Abstract
Placental fatty acid oxidation (FAO) is impaired and lipid storage is increased in pregnancy states associated with chronic oxidative stress. The effect of acute oxidative stress, as seen in pregnancies complicated with asthma, on placental lipid metabolism is unknown. We hypothesized that induction of acute oxidative stress would decrease FAO and increase esterification. We assessed [3H]-palmitate oxidation and esterification in term placental explants from lean women after exposure to hydrogen peroxide (H2O2) for 4 hours. Fatty acid oxidation decreased 16% and 24% in placental explants exposed to 200 (P = .02) and 400 µM H2O2 (P = .01), respectively. Esterification was not altered with H2O2 exposure. Neither messenger RNA nor protein expression of key genes involved in FAO (eg, peroxisome proliferator-activated receptor α, carnitine palmitoyl transferase 1b) were altered. Adenosine triphosphate (ATP) levels decreased with induction of oxidative stress, without increasing cytotoxicity. Acute oxidative stress decreased FAO and ATP production in the term placenta without altering fatty acid esterification. As decreases in placental FAO and ATP production are associated with impaired fetal growth, pregnancies exposed to acute oxidative stress may be at risk for fetal growth restriction.
Keywords: oxidative stress, placenta, oxidation, mitochondria
Introduction
Placental lipid metabolism strongly affects fetal lipid delivery, which is necessary for fetal growth and neurological development.1–4 Fatty acid oxidation (FAO) produces adenosine triphosphate (ATP) for energy, while fatty acid esterification is required for membrane structure and storage of lipids. Oxidative stress—the imbalance of reactive oxygen species (ROS) and antioxidant defenses5–7—has been shown to impair metabolic processes (ie, FAO, ATP production) that take place in human mitochondria.7 In hepatocytes, induction of oxidative stress decreases FAO due to inhibition of mitochondrial function via regulation of peroxisome proliferator-activated receptor α (PPAR-α) and carnitine palmitoyl transferase 1b (CPT-1b).8,9 In the placenta, oxidative stress secondary to a hypoxic environment has been associated with decreased mitochondrial electron transport chain expression,10 suggesting that oxidative stress directly impairs placental mitochondrial function.
While both oxidative stress and alterations in lipid metabolism have been observed in the placenta under chronic adverse pregnancy conditions (ie, obesity, diabetes),11–15 the effect of acute oxidative stress on placental lipid metabolism and energy production is unknown. Several complications expose women to acute oxidative stress in pregnancy. Asthma affects up to 8% of pregnant women16 and, if poorly controlled, can lead to increased small for gestational age infants.16,17 In addition, respiratory decompensation in cases of pneumonia or pyelonephritis can lead to development of acute respiratory distress syndrome in pregnancy.16 Hypoxic events such as these are associated with development of ROS,10 and the effect of these ROS in the acute setting on fatty acid metabolism and energy production in the placenta is not known. We hypothesized that acute oxidative stress would lower FAO and increase esterification by impairing mitochondrial function in the placenta.
Methods
Study Design
Placentas were obtained from uncomplicated lean (prepregnancy body mass index [BMI] < 25 kg/m2) pregnant mothers who delivered at our institution by scheduled, nonlabored cesarean section at term (≥37 weeks’ gestation) after an overnight fast. Placentas were collected after written consent was obtained. Patients were excluded if they were overweight/obese (BMI ≥ 25), smokers, had multifetal gestations, fetal anomalies, active hepatitis/HIV, maternal history of drug use (heroin, cocaine, crack, LSD, or methamphetamines), maternal history of alcohol abuse, maternal rheumatological/chronic inflammatory states, or chorioamnionitis (diagnosed by culture). Approval of this study was obtained by MetroHealth’s institutional review board prior to recruitment.
Placental Explant Preparation
Placental explants were collected within 10 minutes of delivery and cultured as previously described.13 Explants were chosen as the initial model system due to their proximity to whole tissue (eg, maintenance of intercellular connections/communication). They were taken from the maternal face of the placenta, avoiding calcified or underperfused cotyledons. Several full-depth samples were collected randomly from different areas of the placenta encompassing multiple cotyledons; decidua and chorion were removed, and they were placed in warm DPBS. Tissue was then dissected into smaller pieces under sterile conditions and placed into net wells, 4 per well, each explant representing different cotyledons. They were incubated in 1.5 mL of DMEM:F12 media (supplemented with 10% fetal bovine serum [FBS]) at 37°C (95% air, 5% CO2) for 30 minutes.
