Abstract
BACKGROUND
Primitive neuroectodermal tumors are rare, highly malignant small round cell tumors belonging to the Ewing sarcoma family. The purpose of this article is to present clinical manifestation, histology, treatment, and prognosis of two primitive neuroectodermal tumors (PNETs) in extremely rare anatomic locations, the abdominal wall and vulva.
CASE SUMMARY
Case 1 was a 66-month-old girl with lesions on the abdominal wall; tumor size was about 3.4 cm × 6.1 cm × 2 cm. The patient underwent radical resection of the tumor. After the operation, an alternating vincristine, doxorubicin, and cyclophosphamide/ifosfamide and etoposide (IE) regimen was given for eight cycles, and the patient survived for 66 mo without progression. Case 2 was a 40-month-old girl, with a vulvar lesion; tumor size was about 3.3 cm × 5 cm × 2.5 cm. The tumor was partially resected by surgery. The family left treatment after two cycles of vincristine, pirarubicin, and cyclophosphamide/IE chemotherapy, and the patient died at home six months after surgery.
CONCLUSION
PNET is a rare, fast-growing, highly malignant tumor that requires histologic and molecular analyses for exact diagnosis, and multimodal treatment is required to achieve a good prognosis.
Keywords: Primitive neuroectodermal tumor, Therapy, Prognosis, Case report
Core tip: Primitive neuroectodermal tumors (PNETs) are rare undifferentiated tumors with similar biological characteristics. They belong to the Ewing sarcoma family, accounting for 4% to 17% of all pediatric soft tissue tumors. PNETs usually occur in children and young adults under 25 years of age. We retrospectively analyzed two PNET cases at the First Affiliated Hospital of Guangxi Medical University from May 2012 to June 2014. Both patients were female with an age of onset at 66 and 40 mo. Both patients were provided inpatient visits, outpatient medical records, and telephone follow-ups for more than one year. In this report, we describe in detail the clinical manifestations, treatment protocols, pathological findings, and patient prognoses. This report provides an in-depth analysis of two cases of PNET at rare sites.
INTRODUCTION
Primitive neuroectodermal tumors (PNET) are rare undifferentiated tumors with similar biological characteristics. They belong to the Ewing sarcoma family, accounting for 4% to 17% of all pediatric soft tissue tumors. Ewing sarcoma PNETs (ES/PNETs) usually occur in children and young adults under 25 years of age[1-3]. There are two main types according to cell location and origin: Central PNET (cPNET) and peripheral PNET (pPNET). The cPNETs are derived from the neural tube and mainly involve the brain and spinal cord, while the pPNETs are derived from the neural crest and occur outside the central nervous system, and often involve the sympathetic nervous system or soft tissue and bone[4]. We report on two patients presenting with PNETs located in the abdominal wall and vulva. As far as we know, only 13 cases of abdominal wall PNET and 37 cases of vulvar PNET have been reported.
We retrospectively analyzed these two PNET cases at the First Affiliated Hospital of Guangxi Medical University from May 2012 to June 2014. Both patients were female with an age of onset at 66 and 40 months. Both patients were provided inpatient visits, outpatient medical records, and telephone follow-ups for more than one year (Table 1). In this case report, we describe in detail the clinical manifestations, treatment protocols, pathological findings, and patient prognoses. This report provides an in-depth analysis of two cases of PNET at rare sites.
Table 1.
Follow-up data of two cases of primitive neuroectodermal tumor patients
| Case | Age (mo) | Position | Size (cm) | Therapy | Survival (mo) | Outcome |
| 1 | 66 | Abdominal wall | 3.4 × 6.1 × 2.0 | S + Ch (VDC/IE) | 66+ | Survival |
| 2 | 40 | Vulva | 3.3 × 5.0 × 2.5 | S + Ch (VAC/IE) | 14 | Death |
F: Female; S: Surgery; Ch: Chemotherapy.
CASE PRESENTATION
Chief complaints
Case 1: A 66-month-old girl with no traumatic medical history presented with reported abdominal pain two months ago.
Case 2: A 40-month-old girl presented with a mass (the size of a broad bean) at the external vaginal orifice, accompanied by intermittent hemorrhage.
History of present illness
Case 1: Two months ago, the girl began to present with reported abdominal pain without any obvious causes.
Case 2: Eight months ago, the girl began to present with a mass (the size of a broad bean) at the external vaginal orifice, which was grown up and accompanied by intermittent hemorrhage.
History of past illness
Case 1: The patient has no special past history.
Case 2: The patient was diagnosed with pPNET via pathological biopsy at another hospital. But she did not receive surgical interventions.
Personal and family history
Both of the two patients had no significant personal or family history.
Physical examination upon admission
Case 1: Clinical examination revealed a large mass in her right abdomen approximately 5 cm × 5 cm.
Case 2: Physical examination revealed a 3 cm × 3 cm × 1 cm, red, irregular, soft mass located on the external vaginal orifice; no other symptoms were noted.
Laboratory examinations
Case 1: The patient had no significant laboratory test result.
Case 2: Laboratory examination revealed a hemoglobin level of 118 g/L (normal range: 120-160 g/L), blood platelet count of 422 × 109/L (normal range: 125 × 109/L to 355 × 109/L), neutrophil percentage of 20.9% (normal range: 40%-75%), neuronspecific enolase level of 61.96 ng/mL (normal range: 0.00-23.00), international normalized ratio of 0.75 (normal range: 0.80-1.40), and prothrombin time of 8.90 s (normal range: 9.00-15.00 s).
