Abstract
Osteomas are benign osteogenic neoplasms caused by proliferation of mature compact or cancellous bone. Clinically they may be classified as peripheral, central or extraskeletal. Osteomas usually involve the craniofacial region with mandible being the most common site. Central osteoma of the jaws is a very rare entity with only 13 cases reported in the literature till date of which only five cases occurred in the maxilla. Here we present a case of a large central osteoma of anterior maxilla associated with an impacted tooth, the first of its kind and a review of literature.
Keywords: Central osteoma, Osteoma, Benign, Jaw, Maxilla
Introduction
Osteomas are considered to be benign slow growing bony neoplasms of central, peripheral or soft tissue origin. The exact etiology is unknown but multiple mechanisms of development have been described in the literature. Although peripheral craniofacial osteomas have been frequently reported, central osteomas are truly a rare entity with only 13 cases reported in the jaws. The unusual clinical presentation and radiographic features make this lesion difficult to diagnose. The presence of an associated impacted tooth in the same region further complicates the diagnosis. Here we present an unusual case of a maxillary central osteoma with some insight to its probable etiology and mechanism of development along with the literature review.
Case Report
A 20 year old male patient reported to the Department of Oral Medicine and Radiology at our institute with a chief complaint of a painless swelling in the front left region of the upper jaw since 8 months. Patient gave a history of trauma due to fall from stairs with an impact on the left upper front jaw region 10 months ago and subsequent exfoliation of a tooth in the same region. The impact was not followed by any nasal or oral bleeding. The swelling was about the size of 1 × 1 cm when first noted by the patient and had a constant growth since then. The patient had no history of any dyspnea or change in voice.
On extraoral examination a diffuse swelling of the left maxillary anterior alveolar segment of approximate size 4 × 3 cm causing elevation of upper lip on left side and downward slanting of left corner of mouth was evident (Fig. 1a, b). The overlying skin was normal and the swelling was hard, afebrile and non-tender on palpation with smooth well defined borders. Submental and submandibular lymph nodes were not palpable. No other deformity of face was detected.
Fig. 1.
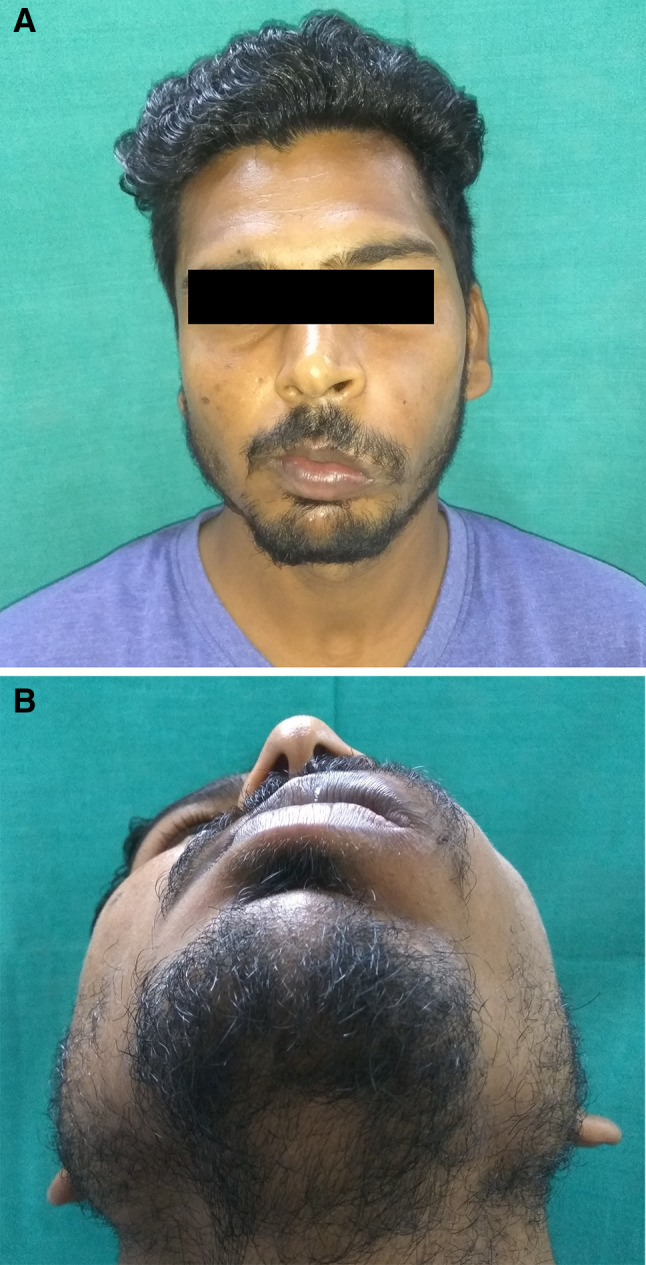
a and b Clinical aspect of maxillay central osteoma showing noticeable extraoral swelling of the left anterior maxilla
On intraoral examination a well-defined swelling of size 5 × 3 cm in the left anterior maxilla involving the alveolar segment and the facial surface of maxillary bone with obliteration of the labial and buccal vestibule was noted. It extended mesially from distal aspect of left central incisor to mesial aspect of left first premolar and superiorly from 1 cm below the infraorbital margin to inferiorly upto the incisal margin (Fig. 2a). There was expansion of the alveolar bone on the palatal and buccal side with resultant palatal displacement of left lateral incisor and slight buccal displacement of the left first premolar (Fig. 2b). The left maxillary canine was missing. The overlying mucosa was blanched with few areas of hyperpigmentation. The oral hygiene status of the patient was average, having stains and calculus. On palpation the swelling was bony hard, non-tender and non-pulsatile. No signs of paresthesia were noted.
