Abstract
Background:
Oxidative stress is associated with poor perinatal outcomes. Little is known regarding the longitudinal levels of oxidative stress in the perinatal period or the correlation between maternal and neonatal oxidative stress levels.
Objective:
Describe and compare oxidative stress, specifically superoxide, superoxide dismutase, catalase, and glutathione levels, over the perinatal period.
Study Design:
Longitudinal descriptive design using a convenience sample of medically high- and low-risk pregnant women (n = 140) from a maternal–fetal medicine and general obstetrics practice, respectively. Blood was obtained from women at 12–20 and 24–28 weeks’ gestation and during labor, from the umbilical cord at birth, and from neonates at 24–72 hr after birth. Levels of superoxide were measured using electron paramagnetic resonance (EPR) spectroscopy; antioxidants (superoxide dismutase, catalase, and glutathione) were measured using commercial assay kits. Relationships between oxidative stress levels at different time points were examined using nonparametric methods. Pregnancy outcome was collected.
Results:
Demographic variables, outcome variables, and oxidative stress levels in maternal blood, cord blood, and infants differed between medically high- and low-risk women. Descriptive patterns for oxidative stress measures varied over time and between risk groups. Significant correlations between time points were noted, suggesting intraindividual consistency may exist throughout the perinatal period. However, these correlations were not consistent across each medical risk group.
Conclusion:
EPR spectroscopy is a feasible method for the perinatal population. Results provide new information on perinatal circulating superoxide levels and warrant further investigation into potential relationships between prenatal and neonatal physiologic dysregulation of oxidative stress.
Keywords: redox, superoxide, perinatal, antioxidants, allostatic load
Etiologies of poor perinatal outcomes such as preterm birth (PTB) and neonatal complications (e.g., chronic lung disease) remain unclear. Experts agree that these outcomes are multifactorial, with contributing factors that include biological, psychological, and sociodemographic risk profiles (Haas et al., 2015; Hux, Catov, & Roberts, 2014). PTB and major neonatal complications occur across all populations in the United States; however, the incidence is connected with sources of chronic stress such as race, socioeconomic status, and maternal risk behaviors (Centers for Disease Control and Prevention, 2016). Thus, investigating PTB and neonatal complications using biopsychosocial theoretical frameworks may be necessary to further understand, predict, and decrease their incidence.
Allostatic load (AL) is a biopsychosocial concept that represents the cumulative damage in multiple body systems caused by excessive and/or chronic activation of allostasis, the physiologic responses that maintain homeostasis during change (McEwen, 1998). AL has emerged as a theoretical framework for clinical research in the perinatal population (i.e., pregnant women and neonates; Giurgescu, 2009; Glynn, Schetter, Hobel, & Sandman, 2008; Latendresse, 2009; Wallace & Harville, 2012). The model of AL and complications of prematurity (Moore, Berger, & Wilson, 2014) suggest that the general stress of prematurity (i.e., environmental, physical, and psychological factors) causes a constant state of allostasis in preterm infants. Depending on whether the physiologic response pattern is adaptive or maladaptive, allostasis may lead to chronic physiologic dysregulation and AL, operationalized as complications of prematurity.
Our recent research findings support this theoretical model (Dietze, Rose, & Moore, 2016; Moore et al., 2013) but also highlight the role of maternal physiologic dysregulation, which was not described in the original model. Thus, the current study expands on the original theoretical model and proposes that maternal physiologic dysregulation is directly related to physiological dysregulation in the neonate. We propose that exposure to maternal physiologic dysregulation in utero predisposes the neonate to maladaptive physiologic response patterns and exacerbates existing maladaptive patterns, leading to physiologic dysregulation and AL in the neonate, resulting in complications of prematurity.
Researchers have operationalized AL using several different biological parameters including oxidative stress. Oxidative stress refers to the imbalance between free radicals and antioxidants. Free radicals, also called reactive oxygen species, are essential for biological homeostasis (Burton & Jauniaux, 2011; Schieber & Chandel, 2014). The most abundant endogenous reactive oxygen species in the body is superoxide (; Burton & Jauniaux, 2011). This one-electron reduction of molecular oxygen is mainly generated from inefficient leakage of electrons along the mitochondria electron transport chain. Endogenous and exogenous antioxidants achieve equilibrium by directly scavenging reactive oxygen species and/or repairing damage from reactive oxygen species. Common antioxidants include dietary vitamins (e.g., vitamin C), small-molecule thiols (e.g., glutathione), and cellular enzymes (e.g., superoxide dismutase [SOD], catalase [CAT]). Excessive reactive oxygen species and/or insufficient antioxidants create the imbalance in the reduction–oxidation (redox) environment known as oxidative stress. The resulting unstable redox environment can lead to detrimental conditions such as irreversible cellular damage, protein alterations, apoptosis from lipid peroxidation, and DNA oxidation (Burton & Jauniaux, 2011; Moore, Ahmad, & Zimmerman, 2018; Schieber & Chandel, 2014).
