Abstract
Acute respiratory distress syndrome (ARDS) features an exudative phase characterized by alveolar damage, lung edema and exacerbated inflammatory response. Given their anti‐inflammatory properties, the potential therapeutic effect of corticosteroids has been evaluated in ARDS clinical trials and experimental models of ALI. These studies produced contradictory results. Therefore, our aim was to investigate the effects of dexamethasone in an animal model of bleomycin‐induced acute lung injury and then to determine if the lack of response could be related to an impairment in repair ability of alveolar epithelial cells after injury. NMRI mice were challenged with bleomycin and then treated daily with dexamethasone or saline. Bronchoalveolar lavages (BAL) and lungs were collected for assessment of the inflammatory response and wet/dry ratio (lung edema) and for histological analyses. The effect of bleomycin and dexamethasone on wound repair was also evaluated in vitro on primary alveolar epithelial cell (ATII) cultures. Our data first showed that dexamethasone treatment did not reduce the weight loss or mortality rates induced by bleomycin. Although the TNF‐α level in BAL of bleomycin‐treated mice was reduced by dexamethasone, the neutrophil infiltration remained unchanged. Dexamethasone also failed to reduce lung edema and damage scores. Finally, bleomycin elicited a time‐ and dose‐dependent reduction in repair rates of ATII cell cultures. This inhibitory effect was further enhanced by dexamethasone, which also affected the expression of β3‐ and β6‐integrins, key proteins of alveolar repair. Altogether, our data indicate that the inability of dexamethasone to improve the resolution of ALI might be due to his deleterious effect on the alveolar epithelium repair.
Keywords: Acute lung injury, acute respiratory distress syndrome, bleomycin, dexamethasone, edema, inflammation, repair
Our data indicates that the inability of dexamethasone to improve the resolution of bleomycin outcomes, in particular, mortality rates, neutrophil infiltration and lung edema, may be due to the remaining alveolar damage observed at day 7 and repair impairment induced by dexamethasone.

Introduction
Acute respiratory distress syndrome (ARDS) (Ranieri et al. 2012; Fitzgerald et al. 2014), a severe form of respiratory failure, remains one of the leading causes of mortality both in adults and children in intensive care units. Various disorders, either direct (e.g., pneumonia, gastric content aspiration) or indirect (e.g., sepsis, trauma), are associated with the development of ARDS (Monahan 2013; Ware and Matthay 2000). However, regardless of the causes, ARDS features overlapping exudative, proliferative and fibrotic phases. The acute exudative phase is characterized by extensive alveolar epithelial and endothelial damage, eliciting alveolar edema, neutrophil infiltration, high levels of chemokines/cytokines and decreased lung compliance (Ware and Matthay 2000; Monahan 2013). Collagen deposition and fibroproliferation, competing with epithelial repair, can rapidly progress toward irreversible pulmonary fibrosis, ultimately leading to respiratory failure (Ware and Matthay 2000; Shimabukuro et al. 2003; Ranieri et al. 2012). Therefore, the resolution of the acute phase is pivotal for ARDS recovery.
Although improvements in mechanical ventilation procedures have been associated with increased survival over the last decades (Ranieri et al. 2012; Fitzgerald et al. 2014), mortality rates (30–45%) still remain unacceptably high, and effective, noninvasive pharmacological therapies are needed. Because the inflammatory response is a key determinant of ARDS, several studies have evaluated the efficiency of anti‐inflammatory therapies. One of the most studied therapy has been systemic corticosteroids. Clinical trials on ARDS patients and subsequent meta analyses (Steinberg et al. 2006; Foster 2010; Yehya et al. 2015; Meduri et al. 2016; Kimura et al. 2016; Tongyoo et al. 2016; Standiford and Ward 2016; Yang et al. 2017; Fan et al. 2018) as well as experimental studies in animal models of acute lung injury (ALI) (Chen et al. 2006; Leite‐Junior et al. 2008; Wang et al. 2008; Xu et al. 2009; Yubero et al. 2012; Hegeman et al. 2013; Engel et al. 2015) have indicated that glucocorticoids (e.g., methylprednisolone, dexamethasone, hydrocortisone) elicit variable effects as a function of the dose, the route of administration, the time to treatment initiation and duration, the age of the patient, the cause of ARDS/type of ALI model and/or the measured outcomes. Therefore, the available evidence for glucocorticoids benefits in ARDS is conflicting and the use of these treatments in ARDS patients are still subject to debate (Bein et al. 2016; Bihari et al. 2016; Seam and Suffredini 2016; Thompson and Ranieri 2016; Mac and McAuley 2017; Meduri and Siemieniuk 2017; Bos et al. 2018).
The goal of our study was first to dissect the effect of daily treatments with dexamethasone in an experimental model of ALI in mice. We opted for the well‐characterized model of bleomycin‐induced lung injury and focused our study on the acute exudative phase, featuring an endothelial/alveolar epithelial damage, lung edema, neutrophil infiltration and decreased lung function (over a 7‐day period), before establishment of fibrosis (day‐12–21) (Matute‐Bello et al. 2008; Saito et al. 2008; Goto et al. 2010). The effect of the dexamethasone treatment was evaluated by assessing mouse survival, lung edema, inflammatory response and alveolar injury after the bleomycin challenge. Our data indicated that dexamethasone reduced TNF‐α levels but failed to improve mouse survival or to reduce lung edema, neutrophil infiltration and injury scores after bleomycin. We then hypothesized that dexamethasone may impair the repair ability of alveolar epithelial cells after injury. We thus analyzed the effect of bleomycin and dexamethasone in vitro on the wound healing of primary alveolar epithelial cell cultures and showed that dexamethasone worsened the deleterious effect of bleomycin on the repair rates.
Materials and Methods
In vivo experimental design
Wild‐type NMRI mice (Naval Medical Research Institute, Bethesda, Maryland, USA) were kindly gifted by E. Hummler (Lauzanne, Switzerland). All procedures were conducted according to the Canadian Council on Animal Care (CCAC), and the experimental protocol was approved by the Institutional Animal Protection Committee (CIPA) of the Centre de Recherche du Centre hospitalier de l'Université de Montréal (CRCHUM). Animals were sheltered under standard conditions with food and water provided ad libitum. Experiments were conducted on 7‐ to 10‐week‐old male mice, randomly divided into 4 groups: Ctl (control, instillation of saline and daily intra‐peritoneal (i.p.) treatment with saline), Bleo (instillation with bleomycin and daily i.p. treatment with saline), Dex (instillation with saline and daily i.p. treatment with dexamethasone) and Bleo + Dex (instillation with bleomycin and daily i.p. treatment with dexamethasone). More precisely, mice were anesthetized at day 1 with a solution (0.01 mL/g) of 13% ketamine (100 mg/mL) and 1.3% xylazine (20 mg/mL) in 0.9% saline. Then, animals were instilled intratracheally (i.t.) with saline (0.9%, 50 µL) or bleomycin (MaynePharma Canada, QC, Canada, 4 U/kg, 50 µL) following a modified non‐surgical and non‐damaging method (Guilbault et al. 2005). Immediately after instillation of saline or bleomycin, mice were treated with dexamethasone (Sandoz Canada, QC, Canada, 0.5 mg/kg, 100 µL) or vehicle (saline 0.9%, 100 µL) by i.p. Treatments (saline or dexamethasone) were repeated daily and outcomes (see below) were measured 3, 7 and/or 12 days after the bleomycin challenge, corresponding to the development of acute lung injury, as described in the literature (Matute‐Bello et al. 2008; Saito et al. 2008; Goto et al. 2010). As supplementary experiments (Fig. S1), a group was treated with methylprednisolone (NovoPharm, QC, Canada, 1 mg/kg, 100 µL, daily, i.p. treatment).