Fig. 2.
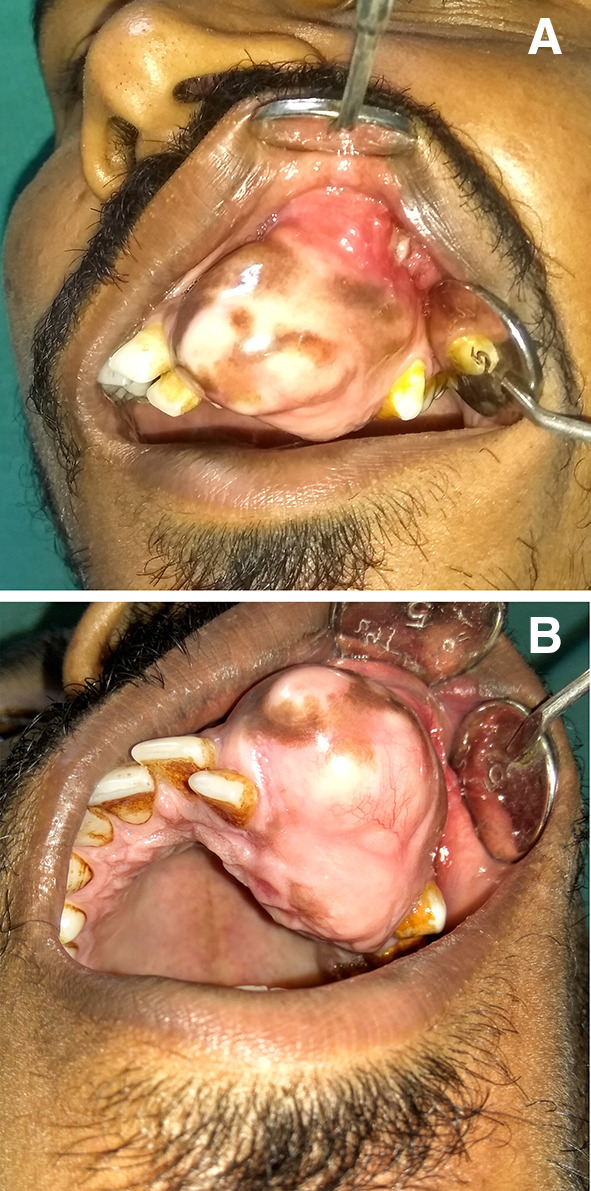
a and b Intraoral aspect of the lesion showing well defined swelling with bicortical expansion, displaced maxillary left lateral incisor and missing canine
Maxillary occlusal view revealed a well defined radiopaque mass in the left maxillary alveolar segment extending from left maxillary lateral incisor to second premolar with cotton wool type internal appearance and small flecks of radiolucent areas associated with impacted left maxillary canine (Fig. 3). There was an absence of any radiolucent rim surrounding the radiopaque mass. Orthopantomograph revealed an ill defined radiopaque lesion in the left anterior maxillary region with mesial displacement of left central and lateral incisor and an impacted left maxillary canine which was displaced superiorly (Fig. 4). No root resorption was seen. Contrast enhanced computed tomography images revealed a well defined hyperdense lesion in the left anterior maxilla showing bicortical expansion in the lower alveolar region (Fig. 5b) and only buccal cortical expansion and an impacted canine in the superior region of the lesion (Fig. 5a).
Fig. 3.
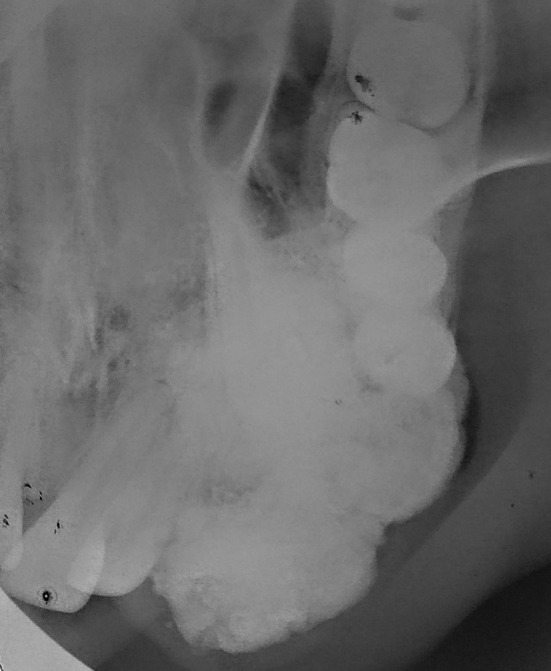
Maxillary anterior occlusal radiograph showing well defined radiopaque mass with cotton wool type appearance
Fig. 4.