Oxidative stress is relevant in the perinatal population as multiple researchers have identified relationships between oxidative stress and poor perinatal outcomes such as PTB and neonatal complications (Ferguson et al., 2015; Menon et al., 2011; Rosanna & Salvatore, 2012). However, some studies have reported conflicting or inconsistent findings (Moore et al., 2018). Our ability to synthesize results is limited because investigators have used a variety of methods to measure oxidative stress across studies. Few clinical studies have used electron paramagnetic resonance (EPR) spectroscopy to measure oxidative stress (Moore et al., 2018). EPR is an advanced method that bench scientists consider to be the gold standard for measuring free radicals (Hawkins & Davies, 2014), yet it has had limited use in perinatal research. Rather, perinatal researchers thus far have concentrated on nonspecific biomarkers of oxidative stress, such as 8-hydroxydeoxyguanasine (OHdG) and isoprostanes, and have concluded that oxidative stress has occurred based on fragments of damaged DNA or protein, respectively. However, these nonspecific biomarkers do not identify specific free radicals and antioxidants involved in the destructive mechanisms occurring at the cellular level. Failure to identify and understand these destructive mechanisms inhibits our ability to predict problems and to develop successful interventions to prevent PTB and other neonatal complications.
Understanding relationships between maternal oxidative stress and neonatal oxidative stress is also essential for advancing knowledge of perinatal complications. Research incorporating the AL model and using oxidative stress measurements as AL parameters is warranted to further understand the relationship between maternal physiologic dysregulation from oxidative stress and neonatal outcomes. Few research studies have measured AL parameters of oxidative stress by quantifying free radicals, specifically superoxide ( ), using EPR methods in the perinatal population (Sikkema et al., 2001). Antioxidants, specifically CAT, SOD, reduced glutathione (GSH), and oxidized glutathione (GSSG), are present throughout pregnancy (Lekharu, Pradhan, Sharma, & Sharma, 2014; Sharma et al., 2006; Yüksel & Yigit, 2015). However, a gap in knowledge exists in the longitudinal description of , CAT, SOD, GSH, and GSSG as AL parameters of oxidative stress.
The purpose of the present study was to describe oxidative stress levels throughout the perinatal period: during pregnancy (12–20 weeks’ gestation, 24–28 weeks’ gestation, labor) from the pregnant women, from the umbilical cord at birth, and from the newborn infant at 24–72 hr after birth. The specific aims of the study were to (1) determine the feasibility of using EPR in the perinatal population, (2) describe and compare medically high-risk and medically low-risk pregnancies on demographic variables (race, ethnicity, and type of insurance) and outcome variables (maternal preeclampsia [PE], PTB, neonate’s gestational age at birth, and neonate’s birth weight) and AL parameters of oxidative stress over time, (3) examine within-subject and within-group and between-group differences in AL parameters of oxidative stress over time, and (4) determine internal consistency of AL parameters over time.
Materials and Methods
We used a prospective, correlational, longitudinal design. The university’s institutional review board approved the study, and participants gave informed, written consent. We recruited a convenience sample of 140 pregnant women from a maternal–fetal medicine practice group (medically high risk, MFM; n = 70) and a general obstetrics practice group (medically low risk, GOP; n = 70) associated with a Midwestern academic tertiary perinatal center. The center is located in an urban setting and is affiliated with an on-site labor and delivery unit, newborn nursery, and Level III neonatal intensive care unit. Pregnant women at between 12 and 20 weeks’ gestation were consecutively recruited during obstetrical visits to the MFM and GOP practice groups. Classification of MFM and GOP participants was based solely on the practice group from which the participant was recruited.
Inclusion criteria for the pregnant women included <20 weeks’ gestation at recruitment and the intention to deliver at the on-site labor and delivery unit. Our exclusion criterion was known congenital anomalies of the fetus at time of consent. Staff at the practices identified eligible patients and established ethical access before a member of the research team approached the patient. The research team member discussed the consent form and procedures for the study with potential participants. Prior to obtaining informed consent, the team member verified patient understanding by using the teach-back method, where the eligible patient describes the study and their expectations using their own words.
After enrollment, blood from the pregnant woman was obtained for research purposes or in conjunction with a blood draw for clinical purposes prior to 20 weeks’ gestation (T1). We chose the broad range of possible timing for baseline blood samples to account for the variability in initial prenatal visits in this population. Blood draws were coordinated with routine laboratory procedures for the remaining specified times: from the pregnant woman at 24–28 weeks’ gestation (T2) and upon her admission to the labor and delivery unit for labor (T3), from the umbilical cord at birth (T4), and from the neonate at 24–72 hr after birth (T5).
We collected demographic (race, ethnicity, and type of insurance) and pregnancy outcome data (maternal PE, PTB, neonate’s gestational age at birth, neonate’s birth weight) from the electronic health records of the mother and neonate after delivery.
Measures
We measured AL parameters of oxidative stress from the blood collected during the perinatal period at the five time points described above. was measured in whole blood using EPR spectroscopy as previously described (Ahmad, Temme, Abdalla, & Zimmerman, 2016). Briefly, after blood collection, the sample was transported directly to the laboratory for analyses, so that processing occurred approximately 30 min after collection. EPR processing included incubating part of the whole blood for an additional 30 min at 37°C with a superoxide-sensitive EPR spin probe, 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine, then freezing it in liquid nitrogen. Oxidation of these spin probes results in the formation of stable nitroxide radicals that can be detected by EPR spectroscopy. The amount of nitroxide formed is directly proportional to the concentration of the free radical with which it reacted, in this case . Therefore, the concentration of in blood was determined by analyzing the amplitude of the EPR spectra. All EPR measurements were performed with a Bruker eScan EPR spectrometer (Bruker BioSpin GmbH, Rheinstetten/Karlsruhe, Germany) and expressed as EPR arbitrary units.
