amount of eosinophilic cytoplasm, uniform round to ovoid pale nuclei with powdery chromatin, and inconspicuous nucleoli. Nuclear grooves and intranuclear cytoplasmic pseudo inclusions were identified in many of the nuclei (Fig. 2c, d). Mitoses were rare, and necrosis or calcification were not seen. On immunohistochemistry (Fig. 2e–h), tumor cells were immunopositive for pancytokeratin, epithelial membrane antigen (EMA), CK7, CK19 (focal), and S-100 (focal), while they were negative for CK5/6, p40, CK20, CD117, DOG1, TTF-1, thyroglobulin, and bcl-2. Ki-67 labelling index was low (3–4%). Based on the histomorphological and immunohistochemical features, a diagnosis of Nasopharyngeal papillary adenocarcinoma was made. Ultrasonographic examination of the thyroid did not reveal any lesion. Whole body PET CT showed a large, metabolically active, heterogeneously enhancing soft tissue mass lesion in the nasopharynx; there was no uptake in the neck, and no evidence of distant metastasis (Fig. 1f). The patient underwent excision of the tumor 3 months later. Histological examination revealed similar features as the biopsy (Fig. 2e, f). Post-operative period was uneventful, and the patient is on follow up with no evidence of disease 8 months later.
Fig. 2.
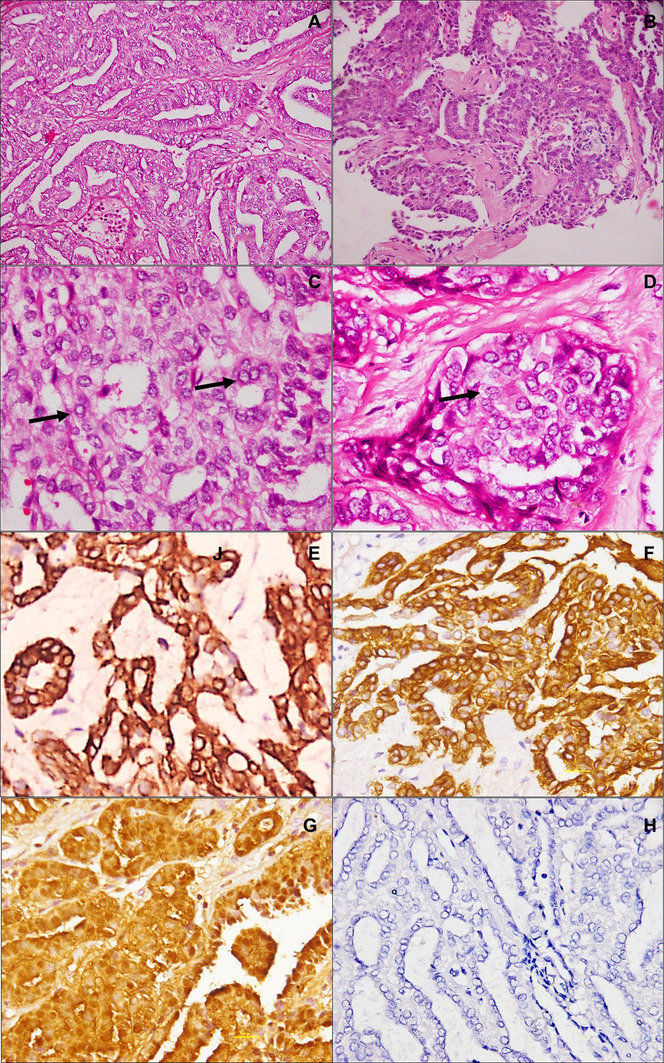
Histopathological features: Nasopharyngeal biopsy showed a tumor with papillary and glandular architecture (a; HE, ×200). Some of the papillae show hyalinized cores (b, HE, ×200). Intranuclear inclusions (c; HE, ×400) and nuclear grooves (d; HE, ×400) are seen. Tumor cells are positive for pancytokeratin (e; IHC, ×400), CK7 (f; IHC, ×400), and S100 (g; IHC, ×400), and negative for TTF-1 (h; IHC, ×200)
Discussion
NPAC was first described by Wenig et al. as a low grade malignant tumor of the nasopharynx, following which 41 cases have been reported in English literature, and around ten cases in Chinese literature [6]. Based on its characteristic morphological features and indolent clinical behaviour, it was recognized as a distinct entity by the WHO classification of tumors of the head and neck in 2005, under malignant epithelial neoplasms of the nasopharynx. It occurs over a wide age range from the first to seventh decades of life (age range: 9–64 years), with a median age of 34 years, and shows an almost equal gender distribution (M:F = 1.05) [2, 7–9]. Patients present with symptoms of nasal obstruction and epistaxis [10, 11]. Increasing awareness of NPAC brings unusual presentations of this tumor into the literature [3, 12]. Most tumors are located in the posterior and lateral walls of nasopharynx, and on the nasopharyngeal roof, where they occur as nodular or polypoid exophytic growths [5, 8–11, 13].