Oxidative Stress Induction
8-Hydroxy-2’-deoxy guanosine (8OH-dG) production, an indicator of cellular oxidative stress, was measured in the media of untreated placental explants of obese women (BMI ≥ 30 kg/m2; N = 4) after 4-hour incubation. These were considered physiologically relevant levels of oxidative stress to target in the development of our experimental model. To determine the concentrations of hydrogen peroxide (H2O2) needed to induce comparable levels of oxidative stress in placental explants of lean women (N = 5), 8OH-dG production was measured following a 4-hour incubation with a range of H2O2 (0, 200, and 400 μM). The concentration of 8OH-dG was measured using the StressXpress DNA Damage (8-OHdG) ELISA kit (StressMarq Biosciences, Victoria, British Columbia), per manufacturers guidelines, and normalized to milligrams of tissue.
The placentas of lean women treated with 200 μM of H2O2 reached similar 8OH-dG levels as the placentas from obese women (Supplemental Figure 1). For the remaining experiments, 200 and 400 μM of H2O2 were used to determine dose–response effects. Following dissection, explants were initially equilibrated in DMEM:F12 media without H2O2 for 30 minutes. Media was removed and fresh DMEM:F12 media (supplemented with 1% Pen-Strep, and H2O2) was added. Each concentration of H2O2 was repeated in triplicate with final concentrations of 0, 200, and 400 µM. Explants were incubated with H2O2 for 4 hours at 37°C (95% air, 5% CO2).
Placenta Fatty Acid Metabolism
Fatty acid oxidation assays (N = 12) were performed in vitro with placental explants as described previously, with some modifications.18 Briefly, explants from 12 placentas were incubated in culture medium supplemented with 10% FBS and 1% penicillin/streptomycin in the presence of 1.25% fatty acid-free body surface area (BSA), 0.1 mmol/L unlabeled palmitate, and 18 500 Bq/mL [3H]-palmitate for 18 hours at 37°C under 5% CO2. After 18 hours of incubation with [3H]-palmitate as described earlier, the medium was collected, and tritiated water ([3H]2O), representing the oxidized palmitate, was determined by the phase equilibration method.19 Data were calculated as nmol palmitate/mg protein/hour.
Fatty acid esterification in placental explants (N = 12) was determined as described previously.18 As described above, the explants were incubated for 18 hours with [3H]-palmitate. At the end of the incubation period, explants were washed with ice-cold phosphate-buffered saline (PBS) and homogenized in 200 μL of high-performance liquid chromatography-grade acetone. Following total cellular lipid extraction, radioactivity in a 100 μL aliquot representing nonoxidized (largely esterified or stored) palmitate was counted on a Beckman LS3801 Liquid Scintillation Counter (Beckman Coulter, Brea, California). An additional aliquot was used to determine total proteins using the bicinchoninic acid method (Sigma, St Louis, Missouri). Esterification was calculated as nmol palmitate/mg protein/hour. The [9, 10-[3H]]-palmitic acid was from Movarek Biochemicals (Brea, California) and fatty acid-free BSA was from Sigma.
Gene Expression Analysis
To assess the impact of oxidative stress on expression of genes regulating FAO, quantitative polymerase chain reaction (PCR) was performed in N = 12 sets of experiments. Total RNA (N = 12) was extracted from ∼10 mg of placental tissue using TRIzol reagent (Invitrogen, Carlsbad, California) as per manufacturer’s guidelines. Gene expression was monitored by real-time PCR using a Roche thermal cycler (Roche Applied Science, Indianapolis, Indiana) with Lightcycler Fast-start DNA Sybr Green 1 master mix (Roche) as previously described.15 Gene-specific primers were designed to analyze the expression of key genes involved in FAO: CPT-1b and PPAR-α. Primer sequences are shown in Supplemental Table 1. For each primer pair, a standard curve including no template control and unknowns was run in triplicate. The melt curve of the resulting amplicon was analyzed to ensure that a single product was detected for each replicate, and data were analyzed using Roche LightCycler 480 software (version 1.5.1) as described previously.18 L19 was used as a reference gene. Values were expressed as a ratio of the gene of interest:reference gene in each sample.
Western Blotting
To assess the effect of oxidative stress on placental lipid metabolism protein expression, Western blotting was performed in a subset of samples (n = 8). Briefly, 25 μg of total protein was separated on a 10% SDS-polyacrylamide gel and electrotransferred onto a nitrocellulose membrane. After blocking the membrane with 5% milk PBS-T buffer, it was incubated with primary antibody rabbit anti-PPARα (1:500 [sc-9000]; Santa Cruz Biotechnology, Dallas, Texas) or anti-CPT-1b (1:500 [134988]; Abcam, Cambridge, Massachusetts) overnight. After washing with PBS-T, the membrane was exposed to the anti-rabbit secondary antibody (1:2500 to 1:4000 [sc-2004]; Cell Signaling Technology, Danvers, Massachusetts) for 1 hour at room temperature. β-actin was the housekeeping protein used to normalize the data for placenta. Values were expressed as the ratio between protein of interest and housekeeping protein expression. Image was detected using enhanced chemiluminescence (GE Life Sciences, Marlborough, Massachusetts) and densitometry performed using NIH Image J software (version 1.48).