The remaining blood was centrifuged, and the red blood cell lysate was divided into aliquots, frozen, and stored in a −70 C freezer for batch processing of antioxidants (i.e., SOD, CAT, GSH, and GSSH) using commercially available assays (SOD Assay Kit-WST [DOJINDO, Inc., Rockville, MD], OxiSelect Catalase Activity Assay Kit [Cell Biolabs, Inc., San Diego, CA], GSSG/GSH Quantification kit [DOJINDO, Inc., Rockville, MD, USA]) per the manufacturer instructions.
Statistical Analyses
We completed a power analysis for Specific Aim 3 of the project using a two-sided significance level of .01 to informally adjust for multiple tests, power of 80%, and a moderate effect size (.45) using G*Power Version 3.1.2 (Franz Faul, Universitat Kiel, Germany, 2009). The null hypothesis was that no correlation exists between the AL parameters over time. We calculated that we would need 50 pregnant women to detect a correlation of .45. To accommodate a potential attrition rate of 40%, we recruited 70 women for each group.
We used SPSS Version 25 (SPSS Inc., Chicago, IL) for statistical analysis. Descriptive statistics and parametric tests (t tests and χ2) were used to describe and compare demographic and outcome variables between the MFM and GOP groups. Because the biological AL parameters of oxidative stress (i.e., and antioxidants) were not normally distributed, nonparametric tests were used to (1) assess the feasibility of using EPR in the perinatal population, (2) compare oxidative stress levels between the MFM and GOP groups using Mann–Whitney U tests, (3) compare oxidative stress levels over time and via post hoc analyses between time points within the MFM and GOP groups using Wilcoxon signed rank tests, and (4) determine internal consistency over time for each AL parameter of oxidative stress using Spearman’s correlations. p Values for post hoc pairwise tests (Aim 3) and correlations (Aim 4) are reported after Bonferroni adjustments for multiple comparisons.
Results
Specific Aim 1
A research team member approached 176 eligible pregnant women (n = 80, MFM; n = 96, GOP) about participating in the present study, 140 of whom (n = 70, MFM; n = 70, GOP) consented to participate between August 2014 and October 2016. We obtained data from 130 neonates, including three sets of twins. We attempted to collect 706 samples in total, including an additional three cord blood and three neonatal blood specimens from the twin deliveries. Missing data were from miscarriages (n = 5), withdrawals (n = 2), delivering off-site (n = 6), and data collection protocol errors primarily from missed sample collection (n = 190, 27%; T1 [n = 15], T2 [n = 44], T3 [n = 46], T4 [n = 40], and T5 [n = 45]). An additional 194 samples were missing for EPR analyses because we were unable to process the samples within 1 hr after collection (T1 [n = 4], T2 [n = 29], T3 [n = 66], T4 [n = 68], and T5 [n = 27]). We thus analyzed 316 samples (45%) for superoxide using EPR and 510 samples (72%) for antioxidants.
Specific Aim 2
Demographic variables and outcome variables for the MFM and GOP pregnant women are shown in Table 1. Maternal race exhibited slightly different distributions between MFM and GOP pregnant women (p = .07). Cesarean delivery mode (p = .001) and the incidence of maternal PE (p = .005) and PTB (p = .001) were significantly higher in MFM pregnant women compared to the GOP pregnant women. We identified no other differences in demographic or outcome variables between the two groups.
Table 1.
Descriptive Statistics of Entire Sample, Maternal–Fetal Medicine (MFM), and General Obstetrics Practice (GOP) Pregnant Women.
| Variable | Total Sample | MFM | GOP |
|---|---|---|---|
| N = 140 | n = 70 | n = 70 | |
| Mean ± SD or n (%) | Mean ± SD or n (% MFM) | Mean ± SD or n (% GOP) | |
| Demographic | |||
| Maternal age (years) | 28.8 ± 5.1 | 29.4 ± 5.2 | 28.3 ± 5.0 |
| Maternal racea | |||
| White | 94 (67.1) | 53 (75.7) | 41 (58.6) |
| Black | 26 (18.6) | 9 (12.9) | 17 (24.3) |
| Other | 19 (13.6) | 7 (10.0) | 12 (17.1) |
| Unknown | 1 (0.7) | 1 (1.4) | 0 |
| Maternal ethnicity | |||
| Non-Hispanic | 124 (88.6) | 62 (88.6) | 62 (88.6) |
| Hispanic | 15 (10.7) | 7 (10.0) | 8 (11.4) |
| Unknown | 1 (0.7) | 1 (1.4) | 0 |
| Private insurance | |||
| Yes | 74 (52.9) | 34 (48.6) | 40 (57.1) |
| Unknown | 7 (5.0) | 4 (5.7) | 3 (4.3) |
| Outcome | |||
| Delivery modeb | |||
| Vaginal | 93 (70.5) | 38 (57.6) | 55 (83.3) |
| Cesarean | 39 (29.5) | 28 (42.4) | 11 (16.7) |
| Preeclampsiac | 17 (12.8) | 14 (20.6) | 3 (4.6) |
| Preterm birthd | 39 (29.8) | 29 (42.6) | 10 (15.9) |
| Gestational age at birth (weeks)e | 38 3/7 ± 2 | 37 4/7 ± 2 | 39 2/7 ± 1 |
| Birth weight (g)e | 3,277.4 ± 611.7 | 3,158.5 ± 653.3 | 3,400.0 ± 543.8 |
Note. We used χ2 and t tests to test for significant differences between the MFM and GOP groups. See notes below. Of the N = 140 pregnant women who started the study, we followed n = 134 until delivery. There were n = 5 miscarriages, n = 2 who withdrew before delivery, n = 6 who delivered off-site, and n = 130 infants born on-site, including n = 3 sets of twins. We collected data on N = 140 mothers (n = 70: MFM, n = 70: GOP) for the demographic variables. Sample sizes for outcome measures as indicated.
a p = .071 using χ2 tests. b n = 66 in each group; p = .001. c n = 68 MFM, 65 GOP; p = .005. dPreterm birth defined as ≤38 weeks’ gestation at birth; n = 68: MFM, 63 GOP; p = .001. e n = 66 MFM, 64 GOP.