Imaging examinations
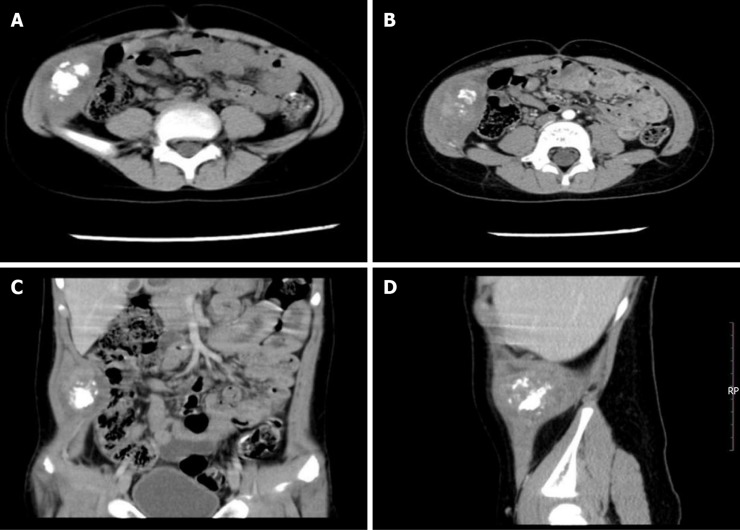
Case 1: Computed tomography (CT) showed a fusiform soft tissue density between the abdominis obliquus internus musculus and the abdominal transverse muscle in the right inferior abdominal, with a density measuring about 3.4 cm × 6.1 cm with irregular patchy calcification, evenly enhanced in the middle of the right lower abdominal wall (Figure 1). No evidence of metastatic disease was uncovered after a complete examination.
Figure 1.
Computed tomography evaluation of Case 1. A: Axial computed tomography image showing a fusiform soft tissue density between the abdominis obliquus internus musculus and the abdominal transverse muscle in the right inferior abdomen; the middle of the mass has irregular patchy calcification; B: Enhanced image showing uniform enhancement of the mass; C and D: The mass involving the musculus transversus abdominis.
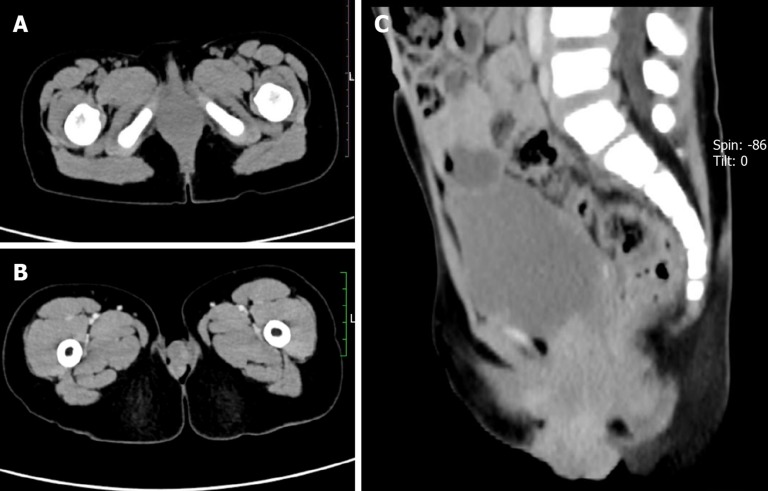
Case 2: Pelvic CT showed an irregular soft tissue density in the patient’s vagina with some protruding lesions ranging over an area of about 3.3 cm × 5 cm × 2.5 cm. A boundary was not clear, nor was the inner wall of the normal vagina, and the enhancement scanning showed that the lesion was enhanced (Figure 2). No evidence of distant metastases was revealed upon head, chest, and abdominal CT.
Figure 2.
Computed tomography evaluation of Case 2. A: Axial computed tomography image showing an irregular soft tissue density in the vagina; B: Enhanced image showing non-uniform lesion enhancement; C: Sagittal image showing partial lesion protruding into the vagina.
TREATMENT
Case 1
During surgery to remove the mass, a gray-white mass measuring approximately 4 cm × 3 cm × 2 cm was excised from between the abdominis obliquus internus musculus and the abdominal transverse muscle. The mass was tough and unencapsulated, with a basal portion adhered to the abdominal transverse muscle. There was no invasion of the abdominal transverse muscle membrane. Incision of the tumor revealed calcification and a yellow, turbid liquid.
Case 2
During the surgery, a mass protruding into the vulva about 5 cm × 3 cm × 3 cm in size was visualized, presenting as red, irregular, soft tissue, leading inward to the vagina but clearly separated from the cervix with no cervical invasion. The tumor filled the vaginal orifice, invaded the hymen and urethral orifice, and covered the external urethral orifice. Partial resection was conducted due to the many invasion sites of tumor and the difficulty of complete resection. The operation was completed without complication.
FINAL DIAGNOSIS
Case 1
Histologic examination revealed small round cells with a high nuclear cytoplasmic ratio. Immunohistochemistry showed that the tumor cells were positive for CD99 and Synaptophysin (Syn), and negative for CK, LCA, chromogranin A (CgA), vimentin, CD3, CD20, and CD56 (Figure 3). EWSR1 gene rearrangement was positively confirmed by fluorescent in situ hybridization (FISH) analysis (Figure 4). The patient was thereby diagnosed with pPNET.
Figure 3.
Case 1. A and B: Small round cells with large nuclei and little cytoplasm showing the characteristic perivascular rosette of primitive neuroectodermal tumors (H and E, 100× and 200×); C: Tumor cells positive for CD99 (DAB, 200×); D: Diffuse staining for Syn (DAB, 200×).
Figure 4.
Fluorescent in situ hybridization in Case 1. The 5'-terminus of the EWSR1 gene was labeled with red dye, and the 3'-terminus labeled with green dye. Results showed that the red and green signals were isolated from tumor cells, suggesting translocation of the EWSR1 gene (DAPI, 1000×).
Case 2
Pathological examination demonstrated round and oval tumor cells in the shape of flakes or beams; cells were abnormal, and mitosis was apparent. On immunohistochemical analysis, the tumor cells were positive for CD99 and vimentin and negative for desmin, CgA, Syn, CD56, NSE, CK, LCA, myogenin, and actin. FISH analysis revealed heterotopic changes in the EWSR1 gene (Figure 5). The diagnosis was pPNET. As the patient's sections were loaned out, we are unable to show the pathology images.
Figure 5.
Fluorescent in situ hybridization in Case 2. The separation of the red and green signals indicates that the EWSR1 gene is translocated. Red arrows indicate the translocation change of the EWSR1 gene (DAPI, 1000×).