Adenosine Triphosphate Production in Primary Trophoblast Cells
Adenosine triphosphate production was measured in response to H2O2 exposure as an assessment of mitochondrial function. Human trophoblast cells were isolated from an additional 7 placentas of women who met the above inclusion criteria as cumulative ATP production over the exposure period could not be measured in explants using the available technology. Trophoblasts were isolated by sequential trypsin and DNase digestion followed by gradient centrifugation as described previously.18,20 Cells were seeded onto 96-well plates at a density of 150 000 cells/well and cultured overnight in Iscoves’s modified DMEM culture medium supplemented with 10% FBS and 1% penicillin/streptomycin and maintained at 37°C under 5% CO2. Primary trophoblast ATP concentration and cytotoxicity were evaluated using the Promega Mitochondrial ToxGlo Assay (Madison, Wisconsin) per the manufacturer’s specifications. Adenosine triphosphate detection reagent, ATPase inhibitors, and thermostable Ultra-Glo luciferase were added to each well after 4 hours of H2O2 (0-800 µM). Cells were lysed and luminescence was generated proportional to the amount of ATP produced. The plate was placed on an orbital shaker for 5 minutes, and ATP concentration was determined by measuring luminescence with a Perkin Elmer EnSpire 2300 Multilabel Reader (Shelton, Connecticut).
Statistical Analysis
Data in Table 1 are presented as means with interquartile range. All figures are presented as mean ± standard error of the mean unless noted otherwise. Results for the messenger RNA (mRNA) data were expressed in arbitrary units and normalized to the housekeeping gene (L19). Protein quantification of