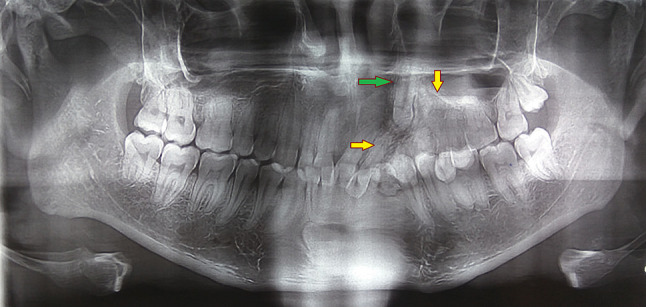
Orthopantomogram showing ill defined radiopaque mass in left anterior maxilla (yellow arrows) associated with a vertically impacted canine (green arrow)
Fig. 5.
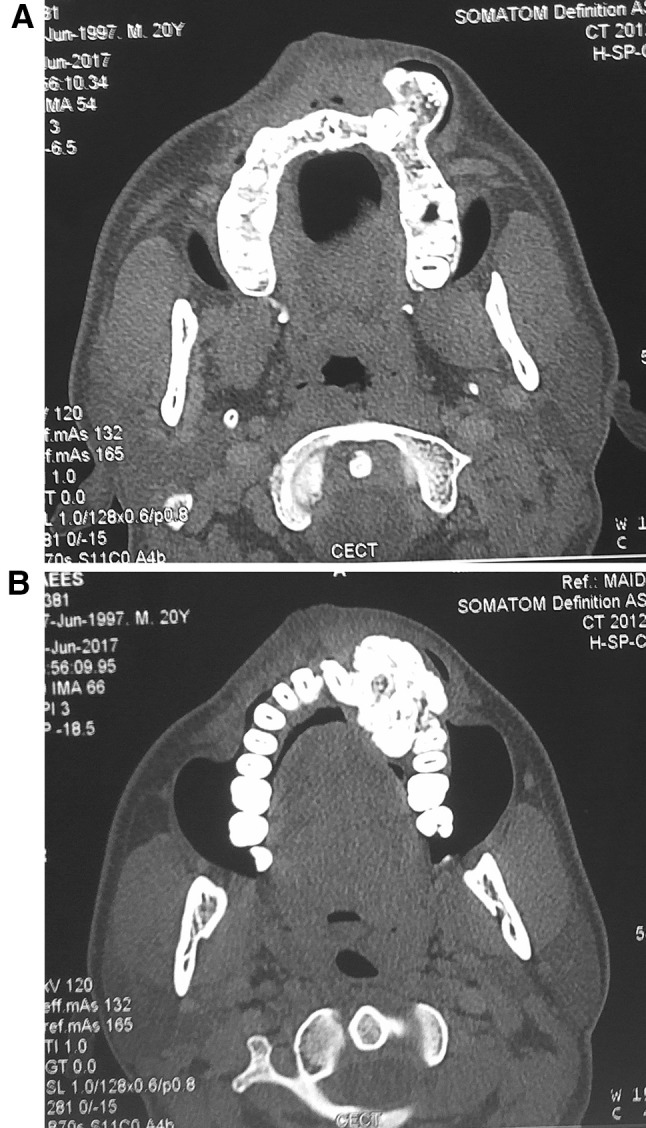
a and b Computed tomographic images showing well defined bony mass with no significant contrast enhancement, intact buccal and lingual cortices along with impacted left maxillary canine, bicortical expansion and normal marrow space
The patient had normal serum alkaline phosphatase levels and other blood parameters were also within normal range. An incisional bone biopsy was then performed. Histopathological examination of the decalcified tissue showed sheets of compact bone with few osteocytes within the lacunae and osteoblasts lining the trabaculae (Fig. 6a, b). The osteoblasts did not show any mitotic activity. Intervening minimal marrow tissue was fibro fatty in nature and thus a diagnosis of compact osteoma was arrived at. In view of a solitary lesion as seen clinically and radiographically on CT and absence of any gastric or intestinal symptoms possibility of Gardener’s syndrome was ruled out and colon endoscopy for intestinal polyps was not advised.
Fig. 6.
