Abstract
BACKGROUND
Cardiac injury may occur after acute pathology of central nervous system (CNS) without any evidence of primary cardiac diseases. The resulting structural and/or functional changes are called cerebrocardiac syndrome (CCS). The great majority of studies have been performed in patients with subarachnoid hemorrhage (SAH), while CCS data after intracerebral hemorrhage (ICH) are rare. It may cause diagnostic and therapeutic pitfalls for the clinician due to a lack of specific clinical manifestations and diagnostic methods. Understanding the underlying pathophysiological and molecular mechanism(s) following cerebrovascular incidents will help to implement prevention and treatment strategies to improve the prognosis.
CASE SUMMARY
A 37-year-old man with a history of hypertension presented to our department on an emergency basis because of a sudden dizziness and left limb weakness. Cerebral computed tomography (CT) suggested ICH in the occipital and parietal lobes, and the chosen emergency treatment was hematoma evacuation. Left ventricular (LV) dysfunction occurred after the next 48 h and the electrocardiogram (ECG) showed non-ST elevation myocardial infarction. CCS was suspected first in the context of ICH due to the negative result of the coronary CT angiogram.
CONCLUSION
Misinterpretation of ischemic-like ECGs may lead to unnecessary or hazardous interventions and cause undue delay of rehabilitation after stroke. Our objective is to highlight the clinical implications of CCS and we hope the differential diagnoses will be considered in patients with acute CNS diseases.
Keywords: Stroke, Intracerebral hemorrhage, Cerebrocardiac syndrome, Cardiac insufficiency, Non-ST elevation myocardial infraction, Case report, Neurogenic stunned myocardium
Core tip: Acute stroke (AS)-induced cardiac injury is of utmost clinical importance. The data of cerebrocardiac syndrome (CCS) after intracerebral hemorrhage are few. We report a case of ischemic electrocardiogram changes in acute cerebral hemorrhage and systematically review the mechanisms and characteristics of CCS in the setting of AS and raise awareness of CCS among physicians.
INTRODUCTION
Acute stroke (AS)-induced cardiac injury is of utmost clinical importance. Adverse cardiac events are the second leading cause of death during the acute phase after stroke and are important determinants for long-term survival[1]. AS can induce cardiac injury which presents with elevated concentrations of cardiac biochemical markers, ST-segment deviations, T wave inversion, Q waves, and abnormal left ventricular (LV) function. No definite guidelines are currently available regarding the management of cerebrocardiac syndrome (CCS). This case illustrates the marked electrocardiogram (ECG) changes that are seen with intracranial hemorrhage and that can lead to an erroneous diagnosis of acute myocardial ischemia. It is crucial to interpret cardiac injury in the context of the clinical presentation. The aim of our systematic review is to summarize the mechanisms and characteristics of CCS in the setting of AS and raise awareness of CCS among physicians.
CASE PRESENTATION
Chief complaints
A 37-year-old male patient with a background of hypertension was admitted to our emergency department with sudden dizziness and left limb weakness. Non-jet vomiting occurred before transfer to our hospital for further management, and the vomitus was gastric content. He reported no trauma, fever, or coma, he was a non-smoker, and he had no history of drug use or cardiac events. The patient was immediately subjected to a non-contrast computed tomography (CT) scan of the brain, which revealed hemorrhage in the occipital and parietal lobes. The diagnosis of intracerebral hemorrhage (ICH) was made. He developed sudden onset chest tightness, shortness of breath, and occasional chest pain behind the sternum around 48 h after emergency hematoma evacuation. The pain was stuffy in nature and did not radiate to other places. His symptoms continued to worsen gradually, until breath sounds were observed when the patient was sitting up. There were no severe symptoms of dyspnea, cough, hemoptysis or sputum (a characteristic pink frothy sputum is often produced).
History of present illness
The patient’s medical history was only hypertension; he had not been treated regularly, and the blood pressure control was unknown. There was no other history of illness or medication.
Physical examination
The patient had warm extremities with hemodynamically stable blood pressure maintained at 128/73 mmHg and a heart rate of 77 beats per minute (bpm) on initial assessment. He was breathing smoothly on room air with clear lungs. His cardiac examination was normal and did not show any murmurs or gallops.
The physical examination was unremarkable, except for the low left limb muscle tension and the third limb muscle strength. The physical examination was carried out when the patient suffered from chest tightness. On further examination, his heart rate was faster than 100 bpm and his blood pressure had risen to 150/90 mmHg; other general conditions were stable. However, when the patient was breathing in a sitting position, auscultation revealed abnormal breath sounds, which manifested as obvious bilateral bubbling rales in the lower part of the lungs.
Laboratory examinations
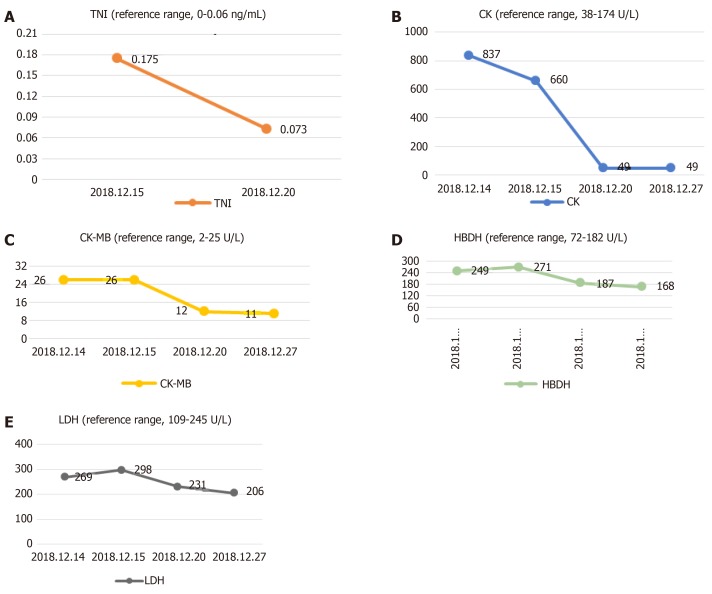
The serum level of creatine kinase (CK) was 837 U/L (reference range, 38-174 U/L), that of CK isoenzyme was 26 U/L (reference range, 2-25 U/L), lactate dehydrogenase (LDH) was 269 U/L (reference range, 109-245 U/L) and hydroxybutyrate dehydrogenase (HBDH) was 249 U/L (reference range, 72-182 U/L) at admission; other blood values were within the normal range. When the symptoms of chest tightness worsened, the patient’s blood was immediately checked for any sign of heart failure (HF). The laboratory examination showed the concentration of N-terminal pro b-type natriuretic peptide (NT-proBNP) was 493 pg/mL (reference range, < 85 pg/mL). Myocardial enzyme levels, including cTnI, did not exhibit normal values after serial determination: the cTnI level was increased to 0.175 ng/mL in the resting state (reference range, < 0.06 ng/mL), the CK level was 660 U/L, the CK-MB level was 26 U/L, LDH was 298 U/L, and HBDH was 271 U/L. Repeated measurements 5 d later showed the values had decreased: CPK, 49 U/L; CK-MB, 12 U/L; LDH, 231 U/L; and HBDH, 187 U/L (Figure 1).