Table 2 provides the and antioxidant levels at each time point by group. We found many significant differences when we compared and antioxidant levels between MFM and GOP pregnant women. At T1, levels of CAT, SOD, and GSH were significantly lower in MFM pregnant women compared to GOP pregnant women. At T2, levels of were significantly higher and CAT and GSH were significantly lower in MFM pregnant women compared to GOP pregnant women. At T3, GSH levels and CAT levels were significantly lower for MFM pregnant women compared to GOP pregnant women. At T4, CAT levels were significantly lower for MFM pregnant women compared to GOP pregnant women. CAT levels (p < .001) were significantly lower in neonates born to women in the MFM group compared to neonates born to women in the GOP group. In light of these findings, we completed subsequent analyses for Aims 2 and 3 separately for the MFM and GOP groups.
Table 2.
Mean (SD) Measures of Oxidative Stress by Group (Maternal–Fetal Medicine [MFM] and General Obstetric Practice [GOP]) and Time Point.
| Measure | 12–20 Weeks | 24–28 Weeks | Labor | Umbilical Cord | Newborn | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MFM | GOP | MFM | GOP | MFM | GOP | MFM | GOP | MFM | GOP | |
| (A.U.) | 9.89 × 105
(8.64 × 105) |
8.58 × 105
(3.55 × 105) |
9.68 × 105
(4.02 × 105)* |
7.67 × 105
(3.49 × 105) |
1.06 × 106
(5.57 × 105) |
1.03 × 106
(5.08 × 105) |
1.49 × 106
(4.84 × 105) |
1.27 × 106
(4.49 × 105) |
2.51 × 105
(1.30 × 105) |
2.21 × 105
(8.46 × 104) |
| CAT (U/ml) | 1.80 × 104
(8.39 × 103)*** |
9.40 × 104
(5.74 × 104) |
2.31 × 104
(2.19 × 104)*** |
6.22 × 104
(4.37 × 104) |
3.54 × 104
(4.81 × 104)*** |
4.84 × 104
(2.27 × 104) |
2.24 × 104
(3.15 × 104)*** |
4.17 × 104
(1.58 × 104) |
3.12 × 104
(3.87 × 104)*** |
4.06 × 104
(1.86 × 104) |
| SOD (U/ml) | 8.29 × 104
(6.67 × 104)*** |
1.78 × 105
(1.91 × 105) |
8.24 × 104
(6.14 × 104) |
1.05 × 105
(7.65 × 104) |
8.65 × 104
(6.83 × 104) |
1.33 × 105
(1.80 × 105) |
9.70 × 104
(1.13 × 105) |
1.46 × 105
(2.02 × 105) |
9.05 × 104
(8.22 × 104) |
1.06 × 105
(1.48 × 105) |
| GSH (nmol/ml) | 1.53 × 103
(8.15 × 102)*** |
1.89 × 103
(4.83 × 102) |
1.68 × 103
(1.24 × 104)** |
1.88 × 103
(7.62 × 102) |
1.51 × 103
(7.48 × 102)*** |
2.04 × 103
(9.20 × 102) |
1.79 × 103
(8.64 × 102) |
2.21 × 103
(1.34 × 103) |
1.85 × 103
(1.12 × 103) |
2.06 × 103
(1.34 × 103) |
| GSSG (nmol/ml) | 48.7 (59.5) | 39.0 (13.4) | 46.7 (68.5) | 38.1 (16.8) | 95.4 (128.0) | 79.5 (93.9) | 86.4 (110.4) | 80.9 (111.5) | 82.0 (100.6) | 90.7 (125.9) |
| GSH/GSSG (nmol/ml) | 73.4 (85.2) | 53.3 (20.7) | 52.8 (44.7) | 63.0 (53.5) | 45.2 (45.8) | 46.2 (31.3) | 75.6 (136.7) | 53.9 (46.3) | 53.8 (84.2) | 56.2 (61.0) |
Note. Testing for differences in oxidative stress levels between the groups at each time point was via Mann–Whitney U tests. A.U. = arbitrary units; CAT = catalase; GSH = reduced glutathione; GSSG = oxidized glutathione; = superoxide; SOD = superoxide dismutase.
*p < .05. **p < .01. ***p ≤ .001.
We also looked at and antioxidant levels by race and delivery mode, the demographic variables that differed between the MFM and GOP groups. CAT levels at T1 and T4 were significantly lower in White women than in non-White women (p = .001and .029, respectively). SOD levels were significantly higher in newborn infants born to women who self-identified their race as “other” compared to the remaining women (p = .032), and the GSH/GSSG ratio was significantly higher in newborn infants born to Black women than in non-Black women (p = .003). Women with vaginal deliveries had higher CAT levels at T1 (p = .012) and higher SOD levels at T1 (p = .019) and T2 (p = .021) than women who delivered via Cesarean. Exploration of potential differences in oxidative stress levels by outcome variables, specifically PE, diabetes mellitus status, and PTB is beyond the scope of this article, but we will discuss it in later publications.
