Abstract
BACKGROUND
Leiomyosarcoma of the stomach is extremely rare, and only 13 cases have been reported in the literature. Before the advent of KIT immunohistochemistry, gastrointestinal stromal tumors (GISTs) were misdiagnosed as leiomyomas and leiomyosarcomas. Leiomyosarcoma rarely occurs in organs besides the uterus and is rarely located in the stomach.
CASE SUMMARY
A 57-year-old woman presented with the symptom of melena over a one-month period. She had suffered weight loss, weakness, nausea and vomiting for fifteen days. At a local hospital, computed tomography showed a very large mass in the stomach, and the results of endoscopic examination and histopathological diagnosis were unclear. She received transfusion therapy and was transferred to our hospital. Upon arrival at our hospital, the patient was anemic. She denied any family history and had no specific past history. No signs of pulmonary metastasis were found on chest radiographs. Magnetic resonance imaging and computed tomography confirmed a very large tumor in the stomach, and no visible signs of metastatic disease were found. On October 30, 2013, the patient underwent resection of the stomach tumor and did not undergo any adjuvant treatment. The margins were negative and she had an uneventful recovery and was discharged after 12 d. One year after surgery, the patient died at home, and the cause of death were gastrointestinal obstruction and malnutrition. During that time, she was treated with Chinese medicine but the effect was not ideal. Because of gastrointestinal obstruction, the patient did not receive any re-examination.
CONCLUSION
Surgical resection is the standard treatment for gastric leiomyosarcoma. The diagnosis of this tumor mainly depends on histopathological examination. This case may suggest the aggressive behavior and poor prognosis of this tumor.
Keywords: Leiomyosarcoma, Stomach, Case report, KIT, Gastrointestinal stromal tumor, Targeted next-generation sequencing
Core tip: Leiomyosarcoma of the stomach is extremely rare, and only 13 cases have been reported in the literature. We herein report one case and review the literature. This case might contribute to improving our understanding of the etiology, diagnosis, treatment strategies, and outcome of gastric leiomyosarcoma. This report can also serve as a reminder to gastroenterologists, surgeons, and pathologists who encounter gastric leiomyosarcoma in their clinical practice.
INTRODUCTION
Leiomyosarcoma of the stomach is a malignant tumor that originates from the stomach. Leiomyosarcoma of the stomach is extremely rare, and most cases reported in the “pre-KIT era” as leiomyosarcomas of the stomach were actually gastrointestinal stromal tumors (GISTs) of the stomach. Only 13 well-described cases of gastric leiomyosarcoma have been reported in English language literature since the early 2000s[1-13]. Due to the low incidence of this tumor, clinicians still have not formed a therapeutic consensus. Currently, the standard treatment is surgical resection of the tumor. Herein, we report one case of gastric leiomyosarcoma. The patient was referred to our hospital with the symptom of melena. Image examination confirmed a very large tumor in the stomach. The patient underwent surgical resection and had an uneventful recovery. The margins were negative and she did not undergo any adjuvant therapy. Histopathological morphology, immunohistochemistry and next-generation sequencing confirmed the presence of gastric leiomyosarcoma. The patient died within one year and the cause of death were gastrointestinal obstruction and malnutrition. We also discuss the clinical features, etiology, symptoms, diagnosis, prognostic factors and treatment strategies of gastric leiomyosarcoma in this report.
CASE PRESENTATION
Chief complaints
A 57-year-old woman presented with the symptom of melena and she also suffered weight loss, weakness, nausea and vomiting.
History of present illness
The patient presented with the symptom of melena over a one-month period. She had suffered weight loss, weakness, nausea and vomiting for fifteen days.
History of past illness
The patient had no special previous medical history.
Personal and family history
She denies any family history and has no specific past history.
Physical examination upon
During the physical examination, no special physical signs were found.
Laboratory examinations
In details see Table 1.
Table 1.