OUTCOME AND FOLLOW-UP
Case 1
One week after the operation, the patient began treatment with an alternating VDC/IE regimen for eight cycles of chemotherapy. VDC was provided as vincristine (1.5 mg/m2), doxorubicin (37.5 mg/m2), and cyclophosphamide (1.2 g/m2); IE was ifosfamide (1.0 g days 1, 2, 3, 4, and 5) and etoposide (50 mg days 1, 2, 3, 4, and 5). The patient was followed for 66 mo with no relapse or metastatic disease, and currently attends school and functions normally. No treatments followed the eight cycles of chemotherapy.
Case 2
The patient received chemotherapy via an alternating VAC/IE regimen three weeks after surgery, as follows: VAC was vincristine (1 mg on day 1), pirarubicin (40 mg on day 1), and cyclophosphamide (700 mg on day 1); IE was ifosfamide (1.0 g on days 1, 2, 3, 4, and 5) and etoposide (50 mg on days 1, 2, 3, 4, and 5). The patient left treatment after two cycles of chemotherapy and died at home 14 months after diagnosis, approximately six months after surgery. Cause of death was spread of the tumor throughout the body.
DISCUSSION
PNETs originate from the neuroectoderm and are highly malignant tumors with small round cells, first elucidated by Hart and Earle[5] in 1973. In 2013, the WHO pathological classification system updated and removed any differences between PNET and Ewing sarcoma because they demonstrate the same biological characteristics, reclassifying PNET as a member of Ewing sarcoma tumor family[6]. Campbell et al[7] compared the epidemiology, clinical features, and outcomes of patients with 3575 reported cases of Ewing sarcoma or PNET over a 40-year period, and proved that the classification by WHO was correct. PNET is known to be a rare disease that rarely occurs in the skin or subcutaneous tissue[8]. We reviewed the literature published over an extended period, and found only 13 cases of PNET in the abdominal wall (Table 2) with an average patient age of 27.5 years and maximum age of 65 years, with the youngest being 2 years. The abdominal wall PNET that we examined in this study is the second youngest of all such patients. Only 37 cases of PNET in the vulva were uncovered (Table 3) with an average age of 24.4 years and a maximum age of 65 years. The patient with vulvar PNET examined for this study was only 40 months of age and is the youngest case of vulvar PNET presently reported.
Table 2.
Thirteen reported cases of primitive neuroectodermal tumor in the abdominal wall
| Ref. | Age | Sex | Size (cm) | Immuno-histo-chemistry | Molecular/cyto-genetic analysis | MATOD | Therapy | Follow-up | Relapse | Outcome |
| Roncati et al[9], 2015 | 45 yr | M | 1.5 | NSE, CD99 (+) | FISH (+) | NA | NA | NA | NA | NA |
| Riccardi et al[18], 2010 | 15 yr | M | 2.5-3.0 | CD99, NB84a, vimentin (+) | FISH, RT-PCR (-) | No | S + Ch | NA | NA | NA |
| Betal et al[19], 2009 | 61 yr | F | NA | CD99, CD56, cytokeratins, S100 (+) | NA | No | S + Ch (6 cycles of VDC) | NA | No | NA |
| Taylor et al[8], 2000 | 33 mo | M | 3.5 × 3.5 × 2.5 | CD99 (+) | NA | No | S | 10 yr | No | Survival |
| Soma et al[20], 2015 | 21 yr | F | 6 × 4 | CD 99, vimentin (+) | NA | No | S + Ch (VDC) | 6 mo | No | Survival |
| Somers et al[21], 2004 | 16 yr | F | 1.5 | CD99, CD56, S100 (+) | FISH and RT-PCR (-) | No | Before metastasis: S + Ch (6 cycles of VAC); after metastasis: S + RT + CT (1 cycle of IE + CBP) | NA | Yes | Death |
| Savić et al[22], 2017 | 15 yr | M | 3.8 × 2.6 × 3.7 | CD99, vimentin, synaptophysin (+) | RT-PCR (+) | No | S + Ch (VAC) + RT | 8m | No | Survival |
| Askri et al[23], 2008 | 35 yr | F | 6.5 × 4 | CD99 (+) | NA | NA | S + Ch (3 cycles of VDC ) | NA | NA | NA |
| Gurria et al[24], 2011 | 23 yr | F | 14 × 10 × 7 | CD99, PAS (+) | NA | No | S + RT + Ch (VAC/IE) | 8 mo | No | Survival |
| Aydinli et al[25], 2009 | 65 yr | M | 5 | CD99 (+) | NA | NA | S + Ch (6 cycles of VDCE) | 1 yr | No | Survival |
| Wang et al[26], 2017 | 21 yr | F | 5 × 4 | CD99, vimentin, NSE (+) | NA | Yes | S + RT + Ch (VAC) | 7 mo | Yes | Death |
| Zhan et al[27], 2012 | 2 yr | F | 5.0 × 3.8 × 5.1 | positive CD99, NSE, Ki67 (+) | NA | No | S + Ch (CTX + ADM + DDP) | 1 yr | No | Survival |
| Present case, 2019 | 66 mo | F | 3.4 × 6.1 × 2 | CD99, Syn (+) | NA | No | S + Ch (VDC/IE) | 66 mo | No | Survival |
+: Positive; MATOD: Metastatic at the time of diagnosis; S: Surgery; Ch: Chemotherapy; RT: Radiotherapy; NA: Not available; FISH: Fluorescence in situ hybridization; RT-PCR: Reverse transcription-PCR; VAC: Vincristine and actinomycin D, cyclophosphamide; IE: Ifosfamide and etoposide; VDC: Vincristine, doxorubicin, and cyclophosphamide.
Table 3.