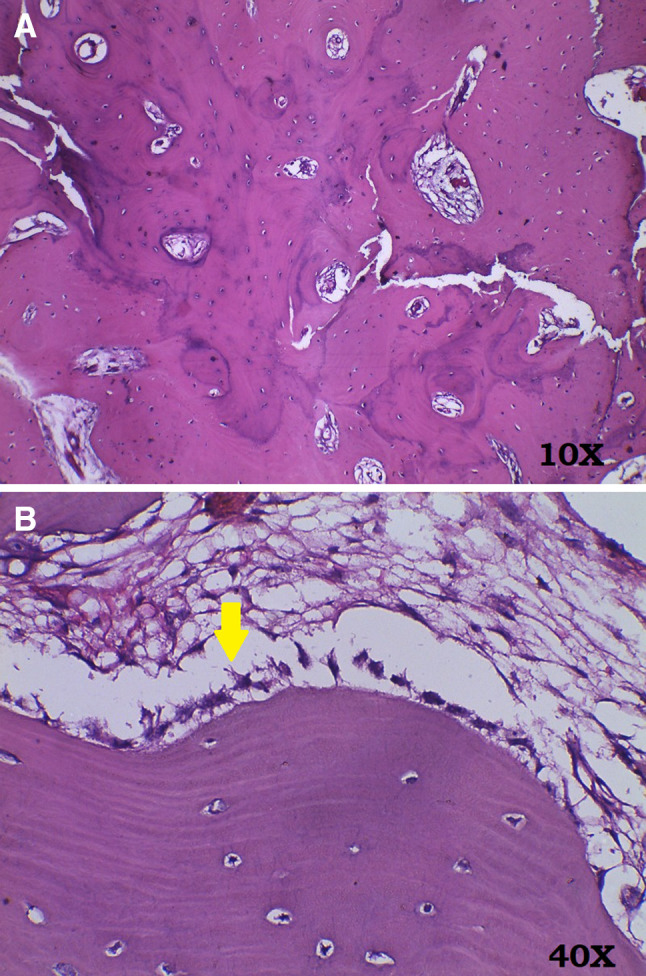
a H&E section from the resected bone (at × 10) showing sheets of compact bone with entrapped tiny irregular marrow spaces containing fibrofatty tissue. b H&E section from the resected bone (at × 40) showing compact bone with entrapped osteocytes. The trabeculae are lined by osteoblasts (yellow arrow)
The patient was planned for a surgical intervention by performing a maxillary anterior segmental ostectomy under general anesthesia. The left maxillary lateral incisor and the impacted canine were also removed along with the resected segment (Fig. 7).
Fig. 7.
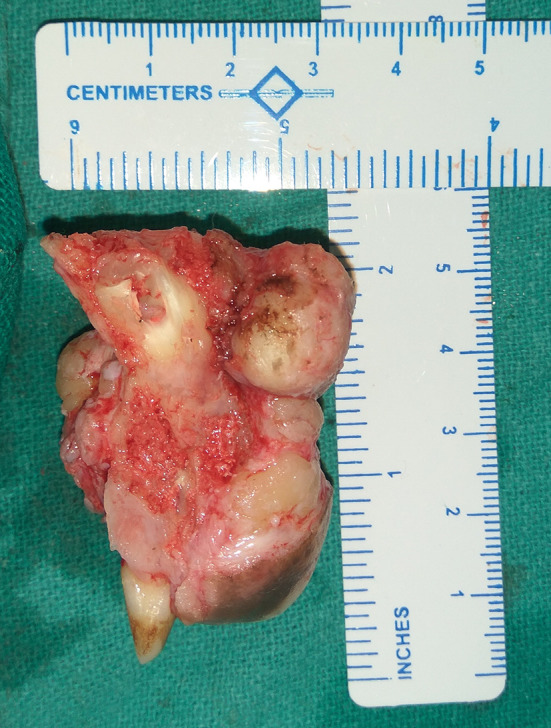
Resected segment in terms of size (approximately 5 × 3.5 cm) showing the associated impacted canine
Discussion
Osteomas are benign slow growing osteogenic tumors mostly arising in the craniofacial region and characterized by the deposition of differentiated and mature either or both cancellous or compact bone [1–4]. They are of three types; peripheral, central or extraskeletal. Peripheral type arises from the periosteum, central type arises from the endosteum and extraskeletal type arises within the muscles or dermis (also known as osteoma cutis) [2, 3]. Osteomas are rarely diagnosed in other bones [3, 4].
Males present with osteomas more commonly than females [1, 3]. The age range for presentation of this lesion is very wide with the average age of diagnosis as 50 years [1, 3]. Among the jaws, mandible is more commonly affected than maxilla with angle and condyle being the most common site followed by the body (molar region) and ascending ramus [5]. Interestingly these are also the sites for various muscle attachments on the mandible. Jaw osteomas are usually asymptomatic with the exception of some large lesions that may cause functional disturbances or neurovascular compression symptoms due to their large size [6]. Condylar osteomas may cause reduced mouth opening, occlusal disharmony, facial asymmetry and even TMJ dysfunction [5].
The exact etiology of osteomas is unknown and is considered to be multifactorial. It may be genetic (as seen in Gardener’s syndrome), related to endocrine disorders (osteoma cutis), trauma or even inflammation [7]. The neoplastic nature of osteomas is doubted due to their limited growth potential and absence of recurrence. However in our case the lesion had a surprisingly fast growth rate with almost fourfold increase in its size in a period of 8 months which to the best of our knowledge has not been seen in any of the earlier cases. Kaplan et al. suggested that a combination of trauma and subsequent muscle traction may play a role in the development of osteomas as trauma causes subperiosteal bleeding that locally elevates the periosteum. The continuous muscle traction accentuates the osteogenic reaction stimulated by local bleeding [8].