Specific Aim 3
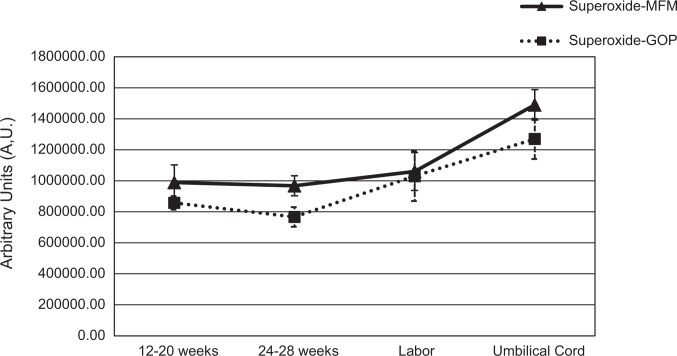
Figures 1 –3 illustrate changes in and antioxidant levels over time for the MFM and GOP groups. As seen in Figure 1, levels changed significantly over time in MFM pregnancies, with post hoc analyses showing a trend toward significance between T1 and T4. We found no significant changes in levels over time in GOP pregnancies. We did not include newborn levels in Figure 1 because the volume of blood for these samples differed from the samples at all other time points, which affected concentration and interpretation of the EPR signal; thus, the results from the neonate samples cannot be compared to maternal samples.
Figure 1.
Superoxide () levels (means and standard errors of the means) over time for maternal–fetal medicine (▴: MFM) and general obstetrics practice (▪: GOP) groups. Changes over time were significant for levels in MFM pregnancies, χ2(4) = 12.8, p = .012, with a trend toward a significant difference from the 12–20 weeks and the umbilical cord time points (z = −2.72, p = .06). There were no significant changes over time in the GOP pregnancies. Reported p values for the pairwise comparisons include the Bonferroni correction.
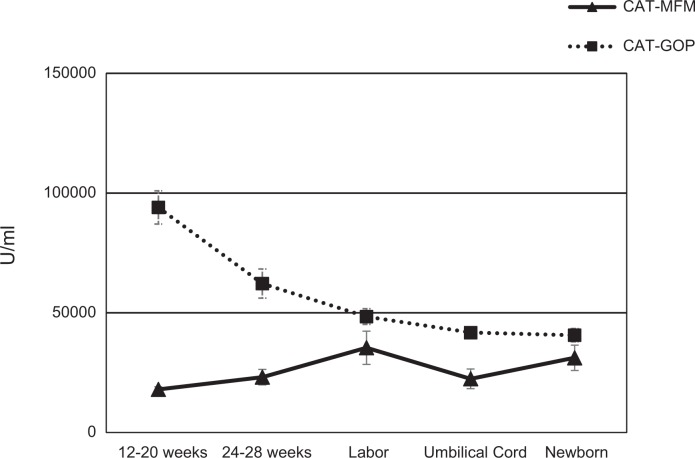
Figure 2.
Catalase (CAT) levels (means and standard errors of the means) and comparisons over time for maternal–fetal medicine (▴: MFM) and general obstetrics practice (▪: GOP) groups. CAT levels exhibited a slight increase over time for MFM pregnancies, χ2(4) = 9.14, p = .058, specifically between blood collected from the mother at labor and that collected from the umbilical cord (z = −3.47, p = .01). CAT significantly decreased over time for GOP pregnancies, χ2(4) = 26.85, p < .001, with specific significant differences between blood obtained at 12–20 weeks and that obtained at all other time points (24–28 weeks [z = −2.92, p = .04], labor [z = −4.92, p = .01], from the umbilical cord [z = −5.58, p = .01], and from the newborn infant [−4.97, p = .01]) and between blood obtained at 24–28 weeks and that obtained from the newborn (z = −2.94, p = .03). Reported p values for the pairwise comparisons include the Bonferroni correction.
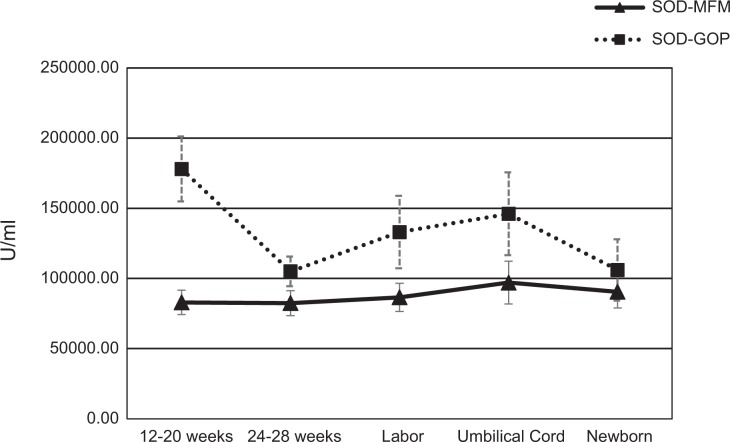
Figure 3.