Figure 1.
Change trend of troponin I, creatine kinase, creatine kinase isoenzyme, hydroxybutyrate dehydrogenase and lactate dehydrogenase. A: Change trend of troponin I; B: Change trend of creatine kinase; C: Change trend of creatine kinase isoenzyme; D: Change trend of hydroxybutyrate dehydrogenase; E: Change trend of lactate dehydrogenase.
Imaging examinations
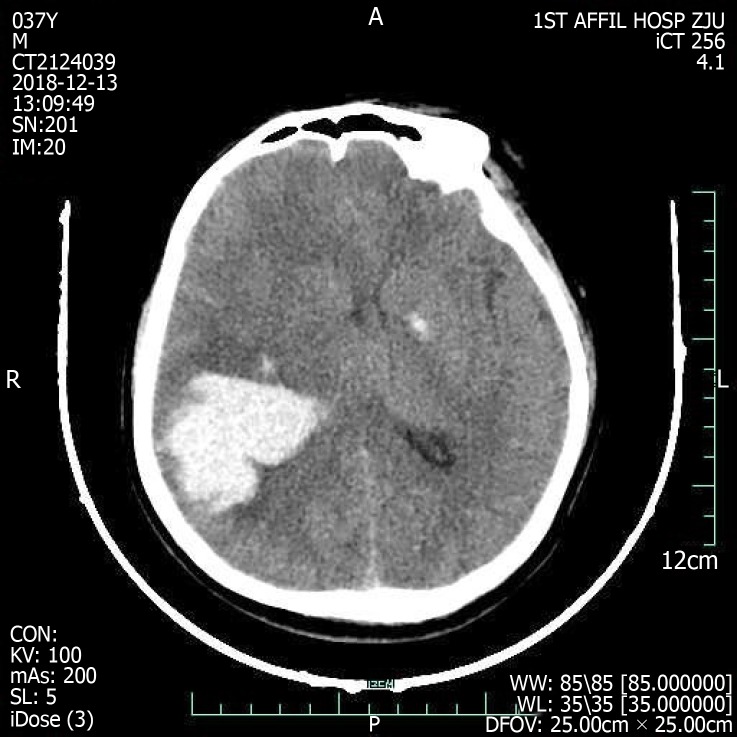
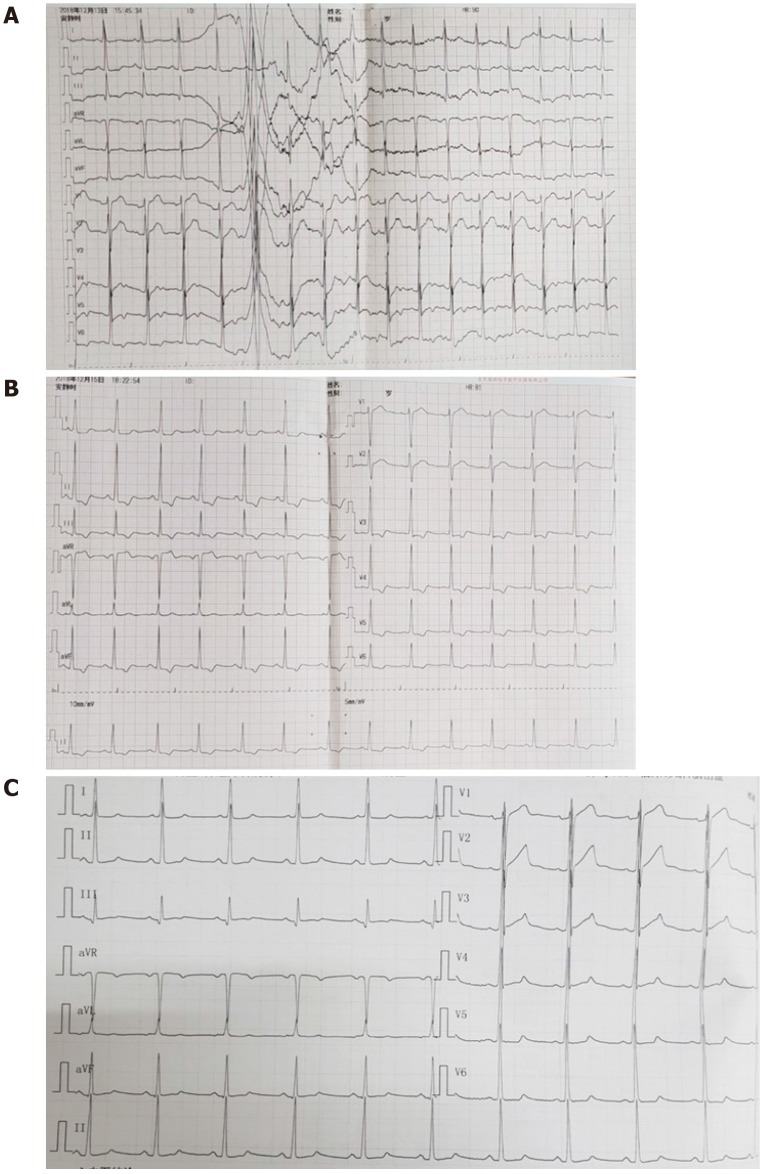
On admission, the emergency cerebral CT image revealed cerebral hemorrhage in the occipital and parietal lobes (Figure 2), while the initial 12-lead ECG (Figure 3A) revealed a normal sinus rhythm and a regular rate within the normal range; however, T wave inversions and ST segment in leads V5 and V6 were already observed.
Figure 2.
Cranial computed tomography evinced cerebral hemorrhage in parietal and occipital lobes dimensions 40 mm × 52 mm × 47 mm.
Figure 3.
Electrocardiographic. A: 12-lead Electrocardiographic findings at presentation; B: Electrocardiographic findings at acute left heart failure; C: Electrocardiographic findings at 11 d after left heart failure.