Thirty-seven reported cases of primitive neuroectodermal tumor in the vulva
| Ref. | Age | Size (cm) | Immunohistochemistry | Molecular/ cytogenetic analysis | MATOD | Therapy | Follow-up | Relapse | Outcome |
| Present case, 2019 | 40 mo | 3.3 × 5 × 2.5 | Vimentin, CD99 (+) | NA | No | S + Ch (VAC/IE) | 14 mo | YES | Death |
| Pei et al[28], 2018 | 33 yr | 0.5 × 0.5 | PAS, CD99, vimentin (+) | EWSR1 gene (+) | Yes | S + RT + Ch | 15 mo | NO | Survival |
| Chiang et al[29], 2017 | 65 yr | NA | CD99, NSE, SYN, CD56, S100, FLI-1 (+) | FISH (+) | NA | NA | NA | NA | NA |
| Kakoti et al[30], 2017 | 16 yr | 15 × 10 | CK, vimentin, CD99, FLI-1 (+) | NA | No | Ch (VDC/IE) | NA | NA | Death |
| Tunitsky-Bitton et al[31], 2015 | 15 yr | 5 | CD99 (+) | RT-PCR (+) | No | S + Ch (VDC/IE 14 cycles) | 29 | NO | Survival |
| Huang et al[32], 2015 | 20 yr | 8 × 10 × 10 | CD99, vimentin, NSE (+) | NA | Yes | S | 2 wk | NA | Death |
| Rekhi et al[33], 2015 | 10 yr | 12 × 8 | MIC2/CD99, FLI-1(+) | FISH (+) | NA | S + Ch (VIME 5 cycles) | 18 mo | YES | Survival |
| Narayanan et al[34], 2014 | 17 yr | 3 × 2 × 2 | MIC2 (+) | NA | NA | S + RT + Ch (VAC/IE) | 22 mo | YES | Death |
| Matsuda et al[35], 2014 | 60 yr | NA | MIC-2, synaptophysin, NSE, neurofilament antibodies (+) | NA | No | S + RT + Ch (VACI) | 48 mo | YES | Survival |
| Xiao et al[36], 2014 | 20 yr | NA | CD99, NSE (+) | NA | NA | Not done | NA | NA | Death |
| Xiao et al[36], 2014 | 36 yr | NA | CD99, Syn, NSE (+) | NA | NA | S + Ch (PEI, 4 cycles; PAC, 2 cycles) | 13 mo | YES | Death |
| Che et al[37], 2013 | 37 yr | 5 × 3.5 × 3; 3 × 2 × 1.2 | CD99, vimentin, FLI-1 (+) | NA | NA | S + Ch (VAC) | 12 mo | YES | Survival |
| Tang et al[38], 2012 | 17 yr | 5.5 × 5 × 5 | CD99 and FLI-1 (+) | NA | NA | S | NA | NA | LS |
| Tang et al[38], 2012 | 25 yr | 2 × 2 × 2 | CD99 and FLI-1 (+) | NA | NA | S | NA | NA | LS |
| Yang et al[39], 2012 | 20 yr | 20 × 10 × 7 | CD99 and NSE (+) | RT-PCR (+) | Yes | Ch | NA | NA | Death |
| Kelling et al[40], 2012 | 18 yr | 1.7 × 9 × 1.5 | CD99 and vimentin (+) | RT-PCR (+) | Yes | S + RT + Ch | 3 mo | NA | Survival |
| Anastasiades et al[41], 2012 | 28 yr | 3 | CD99 (+) | NA | No | S + RT + Ch | 12 mo | YES | Death |
| Dong et al[42], 2012 | 20 yr | 11 × 7.7 × 6.5 | CD99, NSE, CK (AE1/AE3) and Syn (+) | NA | Yes | S | 3 mo | NA | Death |
| Dong et al[42], 2012 | 12 yr | 3.1 | CD99, NSE, CK (AE1/AE3) and Syn (+) | NA | Yes | NA | 13 mo | NA | Survival |
| Dong et al[42], 2012 | 35 yr | NA | CD99 and NSE (+) | NA | Yes | S + Ch | 20m | YES | Death |
| Halil et al[43], 2011 | 14 yr | NA | CD99 (+) | NA | NA | S + RT + Ch | 9 mo | YES | Death |
| Boldorini et al[44], 2010 | 52 yr | NA | CD99, CK(AE1/AE3) and vimentin (+) | FISH (+) | No | S + RT + Ch(VAI/IE) | 12 mo | NO | Survival |
| Dadhwal et al[45], 2010 | 20 yr | 20 × 15 × 10 | CD99 (+) | NA | Yes | S | 20 d | YES | Death |
| Cetiner et al[46], 2009 | 23 yr | 4 × 4 | CD99 and vimentin (+) | RT-PCR (+) | Yes | S + R + Ch (VDC/IE) | 7 yr | NO | Survival |
| Cetiner et al[46], 2009 | 29 yr | NA | CD99 and vimentin (+) | RT-PCR (-) | NA | S + Ch | 51 mo | NO | Survival |
| Fong et al[47], 2008 | 17 yr | 0.7 × 0.6 × 0.2; 2.1 × 1.7 × 1.5 | CD99 and Fli-1 (+) | RT-PCR (+) | No | S + Ch (VDC) | 48 mo | NO | Survival |
| McCluggage et al[48], 2007 | 19 yr | 4 | CD99 and FLI-1 (+) | RT-PCR and FISH (-) | NA | S + Ch | NA | NA | NA |
| McCluggage et al[48], 2007 | 20 yr | 6.5 | CD99 and FLI-1 (+) | FISH (+) | NA | S | NA | NA | Death |
| McCluggage et al[48], 2007 | 40 yr | 3 | CD99, FLI-1 (+) | FISH (+) | NA | S + Ch | 12 mo | NA | Survival |
| Moodley et al[49], 2005 | 26 yr | 4 × 5 | NA | NA | No | Ch + RT | NA | YES | Death |
| Takeshima et al[50], 2001 | 45 yr | 4 (at recurrence) | Neuron specific enolase, vimentin, HBA 71 (+) | NA | No | S | 1 yr (at recurrence) | YES | Survival |
| Lazure et al[51], 2001 | 15 yr | 20 | CD99 (+) | RT-PCR (+) | NA | S + Ch | 7 mo | NO | Survival |
| Vang et al[52], 2000 | 28 yr | 0.9 | CD99 (+) | RT-PCR (+) | NA | S + Ch | 18 mo | NA | Survival |
| Paredes et al[53], 1995 | 29 yr | 5 | Vimentin (+) | NA | NA | S + RT + Ch (6 cycles of VAC) | 8 mo | NO | Survival |
| Nirenberg et al[54], 1995 | 20 yr | 12 | PAS (+) | NA | NA | S + RT + Ch (VA) | 10 mo | YES | Death |
| Scherr et al[55], 1994 | 10 yr | 6.5 × 5.5 × 2.0 | HBA-71 (+) | NA | No | S | NA | NA | NA |
| Habib et al[56], 1992 | 23 yr | 1.5 | CK, EMA (+) | NA | NA | NA | NA | NA | NA |
+: Positive; MATOD: Metastatic at the time of diagnosis; S: Surgery; Ch: Chemotherapy; RT: Radiotherapy; NA: Not available; FISH: Fluorescence in situ hybridization; RT-PCR: Reverse transcription-PCR; PEI: Cisplatin, ifosfamide, and etoposide; PAC: Cisplatin, cyclophosphamide, and actinomycin D; VAC: Vincristine, actinomycin D, and cyclophosphamide; IE: Ifosfamide and etoposide; VDC: Vincristine, doxorubicin, and cyclophosphamide; VIDE: Vincristine, ifosfamide, doxorubicin, and etoposide; VIME: Vincristine, ifosfamide, mesna, and etoposide; LS: Loss to follow-up.