Laboratory examinations
| Test items | Result |
| WBC | 14.61 G/L |
| NEUT | 13.24 G/L |
| HGB | 68 g/L |
| RBC | 2.51 T/L |
| PLT | 379 G/L |
| Na | 128.4 mmol/L |
| Cl | 82.6 mmol/L |
| Ca | 2.01 mmol/L |
| LDH | 303 U/L |
| Fe | 3.3 μmol/L |
| TP | 56.3 g/L |
| ALB | 27.3 g/L |
| CRP | 16.04 mg/dL |
WBC: White blood cell; HGB: Hemoglobin; NEUT: Neutrophil; RBC: Red blood cell; PLT: Platelets; LDH: Lactate dehydrogenase; TP: Total protein; ALB: Albumin; CRP: C-reactive protein.
Imaging examinations
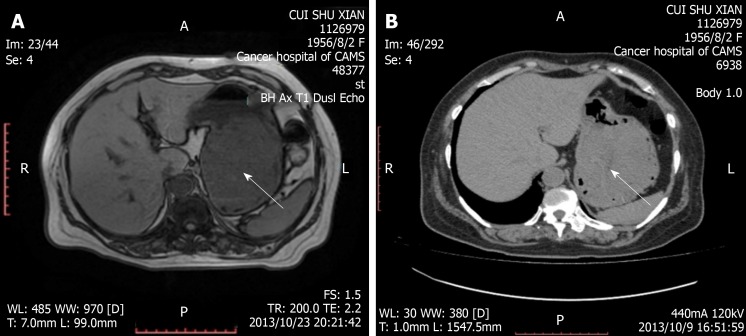
At a local hospital, computed tomography showed a very large mass in the stomach. Our hospital’s magnetic resonance imaging and computed tomography confirmed a very large tumor in the stomach, and no visible signs of metastatic disease were found (Figure 1). No signs of pulmonary metastasis were found on chest radiographs.
Figure 1.
Magnetic resonance imaging and computed tomography confirmed a giant tumor in the stomach (arrow). A: Magnetic resonance imaging; B: Computed tomography.
FINAL DIAGNOSIS
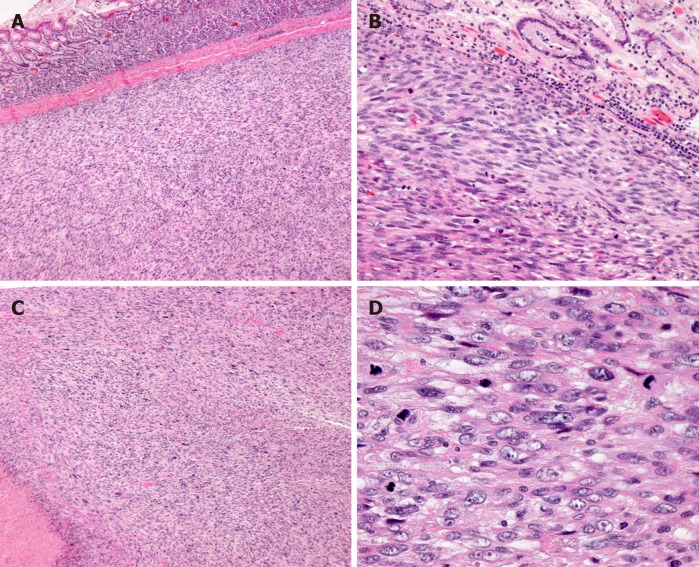
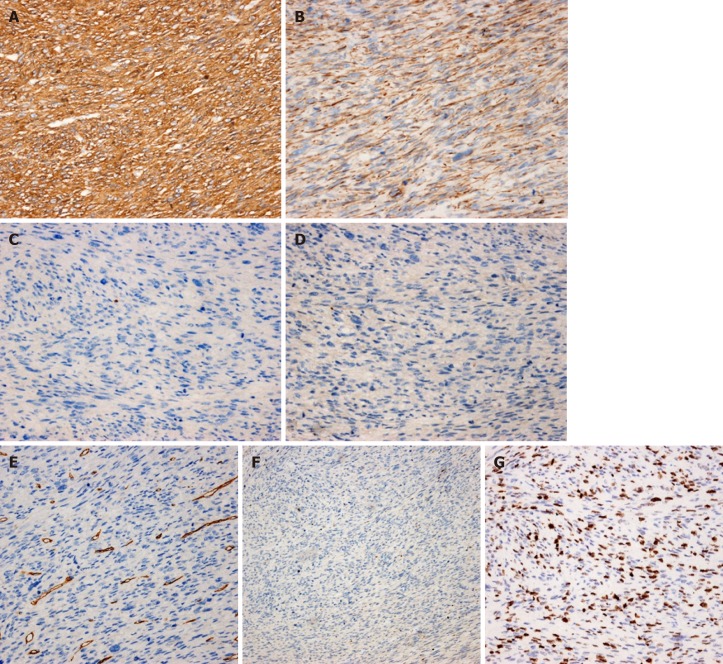
The histopathological diagnosis was gastric leiomyosarcoma, high-grade (differentiation 2, necrosis 1, mitosis 3) (FNCLCC grading system), measuring 13 cm × 13 cm × 5 cm (Figure 2). The tumor had invaded the mucosa to the serosa. The lymph nodes exhibited no metastasis. Immunohistochemical staining showed SMA(3+), desmin(2+), CD117(-), DOG1(-), CD34(-), S-100(-), and Ki-67 index (50%) (Figure 3). For a definitive diagnosis, we performed targeted next-generation sequencing. The genes in the panel were KIT, PDGFRA, SDHA, SDHB, SDHC, SDHD, BRAF, KRAS, NRAS and EGFR, and no mutations were detected in any of the genes.
Figure 2.
H and E stain showing gastric mucosa infiltrated by spindle cell tumor. A: × 40; B: × 200; C, D: With presence of tumor nerosis (lower-left corner of C × 40), and high mitotic activity (D × 400).
Figure 3.
Immunohistochemistry showing the tumor cells diffusely positive for SMA and Desmin, and negative for CD117, DOG1, CD34, S100. Ki-67 index is 50%. A: SMA (× 200); B: Desmin (× 200); C: CD117 (× 200); D: DOG1 (× 200); E: CD34 (× 200); F: S100 (× 100); G: Ki-67 (× 200).
TREATMENT
