Abstract
BACKGROUND
Thyroid gland is an uncommon site for metastases from clear cell renal cell carcinoma (CCRCC) and literature is scarce. Due to the variable and often long lag time before development of metastases in patients with CCRCC, thyroid nodules may be misdiagnosed initially as benign. This systematic review aims at a better understanding of the nature of these metastases.
METHODS
A bibliographic search was performed using PubMed (1990-2019), key words being “renal cell carcinoma, thyroid, kidney cancer, clear cell.” 147 cases were analyzed. The patient’s characteristics assessed were: age, sex, stage, size of metastases, lag time, diagnostic modality, initial symptoms, treatment and outcome in last documented follow up. Binary logistic regression, Spearman’s rho and ANOVA were used to identify differences between the existing variables.
RESULTS
The mean age (± SD) was 64 ± (10) years in males and 64 (± 11) in females. The mean lag time to diagnosis of thyroid metastases was 8.7 (± 6.3) years. Gender distribution of the patients was 46.3% male, 52.4% female. There was a weak correlation between lag time and size of metastases, not statistically significant. Size of metastases was significantly higher in symptomatic patients (6.06 ± 3.51 cm) compared to those with painless mass (4.6 ± 0.29 cm) and asymptomatic ones (3.93 ± 1.99 cm) (P = 0.03). Fine Needle Aspiration was diagnostic in 29.4% of cases, 47.1% were non diagnostic. Most patients (80.3%) underwent thyroid surgery. At 1 year follow up, 55.6% of patients operated were alive versus 35.3% who did not have surgery, though this was not statistically significant (P = 0.1).
CONCLUSION
A larger size of thyroid metastasis was more likely to present with symptomatology. A high index of suspicion is warranted when evaluating thyroid nodules in CCRCC patients. There was no significant difference in outcome between patients who underwent surgery and those who did not. With the wider use of immune check-point inhibitors and tyrosine kinase inhibitors in metastatic CCRCC, surgery may eventually be reserved only for palliative purposes.
Keywords: Clear cell renal cell carcinoma, Thyroid, Metastases, Thyroidectomy
Core tip: Thyroid gland is an infrequent site for metastases from clear cell renal cell carcinoma (CCRCC) and literature regarding this topic is scarce. Due to the variable and often long lag time before development of metastases in patients with CCRCC, thyroid nodules may be misdiagnosed initially as benign. This systematic review aims at a better understanding of the nature of these metastases.
INTRODUCTION
Metastasis to the thyroid gland is an uncommon clinical presentation, with the most common primary tumor being clear cell renal cell carcinoma (CCRCC) in clinical series. Many patients with thyroid metastases present with signs and symptoms similar to those with primary thyroid disease, and occasionally after a long period following initial diagnosis of their primary tumor. Therefore, thyroid metastases may initially be misdiagnosed as benign thyroid nodules. The aim of this systematic review is to provide an overview of published cases of CCRCC with thyroid metastases, help characterize the features of CCRCC metastasizing to the thyroid gland and understand the role of diagnostic and therapeutic approaches in the management of these patients.
MATERIALS AND METHODS
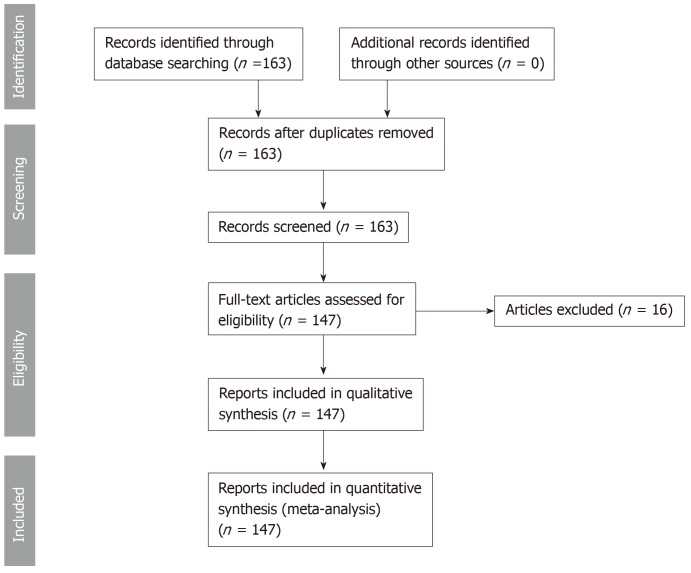
This systematic review was conducted according to the PRISMA guidelines. We searched PubMed, MEDLINE in September 2018 for cases of thyroid metastases from CCRCC between 1990 and 2018 including articles published in English, French, German, Spanish and Japanese. The key words were “renal cell carcinoma, thyroid, kidney cancer, clear cell.” The search strategy is reported in Figure 1. All relevant articles were assessed in full text. A total of 163 cases were obtained. 16 cases were excluded because they were either unavailable in full text, had a primary kidney cancer other than clear cell renal cell carcinoma or due to language barrier. 147 cases were studied. The studied patients’ characteristics were: age, sex, ethnicity, CCRCC stage at initial diagnosis, presence of internal jugular vein thrombosis, lag time between initial diagnosis of CCRCC and detection of thyroid metastases, presence of metastatic sites other than the thyroid gland, diagnostic modality used to confirm the diagnosis of metastatic CCRCC to the thyroid, therapeutic modalities used in treatment, initial presenting symptoms at the diagnosis of the thyroid metastases, presence of thyroid antibodies, thyroid stimulating hormone (TSH) values, airway obstruction, follow-up time after detection of thyroid metastases with the final reported status of the patient (alive versus deceased), status and size of the thyroid metastatic lesions. The characteristics of patient population and different variables are provided in Table 1.