PPAR-α and CPT-1b was expressed in arbitrary units and normalized to β-actin. Friedman repeated-measures analysis of variance and Dunn multiple comparison testing were utilized to assess the effect of increasing H2O2 doses. Statistical analysis was performed using GraphPad Prism (version 7; La Jolla, California). A value of P < .05 was considered statistically significant.
Table 1.
Maternal, Neonatal, and Placental Characteristics.a
| Maternal age, years | 29.0 [11.0] |
| Maternal prepregnancy BMI, kg/m2 | 22.8 [2.4] |
| Gestational weight gain, kg | 14.1 [8.0] |
| Gestational age, weeks | 39 [0] |
| Neonatal birthweight, kg | 3.3 [0.6] |
| Placenta weight, grams | 571 [111] |
Abbreviation: BMI, body mass index.
aData presented as mean [interquartile range], N = 19 except for placental weight (N = 16).
Results
Women recruited had a mean age of 29 years (range: 21-41 years; Table 1), prepregnancy BMI of 22.8 kg/m2; and a gestational weight gain of 14.1 kg (range: 5.9-28.2 kg). The gestational age at delivery was 39 weeks and neonatal birthweight was 3.3 kg (range: 2.7-4.4 kg) in our cohort.
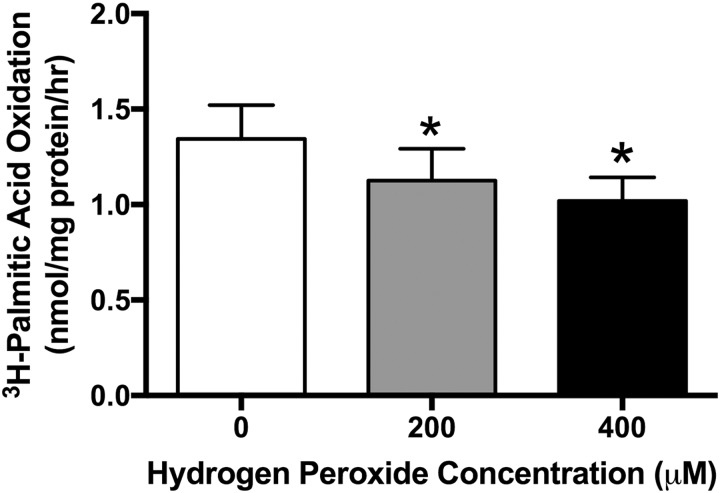
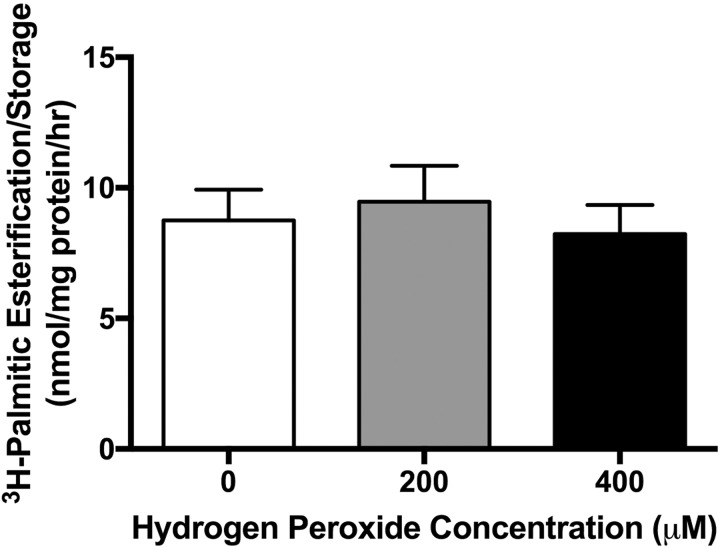
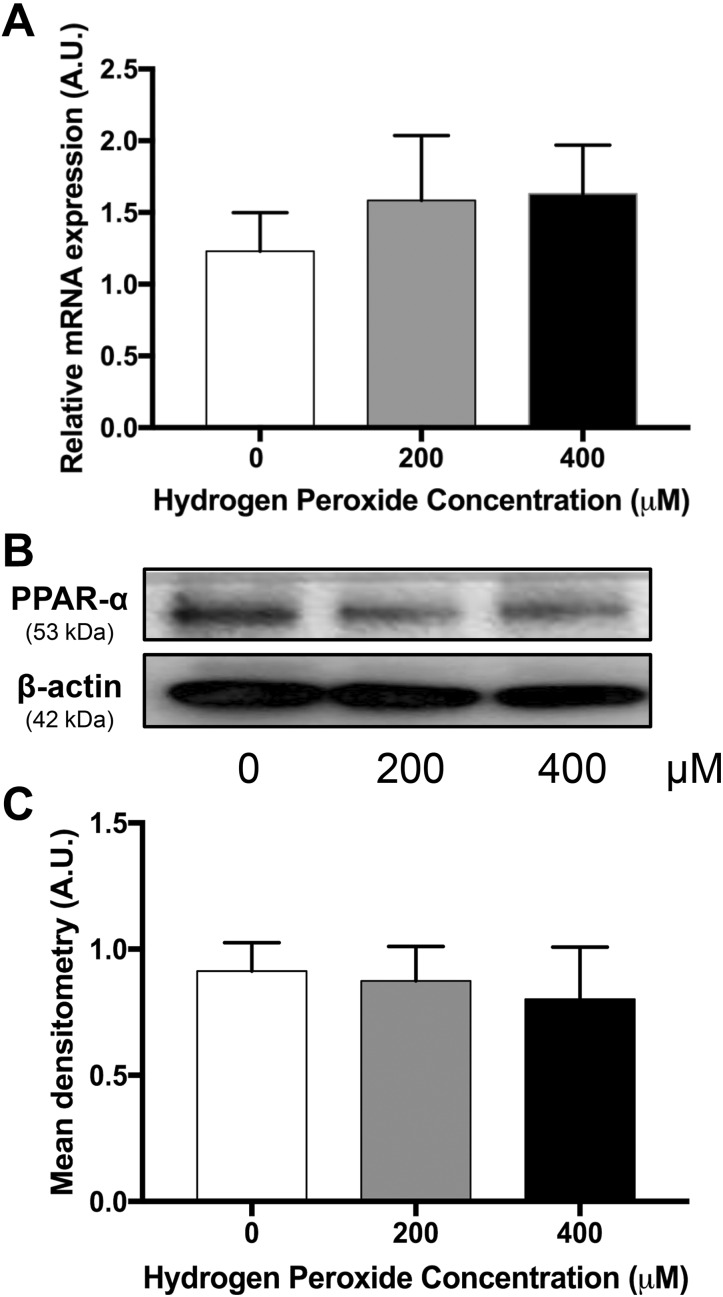
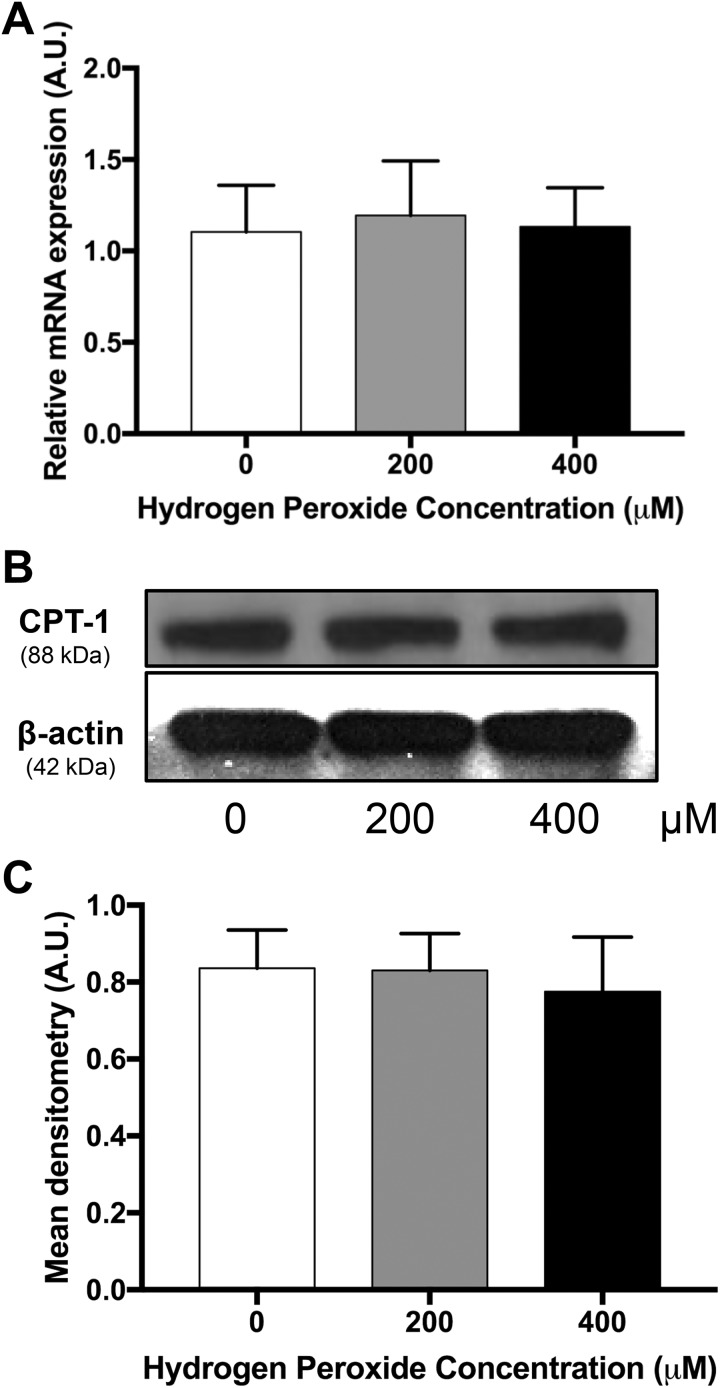
Measured levels of 8OH-dG in placentas from obese women had a mean of 79.38 (standard deviation ± 19.05) (pg/mL)/mg tissue. Mean concentrations of 8OH-dG in placentas from lean women treated with H2O2 were 52.91 (± 13.96), 79.23 (± 23.69) and 64.22 (± 19.04) (pg/mL)/mg tissue for 0, 200, and 400 µM, respectively (Supplemental Figure 1). Hydrogen peroxide concentrations of 200 and 400 µM were chosen to induce oxidative stress in subsequent experiments, as