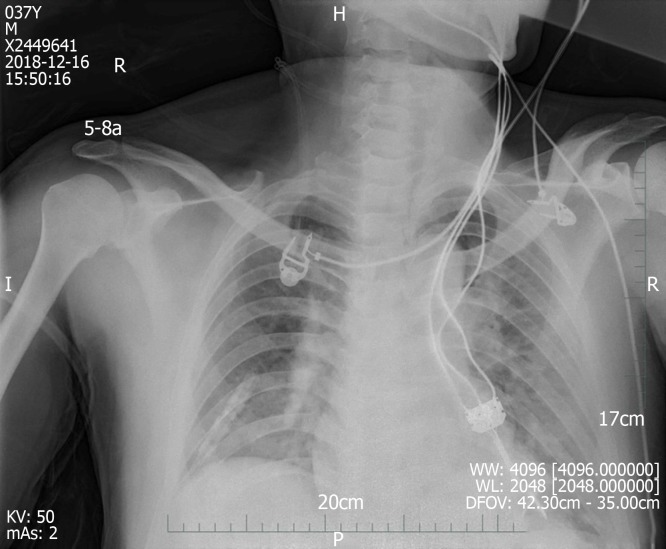
We made another ECG at the onset of chest tightness. It is notable that the ECG demonstrated extensive depression of the ST segment and T wave inversion, suggestive of myocardial injury. At this time, there was a prolongation of the corrected QT interval (QTc, 487 ms) (Figure 3B). But at this time, the chest radiograph showed no bilateral patchy opacities that are typical of pulmonary edema and consistent with the breath sounds when the patient was in a sitting position (Figure 4).
Figure 4.
Chest radiography at 1 d after left heart failure.
In sum, the working hypothesis of a non-ST elevation myocardial infarction (NSTEMI) could be established. Further cardiological investigations, including coronary arteries with CT angiogram (CTA) of the coronary arteries, were needed to make a definite diagnosis. Considering the patient was in acute stage of ICH, coronary angiography was not timely completed.
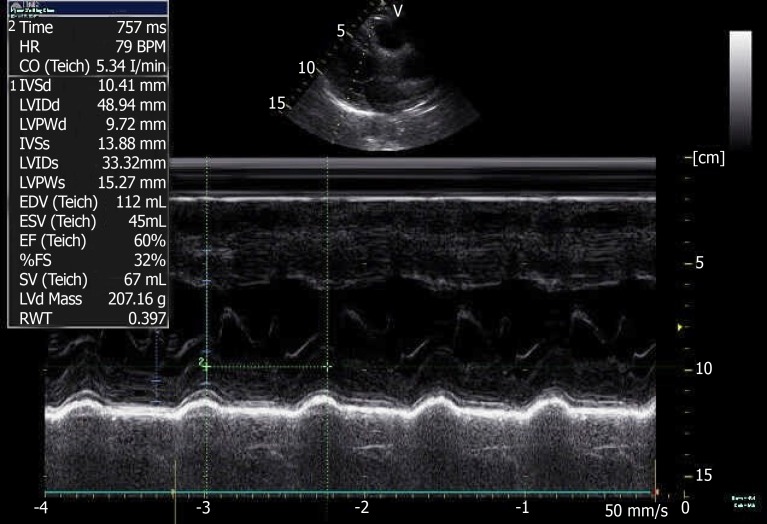
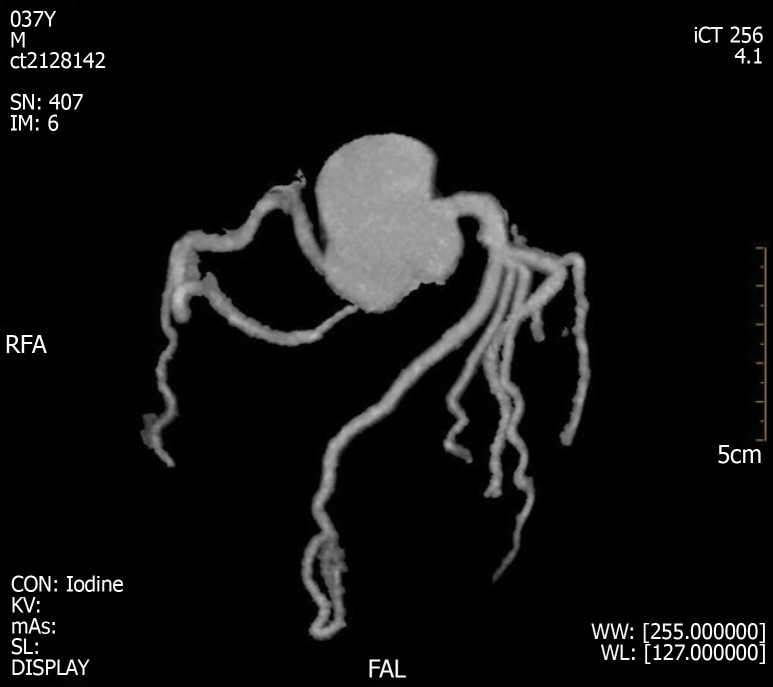
A coronary CTA and echocardiogram (ECHO) were made at 72 h after the initial symptoms when the patient’s chest discomfort disappeared. The ECHO revealed normal sized chambers with an ejection fraction of 60% and showed no significant valvular abnormality or any wall motion abnormality (Figure 5). Coronary arteries did not reveal any sign of obstruction or significant narrowing (Figure 6). Another 12-lead ECG was made 11 d after the patient’s symptoms improved, by this time, the patient had returned to the same state as at admission with the recovery of ICH, but the QTc interval was now prolonged (QTc, 424 ms).
Figure 5.
Color doppler echocardiography at 3 d after left heart failure.
Figure 6.
Coronary computed tomography angiogram results at 3 d after left heart failure.
FINAL DIAGNOSIS
Based on all observations described in the previous sections, we diagnosed the patient with CCS.
TREATMENT
In view of the acute hemorrhage, anticoagulation treatment was not given because the hemorrhagic risk would outweigh the antithrombotic benefits. We reduced the symptoms (and the patient’s discomfort) using a basic symptomatic treatment with vasodilators.
OUTCOME AND FOLLOW-UP
The patient’s eventual neurological outcome was excellent, with resolution of symptoms; the patient was discharged from the hospital and has been in a stable condition until the publication of this report. However, the patient declined further evaluation.