Figure 1.
Systematic review design according to preferred reporting items for systematic reviews and meta-analysis criteria.
Table 1.
Characteristics of patients and different variables in the study
| Characteristics of patients and different variables collected | |
| Age (yr) | mean ± SD |
| Male | 64 ± 10 |
| Female | 64 ± 11 |
| Sex | Percentage (Number of patients) |
| Male | 46.3% (68) |
| Female | 52.4% (75) |
| Initial CCRCC Stage | |
| Stage I CCRCC | 30.6% (19) |
| Stage II CCRCC | 29% (18) |
| Stage III CCRCC | 32.3% (20) |
| Stage IV CCRCC | 8.1% (5) |
| Lag time (years) from initial diagnosis of CCRCC | 8.7 ± 6.35 mean ± SD |
| Size (cm) | |
| Male | 4.87 ± 2.55 |
| Female | 3.84 ± 1.65 |
| Symptoms | |
| Asymptomatic | 21.8% (32) |
| Painless mass | 32.7% (48) |
| Symptomatic | 10.9% (16) |
| Internal Jugular Thrombus | 6.8% (10) |
| Diagnostics | |
| US | 41.2% (35) |
| CT | 20% (17) |
| PET Scan | 15.3% (13) |
| FNA | |
| diagnostic | 29.4% (25) |
| Non-diagnostic | 47.1% (40) |
| Surgery | |
| Total thyroidectomy | 49.0% (72) |
| Subtotal thyroidectomy | 31.3% (46) |
| Follow up period after the surgery (mo) | 26.5 ± 24 |
Numbers represent mean ± SD or percentage and number of patients. CCRCC: Clear cell renal cell carcinoma; US: Ultrasound; CT: Computed tomography; PET Scan: Positron emission tomography scan; FNA: Fine needle aspiration.
Statistical analysis
Descriptive statistics were used; for continuous variables, the mean, median, standard deviation and standard error were calculated. Categorical variables were presented with frequency tables and cross-tabulation matrices. Chi-square test was used for the association of thyroidectomy and subtotal thyroidectomy and the status of the patient (alive/deceased) at 12 mo’ follow-up. We compared the distribution of age and the distribution of lag time of males versus females, using independent samples t-tests. Spearman’s correlation coefficients were used for the correlation of lag time and the size of the metastasis. Analysis of variance was used to compare the distribution of size, across the three symptoms groups and status. Logistic regression was used for the association of lag time and size to the airway obstruction, a binary categorical variable. Statistically significant levels were those with P values < 0.05. The statistical analysis was performed using IBM SPSS (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp).
RESULTS
Out of a total of 143 CCRCC patients with metastases in the thyroid gland, 46.3% were male (n = 68) and 52.4% were female (n = 75). The ratio between male and female was 1:1.13 suggesting a mild female predominance in the studied population.
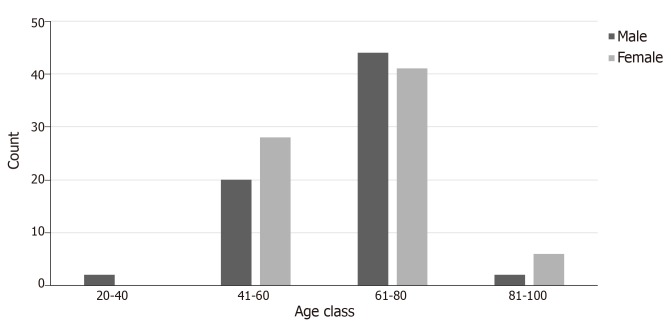
The age of the patients varied from 37 to 87 years with the mean age of males (± standard deviation) being 64 (± 10.36) years and of females 64.23 (± 10.86) years. We compared the distribution of age among male and female patients and we found that the age at which CCRCC was first diagnosed did not differ statistically between the two groups (P = 0.898). The age was divided into 4 categories (20-40, 41-60, 61-80, and 81-100 years, Figure 2).
Figure 2.
Age distribution among patients with clear cell renal cell carcinoma and thyroid metastases.
Ethnicity was documented in only 12.9% (19) of the cases. Of those, 68.4% were Caucasian (13 patients), 10.5% were African American (2 patients) and 21.1% were Asian (4 patients).
The Stage of clear cell renal cell carcinoma at initial diagnosis was reported in 62 cases. Of 62 cases with documented stage at initial diagnosis of CCRCC, 30.6% had Stage I CCRCC at diagnosis (19 patients), 29% had stage II (18 patients), 32.3% had stage III (20 patients) and 8.1% had stage IV (5 patients). However, it is important to note that TNM staging for renal carcinoma has changed since 1990. As an example, T1 staging used to include tumor size less than 2.5 cm and was later changed to ≤ 7 cm.
The mean ± SD lag time from initial diagnosis of CCRCC to discovery of thyroid metastases was 8.7 ± 6.35 years, similar across female and male patients (P = 0.836).
The other non-thyroidal metastatic sites are presented in Table 2. Almost one third, 31.3%, of the patients had lung metastases (25 patients), followed by 17.5% with neck metastases including cervical lymph nodes, muscular, soft tissue structure (14 patients) and other sites. Approximately one fourth of the patients had no other metastases besides in the thyroid gland.