On October 30, 2013, the patient underwent resection of the stomach tumor and did not undergo any adjuvant treatment. The margins were negative and the patient was discharged successfully after 12 d. After discharge, she received Chinese medicine treatment, but the effect was not good.
OUTCOME AND FOLLOW-UP
One year after surgery, the patient died at home, and the cause of death were gastrointestinal obstruction and malnutrition. During that time, she was treated with Chinese medicine but the effect was not ideal. Because of gastrointestinal obstruction, the patient did not receive any re-examination.
DISCUSSION
Before the advent of KIT immunohistochemistry, GISTs were misdiagnosed as leiomyomas and leiomyosarcomas. Since the establishment of KIT immunohistochemistry in the late 1990s, we have realized that primary gastric leiomyosarcoma is extremely rare and reportedly accounts for fewer than 1% of gastric tumors[1,2]. Only 13 well-described cases have been reported in English language literature since 2007[1-13] (Table 2). Before our report, the largest case series of a single cohort of 9 patients with gastric leiomyosarcoma[4] was reported by Rou et al[4].
Table 2.
Review of literature
| Ref. | Age/Sex | Location | Size (cm) | Treatment | R or M | Outcome |
| Sato et al[1] | 74/F | Body | 1.5 | ESD | No | WR in 36 M |
| Hasnaoui et al[2] | 63/F | Cardia | 9 | Total gastrectomy | No | Unknow |
| Mehta et al[3] | 47/M | Body | 13 × 13 × 10 | Total excision of the greater curvature | Yes, liver | Alive in 35 M |
| Rou et al[4] | 48/F | Body | 2 | Chemotherapy | Yes, lung, liver, pancreas | DOD in 12 M |
| Weledji et al[5] | 69/M | Pylorus | 8 | Partial gastrectomy | Unknow | DOD in 7 D |
| Yamamoto et al[6] | 51/M | / | 2.5 | Surgery | No | WR in 18 M |
| Damiano et al[7] | 71/M | Body | 9 × 8 × 3 | Atypical gastroresection | No | WR in 28 M |
| Insabato et al[8] | 51/M | Fundus | 3 | Total gastrectomy | No | WR in 10 M |
| Soufi et al[9] | 16/F | Fundus | / | Subtotal gastrectomy | No | WR in 18 M |
| Masuzawa et al[10] | 29/F | Body | 11 × 9.7 × 3.2 | Distal gastrectomy | No | WR in 8 M |
| Pauser et al[11] | 37/M | Antrum | 1 | Resected endoscopically | No | WR in 36 M |
| Geraci et al[12] | 25/M | Body | 18 × 12 × 7 | Wedge resection | No | WR in 12 M |
| Insabato et al[13] | 65/M | / | 8.5 | Gastrectomy | Yes, lung | DOD in 24 M |
DOD: Death of disease; WR: Without recurrence; R or M: Recurrence or Metastasis; ESD: Endoscopic submucosal dissection.