The mechanisms that underlie PNETs are unclear. Roncati et al[9] found a biological accumulation of copper, chromium, aluminum, and bismuth in an abdominal wall PNET patient who had been applying abdominal skin cream for a long period; interestingly, tests revealed that the cream contained aluminum and bismuth, suggesting that these metals were acting as intracellular carcinogens. The high magnetic resistance of bismuth might have interfered with cell electromagnetic equilibrium, and particularly impacted the electromagnetic balance found in neurons containing ES/PNET cells[10]. It is worth considering whether heavy metals such as aluminum and bismuth cause the formation of the PNET.
PNET differential diagnosis mainly involves the exclusion of other similar round blue cell tumors, as PNETs and those tumors have some of the same morphological characteristics, which can easily lead to misdiagnosis. Immunohistochemical, reverse transcription-polymerase chain reaction (RT-PCR), and fluorescence in situ hybridization (FISH) analyses are necessary for accurate diagnosis. CD99 and vimentin are the most commonly used immunomarkers in the diagnosis of PNET. Both of our cases were CD99-positive, and although Case 1 was negative for vimentin, we did show the translocation of the EWSR1 gene in tumor cells via FISH analysis, confirming the PNET diagnosis. Because both patients' FISH analyses showed EWSR1 gene rearrangement, there was no need to test them by RT-PCR. In recent years, scientists have found high expression levels of cyclin D1 in PNET. A study comparing the expression patterns of cyclin D1 in ES/PNET and rhabdomyosarcoma demonstrated that all PNET patients showed a strong, diffuse, nuclear immune response to cyclin D1, while no expression of cyclin D1 was detected in any patients with rhabdomyosarcoma. Cyclin D1 is another highly sensitive immunological marker for the diagnosis of ES/PNET alongside CD99 and FLI-1 markers, although it is not recommended as an independent diagnostic marker thereof[10].
A main characteristic of PNET[11] is the detection of t (11; 22) (q24; q12) translocation, wherein an EWS-FLI-1 fusion gene is formed. In recent years, scientists have discovered other genetic changes in PNETs. Some researchers found histological and immunohistochemical tumor features consistent with those of PNET in a 15-year-old boy, but no EWSR1 rearrangement was found by FISH and RT-PCR molecular studies. Instead, a translocation of the long arms of chromosomes 18 and 19 was uncovered, resulting in a chromosome t (18; 19) (q23; q13.2) transposition. In addition, some researchers discovered that another translocation in chromosome t (4; 22) (q31n; q12) led to EWSR1-SMARCA5 gene fusion in patients with PNET.
Because PNET is rare and little research has been performed on it, there is not enough epidemiological and evidence-based medical evidence to derive treatment standards. At present, the main chemotherapeutic drugs used in PNET are vincristine (V), doxorubicin (D), cyclophosphamide (C), actinomycin-D (A), ifosfamide (I), and etoposide (E); our methods of treatment are based on these drugs. Regimens of VAC/IE or VDC/IE are commonly used in chemotherapy, but their chemotherapeutic effects are not always satisfactory. Takigami et al[12] reported on a lung PNET case that had been treated with VDC/IE chemotherapy for 5 mo before surgery; after the tumor was resected, it recurred 1.5 mo later. In this case, adriamycin was replaced with actinomycin-D due to a cumulative dose of doxorubicin near 500 mg/m2. Unfortunately, the tumor grew bigger, and the patient began taking pazopanib (800 mg/d); the tumor shrank four weeks later, and the patient survived for five months, eventually dying due to disease spread. When standard treatment fails, pazopanib can be another effective option. A randomized study of 120 cases of metastatic bone Ewing sarcoma and PNET in the Children's Cancer Group and the Pediatric Oncology Group in the United States showed that adding ifosfamide and etoposide to standard therapy does not improve outcomes for patients with bone Ewing sarcoma or PNET with metastases at diagnosis[13], although the addition of ifosfamide and etoposide to standard therapy can improve the prognosis of patients with no metastatic disease at the time of diagnosis[14]. The survival time of Ewing sarcoma patients was determined by whether the tumor had metastasized or not; a five-year survival rate was 33% in the case of tumor metastasis and 70% in those without metastasis. Extraosseous origin was an adverse prognostic factor for Ewing sarcoma[15-17]. A retrospective study of 975 patients with Ewing sarcoma in the European Intergroup Cooperative Ewing Sarcoma Study Group indicated that the presence of metastasis at diagnosis, exceptionally large tumors (volume ≥ 200 mL or largest diameter ≥ 8 cm), primary tumors located in the axial skeleton (especially the pelvis), and a histological response of less than 100% were strongly associated with poor survival in Ewing sarcoma[15]. The two cases we report on here were extraosseous in origin, but neither of them had metastasis at diagnosis, nor were they large tumors located in the axial skeleton. Case 1 completed standardized treatment and survived without progression for 66 mo; we consider that this is a successful treatment. Case 2, however, died 14 mo after diagnosis; extraosseous origin might have been an adverse prognostic factor, but the most likely cause of death was cessation of treatment.