Table 2.
Number of metastatic sites from clear cell renal cell carcinoma other than the thyroid gland in the studied population
| Number | Percent | |
| Pancreas | 13 | 16.3 |
| Neck | 14 | 17.5 |
| Liver | 6 | 7.5 |
| Adrenal | 7 | 8.8 |
| Others1 | 14 | 17.5 |
| Lung | 25 | 31.3 |
Percentages of clear cell renal cell carcinoma metastases in different sites other than the thyroid.
including metastases in the bones, lymph nodes, exocrine glands, esophagus, intestine, subcutaneous, skeletal muscles.
We examined whether the presence of lung metastases had an impact on overall survival, however, the analysis did not show a statistically significant link, as lung metastases were not associated with worsened one year overall survival (P = 0.243).
There were 10 cases (6.8%) which documented the presence or absence of internal jugular (IJ) thrombosis. 7 patients (4.8%) had IJ thrombosis at the time of diagnosis of thyroid metastases while 3 cases (2%) did not have IJ thrombosis on diagnosis.
Symptomatology was reported in 96 cases at the initial diagnosis of renal metastases to the thyroid gland. The majority of cases presented as painless mass 32.7% (48 patients), followed by 21.8% who were asymptomatic (32 patients) and 10.9% were symptomatic (16 patients). The most frequently reported symptom was dysphagia (13 cases), followed by dyspnea (9 cases), hoarseness (6 cases), neck pain (5 cases), cough and stridor (4 cases) and epistaxis (2 cases). Moreover, presence of airway obstruction either when presenting with respiratory symptoms or on radiological imaging was reported in 8.2% patients (12 patients), while 76.2% of patients did not have any clinical or radiological signs of airway obstruction (112 patients) and 15.6% of the cases did not report on the presence or absence of airway obstruction (23 patients).
Size of the metastases in the thyroid gland was reported in several ways, some cases provided accurate information about the number of metastases and size of each lesion, while other cases provided information about the gross nodular enlargement seen on radiological imaging. We used the longest diameter to represent the size of the metastatic lesion.
The size of the metastasis (mean ± SD) between males (4.87 ± 2.55 cm) and females (3.84 ± 1.65 cm) followed the same distribution.
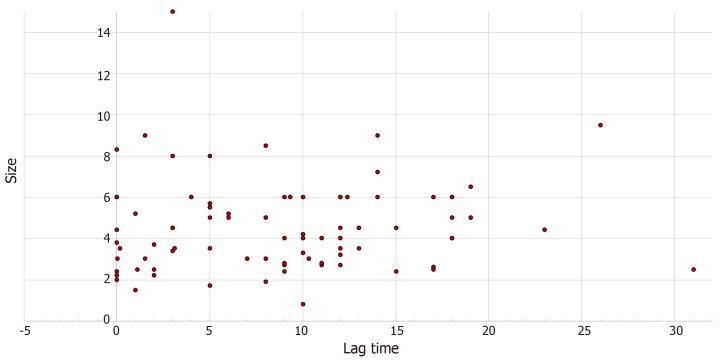
There was a weak monotonic association between lag time and size of metastasis, as shown by the Spearman’s correlation coefficient (r = 0.143, P = 0.1), Figure 3.
Figure 3.
Association of thyroid metastatic lesion size with lag time from clear cell renal cell carcinoma diagnosis to diagnosis of thyroid metastasis (r = 0.143, P = 0.1).
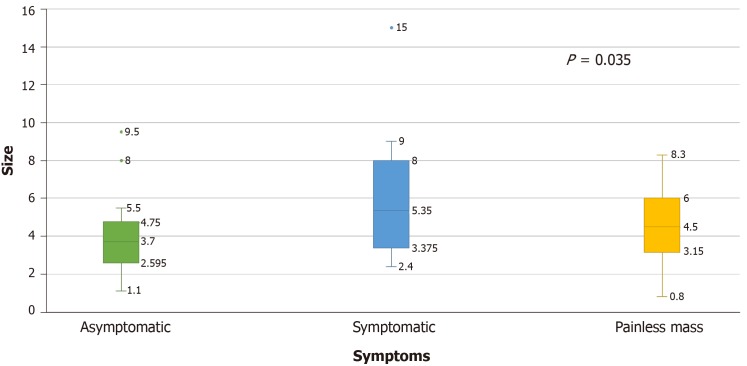
The size of metastases was significantly higher in the symptomatic group (M = 6.06 cm, SD = 3.51), followed by the painless mass group (M = 4.60 cm, SD = 0.29) and asymptomatic group (M = 3.93 cm, SD = 1.99), (P = 0.035) (Figure 4). The size of the metastatic lesions in the thyroid gland appeared to correlate with symptomatology as patients with thyroid lesions over 4 cm were more likely to present with symptoms.
Figure 4.
Mean tumor size in clear cell renal cell carcinoma patients with thyroid metastases depending on their presenting symptoms.
Regarding TSH levels, 28.6% of patient had normal serum levels (42 cases); TSH was high in 2% of the reported population (3 cases) and low in 1.4% of the studied population (2 cases). There was no documentation of TSH levels in 100 cases. There was only one case that mentioned the presence of thyroid antibodies. In 7 cases (4.8%) thyroid antibodies were negative.