DISCUSSION
Cardiac involvement following acute central nervous system (CNS) is common regardless of the condition of cerebral involvement. A prolonged QT interval, ST-segment deviations, T wave changes, increased NT-proBNP, raised cardiac biomarkers and/or abnormal LV function are observed in up to one third of the acute cerebrovascular events[2]. The changes may be similar to those observed in myocardial infarction (MI) but are not necessarily identical to coronary thrombosis. The current study demonstrates that there are two sites associated with stroke-related myocardial injury, that is, the right insular cortex (posterior, medial, and superior areas) and the right inferior parietal lobe[3]. The insular cortex is involved in the control of the cardiac autonomic nervous system, which can cause myocardial damage in stroke patients[4,5]. In contrast to the insula, the link between the right inferior parietal lobe and myocardial injury is currently not known. Timely and accurate differential diagnosis is essential, especially in unconscious patients showing signs similar to those of myocardial ischemia. It may prove useful to continue cardiac examination in patients with signs of cardiac involvement during acute cerebrovascular events in order to exclude concomitant ischemic heart disease. The etiopathological basis of stroke-related myocardial injury is not fully understood. Until recently, neurogenic stunned myocardium (NSM) was defined as the myocardial injury and dysfunction of a sudden onset, occurring after acute brain disease as a result of autonomic disturbances, which may be responsible for the cardiac injury after stroke[6,7]. The clinical features observed include arrhythmia, myocardial necrosis, ECG changes, pulmonary edema, and acute left HF which is not uncommon to progress to cardiogenic shock[8].
Pathology/pathophysiology
Oppenheimer et al[9] induced ECG changes in rats similar to those found after ischemic stroke, supporting the notion of neurogenic cardiac injury. NSM, which can develop in any acute CNS pathology, has a poorly understood pathology[8]. Most models for the etiopathology of NSM involve a subarachnoid hemorrhage (SAH)[10]. ECG changes and elevations in cardiac enzyme levels, indicating autonomic nervous dysfunction, are also observed after ICH, but there is a paucity of data on the occurrence of this condition[11]. The peripheral mechanisms of CCS are generally considered to involve brain-induced autonomic dysfunction, neuroendocrine dysregulation, and systemic inflammatory processes, whereas the central mechanisms are largely unknown.
Autonomic dysfunction and neuroendocrine dysregulation: Laowattana et al[12] put forward the hypothesis of sympathetic overload, that is, central neuropathy leads to autonomic dysfunction and enhances catecholamine release from the neural terminals. This hypothesis explains the cardiac damage described in NSM. Stroke leads to the dysfunction of the autonomic nervous system stimulating the hypothalamus-pituitary-target gland axis, causing sympathetic-adrenal system hyperfunction, enhanced by the stress response, and increased levels of catecholamine in the body, which can act as an index of sympathetic activity in studies of neurological incidents[13-15]. While catecholamines have a positive inotropic effect on the myocardium under normal conditions, oversecretion can lead to hypermetabolism, and hyperdynamic circulation can lead to cardiac disorders. Plasma catecholamines levels can be up to 20 times higher in NSM patients compared to normal individuals[16]. Excessive catecholamine levels can cause cardiac damage through ischemia by increasing the myocardial oxygen demand or causing coronary vasospasms. However, venous plasma catecholamines may not be an accurate index of sympathetic activity[15]. Local and abnormally sharp increases in catecholamine concentration lead to coronary vasoconstriction and microvascular dysfunction and hence to necrosis of cardiac myocytes and changes in contractile function of cardiac myocytes[17]. In addition, cardiac injury can occur in the form of direct cardiotoxic effects due to the local increase in catecholamines[18]. β-adrenergic receptors are stimulated by increased catecholamines levels, which subsequently lead to (over)activation of calcium channels, and release of free radicals and peroxide in cells, and lower adenosine triphosphate levels, accelerating mitochondrial dysfunction and resulting in cardiomyocyte death.
Inflammation-mediated myocardial injury: A rise in stroke-induced inflammation represents the second major NSM induction pathway. It has been shown by some studies that immunologically active components (including cytokines, tumor necrosis factor, adhesion molecules, and bioactive peptides) are produced in the brain and released into the circulation, where they may cause systemic inflammatory response syndrome, leading to dysfunction of most organs, including the heart[19,20]. Conclusive evidence of this phenomenon was observed in patients receiving heart transplants from donors, with higher mortality rates in patients who receive heart transplantation from non-traumatic ICH donors[21]. Further research has shown that cardiac myocytes of transplanted hearts from ICH donors have a higher expression of matrix metalloproteinases, markers of early inflammatory states[22]. Inflammatory reactions can facilitate the smooth entry of enzymes from brain cells into the blood, crossing the blood-brain barrier. In addition, recent studies have shown that the parasympathetic nerve system may also mediate cardiomyocyte damage in stroke by modulating the myocardial inflammatory response through acetylcholine receptors[23].
Although the specific mechanism by which changes to the autonomic nervous system are affected remains unclear, it is known that persistent sympathetic dysfunction with excessive catecholamine release and inflammation can aggravate myocardial injury, increase serum myocardial enzyme levels, and result in cardiac insufficiency[24]. Moreover, most of the stroke patients are treated with diuretic, and eating difficulties can lead to insufficient blood volume and hemorheological and hemodynamic changes, and the abnormal secretion of antidiuretic hormone due to cerebral hemorrhage involving the hypothalamus can lead to electrolyte disturbances (especially hypokalemia and hypomagnesemia), which can affect and aggravate CCS.
A consequence of autonomic imbalance with a concomitant increase in the release of catecholamines and inflammatory factors is the abnormal myocardial contraction and metabolism. Further evidence for neurogenic myocardial injury has come from histopathological findings in animals after occlusion of the middle cerebral artery[25,26]. The hallmark pattern of stroke-related diffuse myocardial damage is micro-islands of necrosis with monocyte infiltration and subendocardial hemorrhage, which is often referred to as myocytolysis[27]. Unlike the delayed coagulative necrosis of the myocardium along the vascular distribution area seen in myocardial ischemia of CAD, myolysis of NSM is concentrated near the nerve endings.
Clinical manifestations
Patients with cardiac involvement can present with chest pain, dyspnea, and syncope in the acute phase, accompanied by ECG changes (such as ST-segment changes and a prolonged QT interval) and laboratory evidence (such as elevated troponin and BNP levels). These non-specific manifestations are similar to those of acute coronary syndrome (ACS). Patients with stroke or ACS have many risk factors in common. Thus, stroke and ACS may occur simultaneously. The typical clinical presentation is absent in stroke patients with myocardial injury, especially in stroke patients with cognitive or language impairment, and many symptoms of myocardial ischemia are less likely to be noticed. Though symptoms of myocardial ischemia may be atypical or concealed by neurological deficits, it is common for hemodynamic instability or even sudden death to occur[28]. Other clinical manifestations are mostly associated with autonomic dysfunction, which can be manifested as sympathetic hyperfunction, including shortness of breath, hypertension, fever, sweating, chills and dilated pupils.