they increased 8OH-dG in placental explants from lean women to physiologically relevant levels. [3H]-palmitate oxidation decreased 16% and 24% in placental explants exposed to 200 (P = .02) and 400 µM H2O2 (P = .01), respectively, following treatment of H2O2 when compared to control (Figure 1). [3H]-palmitate esterification/storage did not change in placental explants treated with 200 µM H2O2 or 400 µM H2O2 when compared to control (Figure 2). Neither PPAR-α mRNA (Figure 3A) and protein (Figure 3B and C) expression nor CPT-1b mRNA (Figure 4A) and protein expression (Figure 4B and C) differed with increasing concentration of H2O2.
Figure 1.
[3H]-Palmitate oxidation in response to oxidative stress (4 hours of H2O2) in placental explants. Data graphed as mean ± standard error of the mean. N = 12. *P < .05 vs 0 µM by Friedman repeated-measures analysis of variance and Dunn multiple comparison testing.
Figure 2.
[3H]-Palmitate esterification/storage in response to oxidative stress (4 hours of H2O2) in placental explants. Data graphed as mean ± standard error of the mean. N = 12. *P < .05 vs 0 µM by Friedman repeated-measures analysis of variance and Dunn multiple comparison testing.
Figure 3.
A, Messenger RNA expression of PPAR-α, a key gene involved in fatty acid oxidation. Data (mean ± standard error of the mean) are expressed as the ratio of PPAR-α: reference gene (L19), N = 11. B, Representative Western blot of PPAR-α in a placenta. C, Densitometry analysis of PPAR-α protein expression, N = 6. *P < .05 vs 0 µM by Friedman repeated-measures analysis of variance and Dunn multiple comparison testing. AU indicates arbitrary units; PPAR-α, peroxisome proliferator-activated receptor α.
Figure 4.