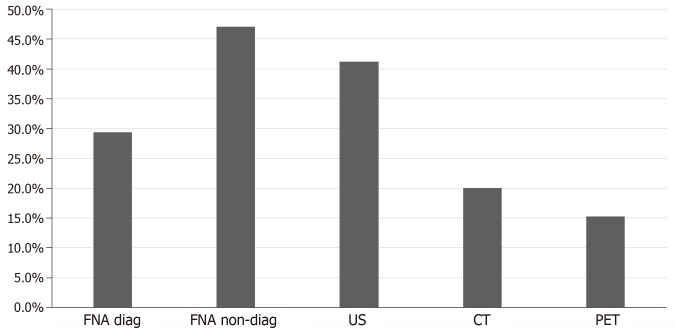
Regarding the tests performed for the diagnosis of CCRCC metastases to the thyroid gland, computed tomography (CT), ultrasound of the neck, positron emission tomography (PET), fine needle aspiration (FNA) and surgery with pathological diagnosis were utilized. Ultrasound was the most frequently used radiological imaging modality when metastases in the thyroid were detected 41.2% (35 cases), followed by CT scan 20.0% (17 cases) and PET scan 15.3% (13 cases). FNA was used in 76.5% of the population (65 cases). Cytology with FNA was diagnostic in 29.4% of the population (25 cases) and was non diagnostic or non-confirmatory in 47.1% of the population (40 cases) as shown in Figure 5. Surgical resection followed by histopathological examination of the thyroid nodule was used in all the studied patient population 100.0% (147 cases).
Figure 5.
Diagnostic modality used for the diagnosis of thyroid metastasis in the studied population. US: Ultrasound; CT: Computed tomography; PET: Positron emission tomography scan; FNA: Fine needle aspiration.
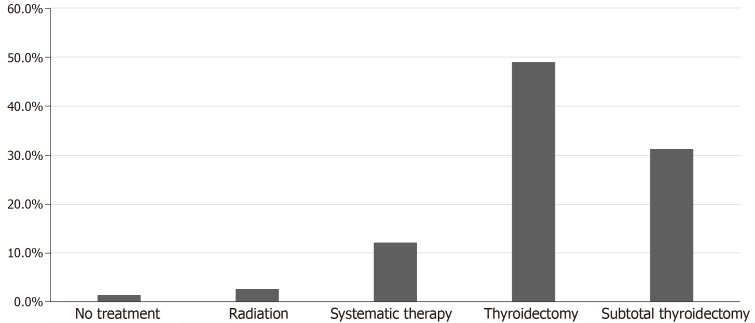
Surgery was the mainstay of treatment in the majority of the studied patients 80.3% (118 patients). Surgery involved either total thyroidectomy 49.0% (72 patients) or subtotal thyroidectomy 31.3% (46 patients). Regarding chemotherapy and targeted therapy, 12.2% of 147 patients received systemic therapy for metastatic CCRCC (18 cases), including tyrosine kinase inhibitors, Interferon-a, interlukin-2, nonspecific chemotherapies and vascular endothelial growth factor inhibitors. Radiotherapy was reported in 2.7% of the studied population (4 cases) and 2 patients did not receive any treatment 1.4% (Figure 6). None of the patients received immune check point inhibitors as these agents were only recently approved for the treatment of metastatic disease.
Figure 6.
Treatment modalities used for thyroid metastases in clear cell renal cell carcinoma patients in the studies population. Systemic therapy included Interferon-a, tyrosine kinase inhibitors, vascular endothelial growth factor inhibitors, alkylating agent (capacitance), interlukin-2, unidentified chemotherapy.
Regarding the impact of surgical intervention (total thyroidectomy or subtotal thyroidectomy) on patient’s outcome, all alive patients who had a follow-up time less than 12 mo (< 12) were excluded from the analysis. The sample consisted of 83 patients. Operated patients (thyroidectomy or subtotal thyroidectomy) and non-operated patients were compared regarding their status (alive or dead) after the 12 mo period. It was found that the majority of the operated patients 55.6% were alive at 1-year follow-up, versus 35.3% of non-operated patients. This suggests that surgery might have an impact on 1-year survival, however this effect was found to be statistically non- significant (P = 0.145).
DISCUSSION
Epidemiology and pathophysiology
The thyroid gland is an infrequent site for metastasis of different primary malignancies. In one study, the estimated prevalence was low accounting for less than 5% when compared to other organs as secondary sites for metastases when assessed in more than 1000 autopsies in 16 different primary malignancies[1]. Another retrospective study in a single institution examining the pathological report over 10 years found that the number of metastatic disease to the thyroid gland accounted only for 0.13% of resected thyroid nodules[2]. Historically, breast adenocarcinoma used to be the most common primary malignancy to metastasize to the thyroid gland as was demonstrated in a large study of more than 16000 autopsies[3]. However, when pooled data from different studies were analyzed, clear cell renal cell carcinoma accounted for the majority of metastatic disease to the thyroid gland[4]. In general, frequency of metastatic clear cell renal cell carcinoma remains low[5,6]. In our study, there was no gender preponderance with a male to female ratio of 1:1.13 similar to previous studies[7]. The pathophysiology underlying the rarity of metastasis to the thyroid gland is not well established and despite the extensive blood supply to the thyroid gland it is uncommon for metastatic disease to develop[8]. One theory behind the rarity of having metastasis to the thyroid gland is the high oxygen and iodine content which prevents the growth of metastatic lesions but there are no original studies examining the microenvironment effect on the enrichment of circulating metastases in the thyroid gland. Metastatic spread from clear cell renal cell carcinoma to secondary sites was found to be more prevalent in patients younger than 55 years old (P < 0.001), however, the scope of this large study (n = 11.000) included all organs as secondary sites for metastases from CCRCC[9]. In our pooled sample, the majority of patients who had thyroid metastases from clear cell renal cell carcinoma were > 55 years old as shown in Figure 2. The presence of non-malignant pathology in the thyroid gland as a risk factor for metastasis is an area of controversy. Heffess et al[10] performed a pathological study in 36 cases of metastatic renal cell carcinoma to the thyroid gland and found presence of pathological non-malignant abnormalities in some patients with CCRCC metastases to the thyroid. This could account for the development of metastases which could be due to a compromise of either the blood supply or cellular metabolism that might lead to low oxygen and iodine content and supports the development of metastatic lesions. The majority of our patients with available TSH levels (89.3%) had normal hormone values and there was no history of prior thyroid disease except for presumed non-toxic multinodular goiter which in the majority of cases was found eventually to be metastatic CCRCC.