Differential diagnosis (Table 1) includes metastatic PTC, papillary adenocarcinomas of salivary gland origin such as polymorphous adenocarcinoma (PMA), and papillary variant of sinonasal intestinal-type adenocarcinoma (ITAC) [14, 15]. NPACs have a morphological resemblance to PTC, owing to their papillary architecture, nuclear features and presence of psammoma bodies. NPAC and PTC are both immunopositive for CK7, and TTF1 positivity has been described in approximately half the NPAC cases reported [15]. However, NPACs do not stain with thyroglobulin, while PTCs are diffusely positive, facilitating distinction between these two entities [3]. Papillary variant of ITAC differs from NPAC in its origin in a sinonasal location rather than in the nasopharynx, lack of nuclear features of PTC, greater nuclear atypia and mitotic activity, presence of mucinous cells, immunopositivity for CK20 and CDX2, and negativity with CK7 and TTF1 [15]. Salivary gland type adenocarcinomas like PMA occur primarily in the palatal region, but may be seen in the nasopharynx on occasion. They are seen in older patients and have a worse outcome, thus requiring adjuvant multimodality therapy unlike NPACs which are cured by surgical excision [16]. They arise in a submucosal location with no transition from the overlying epithelium, show diverse architectural patterns including papillary-cystic, lobular, cribriform and solid patterns, and they lack nuclear clearing. Nojeg et al. reported a case of papillary adenocarcinoma of the nasopharynx in a patient with Turner syndrome. However, the description of the locally destructive nature of this tumor and lack of response to adjuvant radiotherapy raises the possibility that it was of salivary gland origin, rather than a primary low grade NPAC [17].
Table 1.