The most common location for leiomyosarcoma is the retroperitoneum. In addition, large blood vessels, especially the inferior vena cava, comprise a significant proportion of the sites involved in leiomyosarcoma. In addition to these locations, leiomyosarcoma usually appears in the lower extremity, constituting a third group that accounts for 10%-15% of limb sarcomas[14]. Leiomyosarcoma rarely occurs in organs besides the uterus and is rarely located in the stomach[1]. Yamamoto et al[6] reported that among 55 cases of gastrointestinal leiomyosarcoma, only four cases (7.3%) were located in the stomach. According to our statistics, 50% (5/10) of gastric liposarcomas are located in the body of the stomach. In addition, 20% (2/10) of gastric liposarcomas are located in the fundus, with one case in the antrum, one case in the cardia, and one case in the pylorus. The diameters of the tumors described in the literature vary from 1 to 18 cm[1-13]. The origin of gastric leiomyosarcoma is usually between the muscularis propria and muscularis mucosa layers[2]. Agaimy et al[15] reported 85 cases of true smooth muscle neoplasms of the gastrointestinal tract and found that only one case of polypoid leiomyosarcoma arose from the muscularis mucosae of the stomach.
Leiomyosarcoma of the stomach is generally common among adults in their fifties, and men and women share the same risk of this disease[9]. In the literature, most patients are 50-69 years old. However, we are aware of 3 patients under 29 years old, which may suggest that gastric leiomyosarcoma is not truly rare among young people.
The etiology of gastric leiomyosarcoma is not yet clear. Because a diagnosis of primary leiomyosarcoma of the stomach is so rare, little information is available on its clinical characteristics. The tumor usually develops within the gastric wall, and the patient may remain asymptomatic for a long time. Symptoms in patients with leiomyosarcoma of the stomach can range from weakness, epigastric distress, weight loss, nausea and vomiting to upper gastrointestinal tract bleeding. The kind of symptoms depends on the location and size of the tumor and the presence of ulceration. For patients with very large tumors, the main clinical sign may be the presence of a large abdominal mass of unknown origin. In this case, the first clinical sign was melena.
The diagnosis of gastric leiomyosarcomas mainly relies on pathological examination. In general, SMA, desmin, and h-caldesmon are positive in the majority (≥ 70%) of leiomyosarcoma cases[14], and CD117 (KIT), DOG1 and CD 34 are negative. DOG1 is the best marker for GIST, and there have been no gastric leiomyosarcoma cases with positive DOG1 staining[2]. Considering that 10% of GISTs are KIT-negative, gene analysis of KIT or PDGFRA leads to a conclusive diagnosis of gastric leiomyosarcoma. To obtain an accurate diagnosis, we performed targeted next-generation sequencing for KIT, PDGFRA, SDHA, SDHB, SDHC, SDHD, BRAF, KRAS, NRAS and EGFR in our case. Because the patient had no neurofibromatosis or family history, tests for NF1 were ruled out. Computed tomography is the most informative method of examination and can also show secondary lesions in the liver, pancreas, lung, peritoneum, lymph node or other sites. In some studies, in vitro MRI of the fresh, surgically resected tumor was performed to clarify the correlation between radiological and pathological features. More detailed investigations are necessary to evaluate the clinicopathological and radiological characteristics of true gastric leiomyosarcoma[10]. It is difficult to make a precise judgement with endoscopy, and its diagnostic value is unclear. However, endoscopic ultrasonography is very sensitive, with a success rate of up to 97%[16] in the diagnosis of leiomyosarcoma of the stomach[2]. With the guidance of endoscopic ultrasonography, biopsy may be possible, and a histological examination can be performed. Although metastases to the stomach are unusual, Costa et al[17] reported a uterine leiomyosarcoma tumor and its metastasis to the stomach. Thus, the antidiastole between primary gastric leiomyosarcoma and metastatic tumors can be important. The patient’s unique medical history and imageological examination can contribute to finding the primary tumors.