Mice survival rates and weight variation
The mice weight variations were calculated from the measured weights at each time point (days 3, 7 and 12), including before sacrifice of mice reaching the endpoints (see below), and reported as % of the initial weight before the bleomycin challenge. In compliance with the CCAC standards and following daily animal assessment by the CRCHUM animal care personnel, mice reaching endpoints (respiratory failure, prostration, uncontrollable pain, dehydration, or loss of more than 30% of the initial weight), were sacrificed according to the procedure approved by the CRCHUM institutional animal care committee. To avoid a potential bias by studying the animals with the better outcomes, animals reaching endpoints were included in the calculation of the weight variation and survival rates (which were reported as % of the live mice in each group at 3, 7 and 12 days after the beginning of the bleomycin challenge). Subsequent experiments, for the measurement of lung edema, tissue injury and inflammatory response (see below), were performed during the acute exudative phase (day 3 and 7). It has to be noted that at these time points no (day 3) or small (day 7, <10%) mortality rates were observed.
Edema index (wet/dry ratio)
After euthanasia (with 0.02 mL/g, ketamine‐xylazine) on days 3 and 7, the inferior vena cava was severed, the lungs were removed and directly weighed (wet weight). Lungs were heated to 95°C for 24 h to measure the dry weight and then to calculate the wet/dry ratio.
Bronchoalveolar lavages (BAL)
In another series of experiments, BAL were performed after mouse euthanasia (at days 3 and 7, in each condition) by instillation of saline (1 mL) through a catheter and then gentle aspiration. Six repeated BAL (from the same mouse) were collected and pooled on ice before centrifugation (200g, 4°C, 8 min). The supernatants were stored at −80°C until subsequent use to determine protein concentration and TNF‐α levels.
The protein concentration in BAL supernatants was evaluated by the Bradford method (Bio‐Rad Life Science, Mississauga, ON, Canada). TNF‐α levels in BAL supernatant samples were measured by AlphaLISA technology (AL505 C/F, PerkinElmer, Montreal, QC, Canada). Following the manufacturer’s recommendation, experiments were performed in triplicate at room temperature, and TNF‐α concentrations (pg/mL) were estimated from a TNF‐α standard curve (dynamic range from 2.0 to 30,000 pg/mL) after reading with the EnVision‐Alpha Reader (PerkinElmer).
Cell pellets were resuspended in 500 µL of PBS for quantification of the total cell count. The cell suspensions were then diluted at a density of 1 × 106 cells/mL, cytocentrifuged (300 rpm, 3 min, Shandon Cytospin 3 Centrifuge, Block Scientific, NY, USA) onto glass slides (4 × 104 cells/slide) and stained with Hema‐3® (Fisher, US). The differential cell count (number of neutrophils, macrophages, lymphocytes and eosinophils (reported as percentage) among a total of 400 leukocytes) was then determined.
Histological analysis and lung damage severity scores
Mice lungs, collected 7 days after the initial bleomycin challenge, were fixed by immersion in a 10% formalin solution, embedded in paraffin and the sections were stained with hematoxylin and eosin according to standard protocols at the Institut de Recherche en Immunologie et en Cancérologie (IRIC, Université de Montréal). Blind histological analysis was performed by Dr. Louis Gaboury (pathologist, Histology and Molecular Pathology research unit, University of Montréal), who defined a qualitative severity score (from 0 to 4), adapted from a well‐recognized scoring system to evaluate experimental ALI in animals (Matute‐Bello et al. 2011) and incorporating the following criteria: the presence of mononucleated cells/macrophages, polymorphonuclear, fibrinous exudate/hyaline membranes, widening of the septae, regenerative atypias/karyomegaly, intraalveolar hemorrhage, pneumocyte sloughing/cell debris, bronchial exudate, congestion/edema and consolidation. The results were expressed as the percentage of mice with each severity grade (from 0 to 4) of lung damage within the treatment group. Specimens were photographed using a digital camera (DP71) mounted on an Olympus BX61 microscope.
Alveolar epithelial cell (ATII) isolation and primary culture
Alveolar epithelial cells (ATII) were isolated from adult male Sprague‐Dawley rats (6–7 weeks, Charles‐River, St‐Constant, QC) according to a well‐established protocol (Dagenais et al. 2006; Bardou et al. 2012; Girault et al. 2015). Briefly, lungs were washed with a physiological solution to remove excess blood cells and alveolar macrophages. Then, the lungs were digested with 160 U/rat elastase solution (Worthington Biochemical, Lakewood, N.J. USA) and minced, and the resulting cell suspension was filtered. Alveolar cells were purified using a differential adherence technique (Dobbs et al. 1986), which enhances the purity of the ATII cell pool by up to 86% (Brochiero et al. 2004). The freshly isolated ATII cell suspension was then seeded on 6 or 12‐well cell culture clusters (Corning) and cultured in minimum essential medium (MEM, Invitrogen, Burlington, ON, Canada) supplemented with 10% FBS (Invitrogen, Canada), 0.2% NaHCO3 (Sigma‐Aldrich), 0.01 mol/L HEPES (Thermo‐Fisher Scientific Inc.), 2 mmol/L l‐glutamine (Invitrogen, Canada), 0.08 mg/L gentamicin (Life Technologies) and Septra (Aspri Pharma Canada, Canada, 3 g/mL trimethoprim and 17 g/mL sulfamethoxazole) at 37°C with 5% CO2 in a humidified incubator. This medium was replaced by MEM + 10% FBS without Septra at day 4, as previously described (Dagenais et al. 2006; Leroy et al. 2006; Dagenais et al. 2013; Girault et al. 2015). Well clusters with ATII cells from the same animal were then randomly divided into the different experimental conditions (treatments, see below) and experiments repeated on cell cultures from at least 4 animals (as indicated in the figure legend).
Wound‐healing assays
At day 2 of primary culture, ATII cell monolayers were treated, or not, for 24 h with dexamethasone (100 nmol/L in MEM medium supplemented with FBS and Septra (see above)). At day 3, mechanical injuries with a P10 Gilson pipette tip (6 wounds per Petri dish) were performed according to a well‐established, highly reproducible technique (Maillé et al. 2011; Ruffin et al. 2016; Adam et al. 2018). Immediately after injury (T0), the monolayers were then washed (with MEM + FBS without Septra) to remove detached cells, and the injured monolayers were treated with bleomycin (Bleo, 12.5–200 mU/mL), dexamethasone (Dex, 100 nmol/L), a combination of bleomycin and dexamethasone (Bleo + Dex) or vehicle (Ctl, saline 0.9%). Monolayers were photographed with a NIKON camera under light microscopy at T0, T24 h, T36 h and T48 h after injury. A mark under the Petri dishes allowed us to photograph the wounds at exactly the same place at every time point. After analysis with ImageJ software (NIH, Bethesda, MD, USA), the wound area was measured, and the wound healing rates (μm2/h) were calculated. Wound healing assays on cell monolayers provide insight into the initial repair processes engaged after injury (mainly cell migration and proliferation).