In this paper, two extremely rare cases of PNET presenting a primary location in the abdominal wall and vulva are presented. A limitation of this report is the small number of cases reported on, which is due to the rarity of PNETs. We also only analyzed the specific diagnostic methods, pathological results, treatment plans, and follow-up. As survival analysis of the disease and prognostic indicators were lacking, specific tumor staging and treatment criteria could not be provided. When diagnosing PNET, immunohistochemistry is often not enough to provide us with satisfactory diagnostic information, and follow-up by FISH or RT-PCR can make the diagnosis more persuasive. For the treatment of PNET, we chose mass resection by surgery when conditions permit, followed by alternating VAC/IE or VDC/IE chemotherapy. We are increasing our efforts to collect more case data and improve diagnostic parameters for the treatment of PNET. Despite these limitations, we hope that our case report will help inform future clinical work.
Footnotes
Informed consent statement: The patients provided informed consent for publication of the case.
Conflict-of-interest statement: The authors of this manuscript have no conflicts of interest to disclose.
CARE Checklist (2016) statement: The manuscript was revised according to the CARE checklist.
Manuscript source: Unsolicited manuscript
Peer-review started: July 1, 2019
First decision: July 31, 2019
Article in press: September 12, 2019
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lim SC S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Liu JH
Contributor Information
Qiong-Qian Xu, Department of Pediatric Surgery, the First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Wen-Wen Xing, Department of Pathology, the First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Gang Chen, Department of Pathology, the First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Yi-Wu Dang, Department of Pathology, the First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Yi-Ge Luo, Department of Pediatric Surgery, the First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Peng Chen, Department of Pediatric Surgery, the First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Song-Wu Liang, Department of Pediatric Surgery, the First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Jia-Bo Chen, Department of Pediatric Surgery, the First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China. cjb1205@163.com.
References
- 1.Celli R, Cai G. Ewing Sarcoma/Primitive Neuroectodermal Tumor of the Kidney: A Rare and Lethal Entity. Arch Pathol Lab Med. 2016;140:281–285. doi: 10.5858/arpa.2014-0367-RS. [DOI] [PubMed] [Google Scholar]
- 2.Smoll NR. Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs) Cancer. 2012;118:1313–1322. doi: 10.1002/cncr.26387. [DOI] [PubMed] [Google Scholar]
- 3.Windfuhr JP. Primitive neuroectodermal tumor of the head and neck: incidence, diagnosis, and management. Ann Otol Rhinol Laryngol. 2004;113:533–543. doi: 10.1177/000348940411300705. [DOI] [PubMed] [Google Scholar]
- 4.Park JY, Lee S, Kang HJ, Kim HS, Park SY. Primary Ewing's sarcoma-primitive neuroectodermal tumor of the uterus: a case report and literature review. Gynecol Oncol. 2007;106:427–432. doi: 10.1016/j.ygyno.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 5.Hart MN, Earle KM. Primitive neuroectodermal tumors of the brain in children. Cancer. 1973;32:890–897. doi: 10.1002/1097-0142(197310)32:4<890::aid-cncr2820320421>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.WHO. 2013. International Classification of Disease for Oncology (ICD-O), World Health Organization, Geneva, Switzerland, 3rd edition. [Google Scholar]
- 7.Campbell K, Shulman D, Janeway KA, DuBois SG. Comparison of Epidemiology, Clinical Features, and Outcomes of Patients with Reported Ewing Sarcoma and PNET over 40 Years Justifies Current WHO Classification and Treatment Approaches. Sarcoma. 2018;2018:1712964. doi: 10.1155/2018/1712964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor GB, Chan YF. Subcutaneous primitive neuroectodermal tumour in the abdominal wall of a child: long-term survival after local excision. Pathology. 2000;32:294–298. [PubMed] [Google Scholar]
- 9.Roncati L, Gatti AM, Capitani F, Barbolini G, Maiorana A, Palmieri B. Heavy Metal Bioaccumulation in an Atypical Primitive Neuroectodermal Tumor of the Abdominal Wall. Ultrastruct Pathol. 2015;39:286–292. doi: 10.3109/01913123.2015.1013655. [DOI] [PubMed] [Google Scholar]
- 10.Cifra M, Fields JZ, Farhadi A. Electromagnetic cellular interactions. Prog Biophys Mol Biol. 2011;105:223–246. doi: 10.1016/j.pbiomolbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Akazawa M, Saito T, Ariyoshi K, Okadome M, Yokoyama R, Taguchi K. Adjuvant chemotherapy for a primitive neuroectodermal tumor of the uterine corpus: A case report and literature review. J Obstet Gynaecol Res. 2018;44:2008–2015. doi: 10.1111/jog.13753. [DOI] [PubMed] [Google Scholar]