A, Messenger RNA expression of CPT-1b a key gene involved in fatty acid oxidation. Data (mean ± standard error of the mean) are expressed as the ratio of CPT-1b: reference gene (L19), N = 11. B, Representative Western blot of CPT-1b in a placenta. C, Densitometry analysis of CPT-1b protein expression, N = 8. *P < .05 vs 0 µM by Friedman repeated-measures analysis of variance and Dunn multiple comparison testing. AU indicates arbitrary units.
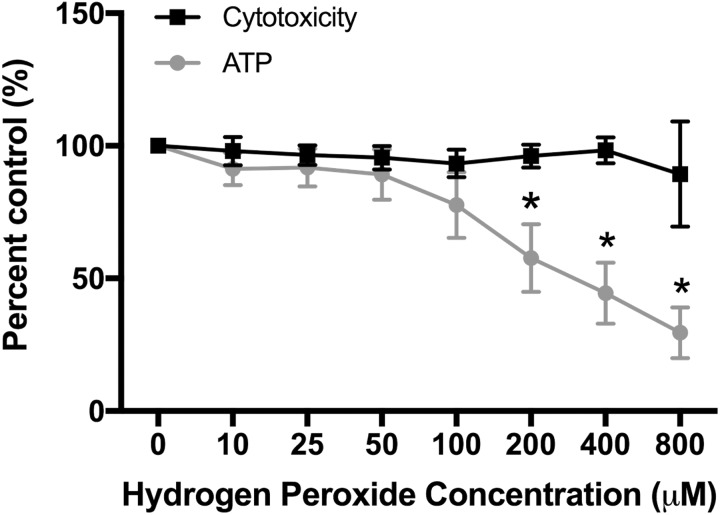
Adenosine triphosphate production decreased with increasing H2O2 exposure in primary trophoblast cells (P < .0001; Figure 5). Decreases of 42%, 56%, and 71% were seen with H2O2 concentrations of 200, 400, and 800 µM, respectively. Cytotoxicity did not increase significantly with increasing H2O2 concentrations (Figure 5).
Figure 5.
Adenosine triphosphate production and cytotoxicity in response to oxidative stress in primary trophoblast cells. N = 7. *P < .05 vs 0 µM by Friedman repeated-measures analysis of variance and Dunn multiple comparison testing.
Discussion
Our primary finding was that acute oxidative stress impairs lipid metabolism and mitochondrial function in the term placenta. Oxidative stress directly decreased placental FAO and ATP production, highlighting the importance of minimizing clinical scenarios in which acute oxidative stress can affect the growing fetus.
We found that placental FAO was decreased following induction of oxidative stress after 4 hours of H2O2 exposure. Chronic oxidative stress has been linked to lower FAO in the placenta and other tissues. Pettinelli et al showed that increased levels of ROS caused decreased FAO in the liver.8 In cases of obesity and diabetes in pregnancy, oxidative stress has been shown to be increased11,21 and these pregnancy states have also been associated with decreased placental FAO.3,15,22,23 However, until now, the direct effect of acute oxidative stress on lipid metabolism in the placenta has not been studied.
We found no changes in [3H]-palmitate esterification (storage in total lipids), nor decreases in CPT-1b mRNA or protein expression in response to 4-hour exposure to H2O2; thus, our results indicate that impaired FAO was not secondary to increases in esterification in our model. In the short term, the lack of response of placental rates of FA esterification and storage to oxidative stress may be due to decreased fatty acid uptake secondary to lower FAO, thus decreasing the cellular drive for uptake of fatty acids as demonstrated by Perazzolo et al4 If sustained (chronic oxidative stress), these initial impairments in placental FAO may result in intracellular fatty acid accretion. This idea is consistent with findings in placentas of obese women: genes involved in FAO are decreased in early gestation, before any changes in esterification and storage genes are detected,24 whereas at term, placental FAO genes remain decreased and esterification and lipid storage is elevated.15
To further understand the potential mechanisms leading to decreased FAO, we evaluated PPAR-α and CPT-1b. Peroxisome proliferator-activated receptor α is a nuclear receptor and key transcription factor involved in FAO in a variety of tissues, including the placenta.12,25–27 Carnitine palmitoyl transferase 1b is regulated by PPAR-α and is an enzyme that facilitates transfer of fatty acids into the mitochondria to enter β-oxidation.8,15 Our model demonstrated that oxidative stress induction, within the first few hours, does not alter FAO via changes in PPAR-α or CPT-1b gene or protein expression. The acute nature of our model may explain these discrepancies in comparison to chronic disease states.