ATII cell apoptosis
ATII cells were collected after a 24 h treatment with bleomycin (Bleo, 50 mU/mL), dexamethasone (Dex, 100 nmol/L), a combination of bleomycin and dexamethasone (Bleo + Dex) or vehicle (Ctl, saline 0,9%) and then caspase‐3/7 activity was determined by luminescent assay (630/595 nm) using the Caspase Glo kit (Promega), following the manufacturer instructions.
Immunoblotting
Freshly isolated primary ATII cells were seeded (2.5 × 106 cells/well) in 6‐well cell culture clusters (Costar; Corning, Corning, NY). At day 4, ATII cell monolayers were treated with bleomycin (Bleo, 50 mU/mL), dexamethasone (Dex, 100 nmol/L), a combination of bleomycin and dexamethasone (Bleo + Dex) or vehicle (DMSO) and total proteins were extracted 24 h later. Briefly, ATII cell monolayers were washed twice with PBS, scraped with 200 µL/well lysis buffer (NaCl 150 mmol/L, EDTA 5 mmol/L, TRIS 50 mmol/L, Triton X‐100 1%, pH 7.5, plus a cocktail of proteases/phosphatases inhibitors (Sigma)). The suspension was centrifuged at 13,000 rpm for 10 min at 4°C. Supernatants were collected and protein content was evaluated by the Bradford method. Sample proteins were denatured at 95°C for 10 min, separated by SDS‐PAGE (10%) and transferred to a PVDF membrane. PVDF membranes were first blocked with 5% powdered milk in Tris‐buffered saline + 0.1% Tween 20 (TBST) for 1 h at room temperature and then washed three times with TBST before overnight incubation (at 4°C) with anti‐β3‐integrin (Abcam, dilution 1:1000, TBST + 5% Bovine serum albumin (BSA, Sigma)), anti‐β6‐integrin (Santa Cruz/Millipore, dilution 1:1000, TBST + 5% BSA), anti‐β‐actin (Sigma, 1:1000, TBST + 5% BSA) or anti‐pan‐actin (Cell signaling, 1:1000, TBST + 5% BSA) antibodies. Membranes were then washed with TBST and incubated with horseradish peroxidase‐labeled secondary antibody (goat anti‐rabbit (Santa Cruz/Cell signaling), mouse anti‐goat (Santa Cruz) and goat anti‐mouse (Sigma/Abcam), dilution 1:1000, TBST + 5% powdered milk). Membranes were rinsed (TBST, 3 × 15 min) and incubated with a luminescent reactive Immun‐Star WesternC Kit (Bio‐Rad Laboratories Inc.) or Western Lightning Plus‐ECL (PerkinElmer). The intensity of each band was measured with a ChemiDoc system (BioRad Laboratories Inc.), quantified with the Image Lab program (BioRad Laboratories Inc.) and normalized to the actin signal. Protein expression is presented as a percentage of the control condition (100%).
Statistical analysis
Mice survival rates (Fig. 1B) are presented as percentages (%) of living mice compared to the initial group for each treatment group, while lung damage scores (Fig. 4B) are presented as a repartition of mice with each damage score (expressed as % of the total number of mice in each treatment group). All other data are presented as means ± standard error of the mean (SEM). Graphs and statistical analyses were performed with GraphPad Prism 5 software (CA, USA). Agostino/Pearson normality tests were first performed, followed by statistical tests, adapted to each type of experiment, as specified in figure legends. P values are also indicated for each series of experiments (P values < 0.05 (*) were considered significant).
Figure 1.
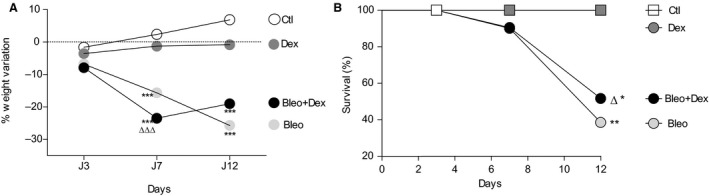
Effect of dexamethasone on body weight and survival after bleomycin‐induced acute lung injury in mice. After i.t. instillation (at day 1) of saline (0.9%, 50 μL) or bleomycin (Bleo, 4 U/kg, 50 μL), male NMRI mice were treated (i.p. injection) daily with saline (0.9%, 100 μL) or dexamethasone (Dex, 0.5 mg/kg, 100 μL) for 3, 7 or 12 days. Weight variations (A, n = 3–21) as a function of time and percentage (%) of mouse survival (B, n = 6–20) were compared between the four groups: Ctl (saline (i.t.)/saline (i.p.)); Bleo (bleo (i.t.)/saline (i.p.)); Dex (saline (i.t.)/dex (i.p.)) and Bleo + Dex (bleo (i.t.)/dex (i.p.)). Values are means ± SEM, **P < 0.01, ***P < 0.001 versus control condition at the same time point, ∆∆P < 0.01, ∆∆∆P < 0.001 versus Dex condition at the same time point. 1‐way ANOVA (Agostino/Pearson normality positive tested, P < 0.0001) and Bonferroni post hoc test (A, day 3), 1‐way ANOVA; Kruskal–Wallis test and Dunn’s post hoc test (A, day 7 and 12). Comparison of survival curves was made with a Log‐rank (Mantel‐Cox) test which generated a Chi square, **P < 0.01 Bleo versus Ctl curve, *P < 0.05 Bleo + Dex versus Ctl curve and ∆ P < 0.05 Dex versus Bleo + Dex curve (B)
Figure 4.
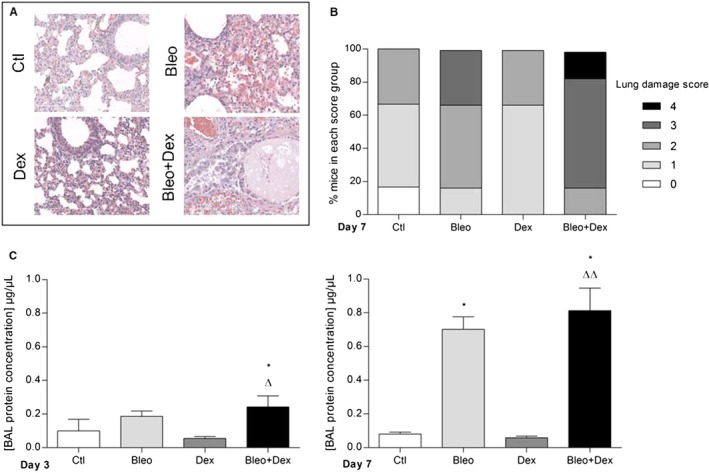
Effect of dexamethasone on alveolar epithelial damage after bleomycin‐induced acute lung injury in mice. Histological sections of mouse lungs stained with hematoxylin‐eosin (Magnification × 20. Scale: 200 µm, A) and repartition of lung damage scores at day 7 (B, n = 6–8) are presented for each group (Ctl (saline (i.t.)/saline (i.p)); Bleo (bleo (i.t.)/saline (i.p.)); Dex (saline (i.t.)/dex (i.p.)) and Bleo + Dex (bleo (i.t.)/dex (i.p.)). The concentration of proteins in BAL (μg/μL, C, n = 5–6) was measured at day 3 (left panel) and 7 (right panel). Values are means ± SEM, *P < 0.05 versus Ctl condition, ∆P < 0.05, ∆∆P < 0.01 versus Dex condition. 1‐way ANOVA; Kruskal–Wallis test and Dunn’s post hoc test
Results
While progressive weight gain was observed in the control (Ctl) group as a function of time (at days 3, 7 and 12), lung delivery of bleomycin (Bleo, 4 U/kg) caused a significant weight loss at days 7 and 12 (Fig. 1A). However, daily (i.p.) treatment with dexamethasone (0.5 mg/kg) in mice with bleomycin‐induced lung injury (Bleo + Dex group) did not prevent weight loss.