- 12.Takigami A, Yamasawa H, Kurosaki A, Sakamoto N, Onuki T, Mato N, Tetsuka K, Endo S, Niki T, Bando M, Hagiwara K. Pazopanib Confers a Progression-free Survival in a Patient with Ewing's Sarcoma/Primitive Neuroectodermal Tumor of the Lung. Intern Med. 2019;58:1335–1339. doi: 10.2169/internalmedicine.1549-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miser JS, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, Donaldson SS, Moore S, Rausen AR, Vietti TJ, Grier HE. Treatment of metastatic Ewing's sarcoma or primitive neuroectodermal tumor of bone: evaluation of combination ifosfamide and etoposide--a Children's Cancer Group and Pediatric Oncology Group study. J Clin Oncol. 2004;22:2873–2876. doi: 10.1200/JCO.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 14.Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, Donaldson SS, Moore S, Rausen AR, Vietti TJ, Miser JS. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 15.Cotterill SJ, Ahrens S, Paulussen M, Jürgens HF, Voûte PA, Gadner H, Craft AW. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol. 2000;18:3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 16.Paulussen M, Ahrens S, Burdach S, Craft A, Dockhorn-Dworniczak B, Dunst J, Fröhlich B, Winkelmann W, Zoubek A, Jürgens H. Primary metastatic (stage IV) Ewing tumor: survival analysis of 171 patients from the EICESS studies. European Intergroup Cooperative Ewing Sarcoma Studies. Ann Oncol. 1998;9:275–281. doi: 10.1023/a:1008208511815. [DOI] [PubMed] [Google Scholar]
- 17.Jiang S, Wang G, Chen J, Dong Y. Comparison of clinical features and outcomes in patients with extraskeletal vs skeletal Ewing sarcoma: an SEER database analysis of 3,178 cases. Cancer Manag Res. 2018;10:6227–6236. doi: 10.2147/CMAR.S178979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riccardi GF, Stein C, de la Roza G, Damron TA. Newly described translocation (18;19)(q23;q13.2) in abdominal wall soft-tissue tumor resembling Ewing sarcoma/primitive neuroectodermal tumor. Cancer Genet Cytogenet. 2010;201:1–5. doi: 10.1016/j.cancergencyto.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Betal D, Shaygi B, Babu R, Jamil K, Sainsbury RJ. Primitive Neuroectodermal Tumour (PNET) in subcutaneous abdominal wall: a case report. Int Semin Surg Oncol. 2009;6:10. doi: 10.1186/1477-7800-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soma S, Shetty SK, Bhat S. PNET of the Abdominal Wall: A Rare Presentation. J Clin Diagn Res. 2015;9:XD01–XD02. doi: 10.7860/JCDR/2015/14562.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somers GR, Shago M, Zielenska M, Chan HS, Ngan BY. Primary subcutaneous primitive neuroectodermal tumor with aggressive behavior and an unusual karyotype: case report. Pediatr Dev Pathol. 2004;7:538–545. doi: 10.1007/s10024-004-2024-6. [DOI] [PubMed] [Google Scholar]
- 22.Savić Đ, Đ uričić SM, Miličković M, Đokić D, Grujić B, Vukadin M, Samardžija G. Extraskeletal Ewing sarcoma in the anterior abdominal wall. Srp Arh Celok Lek. 2018;146:207–210. [Google Scholar]
- 23.Askri A, Farhat LB, Ghariani B, Rabeh A, Dali N, Said W, Hendaoui L. Extraskeletal Ewing sarcoma of the abdominal wall. Cancer Imaging. 2008;8:156–158. doi: 10.1102/1470-7330.2008.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurria JP, Dasgupta R. Rhabdomyosarcoma and Extraosseous Ewing Sarcoma. Children (Basel) 2018;5 doi: 10.3390/children5120165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aydinli B, Ozturk G, Yildirgan MI, Polat KY, Basoglu M, Gundogdu C, Kantarci M, Akgun M. Extraskeletal Ewing's sarcoma in the abdominal wall: a case report. Acta Oncol. 2006;45:484–486. doi: 10.1080/02841860500400987. [DOI] [PubMed] [Google Scholar]
- 26.Wang CM, Zeng JT, Zhang YL. Primary neuroectodermal tumor of abdominal wall: a case report and related literature review. Chongqing Yixue Zazhi. 2017;46:2408–2409. [Google Scholar]
- 27.Zhan JH, Luo XR, Guan ZW, Hu XL, Bao GQ, Liu Y. A case of primitive neuroectodermal tumor in the abdominal wall. Zhonghua Xiao’er Waike Zazhi. 2012;33:719–720. [Google Scholar]
- 28.Pei Y, Zhang BZ, Xu GC, Xiao JJ. A case of vulva with pelvic metastasis of Ewing sarcoma and review of literature. Shanxi Yike Daxue Xuebao. 2018;49:325–327. [Google Scholar]
- 29.Chiang S, Snuderl M, Kojiro-Sanada S, Quer Pi-Sunyer A, Daya D, Hayashi T, Bosincu L, Ogawa F, Rosenberg AE, Horn LC, Wang L, Iafrate AJ, Oliva E. Primitive Neuroectodermal Tumors of the Female Genital Tract: A Morphologic, Immunohistochemical, and Molecular Study of 19 Cases. Am J Surg Pathol. 2017;41:761–772. doi: 10.1097/PAS.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakoti LM, Sharma JD, Kataki AC, Barmon D. Primary Ewing Sarcoma of Vulva: A Case Report and a Review of Literature. Indian J Gynecol Oncol. 2017;15:15. [Google Scholar]
- 31.Tunitsky-Bitton E, Uy-Kroh MJ, Michener C, Tarr ME. Primary Ewing Sarcoma Presenting as a Vulvar Mass in an Adolescent: Case Report and Review of Literature. J Pediatr Adolesc Gynecol. 2015;28:e179–e183. doi: 10.1016/j.jpag.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Huang YR, Kang JL, Li QT. Case of cranial Metastasis of primitive Neuroectodermal tumor of vulva. Guangdong Yixue. 2015;36:3096. [Google Scholar]
- 33.Rekhi B, Chinnaswamy G, Vora T, Shah S, Rangarajan V. Primary Ewing sarcoma of vulva, confirmed with molecular cytogenetic analysis: A rare case report with diagnostic and treatment implications. Indian J Pathol Microbiol. 2015;58:341–344. doi: 10.4103/0377-4929.162869. [DOI] [PubMed] [Google Scholar]