Clinical evaluation
An increment in cardiac troponin (cTn) levels above the upper reference limit, abnormal LV function, increased NT-proBNP levels, ST-segment deviations, and a prolonged QT interval have been reported in up to one-third of patients with acute cerebrovascular events[2]. Based on the literature these signs may represent an acute release of catecholamine and inflammatory markers, provoked by the cerebrovascular events itself and not by coronary thrombosis[29,30].
Despite the variety of diagnostic tests available, the ECG remains the mainstay in the initial. The 12-lead ECG is an integral part of the diagnostic procedures of patients with acute chest discomfort. Patients with a history of CAD and hypertension are more likely to exhibit ECG alterations after acute encephalopathy[31]. Among unselected AS patients, repolarization and ischemic-like ECG changes are regular, being present in more than 90% of unselected patients, but the prevalence was much lower after exclusion of preexisting cardiac diseases (approximately 30%-40%)[32].
Myocardial ischemic-like ECG changes include ST-segment deviations, T wave inversion, and Q-waves. The earliest manifestations of myocardial ischemia typically involve T waves and the ST segment. It is believed that ECG changes in CCS most often represent preexisting ischemic cardiac disease[32]. Indeed, ST inversions, suggestive of myocardial ischemia, are significantly more common in patients with elevated troponin levels[33]. In contrast to ECG changes due to ACS, neurogenic cardiac injury occurs later, reaching a maximum within one week after disease onset, mostly within 48 h, evolves over several days, and disappears in 2 wk with the improvement of the stroke[27,34,35]. Therefore, the mainstream view is that these ECG abnormalities are reversible, transient, and not related to CAD. Although most ECG changes are benign and do not require treatment, it is very important to exclude the causes of cardiovascular changes, especially for high-risk patients with cardiovascular diseases.
Despite the high sensitivity, ST-segment deviation has poor specificity since it may be observed in many non-cardiac conditions. The interpretation of ECGs of these patients may be a real challenge. In this setting, some aspects such as clinical features and biomarkers of myocardial necrosis may play an important role. Cardiac biochemical markers of NSM include CK, CK-MB, troponin, and BNP. Unlike acute MI, in which CK-MB peaks and decreases within the first 24 h of coronary artery occlusion, stroke-induced elevation of CK-MB levels occurs more gradually and peaks at much lower levels[34,36]. However, because of many confounding factors affecting CK-MB levels, it is generally believed that the increase of CK-MB in stroke patients is non-cardiac in origin[37]. Cardiovascular detection should be strengthened to identify any ACS events in patients with elevated CK-MB levels.
cTn is sensitive and specific marker for myocardial damage and for risk stratification of patients with ACS[38]. In patients with suspected ACS, cTn (cTnT and cTnI) are superior to all other markers, including the ECG, in diagnosing myocardial necrosis[39,40]; it is only expressed in cardiac myocytes, it is rapidly released from myocytes following myocardial damage, and it persists for several days. About 88% of patients with elevated troponin levels have suffered a stroke within the right middle cerebral artery territory involving the insular cortex[3]. These minor releases of cTn might be due to transient and reversible myocyte necrosis and subendocardial hemorrhages in scattered of microlesions, which is called myocytolysis, but not due to coronary thrombosis[29,30]. Although in some patients with AS, increased troponin concentrations might indicate coronary ischemia, in others, it is almost certainly is indicative of neurogenic-induced cardiac injury[41]. There is currently no test available to distinguish whether elevated troponin is due to concurrent exacerbation of CAD or neurogenic cardiac involvement. In patients with cardiac injury after stroke, most cTn concentrations in CCS are modest, even though an obvious abnormal contraction is observed[8]. On the contrary, cTn levels typically reach concentrations of up to 50 times the upper reference limit when measured 18-24 h after ACS[42]. This finding helps to differentiate NSM from MI to some extent. Hence, troponin levels might be useful to identify AS patients who need early treatment for CAD and more intensified secondary prevention.
Patients with stroke or ACS have many risk factors in common, especially patients with ischemic stroke (IS). When using both cTnT levels are above the diagnostic limit and ST-T changes satisfy the diagnostic criteria of acute MI, stroke and acute MI were found simultaneously in 3% of patients in an IS cohort, but patients with a history of CAD were excluded[43]. The probability of ICH remains unclear. A prospective study has shown that most AS patients with elevated cTn levels have fewer coincident ACSs than those with NSTEMI, and about half of them show no evidence of obstructive CAD[44]. In addition, although they have similar baseline troponin levels and GRACE scores (both correlating with severity of coronary lesions and TIMI flow rates in ACS patients), the overall burden of coronary lesions is lower in AS patients than in NSTE-ACS patients[44].
LV systolic dysfunction can present in AS[41]. A regional wall motion abnormality is most likely to be present within the first two days[45]. Despite similarities to MI, the regions of contractile dysfunction and abnormalities in myocardial sympathetic innervation still demonstrate normal perfusion[46]. Most of the cardiac injury following stroke is completely reversible. In the majority of cases, the return of normal LV function is seen within 5-14 d, sometimes several weeks[47]. Some view acute myocardial ischemic-like changes after stroke as a stress cardiomyopathy similar to Takotsubo cardiomyopathy which presents with ST-segment changes, increased troponin concentrations and hypokinesis or akinesia of the apex LV[8,47,48]. Unlike Takotsubo cardiomyopathy, the most common echogenic abnormalities include hypokinesis or akinesia of basal and intraventricular segments with sparing of the apical or global hypokinesis of the LV[10,47].
Management
Since the mechanism underlying CCS has been described only recently, there is no clear guideline for its management. Most of the suggestions are to monitor vital signs, cardiovascular functions, and the neurologic status of stroke patients by a dual cardio-cerebral approach in the first 3-5 d[34]. According to the recommendations of the American Heart Association/ American Stroke Association for early treatment of AS patients, continuous ECG monitoring and assessment of LV function (ECHO) and cardiac injury biomarkers are crucial for cardiac monitoring, especially during the first 24 h[49]. It helps to screen for any myocardial damage to prevent sudden cardiac death.