A 100% survival rate was observed in the control (Ctl) and dexamethasone (Dex) groups at each time points, whereas the bleomycin challenge (Bleo and Bleo + Dex groups) induced an increasing mortality rate (P < 0.05). Dexamethasone treatment (Bleo + Dex) did not significantly improve the survival (Fig. 1B).
In subsequent experiments, the effect of dexamethasone on the bleomycin outcomes was measured during the acute exudative phase, i.e., at 3 and 7 days. We first evaluated the levels of the TNF‐α cytokine, which plays a key role in alveolar epithelial damage and dysfunction in acute lung injury (Patel et al. 2013). We found that exposure to bleomycin was associated with a significant increase in TNF‐α in the BAL collected at day 3 (Bleo and Bleo + dex group, Fig. 2A), and that the TNF‐α levels in the Bleo + Dex group were significantly lower compared to the Bleo group on day 3. The same trend was observed at day 7, although the variations were not statistically significant.
Figure 2.
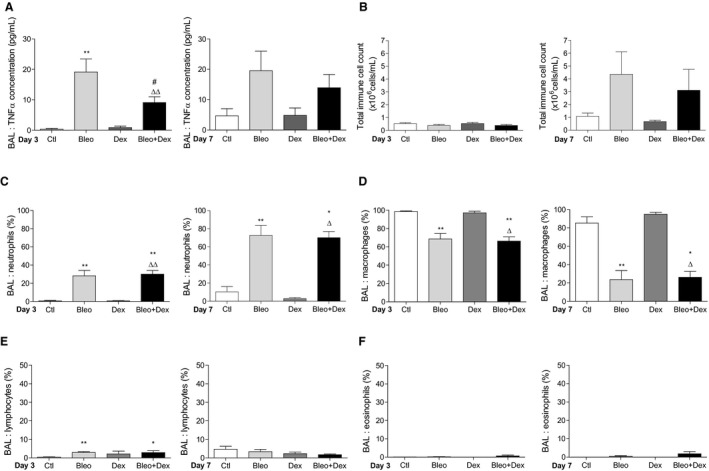
Effect of dexamethasone on the inflammatory response after bleomycin‐induced acute lung injury in mice. A. Levels of TNF‐α (pg/mL) detected by ELISA in BAL collected at day 3 (n = 6, left panel) and 7 (n = 5–7, right panel). Total immune cell counts (B) and differential cell count (% of neutrophils (C), macrophages (D), lymphocytes (E) and eosinophils (F)) in BAL collected from mice 3 (left panel) or 7 (right panel) days after the bleomycin challenge (4 U/kg, 50 μL i.t., n = 4–7). Values are means ± SEM, *P < 0.05, **P < 0.01 versus Ctl condition, #P < 0.05 versus Bleo condition, ∆P < 0.05, ∆∆P < 0.01 versus Dex condition. Non‐parametric t‐test (Mann–Whitney, panel A) or 1‐way ANOVA; Kruskal–Wallis test and Dunn’s post hoc test (panels B, C, D, E, F)
Elevated total leukocyte cell counts in BAL were observed at day 7 in the Bleo and Bleo + Dex conditions (Fig. 2B). Differential cell count then revealed that compared to that in the control group, a significant increase in the proportion of neutrophils was observed in the BAL of mice challenged with bleomycin (Fig. 2C). The proportion of neutrophils was similar in the Bleo + Dex group. This increase in neutrophils induced by bleomycin was associated with a parallel decrease in the proportion of macrophages in both the Bleo and Bleo + Dex groups (Fig. 2D). The proportion of lymphocytes and eosinophils were lower than 5% in all experimental conditions (Fig. 2E and 2F).
Edema flooding was also associated with the bleomycin‐induced damage of the alveolar epithelial/endothelial barriers. Indeed, at day 7 the wet/dry (W/D) ratio is significantly higher in bleomycin‐challenged mice (6.1 ± 0.2), compared to the control condition (4.6 ± 0.1) (Fig. 3, right panel). Moreover, dexamethasone treatment did not prevent bleomycin‐induced edema. Similarly to dexamethasone, another corticosteroid (methylprednisolone, Fig. S1) did not elicit any beneficial effect on lung edema in bleomycin mice.
Figure 3.
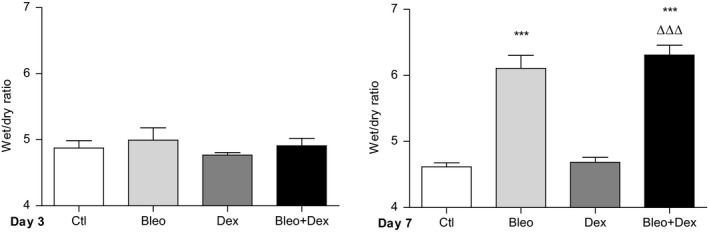
Effect of dexamethasone on edema formation after bleomycin‐induced acute lung injury in mice. Wet‐to‐dry lung weight ratios (edema index) were measured 3 (n = 12, left panel) and 7 (n = 14–22, right panel) days after initial instillation (day 1) of saline (Ctl, 0.9%) or bleomycin (Bleo, 4 U/kg) and daily treatments (i.p. administration) with saline (0.9%) or dexamethasone (Dex, 0.5 mg/kg, 100 μL). Values are means ± SEM, ***P < 0.001 versus Ctl condition, ∆∆∆P < 0.001 versus Dex condition. 1‐way ANOVA; Kruskal–Wallis test and Dunn’s post hoc test
Because the observed inability to clear the lung edema could be due to persistent alveolar damage in the presence of bleomycin and dexamethasone, histological analyses (Fig. 4) were then performed. Substantial lung injury was observed in the presence of bleomycin, with injury scores of 2 and 3 in 50% and 33.3% of mice, respectively (Fig. 4B). Mice in the Bleo + Dex group exhibited severe injury scores [2 (16.7%), 3 (66.7%) and 4 (16.7%)]. Alveolar damage was also associated with a significant increase in the protein concentration in the BAL of mice as early as day 3 in the Bleo + Dex group and at day 7 in the Bleo condition (Fig. 4C).
We then hypothesized that this persistent alveolar damage could be due to a deleterious effect of bleomycin and dexamethasone on the repair capacity of alveolar cells. To test this hypothesis, we then performed a series of wound healing assays on primary alveolar epithelial (ATII) cell cultures. As depicted in Figure 5A, a time‐ (over a 48 h period after injury) and dose‐ (12.5 to 200 mU/mL) dependent inhibition of the wound healing rates was observed in the presence of bleomycin. Because a dose of 50 mU/mL significantly decreased the repair rates at every time point, this concentration was used in subsequent experiments (Fig. 5B–D). Our data showed that a 24 h treatment with dexamethasone (100 nmol/L, a dose previously shown to elicit biological effects on ATII cells (Champigny et al. 1994; Dagenais et al. 2006)) alone significantly reduced the wound healing rates (21,304 ± 1328 vs. 31,523.1 ± 1519 µm2/h in control condition) and further dampened the repair rates measured in the presence of bleomycin (23,642.9 ± 1484 µm2/h and 13,671 ± 2416 µm2/h in Bleo and Bleo + Dex, respectively).
Figure 5.