Differential diagnosis of Nasopharyngeal papillary adenocarcinoma
| Nasopharyngeal papillary adenocarcinoma | PTC | PMA | Intestinal type adenocarcinoma-papillary variant | |
|---|---|---|---|---|
| Morphological features | Transition from overlying epithelium; Papillary and glandular architecture; Nuclear features of PTC; psammoma bodies+/ | Papillary and glandular architecture; Nuclear features of PTC; psammoma bodies± | Submucosal, separate from overlying epithelium; Multiple architectural patterns; Perineural invasion | Sinonasal location; Tubulo-papillary architecture; Mucinous/goblet cells; no nuclear features of PTC; Greater nuclear atypia |
| CK 7 | + | + | + | −/+ |
| CK 5/6 | − | − | ± | − |
| CK19 | + | + | − | + |
| CK 20 | − | − | − | + |
| TTF-1 | ± | + | − | − |
| Thyroglobulin | − | + | − | − |
| S-100 | ± | − | + | − |
| PAX8 | − | + | − | − |
| CDX-2, villin | − | − | − | + |
| Bcl2 | − | + | − | |
| Vimentin | ± | − | + | − |
| CD15 | − | + | − | −/+ |
| DOG1 | − | − | − | − |
The etiopathogenetic mechanisms responsible for development of NPAC remain to be elucidated. The role of Epstein Barr virus (EBV) in causation of nasopharyngeal carcinoma, particularly of the non-keratinizing histology, is well established. With this rationale, few authors have performed in situ hybridization for EBV in their cases of NPAC and failed to demonstrate the same, indicating that EBV has no role in the pathogenesis of these tumors [8, 15, 18, 19]. Considering the morphological similarity to PTC, Oide et al. performed sequencing for mutations in BRAF (exons 11 and 15) and N-RAS (codon 61) genes which are known to be mutated in PTC in a case of NPAC. However, the results were negative and they concluded that, although morphologically similar, NPAC and PTC do not share the same molecular pathogenesis [9]. Similarly, Oishi et al. reported negative immunohistochemical staining with BRAFV600E mutant antibody [13]. Exposure to radiation is a known risk factor for development of PTC. Our patient developed NPAC shortly after receiving radiation for a brain tumor. We therefore suggest that exposure to radiation could be a shared pathogenetic factor between these two histologically similar tumors. Our patient is the first reported case of NPAC to have prior exposure to radiation; therefore, more such reports are necessary to corroborate the role of radiation exposure with NPAC carcinogenesis.
NPAC is a slow-growing neoplasm whose prognosis is excellent, as compared to other nasopharyngeal adenocarcinomas with papillary architecture [2]. Surgical excision appears to be the treatment of choice [15]. No evidence of local recurrence or distant metastasis following complete excision has been described in cases reported in literature, with a median follow up period of 2 years (range: 3 months–15 years) [15]. Adjuvant radiotherapy has been suggested for incompletely resected tumors [6, 20]; however, its role is not established as these well differentiated tumors respond poorly to radiation.
Conclusion
NPAC is an extremely rare entity, the awareness of which is necessary among otorhinolaryngologists and head and neck surgeons in order to determine management strategies, and for prognostication of these patients. Pathologists also should be aware of this unusual neoplasm so as to avoid misdiagnosis as one of its more common morphological mimickers. An appropriate immunohistochemical panel is extremely useful in arriving at the correct diagnosis, and should be employed to supplement the characteristic morphological features. Lastly, patients receiving radiation therapy for malignant brain tumors should be followed up carefully and examined periodically for nasopharyngeal growths.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article is a case report and does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from the patient included in the study.
References