Period of latency and secondary metastatic sites
The distribution of metastatic CCRCC to the thyroid gland appears to be slightly different in the studied population with a male to female ratio of 1:1.13. The recurrence of metastatic disease in clear cell renal cell carcinoma follows an unpredictable distribution and it is not uncommon to have a long latency period of years before the development or detection of metastases[11]. In our study, the mean latency time before the detection of thyroid metastases was 8.7 years (ranging from several weeks to 31 years) which correlates with previous published reports[12,13]. However, the highest risk of metastatic recurrence after surgical nephrectomy of the primary tumor appears to be in the first 5 years with the initial tumor staging predicting the time of recurrence and metastases[14].
We examined whether the presence of lung and thyroid metastases conferred worse prognosis compared to other metastatic sites. However, our analysis did not yield a significant difference. We could not perform the analysis on the other metastatic site in the pooled population due to small sample size. In our search of the literature we found that patients with pancreatic metastasis from renal cell carcinoma had a better median overall survival as demonstrated in the study of Kalra et al[15] which compared overall survival in patient with pancreatic metastases versus patients with non-pancreatic metastases (39 vs 26 mo respectively, P < 0.01) in a cohort of 228 patients[15].
Clinical features
The majority of patients with CCRCC and thyroid metastases present with a painless mass and can be often misdiagnosed initially as non-toxic multinodular goiter. In our study, we noticed a weak correlation between the lag time and the size of thyroid metastases; however, not statistically significant (P = 0.1, Figure 3). Moreover, the size of metastasis correlates with symptoms as increased lesion size correlated with symptomatic disease (P = 0.035). These two observations suggest that high index of suspicion is required as thyroid metastases may take a long time to develop with smaller size metastases not producing symptoms. However, the current guidelines recommend surveillance for up to 5 years given that the highest risk of recurrence of CCRCC is within 3 to 5 years and due to the lack of evidence of cost effectiveness and overall benefit when extending the surveillance period[16]. The most frequent symptoms encountered in our study were dysphagia followed by dyspnea, hoarseness, neck pain, cough, stridor and epistaxis. Other uncommon symptoms include acute respiratory failure[17] and chest pain which were described in anecdotal reports.
Diagnosis
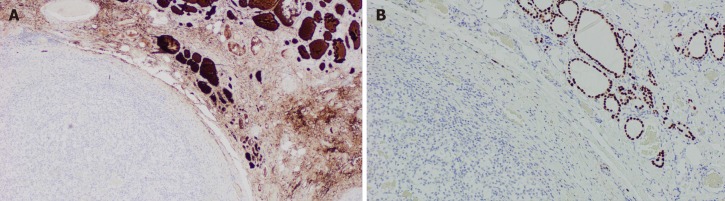
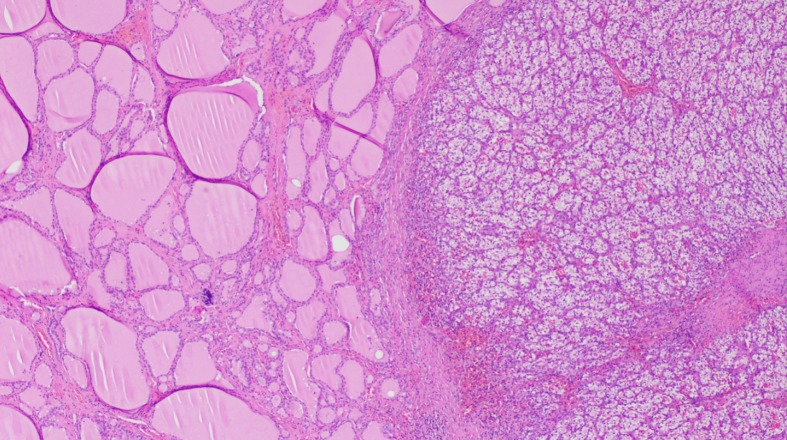
The approach to a newly detected thyroid nodule in patients with prior history of clear cell renal cell carcinoma is similar to the general approach of thyroid nodules. All the cases that were included our systematic review had a definitive diagnosis following surgical resection of the thyroid mass with histopathology (100% cases). Nevertheless, FNA appears to be a reliable and sufficient method of diagnosis when proper immunohistochemical stain (IHC) is used. Despite our findings of the majority of FNA being non diagnostic or inconclusive, prior studies have demonstrated the high sensitivity and specificity of FNA in diagnosing CCRCC metastases to the thyroid gland. Heffess et al[10] suggested that FNA or core needle biopsy might not be sufficient for a proper diagnosis of CCRCC metastases to the thyroid gland and recommended a surgical resection for a definitive diagnosis. However, a multi-institutional study showed a high sensitivity and specificity of FNA in diagnosing CCRCC to the thyroid even without the use of immunohistochemistry and only based on cellular morphology and prior history of kidney cancer (10 cases diagnosed with FNA without the need for surgical resection)[18]. Another case series performed from Mayo Clinic confirmed the high sensitivity and specificity of FNA in the detection of CCRCC metastases to the thyroid gland[19]. The most helpful IHC stains used on FNA specimens for diagnosis of CCRCC metastatic to the thyroid gland are thyroid transcription factor-1 (TTF-1), thyroglobulin (TGB) and carbonic anhydrase IX and their sensitivity and specificity reach 100%[19] as these stains are negative in CCRCC metastases foci in the thyroid gland and positive in primary thyroid malignancies. As an example, Figure 7 illustrates IHC stains for TTF-1 and TGB in a case of CCRCC with thyroid metastases. Histopathology with tissue biopsy will reveal a distinct alteration between the normal thyroid architecture and areas containing clear cell renal carcinoma cells as shown in Figure 8.