- 34.Narayanan G, Rajan V, Puthusseri J, Kattoor J, Soman LV. Primitive Neuroectodermal Tumor of the Vulva in an Adolescent Girl. World J Oncol. 2014;5:220–222. doi: 10.14740/wjon819w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuda M, Ichimura T, Kasai M, Murakami M, Hoshi M, Kawamura N, Sumi T. Primitive neuroectodermal tumor originating in the vulva: A case report. Oncol Lett. 2014;8:187–189. doi: 10.3892/ol.2014.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao C, Zhao J, Guo P, Wang D, Zhao D, Ren T, Yang J, Shen K, Lang J, Xiang Y, Cui Q. Clinical analysis of primary primitive neuroectodermal tumors in the female genital tract. Int J Gynecol Cancer. 2014;24:404–409. doi: 10.1097/IGC.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 37.Che SM, Cao PL, Chen HW, Liu Z, Meng D. Primary Ewing's sarcoma of vulva: a case report and a review of the literature. J Obstet Gynaecol Res. 2013;39:746–749. doi: 10.1111/j.1447-0756.2012.02019.x. [DOI] [PubMed] [Google Scholar]
- 38.Tang X, Wang P, He Y, Yang F, Li L, Wang H, Wang QL, Yao XY, Yang KX. [Primitive neuroectodermal tumor in female genital tract: a clinicopathologic study] Zhonghua Bing Li Xue Za Zhi. 2012;41:729–732. doi: 10.3760/cma.j.issn.0529-5807.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Guo Q, Yang Y, Zhang J, Lang J, Shi H. Primary vulvar Ewing sarcoma/primitive neuroectodermal tumor: a report of one case and review of the literature. J Pediatr Adolesc Gynecol. 2012;25:e93–e97. doi: 10.1016/j.jpag.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Kelling K, Noack F, Altgassen C, Kujath P, Bohlmann MK, Hoellen F. Primary metastasized extraskeletal Ewing sarcoma of the vulva: report of a case and review of the literature. Arch Gynecol Obstet. 2012;285:785–789. doi: 10.1007/s00404-011-2011-x. [DOI] [PubMed] [Google Scholar]
- 41.Anastasiades EN, El Abiad SA, Chouairy CJ. Ewing sarcoma/primitive neuroectodermal tumor (PNET) of the vulva. Case report and review of the literature. J Med Liban. 2012;60:113–116. [PubMed] [Google Scholar]
- 42.Dong YJ, Wang WZ, Zhong DR. Clinicopathological features of primary peripheral primitive neuroectodermal tumors of vulva: a report of 3 case. Linchuang Yu Shiyan Binglixue Zazhi. 2012;28:98–100. [Google Scholar]
- 43.Halil S, Kucuk M, Arvas M, Aydin O, Calay ZZ. Peripheral primitive neuroectodermal tumor (PNET) of the vulva: a case report. Eur J Gynaecol Oncol. 2011;32:117–118. [PubMed] [Google Scholar]
- 44.Boldorini R, Riboni F, Cristina S, Allegrini S, Valentini S, Muscarà M, Ruspa G. Primary vulvar Ewing's sarcoma/primitive neuroectodermal tumor in a post-menopausal woman: a case report. Pathol Res Pract. 2010;206:476–479. doi: 10.1016/j.prp.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Dadhwal V, Bahadur A, Gupta R, Bansal S, Mittal S. Peripheral neuroectodermal tumor of the vulva: a case report. J Low Genit Tract Dis. 2010;14:59–62. doi: 10.1097/LGT.0b013e3181b0f8f3. [DOI] [PubMed] [Google Scholar]
- 46.Cetiner H, Kir G, Gelmann EP, Ozdemirli M. Primary vulvar Ewing sarcoma/primitive neuroectodermal tumor: a report of 2 cases and review of the literature. Int J Gynecol Cancer. 2009;19:1131–1136. doi: 10.1111/IGC.0b013e3181acae33. [DOI] [PubMed] [Google Scholar]
- 47.Fong YE, López-Terrada D, Zhai QJ. Primary Ewing sarcoma/peripheral primitive neuroectodermal tumor of the vulva. Hum Pathol. 2008;39:1535–1539. doi: 10.1016/j.humpath.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 48.McCluggage WG, Sumathi VP, Nucci MR, Hirsch M, Dal Cin P, Wells M, Flanagan AM, Fisher C. Ewing family of tumours involving the vulva and vagina: report of a series of four cases. J Clin Pathol. 2007;60:674–680. doi: 10.1136/jcp.2006.040931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moodley M, Jordaan A. Ewing's sarcoma of the vulva--a case report. Int J Gynecol Cancer. 2005;15:1177–1178. doi: 10.1111/j.1525-1438.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 50.Takeshima N, Tabata T, Nishida H, Furuta N, Tsuzuku M, Hirai Y, Hasumi K. Peripheral primitive neuroectodermal tumor of the vulva: report of a case with imprint cytology. Acta Cytol. 2001;45:1049–1052. doi: 10.1159/000328353. [DOI] [PubMed] [Google Scholar]
- 51.Lazure T, Alsamad IA, Meuric S, Orbach D, Fabre M. [Primary uterine and vulvar Ewing's sarcoma/peripheral neuroectodermal tumors in children: two unusual locations] Ann Pathol. 2001;21:263–266. [PubMed] [Google Scholar]
- 52.Vang R, Taubenberger JK, Mannion CM, Bijwaard K, Malpica A, Ordonez NG, Tavassoli FA, Silver SA. Primary vulvar and vaginal extraosseous Ewing's sarcoma/peripheral neuroectodermal tumor: diagnostic confirmation with CD99 immunostaining and reverse transcriptase-polymerase chain reaction. Int J Gynecol Pathol. 2000;19:103–109. doi: 10.1097/00004347-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Paredes E, Duarte A, Couceiro A, Fernandes D, Alves A, Bastos S. [A peripheral neuroectodermal tumor of the vulva] Acta Med Port. 1995;8:161–163. [PubMed] [Google Scholar]
- 54.Nirenberg A, Ostör AG, Slavin J, Riley CB, Rome RM. Primary vulvar sarcomas. Int J Gynecol Pathol. 1995;14:55–62. doi: 10.1097/00004347-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Scherr GR, d'Ablaing G, 3rd, Ouzounian JG. Peripheral primitive neuroectodermal tumor of the vulva. Gynecol Oncol. 1994;54:254–258. doi: 10.1006/gyno.1994.1204. [DOI] [PubMed] [Google Scholar]
- 56.Habib K, Finet JF, Plantier F, Spatz A, Sfoggia D, Fitoussi F. [Rare lesion of the vulva] Arch Anat Cytol Pathol. 1992;40:158–159. [PubMed] [Google Scholar]