To understand our finding of impaired FAO in lieu of alterations in lipid esterification/storage or gene expression, we evaluated mitochondrial function. When mitochondrial function is compromised, ATP production decreases, which has been demonstrated in aging28 and nonalcoholic steatohepatitis.8 We found that ATP production decreased with increasing concentrations of H2O2, while cytotoxicity did not change at the levels of H2O2 used in our FAO assays. Mantena et al has shown that increased oxidative stress in the liver led to decreased ATP levels due to proton leaks across the inner mitochondrial membrane, producing ineffective mitochondria.29 Similarly, in the kidney, oxidative stress led to inner mitochondrial membrane damage, decreasing the proton gradient and leading to decreased ATP production.30,31 While other tissues in the human have produced similar results, the direct effect of acute oxidative stress on ATP production in the placenta has not been previously reported.
The finding of impaired ATP production without detectable alterations in esterification/storage or gene expression changes strongly suggest that decreased FAO is due to mitochondrial dysfunction following acute oxidative stress exposure. Alterations in lipid metabolism and mitochondrial function impair placental effectiveness which ultimately can affect fetal growth throughout pregnancy. Pregnancies complicated by placentas and fetuses that lack the machinery for complete FAO are associated with fetal growth restriction.2 In addition, others have shown that in hypoxic states, placental ATP production is decreased and is associated with impaired fetal growth.10 Clinically, acute oxidative stress events such as an asthma attack or development of acute respiratory distress can now be expected to affect both placental lipid metabolism and energy production, potentially leading to growth restriction. Poorly controlled asthma has been linked to growth restriction17; however, no studies to our knowledge have evaluated the correlation between number of acute oxidative stress events and growth restriction. This would be an interesting future investigation.
A major strength of this study was that placental tissue was collected from lean women at time of scheduled cesarean, as it minimized endogenous oxidative stress which is seen with labor. In addition, we directly measured ATP production in primary trophoblasts after H2O2 exposure, demonstrating that impairment of mitochondria, but not cytotoxicity, may be responsible for impairments in FAO. Limitations of our study include utilizing a single agent to induce oxidative stress and not elucidating the exact mechanism in which oxidative stress affects the mitochondria. In addition, we utilized palmitate for our metabolism experiments, and while this fatty acid is one of the most prevalent, it does not represent all fatty acids. Future studies should assess whether mitochondrial function could be recovered following acute oxidative stress exposure.
In conclusion, acute oxidative stress decreases FAO and mitochondrial function in the term placenta. Multiple exposures to this type of stress may lead to sustained impairments in placental nutrient metabolism. Due to the potential for growth abnormalities from these alterations in placental function, pregnancies complicated by acute oxidative stress should have fetal growth evaluated prenatally.
Supplemental Material
Supplemental Material, supplemental_table1_and_figure for Oxidative Stress Impairs Fatty Acid Oxidation and Mitochondrial Function in the Term Placenta by Megan M. Thomas, Maricela Haghiac, Catalin Grozav, Judi Minium, Virtu Calabuig-Navarro and Perrie O’Tierney-Ginn in Reproductive Sciences
Acknowledgments
The authors are grateful for the support of the participating mothers, the clinical staff of the Department of Obstetrics and Gynecology, and staff of the Clinical Research Unit at MetroHealth Medical Center.
Authors’ Note: Perrie O'Tierney-Ginn is also affiliated with Mother Infant Research Institute, Tufts Medical Center, Boston, MA, USA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NICHD R00HD062841 (PFOG) and the Department of Obstetrics and Gynecology at MetroHealth Medical Center.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine. 2002;19(1):43–55. [DOI] [PubMed] [Google Scholar]
- 2. Shekhawat P, Bennett MJ, Sadovsky Y, Nelson DM, Rakheja D, Strauss AW. Human placenta metabolizes fatty acids: implications for fetal fatty acid oxidation disorders and maternal liver diseases. Am J Physiol Endocrinol Metab. 2003;284(6):E1098–E1105. [DOI] [PubMed] [Google Scholar]
- 3. Mele J, Muralimanoharan S, Maloyan A, Myatt L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am J Physiol Endocrinol Metab. 2014;307(5):E419–E425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG. The influence of placental metabolism on fatty acid transfer to the fetus. J Lipid Res. 2017;58(2):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122(4):369–382. [DOI] [PubMed] [Google Scholar]
- 6. Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Mitochondrial control of cellular life, stress, and death. Circ Res. 2012;111(9):1198–1207. [DOI] [PubMed] [Google Scholar]