Although the patient in this case had a history of direct trauma prior to development of the lesion, this theory of combination of local bleeding and muscle traction does not seem to be true for this case as this is an endosteal osteoma and only the development of periosteal osteoma can be influenced by muscle traction. Moreover, the site of the lesion in the present case was such where the adjacent muscles (muscles of facial expression around nose and mouth) insert into the skin rather than the bone. Kumar Nilesh et al. in their case of solitary central osteoma of anterior mandible in a geriatric patient suggested that a chronic periodontal infection of the mandibular anterior teeth which were later extracted could possibly have triggered the osteoproliferative process [4]. Most peripheral osteomas present within the paranasal sinuses are usually associated with sinus infections while some even develop within a sinus polyp, a finding which does not rules out the role of infection in the development of an osteoma [8]. Another etiological theory based on stimulation of embryological remains states that the membranous and cartilaginous elements present at the sutures of skull bones could stimulate the development of cell rests and subsequent tumor formation [9].
Compiling the evidences seen in earlier published cases and including the present case, it could be hypothesized that it is the stimulation of osteogenic cells (differentiated or embryonic cell rests) by any means like infection, trauma, genetic alteration etc which could lead to the development of an osteoma. The muscle traction theory described by Kaplan seems to hold true only for the development of a peripheral osteoma where it only helps in the deposition of bone by its periosteal pull and is itself not responsible for the development of an osteoma. However this theory does not explain the development of a central osteoma and even peripheral osteomas at other sites. Thus the most probable cause for the development of osteoma in the present case is the stimulation of either the endosteal osteoblasts or the cell rests of Malassez associated with the impacted canine which led to their differentiation to osteoprogenitor cells and subsequent tumor formation. Evidence supportes the epithelial–mesenchymal transition of cell rests of Malassez which are a source of multipotent stem cells [10].
Some osteomas are not solitary and are associated with Gardner’s syndrome. Gardner syndrome is a variant of familial adenomatous polyposis which is of autosomal dominant inheritance pattern and is caused by a mutation of the APC gene on chromosome 5q21 [7–9]. Apart from multiple craniofacial osteomas the other manifestations include multiple epidermoid cysts, desmoid tumors, intestinal polyps and multiple impacted supernumerary teeth. The craniofacial osteomas serve as an insightful marker for this syndrome as they usually precede the development of other symptoms.
Growth potential of peripheral osteomas is usually limited; however, they continue to slowly grow if left untreated. However, some reported cases of peripheral osteoma have reached significantly large dimensions, enough to cause dysphagia and sleep apnoea [11]. The growth rate of this case was an exception to the usual presentation of osteomas described in literature. These few cases showing excessive growth potential and growth rate support their nature as a true neoplasm.
As only 13 cases of solitary central osteomas of jaws have been documented in the literature, the cumulative data is insufficient to state with conviction the prevalent age, sex and location of the lesion. Most central lesions have been reported in the premolar–molar region of the mandible [12]. Only five cases of maxillary involvement have been reported in the medical literature till date (Table 1). Central osteomas arise within the endosteum and hence cause expansion of one or both the cortices. Root displacement can also be seen if the lesion involves a dentulous area as was seen in our case. One case of mandibular central osteoma as reported by Emel Bulut et al. showed root resorption of associated mandibular molar [13]. Some tumors are associated with pain and neurologic disturbances when compression of adjacent nerves is associated with the lesion due to its position and growth [9]. On radiographic assessment the lesion appears as ‘bone within bone’ with both the cortices expanded and intact with the medulla of the lesion confluent with the adjacent normal bone as seen in this case.
Table 1.
Previously reported cases of central osteomas involving the jaws
| Year | Author | Sex | Age | Location |
|---|---|---|---|---|
| Maxilla | ||||
| 1981 | Rajayogeswaranav and Evson | F | 69 | Anterior maxilla |
| 2005 | Firat et al. | M | 15 | Premolar region and sinus |
| 2008 | Kaplan et al. | M | 46 | Premolar/molar region |
| 2011 | Santos et al. | M | 44 | Premolar/molar region and sinus |
| 2014 | Fabrizio Carini et al. | F | 33 | Canine to molar region |
| Mandible | ||||
| 1955 | Hitchin AD, White JW | F | 47 | Premolar/molar region |
| 1970 | Khosla VM | M | 13 | Molar region |
| 1981 | Fritz GW, Deshpande V | F | 22 | Molar region |
| 2005 | Zielinska-Kazamierska B et al. | F | 21 | Premolar/molar region |
| 2008 (three cases) | Kaplan I, Nicolaou Z | F | 52 | Premolar/molar |
| F | 67 | Canine/premolar | ||
| F | 57 | Molar | ||
| 2010 | Bulut E, Ozan B | F | 52 | Molar |
Histologically, osteomas can be classified into three types: ivory or compact, mature or cancellous, and mixed. Ivory osteomas are characterized by hard bone with a thick matrix containing only a small amount of fibrous tissue and minimal marrow. Cancellous osteomas are composed of cancellous bone with intertrabecular hematopoietic bone marrow or fat, whereas mixed osteomas share characteristics from both types [1].
Differential diagnosis of the lesion in the present case include complex odontoma, central ossifying fibroma, osteoid osteoma, osteblastoma, Pindborg’s tumor and low grade osteosarcoma (Table 2).
Table 2.