Currently, the standard treatment for gastric leiomyosarcomas is complete surgical resection of the tumor. We noticed that two patients in the literature underwent endoscopic submucosal dissection. Due to a lack of clinical data, the advantages and disadvantages of this method are unknown. The use of chemotherapy and radiotherapy has been rarely reported in the literature, as leiomyosarcoma of the stomach is extremely rare worldwide. In an imageable patient-derived orthotopic xenograft model, Kawaguchi et al[18] found that a combination of gemcitabine and docetaxel caused the regress of both gastric leiomyosarcoma proliferation and invasion and provided a potential therapy for gastric leiomyosarcomas.
The main prognostic factors for these tumors include their histopathological grade and type, tumor size, evidence of synchronous metastasis and parietal gastric infiltration[9,12]. The 5-year survival rate for patients with leiomyosarcomas is 22%[19]. Unfortunately, there is little data on the prognosis of patients with gastric leiomyosarcomas, and more careful clinical follow-up is advised.
CONCLUSION
Gastric leiomyosarcomas are extremely rare, and little information is available on their clinical characteristics. The diagnosis of this tumor mainly depends on histopathological examination. Differential diagnoses between gastric leiomyosarcomas and GISTs are important. At present, surgical resection is the standard treatment for leiomyosarcoma, and there is no therapeutic consensus. Because leiomyosarcoma is rarely observed in the stomach, its prognosis remains unclear. Our experience suggests that the outcome for gastric leiomyosarcoma is not optimistic.
Footnotes
Informed consent statement: Consent was obtained from patients for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: August 11, 2019
First decision: September 9, 2019
Article in press: October 5, 2019
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arville B, Ashley S S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Qi LL
Contributor Information
Wen-Zhe Kang, Department of Pancreatic and Gastric Surgery, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China.
Li-Yan Xue, Department of Pathology, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China.
Yan-Tao Tian, Department of Pancreatic and Gastric Surgery, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China. tyt67@163.com.
References
- 1.Sato T, Akahoshi K, Tomoeda N, Kinoshita N, Kubokawa M, Yodoe K, Hiraki Y, Oya M, Yamamoto H, Ihara E. Leiomyosarcoma of the stomach treated by endoscopic submucosal dissection. Clin J Gastroenterol. 2018;11:291–296. doi: 10.1007/s12328-018-0838-4. [DOI] [PubMed] [Google Scholar]
- 2.Hasnaoui A, Jouini R, Haddad D, Zaafouri H, Bouhafa A, Ben Maamer A, Ben Brahim E. Gastric leiomyosarcoma and diagnostic pitfalls: a case report. BMC Surg. 2018;18:62. doi: 10.1186/s12893-018-0393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta V, Rajawat M, Rastogi S, Phulware RH, Mezencev R. Leiomyosarcoma of the stomach with metastasis to the liver: a case report with review of the literature. Future Sci OA. 2017;4:FSO264. doi: 10.4155/fsoa-2017-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rou WS, Ju JS, Kang SH, Moon HS, Sung JK, Lee BS, Jeong HY, Song KS. [A case of gastric leiomyosarcoma with multiple metastases] Korean J Gastroenterol. 2015;65:112–117. doi: 10.4166/kjg.2015.65.2.112. [DOI] [PubMed] [Google Scholar]