Superoxide dismutase (SOD) levels (means and standard errors of the means) over time for the maternal–fetal medicine (▴: MFM) and general obstetrics practice (▪: GOP) groups. SOD levels remained stable over time for MFM pregnancies but changed significantly over time for GOP pregnancies, χ2(4) = 11.58, p = .021; specifically, analyses revealed a trend toward a significant difference between blood obtained at 12–20 weeks and that obtained during labor (z = −2.61, p = .09). Reported p values for the pairwise comparisons include the Bonferroni correction.
Figure 2 illustrates CAT levels over time for MFM and GOP groups. CAT levels exhibited a slight increase over time for MFM pregnancies. Post hoc analyses indicated significant differences in CAT levels between T3 and T4. CAT decreased significantly over time for GOP pregnancies. Post hoc analyses indicated significant differences in CAT levels between T1 and T2, T1 and T3, T1 and T4, T1 and T5, and T2 and T5.
SOD levels remained stable over time for MFM pregnancies but changed significantly over time for GOP pregnancies, as illustrated in Figure 3. Post hoc analyses for GOP pregnancies revealed slight differences in SOD levels between T1 and T3 (z = −2.61, p = .09). GSH/GSSG levels varied for both MFM and GOP pregnancies but did not show significant changes over time.
Specific Aim 4
Table 3 shows correlations (rs) for each measure between time points within the MFM and GOP groups, which allow for the determination of consistency for each measure over time. Although we did find significant relationships for individual measures between time points within each group, none were consistent across groups. For example, there was a positive correlation for CAT levels between T1 and T3 in the MFM group (rs = .520, p < .01) but no correlation for this measure between these time points in the GOP group (rs = .097, p = .52). A few of the relationships had moderate correlation coefficients but did not reach statistical significance. For example, in the GOP group, levels between T2 and T3 had a coefficient of more than .4, but this relationship was not statistically significant.
Table 3.
Correlations Between Oxidative Stress Measures of Superoxide (), Catalase (CAT), Superoxide Dismutase (SOD), Reduced Glutathione (GSH), and Oxidized Glutathione (GSSG) Levels at 12−20 Weeks’ Gestation (T1), 24−28 Weeks’ Gestation (T2), Labor (T3), Umbilical Cord at Delivery (T4), and Newborn Infants at 24−72 hr after delivery (NB) in Pregnant Women Receiving Care at a Maternal−Fetal Medicine (MFM) and a General Obstetrics Practice (GOP).
| Oxidative Stress Measure | T1 | T2 | T3 | T4 | NB |
|---|---|---|---|---|---|
| T1 | |||||
| — | MFM (−.221, .21) | MFM (.018, .95) | MFM (−.062, .81) | MFM .053, .75) | |
| GOP (.218, .23) | GOP (−.083, .83) | GOP (−.007, .98) | GOP (−.103, .65) | ||
| CAT | — | MFM (.096, .55) | MFM (.520, <.01) | MFM (.555, <.01) | MFM (.504, .01) |
| GOP (−.109, .45) | GOP (.097, .52) | GOP (.076, .62) | GOP (−.261, .08) | ||
| SOD | — | MFM (.466, .02) | MFM (.269, .10) | MFM (.116, .29) | MFM (.048, .77) |
| GOP (−.148, .30) | GOP (.013, .93) | GOP (−.034, .82) | GOP (−.296, .46) | ||
| GSH | — | MFM (.184, .25) | MFM (.186, .24) | MFM (.003, .99) | MFM (.318, .40) |
| GOP (.200, .172) | GOP (−.133, .38) | GOP (.081, .60) | GOP (.088, .57) | ||
| GSSG | — | MFM (−.552, <.01) | MFM (.057, .73) | MFM (.087, .57) | MFM (−.073, .65) |
| GOP (.127, .39) | GOP (.121, .42) | GOP (−.105, .50) | GOP (−.240, .11) | ||
| T2 | |||||
| MFM (−.221, .21) | — | MFM (−.338, .20) | MFM (−.171, .54) | MFM (−.127, .50) | |
| GOP (.218, .24) | GOP (.600, .29) | GOP (.643, .12) | GOP (−.473, .14) | ||
| CAT | MFM (.096, .55) | — | MFM (.175, .31) | MFM (.096, .56) | MFM (.215, .22) |
| GOP (−.109, .45) | GOP (−.135, .41) | GOP (−.222, .19) | GOP (.034, .84) | ||
| SOD | MFM (.466, .02) | — | MFM (.419, .11) | MFM (.198, .23) | MFM (.291, .09) |
| GOP (−.148, .30) | GOP (−.039, .81) | GOP (.095, .58) | GOP (−.064, .70) | ||
| GSH | MFM (.184, .25) | — | MFM (−.011, .95) | MFM (.046, .78) | MFM (.177, .30) |
| GOP (.200, .17) | GOP (−.506, .01) | GOP (−.114, .52) | GOP (−.143, .41) | ||
| GSSG | MFM (−.552, <.01) | — | MFM (−.249, .14) | MFM (−.290, .07) | MFM (−.203, .23) |
| GOP (.127, .39) | GOP (.417, .09) | GOP (−.031, .86) | GOP (.340, .43) | ||
| T3 | |||||
| MFM (.018, .95) | MFM (−.338, .20) | — | MFM (.718, .13) | MFM (.525, .31) | |
| GOP (−.083, .83) | GOP (.600, .29) | GOP (.200, .80) | GOP (−.900, .37) | ||
| CAT | MFM (.520, <.01) | MFM (.175, .31) | — | MFM (.838, <.01) | MFM (.613, <.01) |
| GOP (.097, .52) | GOP (−.135, .41) | GOP (.421, .07) | GOP (.225, .19) | ||
| SOD | MFM (.269, .10) | MFM (.419, .11) | — | MFM (.164, .31) | MFM (.129, .45) |