Summary of differential diagnosis for maxillary bone forming tumors
| Complex odontoma [14–16] | Osteoid osteoma [20, 21] | Osteoblastoma [22–24] | Central ossifying fibroma [22–24] | Calcifying epithelial odontogenic tumor [25, 26] | Osteosarcoma [27–30] | |
|---|---|---|---|---|---|---|
| Age | 20–30 years | 10–20 years | < 30 years | 20–40 years | 20–40 years |
20–40 years Mean age 36 years |
| Sex | No sex predilection | M > F | M > F | F > M | No sex predilection | M > F |
| Association with trauma | Yes | No | Yes | Yes | No | No |
| Association with impacted tooth | Yes | No | No | No | Yes (occasional) | No |
| Radiographic appearance |
a. Mixed to Radiopaque (radiodensity comparable to tooth, but may appear lucent in initial stages) b. Surrounding radiolucent rim c. Benign, localised and non aggressive |
a. Radiolucent intracortical nidus (< 1 cm) surrounded by large, dense sclerotic zone of cortical thickening b. Benign, localised and non aggressive |
a. Well defined and surrounding reactive sclerosis usually evident b. Usually benign but occasionally locally aggressive |
a. Mixed to radiopaque b. Cortical expansion/root resorption seen occasionally c. Surrounding thin radiolucent rim may appear d. Usually benign but occasionally locally aggressive |
a. Radiolucent to mixed b. Unilocular to multilocular c. Calcific flecks around impacted tooth in a “driven snow” appearance d. Usually benign but occasionally locally aggressive |
a. Radiolucent, mixed or radiopaque b. Sunburst appearance c. Root resorption, cortical destruction/expansion d. Aggressive and invading into surrounding structures |
| Histological appearance | a. Ground sections show disorganised enamel, dentin and pulp tissue in varying proportions |
a. Bony trabaculae and osteoid intermixed in fibous connective tissue rich in vascularity b. Trabaculae lined by numerous osteoblasts c. Tumor nidus present |
Similar to osteoid osteoma except for the tumor nidus |
1. Fibrous to cellular connective tissue with variable amounts of bone, osteoid and/or cementum like material 2. Peripheral osteoblastic rimming |
a. Islands or sheets of polyhedral epithelial cells spread in a fibrous stroma b. Eosinophilic, amyloid like extracellular material often present with surrounding Liesegang ring calcifications |
a. Presence of tumor osteoid b. Anaplastic cells evident |
| Tooth displacement | Yes (may cause) | No | Yes (may cause) | Yes (in large lesions) | Yes (in large lesions) | Yes (mostly) |
| Signs & symptoms | Painless | Pain relieved by NSAIDs | Usually dull pain and tenderness | Painless | Painless | Local swelling, pain, paresthesia and ulceration |
Odontomas are hamartomas of hard tissue of dental origin with most of them arising in the second to third decade of life. Compound odontomes usually develop in the earlier age group than the complex odontomes. The radiographic appearance of odontomes varies from mixed to radiopaque depending on its developmental stage. Compound odontomes appear as well defined irregular tooth like radiopacities of varying sizes with a surrounding radiolucent band. They may be single or multiple in number and rarely exceed 30 mm in size, thus making only complex odontoma as a possible differential diagnosis. Mature complex odontomas appear as irregular radiopaque masses with a thin radiolucent band surrounding them, the absence of which helped in ruling out complex odontoma as a possible diagnosis in this case. This was corroborated on histological examination where no dental tissues were observed [14–16].
Central ossifying fibroma (COF), earlier termed as ‘cementifying ossifying fibroma’ is a benign fibro-osseous lesion. It has peak incidence at around 20–40 years of age with the lesion mostly occurring in mandible and affecting females more than males. Radiographically they appear as unilocular or multilocular radiolucencies having well defined corticated borders with or without multiple radiopaque foci or flecks of varying radiodensities and sizes depending upon its maturity. When totally calcified, it may appear as completely radiopaque. With sufficient growth they may cause cortical expansion, tooth displacement and even resorption. The main distinguishing features of COF from central osteoma is its surrounding radiolucent band and its well delineated and corticated borders [17–19].
Osteoid osteoma is a benign osteogenic neoplasm with limited growth potential. Mandibular cases have been reported more than those in maxilla. Radiographically, it presents as a well defined round to oval radiolucency called the ‘nidus’ with varying amount of radiopacity in the centre and peripheral reactive sclerosis. It rarely exceeds 2 cm in size. Nocturnal pain which is alleviated by NSAIDs is the most striking feature of this lesion. Cortical and subperiosteal osteoid osteoma do not cause expansion while cancellous or medullary variant may cause an obvious cortical expansion. The possibility of osteoid osteoma was ruled out due to absence of any pain or tenderness, surrounding sclerosis and the large size of the present case [20, 21].
Osteoblastoma is also a benign osteogenic neoplasm mostly affecting the axial skeleton in young adults. Among the jaws mandible is the more common site. It differs from osteoid osteoma in its higher growth potential with most lesions exceeding more than 2 cm in size. They cause pain which is not relieved by NSAIDs along with local swelling and tenderness. Radiographic presentation comprises of well defined radiolucency with areas of patchy radiopacities of varying densities and peripheral sclerosis. A radiolucent band is usually visible in a mature lesion. It is not seen in the aggressive variant. Osteoblastoma was ruled out owing to the absence of pain and any surrounding radiolucent band or halo [22–24].