- 5.Weledji EP, Enoworock G, Ngowe MN. Gastric leiomyosarcoma as a rare cause of gastric outlet obstruction and perforation: a case report. BMC Res Notes. 2014;7:479. doi: 10.1186/1756-0500-7-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto H, Handa M, Tobo T, Setsu N, Fujita K, Oshiro Y, Mihara Y, Yoshikawa Y, Oda Y. Clinicopathological features of primary leiomyosarcoma of the gastrointestinal tract following recognition of gastrointestinal stromal tumours. Histopathology. 2013;63:194–207. doi: 10.1111/his.12159. [DOI] [PubMed] [Google Scholar]
- 7.Damiano G, Di Ganci S, Palumbo VD, Spinelli G, De Luca S, Cudia B, Tomasello G, Lo Monte AI. [Gastric leiomyosarcoma: case report and review of literature] Clin Ter. 2012;163:e181–e184. [PubMed] [Google Scholar]
- 8.Insabato L, Masone S, Campione S, Vigliar E, Staibano S, Tornillo L. Coexistence of primary gastric giant cell-rich leiomyosarcoma and gastrointestinal stromal tumor: report of a very rare combination and review of the literature. Int J Surg Pathol. 2012;20:74–78. doi: 10.1177/1066896911414018. [DOI] [PubMed] [Google Scholar]
- 9.Soufi M, Errougani A, Chekkof RM. Primary gastric leiomyosarcoma in young revealed by a massive hematemesis. J Gastrointest Cancer. 2009;40:69–72. doi: 10.1007/s12029-009-9080-0. [DOI] [PubMed] [Google Scholar]
- 10.Masuzawa N, Kishimoto M, Nishimura A, Ichiba N, Aoki E, Yanagibashi K, Hirota S, Yanagisawa A. Gastric leiomyosarcoma manifesting peculiar findings: radiological-pathological correlation. Pathol Int. 2009;59:306–311. doi: 10.1111/j.1440-1827.2009.02370.x. [DOI] [PubMed] [Google Scholar]
- 11.Pauser U, Grimm H. Intramucosal leiomyosarcoma of the stomach following hereditary retinoblastoma in childhood - a case report and review of the literature. World J Surg Oncol. 2008;6:131. doi: 10.1186/1477-7819-6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geraci G, Pisello F, Sciumè C, Li Volsi F, Platia L, Facella T, Modica G. Unusual acute onset of pedunculated extragastric leiomyosarcoma. Case report. G Chir. 2007;28:265–269. [PubMed] [Google Scholar]
- 13.Insabato L, Di Vizio D, Ciancia G, Pettinato G, Tornillo L, Terracciano L. Malignant gastrointestinal leiomyosarcoma and gastrointestinal stromal tumor with prominent osteoclast-like giant cells. Arch Pathol Lab Med. 2004;128:440–443. doi: 10.5858/2004-128-440-MGLAGS. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher CDM, Bridge JA, Hogendoom PCW. Lyon: IARC Press; 2013. WHO classification of tumours of soft tissue and bone; pp. 111–113. [Google Scholar]
- 15.Agaimy A, Wünsch PH. True smooth muscle neoplasms of the gastrointestinal tract: morphological spectrum and classification in a series of 85 cases from a single institute. Langenbecks Arch Surg. 2007;392:75–81. doi: 10.1007/s00423-006-0092-y. [DOI] [PubMed] [Google Scholar]
- 16.Karila-Cohen P, Petit T, Kotobi H, Merran S. [Gastric leiomyosarcoma] J Radiol. 2004;85:1993–1997. doi: 10.1016/s0221-0363(04)97771-2. [DOI] [PubMed] [Google Scholar]
- 17.Costa M, Ivanova E, Esteves J. Upper gastrointestinal bleeding due to gastric metastasis from a primary uterine leiomyosarcoma. Acta Clin Belg. 2016;71:271–272. doi: 10.1179/2295333715Y.0000000071. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi K, Igarashi K, Murakami T, Kiyuna T, Nelson SD, Dry SM, Li Y, Russell TA, Singh AS, Chmielowski B, Unno M, Eilber FC, Hoffman RM. Combination of gemcitabine and docetaxel regresses both gastric leiomyosarcoma proliferation and invasion in an imageable patient-derived orthotopic xenograft (iPDOX) model. Cell Cycle. 2017;16:1063–1069. doi: 10.1080/15384101.2017.1314406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh CC, Shih CS, Wu YC, Huang BS, Hsu WH, Huang MH, Wang LS. Leiomyosarcoma of the gastric cardia and fundus. Zhonghua Yi Xue Za Zhi (Taipei) 1999;62:418–424. [PubMed] [Google Scholar]