Figure 7.
Illustrates immunohistochemical stains for thyroid transcription factor-1 and thyroglobulin in a case of clear cell renal cell carcinoma with thyroid metastases. A: Immunohistochemically stain showing positive thyroglobulin on right side coinciding with normal thyroid architecture and negative on the left; B: Immunohistochemically stain for thyroid transcription factor-1 which is positive on the right correlating with normal thyroid architecture and negative on the left where the metastatic renal cell carcinoma is present (Magnification x 100).
Figure 8.
Hematoxylin-eosin staining showing benign thyroid tissue architecture (left) and metastatic renal cell carcinoma (right), (Magnification x 100).
Radiological studies including CT and PET scans are used for surveillance of metastatic CCRCC. While CT is considered to have better sensitivity than PET they are often both obtained to maximize the detection of metastasis of renal cell carcinoma[20]. However, metastatic lesions in the thyroid might be difficult to identify in radiological studies such as PET-CT as they can appear as non-specific metabolic activity resembling benign lesions[12].
Ultrasonography can be useful in differentiating CCRCC thyroid metastatic lesions from benign nodules with features of irregularity, solitary nodule, intra-nodule vascularity and venous thrombosis being suggestive of a cancerous/metastatic lesion[21]. In our systematic review, 4.8% of the patients had internal jugular thrombosis which could represent either local tumor invasion of the vascular bed or hypercoagulable state from the metastatic lesions. Other studies have shown that solid nodules with well-defined margins and high vascularity may represent metastatic lesions[22].
Treatment and prognosis
In our systematic review, most patients had surgical resection of their CCRCC metastatic lesions in the thyroid gland with an approach involving either thyroidectomy or subtotal thyroidectomy. Given the rarity of the metastatic disease in this location the studies performed regarding surgical approach included small number of patients without a control arm and the literature regarding the role of surgery is controversial. Usually, surgery is aimed as a palliative treatment and local control of the metastatic disease and should be part of a broader systematic and multidisciplinary approach. Some studies including a small number of patients showed decreased survival with extra thyroid nodal metastatic extension suggesting that surgery might play a role in controlling local recurrence[23]. A case series done by Nixon et al[24] showed the median survival from surgery to death to be 26.5 mo (n = 21) and suggested a role for surgery only to control local disease[24].
Whether there is a difference between total and subtotal thyroidectomy is an area of debate with some studies suggesting no difference in survival between the two surgeries. Beutner et al[25] found that the 5-year survival with total thyroidectomy was better than non-total thyroidectomy but the results were statistically non-significant (P = 0.49) suggesting no improved survival with total thyroidectomy compared to partial thyroidectomy. However, one observation from this study is that median overall survival after thyroidectomy was better in females compared to males (5 years vs 2 years P = 0.003)[25]. A cohort of 8 patients reported a 4-year survival of metastasectomy of thyroid metastases to be 53%[26]. Another retrospective study in Europe (n = 45) showed that the 5-year overall survival rate with thyroid metastasectomy was 51%[27].
The decision about choice of treatment in metastatic renal cell carcinoma depends on different factors including the extent of the disease, metastatic sites of involvement and prognostic risk factors including Kornofsky performance status, time from diagnosis to treatment and laboratory findings. Several guidelines still recommend consideration of metastatectomy within a multidisciplinary approach in patients with metastatic CCRCC regardless of the site if there is a single lesion amenable for resection and without presence of contraindications to surgery[28,29].
Pharmacological therapy including immune-check point blockade and tyrosine kinase inhibitors have made advancement in the treatment of metastatic CCRCC with studies showing prolonged overall survival and progression free survival[30,31]. This could change practice in the future with a medical based approach to metastatic CCRCC rather than surgery, however, more studies are needed to determine the best approach.
Our study offers a comprehensive insight into the characteristics of thyroid metastases from clear cell renal cell carcinoma, include a wide range of patients reported from 1990 to 2018 and had a sufficient number of patients. Limitations include the heterogeneity of studied population and the retrospective nature of the study.