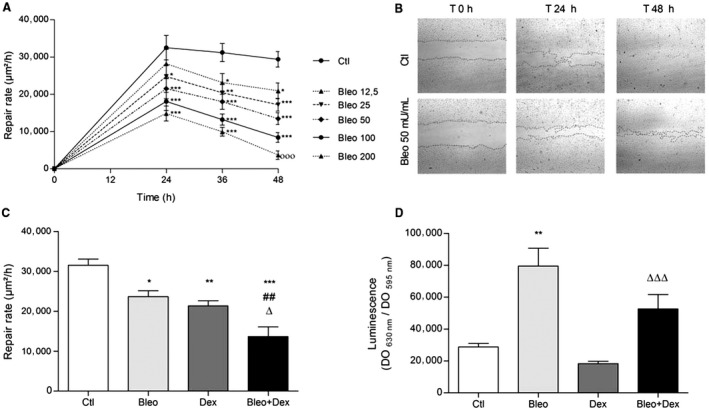
Effect of dexamethasone on the repair rates of primary ATII cell monolayers. (A) Primary rat ATII cell monolayers were injured mechanically, and repair rates (μm2/h) were measured over periods of 24, 36 and 48 h in the control (Ctl) condition (saline, 0.9%) and after treatments with increasing doses of bleomycin (Bleo, 12.5, 25, 50, 100, 200 mU/mL, n = 8). (B) Representative photographs of healing ATII cell monolayers at 0, 24 and 48 h after treatment with saline (0.9%) or bleomycin (Bleo, 50 mU/mL). (C) Repair rates (μm2/h) of ATII cell monolayers in control condition (Ctl, 0.9%), after treatment with bleomycin alone (Bleo, 50 mU/mL, applied at T0), dexamethasone alone (Dex, 100 nmol/L, applied 24 h before injury) and a combination of bleomycin (50 mU/mL, T0) and dexamethasone (100 nmol/L, 24 h before injury) (Bleo + Dex, n = 8). (D) ATII cell apoptosis in control condition (Ctl, 0.9% saline) and after 24 h treatment with bleomycin alone (Bleo, 50 mU/mL), dexamethasone alone (Dex, 100 nmol/L) or a combination of bleomycin (50 mU/mL) and dexamethasone (100 nmol/L) (Bleo + Dex) (n = 10). Values are means ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 versus Ctl condition, ##P < 0.01 versus Bleo condition, ∆P < 0.05 versus Dex condition. 2‐way ANOVA and Bonferroni post hoc test (vs. control at the same time point, panel A), 1‐way ANOVA (Agostino/Pearson normality positive tested, P < 0.0001) and Bonferroni post hoc test (panel C), 1‐way ANNOVA Kruskal‐Wallis test and Dunn’s post hoc test (panel D)
A pro‐apoptotic effect of bleomycin has been established in several cell models (Lee et al. 2005; Wallach‐Dayan et al. 2006). In agreement with these previous reports, a significant increase in caspase 3/7 activity was noted in bleomycin‐treated ATII cells (in Bleo and Bleo + Dex conditions, compared to Ctl and Dex, respectively) (Fig. 5D). Although, ATII cell apoptosis was slightly lower in the presence of Dex (Bleo + Dex, compared to Bleo alone), the decrease was not statistically significant.
The negative effect of dexamethasone on wound healing was associated with a significant decrease in the expression of two key proteins of alveolar repair, i.e., β3‐ and β6‐integrins (37 and 55% decrease, compared to the control condition, when the cells were treated with dexamethasone (Fig. 6)). A significant reduction in β6‐integrin expression was also observed after exposure to bleomycin. The Bleo + Dex combination also induced a decrease in β3‐ and β6‐integrins (although non‐statistically significant for β6‐integrin).
Figure 6.
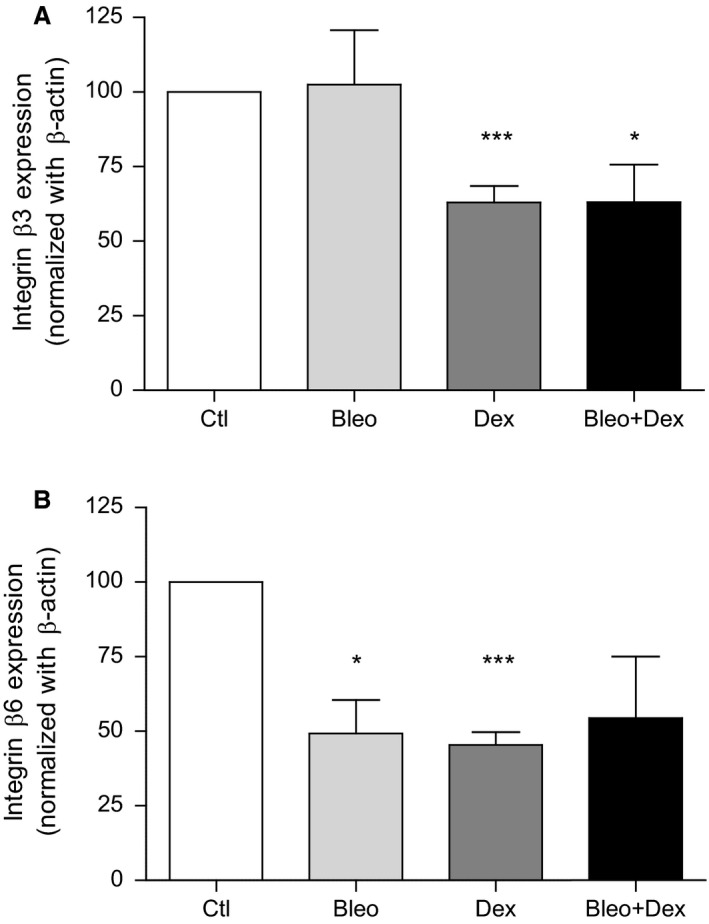
Effect of dexamethasone on β3‐ and β6‐integrin expression in primary ATII cells. Protein expression (% of control condition normalized with actin expression) of β3‐ (upper panel, n = 6–14) and β6‐integrin (lower panel, n = 3–8) in control, bleomycin, dexamethasone and bleomycin + dexamethasone pretreated (24 h) conditions. Values are presented as means ± SEM, one simple t‐test with a theoretical mean of 100%, *P < 0.05 and ***P < 0.001 versus Ctl condition
Discussion
Although dexamethasone treatment efficiently prevented the TNF‐α response after bleomycin‐induced lung injury, our experiments showed that it failed to reduce the neutrophil infiltration in BAL, weight loss and mortality rates, as well as lung edema and injury scores. Our data support the hypothesis that this inability of dexamethasone to improve the resolution of bleomycin outcomes may be due to its damaging effect on the repair capacity of the alveolar epithelium.
Our data first showed that daily treatments with dexamethasone over a 12‐day period failed to prevent weight loss associated with the inflammatory response after the bleomycin challenge. The muscle atrophy induced by glucocorticoids might also be responsible for this inability to gain weight (Schakman et al. 2009; Bodine and Furlow 2015). Our study also demonstrated that the number of mice requesting euthanasia because they reached endpoints was similar in the Bleo and Bleo + Dex experimental groups. These findings are in agreement with previous reports indicating that dexamethasone did not reduce weight loss and/or mortality rates in experimental models of acute lung injury (Koshika et al. 2005; Xu et al. 2009).