Calcifying epithelial odontogenic tumor (CEOT) is an uncommon benign tumor of odontogenic origin mostly occurring in 20–40 years of age without any sex predilection and occasionally associated with an unerupted tooth. Most cases have been found in the posterior mandible. Cortical bone perforation and tooth displacement are common findings in the central variant [25]. Radiographic appearance varies from completely radiolucent to mixed radiopaque and unilocular to multilocular depending on its developmental stage. In later stages multiple flecks of calcific foci appear around the impacted tooth in a characteristic “driven snow” appearance. The margins are usually well defined. Histologically, the tumor comprises of polyhedral epithelial cells arranged in sheets in a fibrous background, Liesegang’s rings and variable amount of amyloid like material [26].
Osteosarcoma is a primary malignant tumor of cells of mesenchymal origin having ability of osteogenic differentiation. The low grade medullary variant is considered as a possible differential diagnosis in the present case. Clinical features include fast expansile growth with pain, paresthesia and tooth mobility. Radiographic appearance may be of a completely radiolucent, mixed or radiopaque lesion with ill defined borders with occasional root resorption and cortical breach due to its aggressive nature. Although the lesion in the present case had a very fast growth rate and also appeared as a radiopaque mass with ill defined borders on the panoramic view, the absence of any symptoms, root resorption, cortical breach or sunburst appearance ruled out its possibility. Moreover, on microscopic examination, the tumor cells showed no evidence of abnormal mitotic activity or pleomorphism [27–30].
Conclusion
Central osteoma in the maxillofacial region is a very rare presentation as evidenced by only 13 cases being reported in the literature worldwide, of which only five cases have been reported in the maxilla prior to the present case. Diagnosis of central osteomas of the maxillofacial region can be daunting task due to their unusual clinical and radiographic presentation. Moreover the earlier given hypotheses for the development of an osteoma might hold true for a peripheral variant but they do not explain the development of a central variant, as seen in the present case. Histology is not able to differentiate between a peripheral or a central variant as both show deposition of mature compact or cancellous bone. Thus the diagnosis of a central osteoma is truly based upon it’s growth characteristics, cortical expansion and tooth displacement if present in a dentulous region. The clinician must thus be well aware of this entity to include it as a differential diagnosis for lesions giving history and clinico-radiological presentation similar to the present case.
Compliance with Ethical Standards
Conflict of interest
There are no conflicts of interest declared by any other author.
Informed Consent
Informed consent taken from the patient.
References
- 1.Georgalas C, Goudakos J, Fokkens WJ. Osteoma of the skull base and sinuses. Otolaryngol Clin N Am. 2011;44:875–890. doi: 10.1016/j.otc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Dell’Aversana Orabona G, Salzano G, Iaconetta G, Piombino P, Ponzo L, Santella A, Astarita F, Solari D, Salzano FA, Califano L. Facial osteomas: fourteen cases and a review of literature. Eur Rev Med Pharmacol Sci. 2015;19:1796–1802. [PubMed] [Google Scholar]
- 3.Larrea-Oyarbide N, Valmaseda-Castellón E, Berini-Aytés L, Gay-Escoda C. Osteomas of the craniofacial region. Review of 106 cases. J Oral Pathol Med. 2008;37:38–42. doi: 10.1111/j.1600-0714.2007.00590.x. [DOI] [PubMed] [Google Scholar]
- 4.Nilesh K, Bhujbal RB, Nayak AG. Solitary central osteoma of mandible in a geriatric patient: report and review. J Clin Exp Dent. 2016;8(2):e219-22. doi: 10.4317/jced.52792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancini JC, Woltmann M, Felix VB, Freitas RR. Peripheral osteoma of the mandibular condyle. Int J Oral Maxillofac Surg. 2005;34(1):92–93. doi: 10.1016/j.ijom.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 6.de Souza NT, Cavalcante RCL, de Albuquerque Cavalcante MA, Hespanhol W, de Oliveira MR, Jr, de Carvalho Ferreira D, de Carvalho Coutinho TM, Gonçalves LS. An unusual osteoma in the mandibular condyle and the successful replacement of the temporomandibular joint with a custom-made prosthesis: a case report. BMC Res Notes. 2017;10(1):727. doi: 10.1186/s13104-017-3060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herford AS, Stoffella E, Tandon R. Osteomas involving the facial skeleton: a report of 2 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(2):e1–e6. doi: 10.1016/j.oooo.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan I, Calderon S, Buchner A. Peripheral osteoma of the mandible: a study of 10 new cases and analysis of the literature. J Oral Maxillofac Surg. 1994;52(5):467–470. doi: 10.1016/0278-2391(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 9.Firat D, Sirin Y, Bilgic B, Ozyuvaci H. Large central osteoma of the maxillary antrum. Dentomaxillofac Radiol. 2005;34(5):322–325. doi: 10.1259/dmfr/15263929. [DOI] [PubMed] [Google Scholar]