ECG changes usually do not require specific treatment, but it is helpful to compare the ECG with a previous ECG. Apart from intensive cardiovascular monitoring, appropriate investigations should be carried to identify other causes or contributors such as cardiac CT or magnetic resonance imaging. Coronary angiography, the key to the diagnosis, is contraindicated during the acute stage of stroke because of the uncertainty about the cause of elevated troponin levels and the high risk of secondary hemorrhagic or procedure-related stroke. At present, doctors are still facing this clinical problem in terms of the best time and method for diagnosis, evaluation, and treatment. According to the existing studies, it is reasonable to believe that the vast majority of signs of myocardial injury during the stroke are due to non-thrombotic conditions. This, however, does not rule out that concomitant CAD and ST-T changes or elevated plasma troponin levels indicated significant atherosclerotic coronary stenosis; therefore, all patients with signs of cardiac involvement during stroke should receive a cardiological follow-up in order to exclude ischemic cardiac disease. Severely affected patients should be evaluated by a cardiologist prior to the initiation of therapy.
Although the best management strategy is subject of debate, most researches agree supportive treatment of stroke-related cardiac abnormalities is preferred. Emerging evidence has shown an association of post-stroke autonomic complications with increases in cardiac adverse events[50]. Treatment focusing on the modulation of underlying neurologic processes in order to maximize recovery is a new therapeutic strategy for stroke management[47,51]. Since NSM can be induced or enhanced by catecholamines, it seems that phosphodiesterase inhibitors may be effective (milrinone alone or combined with dobutamine)[52]. In addition, β-blockers may play a role by providing cardio-protection or reverse myocytolysis if administered shortly after hospital admittance. Levosimendan, which does not increase myocardial oxygen demands but can play a cardioprotective role, is also recommended in severe cases[8]. In the acute stage of stroke, when ensuring effective perfusion of brain tissue, blood pressure may fall too low, increasing the burden on the heart, which is liable to suffer malignant arrhythmia and HF. Considering the pathophysiology mechanism of NSM, statins have been demonstrated to have an anti-inflammatory effect, which could protect against myocardial injury in AS as statins slow down the general inflammatory process[53].
Prognosis
The incidence of poor prognosis in patients with CCS is eight times higher than that in patients without CCS and the mortality rate is four times higher[54]. Higher mortality due to adverse cardiac events occurs throughout the first month after the stroke, especially in patients with small functional deficits[55]. An initially elevated troponin concentration following AS was found to be a powerful predictor of follow-up mortality[56]. Other studies found that elevated plasma troponin levels in AS patients are independently associated with a threefold increase in the risk of death, irrespective of the mechanism underlying the elevated troponin levels[57,58]. In one study, patients with elevated troponin levels exhibited significantly increased mortality rates over the 5-year follow-up period compared to patients with normal troponin levels[59]. TnI levels of 0.5 μg/L or higher are independently associated with poorer outcomes[60].
Patients with AS or ACS, especially patients with IS, have many risk factors in common, to a lesser degree also with ICH patients, whereas the pathogenesis of SAH is substantially different. There are currently no data available on the impact of myocardial damage on ICH outcomes. Prospective studies in patients with ICH focusing on concomitant CAD are urgently needed. In addition, the improvement in neuropathology can lead to rapid improvements in cardiac function and complete recovery, as NSM is a fully reversible condition[8]. This view may change in the face of new evidence regarding transplanted hearts that have signs of fibrosis and vasculopathy[22]; we may have to reconsider the obvious reversibility of dysplastic myocardium. Further research is needed to understand the possibilities and limits of neural plasticity within central autonomic networks.
CONCLUSION
Misinterpretation of ischemic-like ECGs may lead to unnecessary or hazardous interventions and cause undue delay of rehabilitation after stroke. Our objective is to highlight the clinical implications of CCS and we hope the differential diagnoses will be considered in patients with acute CNS diseases.
Footnotes
Informed consent statement: Informed consent was obtained from the patient to publish relevant date.
Conflict-of-interest statement: The authors declare that there is no conflict of interest regarding the publication of this paper.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: June 4, 2019
First decision: September 9, 2019
Article in press: October 5, 2019
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Caceres-Loriga FM S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Qi LL
Contributor Information
Xue-Qi Lin, Department of Cardiovascular, The First Affiliated Hospital of Zhejiang University, Hangzhou 310003, Zhejiang Province, China.
Liang-Rong Zheng, Department of Cardiovascular, The First Affiliated Hospital of Zhejiang University, Hangzhou 310003, Zhejiang Province, China. 1191066@zju.edu.cn.
References
- 1.Prosser J, MacGregor L, Lees KR, Diener HC, Hacke W, Davis S VISTA Investigators. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke. 2007;38:2295–2302. doi: 10.1161/STROKEAHA.106.471813. [DOI] [PubMed] [Google Scholar]
- 2.Jespersen CM, Fischer Hansen J. Myocardial stress in patients with acute cerebrovascular events. Cardiology. 2008;110:123–128. doi: 10.1159/000110491. [DOI] [PubMed] [Google Scholar]
- 3.Ay H, Koroshetz WJ, Benner T, Vangel MG, Melinosky C, Arsava EM, Ayata C, Zhu M, Schwamm LH, Sorensen AG. Neuroanatomic correlates of stroke-related myocardial injury. Neurology. 2006;66:1325–1329. doi: 10.1212/01.wnl.0000206077.13705.6d. [DOI] [PubMed] [Google Scholar]