In conclusion, our systematic review of CCRCC patients presenting with thyroid metastases, we found that larger size of thyroid metastasis was more likely to present with symptomatology. A high index of suspicion is warranted when evaluating thyroid nodules in CCRCC patients. There appeared to be no significant difference in outcome between patients who underwent surgery and those who did not. With the wider use of immune check-point and tyrosine kinase inhibitors in metastatic CCRCC, surgery may eventually be reserved only for palliative purposes.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
PRISMA 2009 Checklist statement: Authors revised the manuscript according to the PRISMA 2009 Checklist.
Manuscript source: Invited manuscript
Peer-review started: May 10, 2019
First decision: September 9, 2019
Article in press: October 15, 2019
Specialty type: Medicine, Research and Experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ieni A, Mogulkoc R, Schietroma M S-Editor: Zhang L L-Editor: A E-Editor: Wu YXJ
Contributor Information
Karam Khaddour, Department of Medicine at Rosalind Franklin University of Medicine and Science, Chicago, IL 60050, United States. Karam.khaddour@gmail.com.
Nadiia Marernych, Department of Medicine at Rosalind Franklin University of Medicine and Science, Chicago, IL 60050, United States.
Wendy L Ward, Northwestern Medicine McHenry, Chicago, IL 60050, United States.
Jerry Liu, Advocate Medical Group Oncology, Crystal Lake, IL 60014, United States.
Theodora Pappa, Department of Medicine at Rosalind Franklin University of Medicine and Science, Chicago, IL 60050, United States.
References
- 1.Budczies J, von Winterfeld M, Klauschen F, Bockmayr M, Lennerz JK, Denkert C, Wolf T, Warth A, Dietel M, Anagnostopoulos I, Weichert W, Wittschieber D, Stenzinger A. The landscape of metastatic progression patterns across major human cancers. Oncotarget. 2015;6:570–583. doi: 10.18632/oncotarget.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papi G, Fadda G, Corsello SM, Corrado S, Rossi ED, Radighieri E, Miraglia A, Carani C, Pontecorvi A. Metastases to the thyroid gland: prevalence, clinicopathological aspects and prognosis: a 10-year experience. Clin Endocrinol (Oxf) 2007;66:565–571. doi: 10.1111/j.1365-2265.2007.02773.x. [DOI] [PubMed] [Google Scholar]
- 3.Berge T, Lundberg S. Cancer in Malmö 1958-1969. An autopsy study. Acta Pathol Microbiol Scand Suppl. 1977:1–235. [PubMed] [Google Scholar]
- 4.Nixon IJ, Coca-Pelaz A, Kaleva AI, Triantafyllou A, Angelos P, Owen RP, Rinaldo A, Shaha AR, Silver CE, Ferlito A. Metastasis to the Thyroid Gland: A Critical Review. Ann Surg Oncol. 2017;24:1533–1539. doi: 10.1245/s10434-016-5683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cesaretti M, Trotta M, Varaldo E, Ansaldo G, Leale I, Borgonovo G. Metastases to the thyroid gland from renal cancer. Tumori. 2013;99:e107–e110. doi: 10.1177/030089161309900327. [DOI] [PubMed] [Google Scholar]
- 6.Sindoni A, Rizzo M, Tuccari G, Ieni A, Barresi V, Calbo L, Cucinotta E, Mallamace A, Trimarchi F, Benvenga S. Thyroid metastases from clear cell renal carcinoma 18 years after nephrectomy. Ann Endocrinol (Paris) 2010;71:127–130. doi: 10.1016/j.ando.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Sindoni A, Rizzo M, Tuccari G, Ieni A, Barresi V, Calbo L, Cucinotta E, Trimarchi F, Benvenga S. Thyroid metastases from renal cell carcinoma: review of the literature. ScientificWorldJournal. 2010;10:590–602. doi: 10.1100/tsw.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willis RA. Metastatic Tumours in the Thyreoid Gland. Am J Pathol. 1931;7:187–208.3. [PMC free article] [PubMed] [Google Scholar]
- 9.Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, Tian Z, Schmitges J, Graefen M, Perrotte P, Menon M, Montorsi F, Karakiewicz PI. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973–980. doi: 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- 10.Heffess CS, Wenig BM, Thompson LD. Metastatic renal cell carcinoma to the thyroid gland: a clinicopathologic study of 36 cases. Cancer. 2002;95:1869–1878. doi: 10.1002/cncr.10901. [DOI] [PubMed] [Google Scholar]
- 11.Ljungberg B, Alamdari FI, Rasmuson T, Roos G. Follow-up guidelines for nonmetastatic renal cell carcinoma based on the occurrence of metastases after radical nephrectomy. BJU Int. 1999;84:405–411. doi: 10.1046/j.1464-410x.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- 12.Cilengir AH, Kalayci TO, Duygulu G, Rezanko TA, İnci MF. Metastasis of Renal Clear Cell Carcinoma to Thyroid Gland Mimicking Adenomatous Goiter. Pol J Radiol. 2016;81:618–621. doi: 10.12659/PJR.899459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calkovsky V, Szepe P, Hajtman A. Solitary intrathyroid metastasis of renal cancer. Bratisl Lek Listy. 2011;112:395–397. [PubMed] [Google Scholar]