- 10.Xiong J, Mrozik K, Gronthos S, Bartold PM. Epithelial cell rests of Malassez contain unique stem cell populations capable of undergoing epithelial-mesenchymal transition. Stem Cells Dev. 2012;21(11):2012–2025. doi: 10.1089/scd.2011.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarsitano A, Marchetti C. Unusual presentation of obstructive sleep apnoea syndrome due to a giant mandible osteoma: case report and literature review. Acta Otorhinolaryngol Ital. 2013;33(1):63–66. [PMC free article] [PubMed] [Google Scholar]
- 12.de Santana Santos T, Frota R, Martins-Filho PR, Melo AR, de Souza Andrade ES, de Oliveira e Silva ED, Avelar RL. Central osteoma of the maxilla with involvement of paranasal sinus. J Craniofac Surg. 2011;22(2):589–591. doi: 10.1097/SCS.0b013e318208555d. [DOI] [PubMed] [Google Scholar]
- 13.Bulut E, Ozan B, Günhan O. Central osteoma associated with root resorption. J Craniofac Surg. 2010;21(2):419–421. doi: 10.1097/SCS.0b013e3181cfa7d7. [DOI] [PubMed] [Google Scholar]
- 14.Boffano P, Zavattero E, Roccia F, Gallesio C. Complex and compound odontomas. J Craniofac Surg. 2012;23(3):685–688. doi: 10.1097/SCS.0b013e31824dba1f. [DOI] [PubMed] [Google Scholar]
- 15.Tuczyńska A, Bartosik D, Abu-Fillat Y, Sołtysik A, Matthews-Brzozowska T. Compound odontoma in the mandible–case study and literature review. Dev Period Med. 2015;19(4):484–489. [PubMed] [Google Scholar]
- 16.Sun L, Sun Z, Ma X. Multiple complex odontoma of the maxilla and the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(1):e11-6. doi: 10.1016/j.oooo.2015.02.488. [DOI] [PubMed] [Google Scholar]
- 17.Eversole LR, Merrell PW, Strub D. Radiographic characteristics of central ossifying fibroma. Oral Surg Oral Med Oral Pathol. 1985;59(5):522–527. doi: 10.1016/0030-4220(85)90096-9. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad M, Gaalaas L. Fibro-osseous and other lesions of bone in the jaws. Radiol Clin North Am. 2018;56(1):91–104. doi: 10.1016/j.rcl.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Woo SB. Central cemento-ossifying fibroma: primary odontogenic or osseous neoplasm? J Oral Maxillofac Surg. 2015;73(12 Suppl):87–93. doi: 10.1016/j.joms.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 20.An SY, Shin HI, Choi KS, Park JW, Kim YG, Benavides E, Kim JW, An CH. Unusual osteoid osteoma of the mandible: report of case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(2):e134-40. doi: 10.1016/j.oooo.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Khaitan T, Ramaswamy P, Ginjupally U, Kabiraj A. A bizarre presentation of osteoid osteoma of maxilla. Iran J Pathol. 2016;11(5):431–434. [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas DR, Unni KK, McLeod RA, O’Connor MI, Sim FH. Osteoblastoma: clinicopathologic study of 306 cases. Hum Pathol. 1994;25(2):117–134. doi: 10.1016/0046-8177(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 23.Salmen FS, Oliveira MR, Navarro CM, Dedivitis RA, Pereira Filho VA, Gabrielli MFR. Aggressive osteoblastoma in the maxilla: unusual lesion in the craniofacial skeleton. J Craniofac Surg. 2017;28(3):794–797. doi: 10.1097/SCS.0000000000003641. [DOI] [PubMed] [Google Scholar]
- 24.Lypka MA, Goos RR, Yamashita DD, Melrose R. Aggressive osteoblastoma of the mandible. Int J Oral Maxillofac Surg. 2008;37(7):675–678. doi: 10.1016/j.ijom.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Chrcanovic BR, Gomez RS. Calcifying epithelial odontogenic tumor: an updated analysis of 339 cases reported in the literature. J Craniomaxillofac Surg. 2017;45(8):1117–1123. doi: 10.1016/j.jcms.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhang A, Chaw SY, Talacko AA, Besly WJ, Savage NW, Monsour PA. Central calcifying epithelial odontogenic tumour in the posterior maxilla: a case report. Aust Dent J. 2016;61(3):381–385. doi: 10.1111/adj.12384. [DOI] [PubMed] [Google Scholar]
- 27.Kassir RR, Rassekh CH, Kinsella JB, Segas J, Carrau RL, Hokanson JA. Osteosarcoma of the head and neck: meta-analysis of nonrandomized studies. Laryngoscope. 1997;107(1):56–61. doi: 10.1097/00005537-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Prabhusankar K, Karande A, Jerry JJ, Rishal Y. Osteosarcoma of the posterior maxilla. J Int Soc Prev Community Dent. 2016;6(Suppl 2):171-4. doi: 10.4103/2231-0762.189762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mardinger O, Givol N, Talmi YP, Taicher S. Osteosarcoma of the jaw: the Chaim Sheba Medical Center experience. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(4):445–451. doi: 10.1067/moe.2001.112330. [DOI] [PubMed] [Google Scholar]
- 30.Bennett JH, Thomas G, Evans AW, Speight PM. Osteosarcoma of the jaws: a 30-year retrospective review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(3):323–332. doi: 10.1067/moe.2000.108274. [DOI] [PubMed] [Google Scholar]