Our results also show that although dexamethasone treatment efficiently dampened the early TNF‐α increase (at day 3), it did not impair the inflammatory response or the injury process. Indeed, the increase in neutrophils, the elevated protein content measured in the BAL, the injury score and the increase in the wet/dry lung weight ratio in the presence of bleomycin on day 7 were not attenuated by dexamethasone, at the tested dose. This persistent neutrophil infiltration most likely contributes to the epithelial injury (Ware and Matthay 2000; Grommes and Soehnlein 2011) observed in our histological analyses. This inability of dexamethasone to protect/improve the alveolar barrier has also been observed in other models of severe acute lung injury (Xu et al. 2009; Yubero et al. 2012; Hegeman et al. 2013; Engel et al. 2015). Indeed, our results are in phase with the variable response observed following dexamethasone treatment of lung‐injured animals. In agreement with our findings, reduced TNF‐α levels were observed in bleomycin + dexamethasone‐treated rats compared to those in the bleomycin condition (Yang et al. 2015). Yubero et al. also reported that intramuscular injection of dexamethasone (1 mg/kg), 1 h after induction of lung injury due to acute pancreatitis in rats, downregulated inflammatory factors but did not reduce leukocyte infiltration (Yubero et al. 2012). However, i.v. administration of dexamethasone at the initiation of ventilation‐induced lung injury in mice has been shown to attenuate both inflammatory mediator expression (KC, MCP‐1, IL‐1β, IL‐6) in lung tissues and neutrophil infiltration in BAL (Hegeman et al. 2013). In contrast, neither cellular infiltration nor cytokine release were inhibited by dexamethasone in a model of acute lung injury induced by the H5N1 virus in mice (Xu et al. 2009) and in ventilated adult sheep with early phase acute respiratory distress syndrome (Engel et al. 2015). Although the variability observed is in part secondary to the outcomes that are measured, the administration of dexamethasone alone does not appear to be efficient in reversing the evolution of lung injury or counteracting its undesirable side effects (Xu et al. 2009; Kohno et al. 2010; Yubero et al. 2012; Hegeman et al. 2013; Engel et al. 2015).
The persistent lung edema and/or inflammatory cell infiltration observed in our model and others (Xu et al. 2009; Yubero et al. 2012; Hegeman et al. 2013; Engel et al. 2015), despite the presence of dexamethasone, may be due to severe and nonresolving alveolar damage, at the measured time points. In agreement with our hypothesis of a deleterious impact of bleomycin and dexamethasone on the repair capacity of the alveolar epithelium, our experiments first show a time‐ and dose‐dependent inhibition of wound repair rates of primary ATII cell cultures by bleomycin. The observed pro‐apoptotic effect of bleomycin, could contribute, at least in part, to this repair impairment. Not only did dexamethasone not reverse the bleomycin‐repair impairment, but it further worsened the repair delay in vitro as well as the injury scores and protein levels in BAL in vivo. In agreement with our results, a deleterious effect of dexamethasone on repair mechanisms has also been shown in other epithelial cells (Liu et al. 2013; Kadmiel et al. 2016). Furthermore, it has been shown that dexamethasone inhibits corneal epithelial wound healing and cell migration (by altering the activity of membrane lamellipodia and filopodia) but promotes tight junction integrity (Kadmiel et al. 2016). The role of corticosteroid‐induced apoptosis on airway epithelial repair impairment has also been studied. A previous study by Dorscheid et al (2006) indicated that the observed airway epithelial cell apoptosis induced by dexamethasone or budesonide was not involved in the decrease in wound repair rates after corticosteroid exposure. At lower concentration, dexamethasone elicited in our study a slight, but non‐significant, decrease in caspase 3/7 activity in ATII cells in the absence or presence of bleomycin.
The decreased β3‐ and β6‐integrin expression that we observed in alveolar epithelial cells may be involved in dexamethasone‐induced repair impairment. However, other mechanisms may also be involved. Indeed, a previous study indicated that the inhibition of airway epithelial repair, cell proliferation and migration by dexamethasone may be mediated, at least in part, by suppression of the MAPK/ERK signaling pathway (Liu et al. 2013). Similarly, a marked inhibition in cell proliferation and migration, associated with decreased levels of Mek1/2‐p‐Erk1/2, has been observed in human airway epithelial cell (16HBE) cultures in the presence of dexamethasone (Jia et al. 2018).
Conclusions
Altogether, our data indicates that the inability of dexamethasone to improve the resolution of bleomycin outcomes, in particular, mortality rates, cell infiltration and lung edema, may be due to the remaining alveolar damage observed at day 7 and repair impairment induced by dexamethasone. Although data from animal models should be taken with caution and never perfectly reflect the physiopathology of ARDS, our study and those previously published suggest that dexamethasone alone is unlikely to be an efficient therapy for acute lung injury. Indeed, there is accumulating evidence that successful treatment of ARDS and lung injury will depend on our capacity to reduce epithelial injury and allow for more rapid restoration of alveolar epithelial integrity and function (Matthay 2014).
Conflict of Interest
The authors declare that they have no competing interests.
Supporting information
Figure S1. Effect of another anti‐inflammatory drug, methylprednisolone, on edema index after bleomycin‐induced acute lung injury in mice. Wet/dry ratios were measured 3 (n = 6, left panel) and 7 days (n = 6, right panel) after instillation of saline (Ctl, 0.9%) or bleomycin (Bleo, 4 U/kg) and daily treatments (i.p. administration) with saline (0.9%) or methylprednisolone (methyl, 1 mg/kg, 100 μL). Values are means ± SEM, **P < 0.01 versus Ctl condition, ∆P < 0.05, ∆∆P < 0.01 versus Dex condition. n = 6. 1‐way ANOVA Kruskal–Wallis test and Dunn’s post hoc test.
Acknowledgments
The authors thank the histopathology core facility and Louis Gaboury of Institut de Recherche en Immunologie et en Cancérologie (IRIC, Université de Montréal) for histological analysis and injury scoring.