- 14.Lam JS, Leppert JT, Figlin RA, Belldegrun AS. Surveillance following radical or partial nephrectomy for renal cell carcinoma. Curr Urol Rep. 2005;6:7–18. doi: 10.1007/s11934-005-0062-x. [DOI] [PubMed] [Google Scholar]
- 15.Kalra S, Atkinson BJ, Matrana MR, Matin SF, Wood CG, Karam JA, Tamboli P, Sircar K, Rao P, Corn PG, Tannir NM, Jonasch E. Prognosis of patients with metastatic renal cell carcinoma and pancreatic metastases. BJU Int. 2016;117:761–765. doi: 10.1111/bju.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart SB, Thompson RH, Psutka SP, Cheville JC, Lohse CM, Boorjian SA, Leibovich BC. Evaluation of the National Comprehensive Cancer Network and American Urological Association renal cell carcinoma surveillance guidelines. J Clin Oncol. 2014;32:4059–4065. doi: 10.1200/JCO.2014.56.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Testini M, Lissidini G, Gurrado A, Lastilla G, Ianora AS, Fiorella R. Acute airway failure secondary to thyroid metastasis from renal carcinoma. World J Surg Oncol. 2008;6:14. doi: 10.1186/1477-7819-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.HooKim K, Gaitor J, Lin O, Reid MD. Secondary tumors involving the thyroid gland: A multi-institutional analysis of 28 cases diagnosed on fine-needle aspiration. Diagn Cytopathol. 2015;43:904–911. doi: 10.1002/dc.23331. [DOI] [PubMed] [Google Scholar]
- 19.Hegerova L, Griebeler ML, Reynolds JP, Henry MR, Gharib H. Metastasis to the thyroid gland: report of a large series from the Mayo Clinic. Am J Clin Oncol. 2015;38:338–342. doi: 10.1097/COC.0b013e31829d1d09. [DOI] [PubMed] [Google Scholar]
- 20.Schöder H, Larson SM. Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med. 2004;34:274–292. doi: 10.1053/j.semnuclmed.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K, Hirokawa M, Yabuta T, Fukushima M, Masuoka H, Higashiyama T, Kihara M, Ito Y, Miya A, Amino N, Miyauchi A. Metastatic carcinoma to the thyroid gland from renal cell carcinoma: role of ultrasonography in preoperative diagnosis. Thyroid Res. 2015;8:4. doi: 10.1186/s13044-015-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song OK, Koo JS, Kwak JY, Moon HJ, Yoon JH, Kim EK. Metastatic renal cell carcinoma in the thyroid gland: ultrasonographic features and the diagnostic role of core needle biopsy. Ultrasonography. 2017;36:252–259. doi: 10.14366/usg.16037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machens A, Dralle H. Outcome after thyroid surgery for metastasis from renal cell cancer. Surgery. 2010;147:65–71. doi: 10.1016/j.surg.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Nixon IJ, Whitcher M, Glick J, Palmer FL, Shaha AR, Shah JP, Patel SG, Ganly I. Surgical management of metastases to the thyroid gland. Ann Surg Oncol. 2011;18:800–804. doi: 10.1245/s10434-010-1408-2. [DOI] [PubMed] [Google Scholar]
- 25.Beutner U, Leowardi C, Bork U, Lüthi C, Tarantino I, Pahernik S, Wente MN, Büchler MW, Schmied BM, Müller SA. Survival after renal cell carcinoma metastasis to the thyroid: single center experience and systematic review of the literature. Thyroid. 2015;25:314–324. doi: 10.1089/thy.2014.0498. [DOI] [PubMed] [Google Scholar]
- 26.Iesalnieks I, Trupka A, Raab M, Glockzin G, Woenckhaus M, Schlitt HJ, Agha A. Renal cell carcinoma metastases to the thyroid gland-8 cases reported. Thyroid. 2007;17:49–52. doi: 10.1089/thy.2006.0176. [DOI] [PubMed] [Google Scholar]
- 27.Iesalnieks I, Winter H, Bareck E, Sotiropoulos GC, Goretzki PE, Klinkhammer-Schalke M, Bröckner S, Trupka A, Anthuber M, Rupprecht H, Raab M, Meyer W, Reichmann F, Kästel M, Mayr M, Braun W, Schlitt HJ, Agha A. Thyroid metastases of renal cell carcinoma: clinical course in 45 patients undergoing surgery. Assessment of factors affecting patients' survival. Thyroid. 2008;18:615–624. doi: 10.1089/thy.2007.0343. [DOI] [PubMed] [Google Scholar]
- 28.Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, Grünwald V, Gillessen S, Horwich A ESMO Guidelines Committee. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:706–720. doi: 10.1093/annonc/mdz056. [DOI] [PubMed] [Google Scholar]
- 29.Motzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, Choueiri TK, Costello BA, Derweesh IH, Fishman M, Gallagher TH, Gore JL, Hancock SL, Harrison MR, Kim W, Kyriakopoulos C, LaGrange C, Lam ET, Lau C, Michaelson MD, Olencki T, Pierorazio PM, Plimack ER, Redman BG, Shuch B, Somer B, Sonpavde G, Sosman J, Dwyer M, Kumar R. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:804–834. doi: 10.6004/jnccn.2017.0100. [DOI] [PubMed] [Google Scholar]
- 30.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, Lee JL, Vasiliev A, Miller WH, Jr, Gurney H, Schmidinger M, Larkin J, Atkins MB, Bedke J, Alekseev B, Wang J, Mariani M, Robbins PB, Chudnovsky A, Fowst C, Hariharan S, Huang B, di Pietro A, Choueiri TK. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, De Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]