- 1.Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11:16–22. doi: 10.1007/s12105-017-0787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pineda``Daboin K, Neto A, Ochoa-Perez V, Luna MA. Nasopharyngeal adenocarcinomas: a clinicopathologic study of 44 cases including immunohistochemical features of 18 papillary phenotypes. Ann Diagn Pathol. 2006;10:215–221. doi: 10.1016/j.anndiagpath.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Horino T, Ichii O, Hamada-Ode K, Matsumoto T, Shimamura Y, Inoue K, Terada Y. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: a case report. Mol Clin Oncol. 2016;5:693–696. doi: 10.3892/mco.2016.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Yan H, Luo Y, Fan T. Low-grade nasopharyngeal papillary adenocarcinoma: a case report and review of the literature. Onco Targets Ther. 2016;9:2955–2959. doi: 10.2147/OTT.S100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajeswari B, Sukumaran Nair RK, Parukuttyamma K, Mathews A. Low-grade papillary adenocarcinoma of nasopharynx with expression of thyroid transcription factor-1: case report and review of literature. Indian J Pathol Microbiol. 2016;59:518–520. doi: 10.4103/0377-4929.191809. [DOI] [PubMed] [Google Scholar]
- 6.Wenig BM, Hyams VJ, Heffner DK. Nasopharyngeal papillary adenocarcinoma: a clinicopathologic study of a low-grade carcinoma. Am J Surg Pathol. 1988;12:946–953. doi: 10.1097/00000478-198812000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Carrizo F, Luna MA. Thyroid transcription factor-1 expression in thyroid-like nasopharyngeal papillary adenocarcinoma: report of 2 cases. Ann Diagn Pathol. 2005;9:189–192. doi: 10.1016/j.anndiagpath.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Fu CH, Chang KP, Ueng SH, Wu CC, Hao SP. Primary thyroid-like papillary adenocarcinoma of the nasopharynx. Auris Nasus Larynx. 2008;35:579–582. doi: 10.1016/j.anl.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Oide T, Kadosono O, Matsushima J, Wu D, Nagashima H, Saigusa H, Masunaga A, Nakatani Y, Hiroshima K. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma with squamous differentiation: a novel histological finding. Hum Pathol. 2017;7(17):30193–30194. doi: 10.1016/j.humpath.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Chu Y, Chung-Tai Y. Nasopharyngeal papillary adenocarcinoma: a case report and clinicopathologic review. Tzu Chi Med J. 2012;24:19–21. doi: 10.1016/j.tcmj.2011.11.003. [DOI] [Google Scholar]
- 11.Huang CH, Chang YL, Wang CP, Wu HP. Positive immunostaining of thyroid transcription factor-1 in primary nasopharyngeal papillary adenocarcinoma. J Formos Med Assoc. 2015;114:473–474. doi: 10.1016/j.jfma.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Petersson F, Pang B, Loke D, Hao L, Yan B. Biphasic low-grade nasopharyngeal papillary adenocarcinoma with a prominent spindle cell component: report of a case localized to the pos- terior nasal septum. Head Neck Pathol. 2011;5:306–313. doi: 10.1007/s12105-011-0252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oishi N, Kondo T, Nakazawa T, Mochizuki K, Kasai K, Inoue T, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: case report and literature review. Pathol Res Pract. 2014;210:1142–1145. doi: 10.1016/j.prp.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Sillings CN, Weathers DR, Delgaudio JM. Thyroid-like papillary adenocarcinoma of the nasopharynx: a case report in a 19- year-old male. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:e258. doi: 10.1016/j.tripleo.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Wei J, Yao X, Wang C. Clinicopathological features of low-grade thyroid-like nasopharyngeal papillary adenocarcinoma. Cancer Res Treat. 2017;49:213–218. doi: 10.4143/crt.2016.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuan EC, Alonso JE, Arshi A, St John MA. Nasopharyngeal adenocarcinoma: a population-based analysis. Am J Otolaryngol. 2017;38:297–300. doi: 10.1016/j.amjoto.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Nojeg MM, Jalaludin MA, Jayalakshmi P. Papillary adenocarcinoma of the nasopharynx–case report and review of the literature. Med J Malaysia. 1998;53:104–106. [PubMed] [Google Scholar]
- 18.Wu PY, Huang CC, Chen HK, Chien CY. Adult thyroid- like low-grade nasopharyngeal papillary adenocarcinoma with thyroid transcription factor-1 expression. Otolaryngol Head Neck Surg. 2007;137:837–838. doi: 10.1016/j.otohns.2007.06.725. [DOI] [PubMed] [Google Scholar]
- 19.Ohe C, Sakaida N, Tadokoro C, Fukui H, Asako M, Tomoda K, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: report of two cases. Pathol Int. 2010;60:107–111. doi: 10.1111/j.1440-1827.2009.02480.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang CP, Chang YL, Chen CT, Yang TH, Lou PJ. Photodynamic therapy with topical 5-aminolevulinic acid as a post- operative adjuvant therapy for an incompletely resected primary nasopharyngeal papillary adenocarcinoma: a case report. Lasers Surg Med. 2006;38:435–488. doi: 10.1002/lsm.20291. [DOI] [PubMed] [Google Scholar]