Physiol Rep, 7(21), 2019, e14253, 10.14814/phy2.14253
Funding Information
This work was supported by the Canadian Institutes of Health Research (CIHR to YB and CIHR grant 230753 and PJT153406 to EB), the Natural Sciences and Engineering Research Council of Canada (NSERC, discovery grant RGPIN‐2016‐04378 to EB), CRCHUM and Université de Montréal (scholarship to EB). MAV also acknowledges studentships from the NSERC and the Fonds de Recherche du Québec‐Santé (FRQ‐S). The CRCHUM is supported by a Centre grant from FRQ‐S. Authors are members of the Respiratory Health Network of Québec. The funders have no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
References
- Adam, D. , Bilodeau C., Sognigbé L., Maillé É., Ruffin M., and Brochiero E.. 2018. CFTR rescue with VX‐809 and VX‐770 favors the repair of primary airway epithelial cell cultures from patients with class II mutations in the presence of Pseudomonas aeruginosa exoproducts. J. Cyst. Fibros. 17:705–714. [DOI] [PubMed] [Google Scholar]
- Bardou, O. , Privé A., Migneault F., Roy‐Camille K., Dagenais A., Berthiaume Y., et al. 2012. K+ channels regulate ENaC expression via changes in promoter activity and control fluid clearance in alveolar epithelial cells. Biochim. Biophys. Acta – Biomembr. 1818:1682–1690. [DOI] [PubMed] [Google Scholar]
- Bein, Thomas , Briegel J., and Annane D.. 2016. Steroids are part of rescue therapy in ARDS patients with refractory hypoxemia: yes. Intensive Care Med. 42:918–920. [DOI] [PubMed] [Google Scholar]
- Bihari, S. , Bailey M., and Bersten A. D.. 2016. Steroids in ARDS: to be or not to be. Intensive Care Med. 42:931–933. [DOI] [PubMed] [Google Scholar]
- Bodine, S. C. , and Furlow J. D.. 2015. Glucocorticoids and Skeletal Muscle. Adv Exp Med Biol. 2015;872:145–76. [DOI] [PubMed] [Google Scholar]
- Bos, L. D. , Martin‐loeches I., and Schultz M. J.. 2018. ARDS: challenges in patient care and frontiers in research. Eur. Respir. Rev. 27:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochiero, E. , Dagenais A., Privé A., Berthiaume Y., and Grygorczyk R.. 2004. Evidence of a functional CFTR Cl(‐) channel in adult alveolar epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 287:L382–L392. [DOI] [PubMed] [Google Scholar]
- Champigny, G. , Voilley N., Lingueglia E., Friend V., Barbry P., and Lazdunski M.. 1994. Na+ channel by steroid hormones. EMBO J. 13:2177–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F. , Gong L., Zhang L., Wang H., Qi X., Wu X., et al. 2006. Short courses of low dose dexamethasone delay bleomycin‐induced lung fibrosis in rats. Eur. J. Pharmacol. 536:287–295. [DOI] [PubMed] [Google Scholar]
- Dagenais, A. , Fréchette R., Clermont M.‐E., Massé C., Privé A., Brochiero E., et al. 2006. Dexamethasone inhibits the action of TNF on ENaC expression and activity. Am. J. Physiol. Lung Cell Mol. Physiol. 291:L1220–L1221. [DOI] [PubMed] [Google Scholar]
- Dagenais, A. , Tessier M. C., Tatur S., Brochiero E., Grygorczyk R., and Berthiaume Y.. 2013. Hypotonic Shock modulates Na+ current via a Cl− and Ca2+/calmodulin dependent mechanism in alveolar epithelial cells. PLoS ONE 8:e74565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs, L. G. , Gonzalez R., and Williams M. C.. 1986. An improved method for isolating type II cells in high yield and purity. Am. Rev. Respir. Dis. 134:141–145. [DOI] [PubMed] [Google Scholar]
- Dorscheid, D. R. , Patchell B. J., Estrada O., Marroquin B., Tse R., and White S. R.. 2006. Effects of corticosteroid‐induced apoptosis on airway epithelial wound closure in vitro. Am. J. Physiol. Lung Cell Mol. Physiol. [Internet] 291:794–801. [DOI] [PubMed] [Google Scholar]
- Engel, M. , Nowacki R. M. E., Boden P., Reiss L. K., Uhlig S., Reynaert N. L., et al. 2015. The effects of dexamethasone and oxygen in ventilated adult sheep with early phase acute respiratory distress syndrome. Lung 193:97–103. [DOI] [PubMed] [Google Scholar]
- Fan, E. , Brodie D., and Slutsky A. S.. 2018. Acute respiratory distress syndromeadvances in diagnosis and treatment. JAMA 319:698–710. [DOI] [PubMed] [Google Scholar]
- Fitzgerald, M. , McAuley D. F., and Matthay M.. 2014. Is there a need for emerging drugs for the acute respiratory distress syndrome? Expert Opin. Emerg. Drugs 19:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, J. R. 2010. Steroids for early acute respiratory distress syndrome: critical appraisal of Meduri GU, Golden E, Freire AX, et al.: Methylprednisolone infusion in early severe ARDS: Results of a randomized controlled trial. Chest 2007; 131:954–963. Pediatr. Crit. Care Med. 11:404–407. [DOI] [PubMed] [Google Scholar]
- Girault, A. , Chebli J., Privé A., Trinh N. T. N., Maillé E., Grygorczyk R., et al. 2015. Complementary roles of KCa3.1 channels and β1‐integrin during alveolar epithelial repair. Respir. Res. 16:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, H. , Ledford J. G., Mukherjee S., Noble P. W., Williams K. L., and Wright J. R.. 2010. The role of surfactant protein A in bleomycin‐induced acute lung injury. Am. J. Respir. Crit. Care Med. 181:1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grommes, J. , and Soehnlein O.. 2011. Contributions of neutrophils to ALI. Mol. Med. 17:293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbault, C. , Martin P., Houle D., Boghdady M. L., Guiot M. C., Marion D., et al. 2005. Cystic fibrosis lung disease following infection with Pseudomonas aeruginosa in Cftr knockout mice using novel non‐invasive direct pulmonary infection technique. Lab. Anim. 39:336–352. [DOI] [PubMed] [Google Scholar]
- Hegeman, M. A. , Hennus M. P., Cobelens P. M., Kavelaars A., Jansen N. J. G., Schultz M. J., et al. 2013. Dexamethasone attenuates VEGF expression and inflammation but not barrier dysfunction in a murine model of ventilator‐induced lung injury. PLoS ONE 8:e57374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, S. , Guo P., Ge X., Wu H., Lu J., and Fan X.. 2018. Overexpression of indoleamine 2, 3‐dioxygenase contributes to the repair of human airway epithelial cells inhibited by dexamethasone via affecting the MAPK/ERK signaling pathway. Exp. Ther. Med. 16:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kadmiel, M. , Janoshazi A., Xu X., and Cidlowski J. A.. 2016. Glucocorticoid action in human corneal epithelial cells establishes roles for corticosteroids in wound healing and barrier function of the eye. Exp. Eye Res. 152:10–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, D. , Saravia J., Rovnaghi C. R., Meduri G. U., Schwingshackl A., Cormier S. A., et al. 2016. Plasma biomarker analysis in pediatric ARDS: generating future framework from a pilot randomized control trial of methylprednisolone: a framework for identifying plasma biomarkers related to clinical outcomes in pediatric ARDS. Front. Pediatr. 4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno, M. , Haramoto M., Nakajima O., Yang L., Hinotsu S., Yokohira M., et al. 2010. Antedrug budesonide by intrapulmonary treatment attenuates bleomycin‐induced lung injury in rats. Biol. Pharm. Bull. 33:1206–1211. [DOI] [PubMed] [Google Scholar]
- Koshika, T. , Hirayama Y., Ohkubo Y., Mutoh S., and Ishizaka A.. 2005. Tacrolimus (FK506) has protective actions against murine bleomycin‐induced acute lung injuries. Eur. J. Pharmacol. 515:169–178. [DOI] [PubMed] [Google Scholar]
- Lee, V. Y. , Schroedl C., Brunelle J. K., Buccellato L. J., Akinci O. I., Kaneto H., et al. 2005. Bleomycin induces alveolar epithelial cell death through JNK‐dependent activation of the mitochondrial death pathway. Am. J. Physiol. ‐ Lung Cell Mol. Physiol. 289:521–528. [DOI] [PubMed] [Google Scholar]