- 4.Oppenheimer S. The insular cortex and the pathophysiology of stroke-induced cardiac changes. Can J Neurol Sci. 1992;19:208–211. [PubMed] [Google Scholar]
- 5.Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]
- 6.Al-Qudah ZA, Yacoub HA, Souayah N. Disorders of the Autonomic Nervous System after Hemispheric Cerebrovascular Disorders: An Update. J Vasc Interv Neurol. 2015;8:43–52. [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein DS. The electrocardiogram in stroke: relationship to pathophysiological type and comparison with prior tracings. Stroke. 1979;10:253–259. doi: 10.1161/01.str.10.3.253. [DOI] [PubMed] [Google Scholar]
- 8.Mierzewska-Schmidt M, Gawecka A. Neurogenic stunned myocardium - do we consider this diagnosis in patients with acute central nervous system injury and acute heart failure? Anaesthesiol Intensive Ther. 2015;47:175–180. doi: 10.5603/AIT.2015.0017. [DOI] [PubMed] [Google Scholar]
- 9.Oppenheimer SM, Cechetto DF. Cardiac chronotropic organization of the rat insular cortex. Brain Res. 1990;533:66–72. doi: 10.1016/0006-8993(90)91796-j. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen H, Zaroff JG. Neurogenic stunned myocardium. Curr Neurol Neurosci Rep. 2009;9:486–491. doi: 10.1007/s11910-009-0071-0. [DOI] [PubMed] [Google Scholar]
- 11.Murthy SB, Shah S, Venkatasubba Rao CP, Suarez JI, Bershad EM. Clinical characteristics of myocardial stunning in acute stroke. J Clin Neurosci. 2014;21:1279–1282. doi: 10.1016/j.jocn.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Laowattana S, Zeger SL, Lima JA, Goodman SN, Wittstein IS, Oppenheimer SM. Left insular stroke is associated with adverse cardiac outcome. Neurology. 2006;66:477–83; discussion 463. doi: 10.1212/01.wnl.0000202684.29640.60. [DOI] [PubMed] [Google Scholar]
- 13.Young CN, Davisson RL. Angiotensin-II, the Brain, and Hypertension: An Update. Hypertension. 2015;66:920–926. doi: 10.1161/HYPERTENSIONAHA.115.03624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marina N, Teschemacher AG, Kasparov S, Gourine AV. Glia, sympathetic activity and cardiovascular disease. Exp Physiol. 2016;101:565–576. doi: 10.1113/EP085713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers MG, Norris JW, Hachniski VC, Sole MJ. Plasma norepinephrine in stroke. Stroke. 1981;12:200–204. doi: 10.1161/01.str.12.2.200. [DOI] [PubMed] [Google Scholar]
- 16.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 17.Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo Syndrome. Circulation. 2017;135:2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121. [DOI] [PubMed] [Google Scholar]
- 18.Mann DL, Kent RL, Parsons B, Cooper G 4th. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992;85:790–804. doi: 10.1161/01.cir.85.2.790. [DOI] [PubMed] [Google Scholar]
- 19.Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. 2017;14:314. doi: 10.1038/nrcardio.2017.33. [DOI] [PubMed] [Google Scholar]
- 20.Christensen H, Johannesen HH, Christensen AF, Bendtzen K, Boysen G. Serum cardiac troponin I in acute stroke is related to serum cortisol and TNF-alpha. Cerebrovasc Dis. 2004;18:194–199. doi: 10.1159/000079941. [DOI] [PubMed] [Google Scholar]
- 21.Tsai FC, Marelli D, Bresson J, Gjertson D, Kermani R, Patel J, Kobashigawa JA, Laks H UCLA Heart Transplant Group. Use of hearts transplanted from donors with atraumatic intracranial bleeds. J Heart Lung Transplant. 2002;21:623–628. doi: 10.1016/s1053-2498(01)00425-9. [DOI] [PubMed] [Google Scholar]
- 22.Yamani MH, Starling RC, Cook DJ, Tuzcu EM, Abdo A, Paul P, Powell K, Ratliff NB, Yu Y, McCarthy PM, Young JB. Donor spontaneous intracerebral hemorrhage is associated with systemic activation of matrix metalloproteinase-2 and matrix metalloproteinase-9 and subsequent development of coronary vasculopathy in the heart transplant recipient. Circulation. 2003;108:1724–1728. doi: 10.1161/01.CIR.0000087604.27270.5B. [DOI] [PubMed] [Google Scholar]
- 23.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 24.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res. 2014;114:1815–1826. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 25.Min J, Farooq MU, Greenberg E, Aloka F, Bhatt A, Kassab M, Morgan JP, Majid A. Cardiac dysfunction after left permanent cerebral focal ischemia: the brain and heart connection. Stroke. 2009;40:2560–2563. doi: 10.1161/STROKEAHA.108.536086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hachinski VC, Smith KE, Silver MD, Gibson CJ, Ciriello J. Acute myocardial and plasma catecholamine changes in experimental stroke. Stroke. 1986;17:387–390. doi: 10.1161/01.str.17.3.387. [DOI] [PubMed] [Google Scholar]
- 27.Korpelainen JT, Sotaniemi KA, Myllylä VV. Autonomic nervous system disorders in stroke. Clin Auton Res. 1999;9:325–333. doi: 10.1007/BF02318379. [DOI] [PubMed] [Google Scholar]
- 28.Sörös P, Hachinski V. Cardiovascular and neurological causes of sudden death after ischaemic stroke. Lancet Neurol. 2012;11:179–188. doi: 10.1016/S1474-4422(11)70291-5. [DOI] [PubMed] [Google Scholar]
- 29.Connor RC. Heart damage associated with intracranial lesions. Br Med J. 1968;3:29–31. doi: 10.1136/bmj.3.5609.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norris JW, Hachinski VC, Myers MG, Callow J, Wong T, Moore RW. Serum cardiac enzymes in stroke. Stroke. 1979;10:548–553. doi: 10.1161/01.str.10.5.548. [DOI] [PubMed] [Google Scholar]
- 31.Togha M, Sharifpour A, Ashraf H, Moghadam M, Sahraian MA. Electrocardiographic abnormalities in acute cerebrovascular events in patients with/without cardiovascular disease. Ann Indian Acad Neurol. 2013;16:66–71. doi: 10.4103/0972-2327.107710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khechinashvili G, Asplund K. Electrocardiographic changes in patients with acute stroke: a systematic review. Cerebrovasc Dis. 2002;14:67–76. doi: 10.1159/000064733. [DOI] [PubMed] [Google Scholar]
- 33.Apak I, Iltumur K, Tamam Y, Kaya N. Serum cardiac troponin T levels as an indicator of myocardial injury in ischemic and hemorrhagic stroke patients. Tohoku J Exp Med. 2005;205:93–101. doi: 10.1620/tjem.205.93. [DOI] [PubMed] [Google Scholar]