| GOP (.013, .93) | GOP (−.039, .81) | GOP (.495, .01) | GOP (.246, .15) | ||
| GSH | MFM (.186, .24) | MFM (−.011, .95) | — | MFM (.653, <.01) | MFM (.501, .01) |
| GOP (−.133, .38) | GOP (−.506, .01) | GOP (.179, .270) | GOP (.264, .12) | ||
| GSSG | MFM (.057, .73) | MFM (−.249, .14) | — | MFM (.835, <.01) | MFM (.295, .065) |
| GOP (.121, .42) | GOP (.417, .09) | GOP (.553, <.01) | GOP (.654, <.01) | ||
| T4 | |||||
| MFM (−.062, .81) | MFM (−.171, .54) | MFM (.718, .13) | — | MFM (.028, .90) | |
| GOP (−.007, .98) | GOP (.643, .12) | GOP (.200, .800) | GOP (.333, .42) | ||
| CAT | MFM (.555, <.01) | MFM (.096, .56) | MFM (.838, <.01) | — | MFM (.686, <.01) |
| GOP (.076, .62) | GOP (−.222, .19) | GOP (.421, .07) | GOP (.175, .32) | ||
| SOD | MFM (.116, .29) | MFM (.198, .23) | MFM (.164, .31) | — | MFM (.326, .03) |
| GOP (−.034, .82) | GOP (.095, .58) | GOP (.495, .01) | GOP (.044, .81) | ||
| GSH | MFM (.003, .99) | MFM (.046, .78) | MFM (.653, <.01) | — | MFM (.209, .16) |
| GOP (.081, .60) | GOP (−.114, .52) | GOP (.179, .27) | GOP (.205, .25) | ||
| GSSG | MFM (.087, .57) | MFM (−.290, .07) | MFM (.835, <.01) | — | MFM (.287, .48) |
| GOP (−.105, .50) | GOP (−.031, .86) | GOP (.553, <.01) | GOP (.412, .16) | ||
| NB | |||||
| MFM (.053, .75) | MFM (−.127, .50) | MFM (.525, .31) | MFM (.028, .90) | — | |
| GOP (−.103, .65) | GOP (−.473, .14) | GOP (−.900, .37) | GOP (.333, .42) | ||
| CAT | MFM (.504, .01) | MFM (.215, .22) | MFM (.613, <.01) | MFM (.686, <.01) | — |
| GOP (−.261, .08) | GOP (.034, .84) | GOP (.225, .19) | GOP (.175, .32) | ||
| SOD | MFM (.048, .77) | MFM (.291, .09) | MFM (.129, .45) | MFM (.326, .03) | — |
| GOP (−.296, .46) | GOP (−.064, .70) | GOP (.246, .15) | GOP (.044, .81) | ||
| GSH | MFM (.318, .40) | MFM (.177, .30) | MFM (.501, .01) | MFM (.209, .16) | — |
| GOP (.088, .57) | GOP (−.143, .41) | GOP (.264, .12) | GOP (.205, .25) | ||
| GSSG | MFM (−.073, .65) | MFM (−.203, .23) | MFM (.295, .65) | MFM (.287, .48) | — |
| GOP (−.240, .11) | GOP (.340, .43) | GOP (.654, <.01) | GOP (.412, .16) | ||
Note. Correlations for oxidative stress measures between each time (T) were analyzed using Spearman’s correlations (rs, p value). Statistically significant correlations (p < .05) after Bonferroni correction are boldfaced.
Discussion
This descriptive study provides new insight on oxidative stress levels throughout the perinatal period in women receiving care at an MFM and a GOP. Race, incidence of PE and PTB, and oxidative stress levels throughout pregnancy differed significantly between the groups; thus, we conducted descriptive and correlational analyses for each group. Each AL parameter of oxidative stress showed variances over time. Levels of appeared to increase over time in both groups, while SOD, GSH, and GSSG levels were variable in both. CAT levels changed over time for both groups, with levels increasing slightly over time in the MFM group and decreasing significantly over time in the GOP group. Internal consistency of antioxidant levels for both MFM and GOP pregnancies were significant later in pregnancy, but we also noted many other correlations throughout pregnancy.
The differences in perinatal outcomes, specifically PE and PTB, between the groups were expected because the pregnancies in the MFM groups were considered high risk. We did not expect to see the significant differences in maternal race that we observed between groups; however, because we recruited women for both from the same tertiary clinic in an urban location. During our study period, more White pregnant women were referred to the MFM practice and included in our MFM group compared to non-White pregnant women. Most MFM pregnant women had previous medical conditions (e.g., history of PE, history of spontaneous abortion, and metabolic disorders) warranting obstetric care from an MFM specialist. These preexisting conditions would explain the higher rate of PTB in the MFM group (43%) compared to the GOP group (16%). However, PTB rates for both groups were higher than they are for the general population in Nebraska (9.1%) and the United States (9.6%; National Center for Health Statistics, 2017). We did expect higher baseline PTB rates for this facility based on known psychosocial risk factors in the GOP group (National Center for Health Statistics, 2017), specifically race, ethnicity, and type of health insurance, but these women were not referred to the MFM practice group based on those risk factors alone. It is unclear whether the women in the MFM group were patients of MFM specialists based on self-referral or referral from another clinician. Future study of investigating referral patterns for medically and socially high-risk pregnant women might inform current practice for standard of care for medically and socially high-risk pregnant women.