- Leite‐Junior, J. H. P. , Garcia C. S. N. B., Souza‐Fernandes A. B., Silva P. L., Ornellas D. S., Larangeira A. P., et al. 2008. Methylprednisolone improves lung mechanics and reduces the inflammatory response in pulmonary but not in extrapulmonary mild acute lung injury in mice. Crit. Care Med. 36:2621–2628. [DOI] [PubMed] [Google Scholar]
- Leroy, C. , Privé A., Bourret J.‐C., Berthiaume Y., Ferraro P., and Brochiero E.. 2006. Regulation of ENaC and CFTR expression with K+ channel modulators and effect on fluid absorption across alveolar epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 291:L1207–L1219. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Zhang M., Niu C., Luo Z., Dai J., Wang L., et al. 2013. Dexamethasone inhibits repair of human airway epithelial cells mediated by glucocorticoid‐induced leucine zipper (GILZ). PLoS ONE 88:e60705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac, S. R. , and McAuley D. F.. 2017. Prolonged glucocorticoid treatment in acute respiratory distress syndrome. Lancet 389:1516–1517. [DOI] [PubMed] [Google Scholar]
- Maillé, E. , Thu N., Trinh N., Privé A., Bilodeau C., Bissonnette É., et al. 2011. Regulation of normal and cystic fibrosis airway epithelial repair processes by TNF‐after injury. Am. J. Physiol. Lung Cell Mol. Physiol. 301:945–955. [DOI] [PubMed] [Google Scholar]
- Matthay, M. A. 2014. Resolution of pulmonary edema thirty years of progress. Am. J. Respir. Crit. Care Med. 189:1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute‐Bello, G. , Frevert C. W., and Martin T. R.. 2008. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 295:L379–L399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute‐Bello, G. , Downey G., Moore B. B., Groshong S. D., Matthay M. A., Slutsky A. S., et al. 2011. An official American Thoracic Society Workshop Report: features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 44:725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meduri, G. U. , and Siemieniuk R. A. C.. 2017. Prolonged glucocorticoid treatment in acute respiratory distress syndrome. Lancet 389:1516–1517. [DOI] [PubMed] [Google Scholar]
- Meduri, G. U. , Bridges L., Shih M. C., Marik P. E., Siemieniuk R. A. C., and Kocak M.. 2016. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients’ data from four randomized trials and trial‐level meta‐analysis of the updated literature. Intensive Care Med. 42:829–840. [DOI] [PubMed] [Google Scholar]
- Monahan, L. J. 2013. Acute respiratory distress syndrome. Curr. Probl. Pediatr. Adolesc. Health Care 43:278–284. [DOI] [PubMed] [Google Scholar]
- Patel, B. V. , Wilson M. R., Dea K. P. O., and Takata M.. 2013. TNF‐induced death signaling triggers alveolar epithelial dysfunction in acute lung injury. J. Immunol. 190:4274–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri, V. M. , Rubenfeld G. D., Thompson B. T., Ferguson N. D., Caldwell E., Fan E., et al. 2012. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307:2526–2533. [DOI] [PubMed] [Google Scholar]
- Ruffin, M. , Bilodeau C., Mailĺe É., LaFayette S. L., McKay G. A., Trinh N. T. N., et al. 2016. Quorum‐sensing inhibition abrogates the deleterious impact of Pseudomonas aeruginosa on airway epithelial repair. FASEB J. 30:3011–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, F. , Tasaka S., Inoue K. I., Miyamoto K., Nakano Y., Ogawa Y., et al. 2008. Role of interleukin‐6 in bleomycin‐induced lung inflammatory changes in mice. Am. J. Respir. Cell Mol. Biol 38:566–571. [DOI] [PubMed] [Google Scholar]
- Schakman, O. , Gilson H., Kalista S., and Thissen J. P.. 2009. Mechanisms of muscle atrophy induced by glucocorticoids. Horm. Res. 72(Suppl 1):36–41. [DOI] [PubMed] [Google Scholar]
- Seam, N. , and Suffredini A. F.. 2016. Steroids are part of rescue therapy in ARDS patients with refractory hypoxemia: we are not sure. Intensive Care Med. 42:924–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro, D. W. , Sawa T., and Gropper M. A.. 2003. Injury and repair in lung and airways. Crit. Care Med. 31(8 Suppl):S524–S531. [DOI] [PubMed] [Google Scholar]
- Standiford, T. J. , and Ward P. A.. 2016. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl. Res. 167:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg, K. P. , Hudson L. D., Goodman R. B., Lee Hough C., Michigan O., Arbor A., et al. 2006. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome the national heart, lung, and blood institute Acute Respiratory Distress Syndrome (ARDS) clinical trials network. N. Engl. J. Med. 354:1671–1684. [DOI] [PubMed] [Google Scholar]
- Thompson, B. T. , and Ranieri V. M.. 2016. Steroids are part of rescue therapy in ARDS patients with refractory hypoxemia: no. Intensive Care Med. 42:921–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongyoo, S. , Permpikul C., Mongkolpun W., Vattanavanit V., Udompanturak S., Kocak M., et al. 2016. Hydrocortisone treatment in early sepsis‐associated acute respiratory distress syndrome: results of a randomized controlled trial. Crit. Care 20:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach‐Dayan, S. B. , Izbicki G., Cohen P. Y., Gerstl‐Golan R., Fine A., and Breuer R.. 2006. Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am. J. Physiol. ‐ Lung Cell Mol. Physiol. 290:790–796. [DOI] [PubMed] [Google Scholar]
- Wang, X. Q. , Zhou X., Zhou Y., Rong L., Gao L., and Xu W.. 2008. Low‐dose dexamethasone alleviates lipopolysaccharide‐induced acute lung injury in rats and upregulates pulmonary glucocorticoid receptors. Respirology 13:772–780. [DOI] [PubMed] [Google Scholar]
- Ware, L. , and Matthay M.. 2000. The acute respiratory distress syndrome. N. Engl. J. Med. 342:1334–1349. [DOI] [PubMed] [Google Scholar]
- Xu, T. , Qiao J., Zhao L., He G., Li K., Wang J., et al. 2009. Effect of dexamethasone on acute respiratory distress syndrome induced by the H5N1 virus in mice. Eur. Respir. J. 33:852–860. [DOI] [PubMed] [Google Scholar]
- Yang, D. , Yuan W., Lv C., Li N., Liu T., Wang L., et al. 2015. Dihydroartemisinin supresses inflammation and fibrosis in bleomycine‐induced pulmonary fibrosis in rats. Int. J. Clin. Exp. Pathol. 8:1270–1281. [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. G. , Lei X. L., and Li X. L.. 2017. Early application of low‐dose glucocorticoid improves acute respiratory distress syndrome: a meta‐analysis of randomized controlled trials. Exp. Ther. Med. 13:1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehya, N. , Servaes S., Thomas N. J., Nadkarni V. M., and Srinivasan V.. 2015. Corticosteroid exposure in pediatric acute respiratory distress syndrome. Intensive Care Med. 41:1658–1666. [DOI] [PubMed] [Google Scholar]
- Yubero, S. , Manso M. A., Ramudo L., Vicente S., and De Dios I.. 2012. Dexamethasone down‐regulates the inflammatory mediators but fails to reduce the tissue injury in the lung of acute pancreatitis rat models. Pulm. Pharmacol. Ther. 25:319–324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of another anti‐inflammatory drug, methylprednisolone, on edema index after bleomycin‐induced acute lung injury in mice. Wet/dry ratios were measured 3 (n = 6, left panel) and 7 days (n = 6, right panel) after instillation of saline (Ctl, 0.9%) or bleomycin (Bleo, 4 U/kg) and daily treatments (i.p. administration) with saline (0.9%) or methylprednisolone (methyl, 1 mg/kg, 100 μL). Values are means ± SEM, **P < 0.01 versus Ctl condition, ∆P < 0.05, ∆∆P < 0.01 versus Dex condition. n = 6. 1‐way ANOVA Kruskal–Wallis test and Dunn’s post hoc test.