- 34.Cheung RTF, Hachinski V. Cardiac effects of stroke. Current Treatment Options in Cardiovascular Medicine. 2004;6:199–207. doi: 10.1007/s11936-996-0014-x. [DOI] [PubMed] [Google Scholar]
- 35.Cheshire WP, Jr, Saper CB. The insular cortex and cardiac response to stroke. Neurology. 2006;66:1296–1297. doi: 10.1212/01.wnl.0000219563.87204.7d. [DOI] [PubMed] [Google Scholar]
- 36.Puleo PR, Guadagno PA, Roberts R, Scheel MV, Marian AJ, Churchill D, Perryman MB. Early diagnosis of acute myocardial infarction based on assay for subforms of creatine kinase-MB. Circulation. 1990;82:759–764. doi: 10.1161/01.cir.82.3.759. [DOI] [PubMed] [Google Scholar]
- 37.Ay H, Arsava EM, Saribaş O. Creatine kinase-MB elevation after stroke is not cardiac in origin: comparison with troponin T levels. Stroke. 2002;33:286–289. doi: 10.1161/hs0102.101544. [DOI] [PubMed] [Google Scholar]
- 38.Katus HA, Remppis A, Neumann FJ, Scheffold T, Diederich KW, Vinar G, Noe A, Matern G, Kuebler W. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991;83:902–912. doi: 10.1161/01.cir.83.3.902. [DOI] [PubMed] [Google Scholar]
- 39.Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP, Fischer GA, Fung AY, Thompson C, Wybenga D, Braunwald E. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335:1342–1349. doi: 10.1056/NEJM199610313351802. [DOI] [PubMed] [Google Scholar]
- 40.Heidenreich PA, Alloggiamento T, Melsop K, McDonald KM, Go AS, Hlatky MA. The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol. 2001;38:478–485. doi: 10.1016/s0735-1097(01)01388-2. [DOI] [PubMed] [Google Scholar]
- 41.Chalela JA, Ezzeddine MA, Davis L, Warach S. Myocardial injury in acute stroke: a troponin I study. Neurocrit Care. 2004;1:343–346. doi: 10.1385/NCC:1:3:343. [DOI] [PubMed] [Google Scholar]
- 42.Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J. 2000;21:1502–1513. doi: 10.1053/euhj.2000.2305. [DOI] [PubMed] [Google Scholar]
- 43.Jensen JK, Kristensen SR, Bak S, Atar D, Høilund-Carlsen PF, Mickley H. Frequency and significance of troponin T elevation in acute ischemic stroke. Am J Cardiol. 2007;99:108–112. doi: 10.1016/j.amjcard.2006.07.071. [DOI] [PubMed] [Google Scholar]
- 44.Mochmann HC, Scheitz JF, Petzold GC, Haeusler KG, Audebert HJ, Laufs U, Schneider C, Landmesser U, Werner N, Endres M, Witzenbichler B, Nolte CH TRELAS Study Group. Coronary Angiographic Findings in Acute Ischemic Stroke Patients With Elevated Cardiac Troponin: The Troponin Elevation in Acute Ischemic Stroke (TRELAS) Study. Circulation. 2016;133:1264–1271. doi: 10.1161/CIRCULATIONAHA.115.018547. [DOI] [PubMed] [Google Scholar]
- 45.Banki N, Kopelnik A, Tung P, Lawton MT, Gress D, Drew B, Dae M, Foster E, Parmley W, Zaroff J. Prospective analysis of prevalence, distribution, and rate of recovery of left ventricular systolic dysfunction in patients with subarachnoid hemorrhage. J Neurosurg. 2006;105:15–20. doi: 10.3171/jns.2006.105.1.15. [DOI] [PubMed] [Google Scholar]
- 46.Banki NM, Kopelnik A, Dae MW, Miss J, Tung P, Lawton MT, Drew BJ, Foster E, Smith W, Parmley WW, Zaroff JG. Acute neurocardiogenic injury after subarachnoid hemorrhage. Circulation. 2005;112:3314–3319. doi: 10.1161/CIRCULATIONAHA.105.558239. [DOI] [PubMed] [Google Scholar]
- 47.Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008;118:397–409. doi: 10.1161/CIRCULATIONAHA.106.677625. [DOI] [PubMed] [Google Scholar]
- 48.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Hemphill JC, 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 50.McLaren A, Kerr S, Allan L, Steen IN, Ballard C, Allen J, Murray A, Kenny RA. Autonomic function is impaired in elderly stroke survivors. Stroke. 2005;36:1026–1030. doi: 10.1161/01.STR.0000160748.88374.ce. [DOI] [PubMed] [Google Scholar]
- 51.Sykora M, Diedler J, Turcani P, Hacke W, Steiner T. Baroreflex: a new therapeutic target in human stroke? Stroke. 2009;40:e678–e682. doi: 10.1161/STROKEAHA.109.565838. [DOI] [PubMed] [Google Scholar]
- 52.Naidech A, Du Y, Kreiter KT, Parra A, Fitzsimmons BF, Lavine SD, Connolly ES, Mayer SA, Commichau C. Dobutamine versus milrinone after subarachnoid hemorrhage. Neurosurgery. 2005;56:21–6l discussion 26-7. doi: 10.1227/01.neu.0000144780.97392.d7. [DOI] [PubMed] [Google Scholar]
- 53.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 54.van der Bilt I, Hasan D, van den Brink R, Cramer MJ, van der Jagt M, van Kooten F, Meertens J, van den Berg M, Groen R, Ten Cate F, Kamp O, Götte M, Horn J, Groeneveld J, Vandertop P, Algra A, Visser F, Wilde A, Rinkel G SEASAH (Serial Echocardiography After Subarachnoid Hemorrhage) Investigators. Cardiac dysfunction after aneurysmal subarachnoid hemorrhage: relationship with outcome. Neurology. 2014;82:351–358. doi: 10.1212/WNL.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 55.Silver FL, Norris JW, Lewis AJ, Hachinski VC. Early mortality following stroke: a prospective review. Stroke. 1984;15:492–496. doi: 10.1161/01.str.15.3.492. [DOI] [PubMed] [Google Scholar]
- 56.James P, Ellis CJ, Whitlock RM, McNeil AR, Henley J, Anderson NE. Relation between troponin T concentration and mortality in patients presenting with an acute stroke: observational study. BMJ. 2000;320:1502–1504. doi: 10.1136/bmj.320.7248.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. 2009;28:220–226. doi: 10.1159/000226773. [DOI] [PubMed] [Google Scholar]
- 58.Newby LK, Jesse RL, Babb JD, Christenson RH, De Fer TM, Diamond GA, Fesmire FM, Geraci SA, Gersh BJ, Larsen GC, Kaul S, McKay CR, Philippides GJ, Weintraub WS. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012;60:2427–2463. doi: 10.1016/j.jacc.2012.08.969. [DOI] [PubMed] [Google Scholar]
- 59.Thålin C, Rudberg AS, Johansson F, Jonsson F, Laska AC, Nygren AT, von Arbin M, Wallén H, Aspberg S. Elevated Troponin Levels in Acute Stroke Patients Predict Long-term Mortality. J Stroke Cerebrovasc Dis. 2015;24:2390–2396. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 60.Batal O, Jentzer J, Balaney B, Kolia N, Hickey G, Dardari Z, Reddy V, Jovin T, Hammer M, Gorcsan J, Schmidhofer M. The prognostic significance of troponin I elevation in acute ischemic stroke. J Crit Care. 2016;31:41–47. doi: 10.1016/j.jcrc.2015.09.018. [DOI] [PubMed] [Google Scholar]