- 8. Pettinelli P, Obregon AM, Videla LA. Molecular mechanisms of steatosis in nonalcoholic fatty liver disease. Nutr Hosp. 2011;26(3):441–450. [DOI] [PubMed] [Google Scholar]
- 9. Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56(4):952–964. [DOI] [PubMed] [Google Scholar]
- 10. Colleoni F, Padmanabhan N, Yung HW, et al. Suppression of mitochondrial electron transport chain function in the hypoxic human placenta: a role for miRNA-210 and protein synthesis inhibition. PloS One. 2013;8(1):e55194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coughlan MT, Vervaart PP, Permezel M, Georgiou HM, Rice GE. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta. 2004;25(1):78–84. [DOI] [PubMed] [Google Scholar]
- 12. Dube E, Gravel A, Martin C, et al. Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta. Biol Reprod. 2012;87(1):14, 11–11. [DOI] [PubMed] [Google Scholar]
- 13. Brass E, Hanson E, O’Tierney-Ginn PF. Placental oleic acid uptake is lower in male offspring of obese women. Placenta. 2013;34(6):503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haghiac M, Yang XH, Presley L, et al. Dietary omega-3 fatty acid supplementation reduces inflammation in obese pregnant women: a randomized double-blind controlled clinical trial. PloS One. 2015;10(9):e0137309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calabuig-Navarro V, Haghiac M, Minium J, et al. Effect of maternal obesity on placental lipid metabolism. Endocrinology. 2017;158(8):2543–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gabbe SN, Jennifer R, Simpson JL, et al. Obstetrics: Normal and Problem Pregnancies. 7th ed Philadelphia, PA: Elsevier; 2017. [Google Scholar]
- 17. Murphy VE, Gibson P, Talbot PI, Clifton VL. Severe asthma exacerbations during pregnancy. Obstet Gynecol. 2005;106(5):1046–1054. [DOI] [PubMed] [Google Scholar]
- 18. Calabuig-Navarro V, Puchowicz M, Glazebrook P, et al. Effect of omega-3 supplementation on placental lipid metabolism in overweight and obese women. Am J Clin Nutr. 2016;103(4):1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hughes SD, Quaade C, Johnson JH, Ferber S, Newgard CB. Transfection of AtT-20ins cells with GLUT-2 but not GLUT-1 confers glucose-stimulated insulin secretion. Relationship to glucose metabolism. J Biol Chem. 1993;268(20):15205–15212. [PubMed] [Google Scholar]
- 20. Varastehpour A, Radaelli T, Minium J, et al. Activation of phospholipase A2 is associated with generation of placental lipid signals and fetal obesity. J Clin Endocrinol Metab. 2006;91(1):248–255. [DOI] [PubMed] [Google Scholar]
- 21. Saben J, Lindsey F, Zhong Y, et al. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35(3):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Visiedo F, Bugatto F, Sanchez V, Cozar-Castellano I, Bartha JL, Perdomo G. High glucose levels reduce fatty acid oxidation and increase triglyceride accumulation in human placenta. Am J Physiol-Endoc M. 2013;305(2):E205–E212. [DOI] [PubMed] [Google Scholar]
- 23. Hastie R, Lappas M. The effect of pre-existing maternal obesity and diabetes on placental mitochondrial content and electron transport chain activity. Placenta. 2014;35(9):673–683. [DOI] [PubMed] [Google Scholar]
- 24. Lassance L, Haghiac M, Leahy P, et al. Identification of early transcriptome signatures in placenta exposed to insulin and obesity. Am J Obstet Gynecol. 2015;212(5):e641–e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Capobianco E, Martinez N, Fornes D, et al. PPAR activation as a regulator of lipid metabolism, nitric oxide production and lipid peroxidation in the placenta from type 2 diabetic patients. Mol Cell Endocrinol. 2013;377(1-2):7–15. [DOI] [PubMed] [Google Scholar]
- 26. Kersten S. Integrated physiology and systems biology of PPARα. Mol Metab. 2014;3(4):354–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lendvai A, Deutsch MJ, Plosch T, Ensenauer R. The peroxisome proliferator-activated receptors under epigenetic control in placental metabolism and fetal development. Am J Physiol-Endoc M. 2016;310(10):E797–E810. [DOI] [PubMed] [Google Scholar]
- 28. Payne BA, Chinnery PF. Mitochondrial dysfunction in aging: much progress but many unresolved questions. Biochim Biophys Acta. 2015;1847(11):1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mantena SK, King AL, Andringa KK, Eccleston HB, Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radical Bio Med. 2008;44(7):1259–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quoilin C, Mouithys-Mickalad A, Lecart S, Fontaine-Aupart MP, Hoebeke M. Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochim Biophys Acta. 2014;1837(10):1790–1800. [DOI] [PubMed] [Google Scholar]
- 31. Videla LA, Rodrigo R, Araya J, Poniachik J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radical Bio Med. 2004;37(9):1499–1507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, supplemental_table1_and_figure for Oxidative Stress Impairs Fatty Acid Oxidation and Mitochondrial Function in the Term Placenta by Megan M. Thomas, Maricela Haghiac, Catalin Grozav, Judi Minium, Virtu Calabuig-Navarro and Perrie O’Tierney-Ginn in Reproductive Sciences