Our findings provide new data on levels measured using EPR spectroscopy throughout pregnancy. Both MFM and GOP pregnancies showed an initial decline in levels followed by a steady incline over time. levels also were consistently higher in MFM pregnancies and neonates compared to GOP pregnancies and neonates. Sikkema et al. (2001) found that placental levels measured using EPR were higher for women with PE (n = 10) compared to a control group of pregnant women without PE (n = 13). Our findings are likely consistent with Sikkema’s findings because more women in the MFM group had PE than those in the GOP group, and they had higher levels of in the cord blood. It remains unclear, however, whether the higher levels were from PE alone or from other confounding factors in the MFM pregnancies. We found no other previous studies that measured circulating using EPR throughout pregnancy and in the neonate.
Our findings on antioxidant levels across the perinatal period were not consistent with those of previous studies. Researchers have reported that CAT, SOD, and GSH levels decreased over time throughout pregnancy (Lekharu et al., 2014; Sharma et al., 2006; Yüksel & Yigit, 2015). In the present study, CAT decreased over time for women in the GOP group yet increased slightly over time for those in the MFM group. SOD, GSH, and GSSG levels were variable over time for both groups in the present study, which is not consistent with previous findings.
We tested the internal consistency of AL parameters of oxidative stress across pregnancy and between maternal blood, cord blood, and neonatal blood. Our findings were similar to those of previous reports. Yüksel and Yigit (2015) reported positive correlations for CAT and SOD levels between blood obtained in the third trimester and cord blood. In the present study, we found that MFM, but not GOP, pregnancies had a significant positive correlation between CAT levels measured at labor and in cord blood, while GOP pregnancies had a significant positive correlation between SOD levels measured at labor and in cord blood. Because we used different units of measure than Yüksel and Yigit used, we cannot compare antioxidant levels between studies directly. Although we found that levels of and antioxidants did not differ significantly between groups for neonates at 24–72 hr of age in the present study despite the difference in the medical risk of their mothers, we did find several significant and near significant correlations between levels in maternal blood at labor and levels in cord and neonatal blood for both groups. These correlations between time points were not consistent between groups, however. Interestingly, the direction of the correlations differed between the MFM and GOP groups at various times. For example, levels were positively correlated between maternal blood at labor and neonatal blood in the MFM group and negatively correlated in the GOP group. Our findings suggest that changes over time in individual biomarkers of oxidative stress warrant further investigation with a larger sample size powered to better detect differences. To our knowledge, no other study has compared levels of antioxidants in maternal or cord blood with neonatal antioxidant levels.
The descriptive findings of the present study alone do not provide conclusive evidence about the effects of maternal AL on neonatal health but do support the revised model of AL and complications of prematurity. The study was not sufficiently powered for our analyses for Aim 3 to detect internal consistency over time. However, the high number of significant correlations between maternal, cord-blood, and neonatal levels of biomarkers of AL suggests that maternal physiologic dysregulation may be a contributing factor to physiologic dysregulation in the neonate. Larger studies are needed to support these findings, and more complex statistical analyses are necessary to identify relationships between AL parameters of oxidative stress and perinatal outcomes and to develop prediction models.
The present study does demonstrate that EPR spectroscopy is a feasible analysis method for the perinatal population, and researchers should consider using this method in future studies of oxidative stress in this population. Although missing specimens were an issue in this study, use of a larger research team that would have members available to cover all hours of the day would make data collection in future studies more successful. Using EPR to directly measure and measuring multiple specific antioxidants will provide a greater understanding of the mechanisms and relationships involved in perinatal AL compared to using only nonspecific biomarkers of oxidative stress. Our results also provide data regarding potential developmental implications of oxidative stress throughout pregnancy because of the high number of correlations between levels measured throughout pregnancy and levels measured in the cord blood and in the neonate. Understanding and recognizing maternal adaptive versus maladaptive activity and antioxidant patterns throughout gestation may provide insight into the redox environment of the developing fetus. Future studies should include longitudinal data points and multiple oxidative stress measurements, including the use of EPR, to provide more comprehensive data that will increase our understanding of oxidative stress in the perinatal period.
The present study had several limitations. Fresh whole blood is required for successful processing for EPR spectroscopy. We considered whole blood to be “fresh” for 60 min after collection based on our previous pilot studies. Due to potential circadian variations, differences in the timing of whole blood processing may have affected our detection of levels. In future studies, researchers should limit the collection and processing of whole blood to specific hours during the day. The need for fresh whole blood resulted in many missing data points, especially blood collected at labor, birth, and from the neonate. Using a sample that included pregnant women from both MFM and GOP clinics meant that there were many confounding variables that made it impossible for us to analyze and report AL parameters of oxidative stress across the entire sample. Missing samples also decreased power for statistical analyses. However, the differences we detected in AL parameters of oxidative stress between the MFM and GOP groups, as well as by maternal race and delivery mode, do provide additional evidence to support the use of precision health care and individualized medicine. A more algorithmic approach to analyzing AL parameters may be necessary to further understand their relationships with perinatal outcomes and identify more personalized screening methods. Finally, both our original and revised models of AL and complications of prematurity are specific to preterm infants. In the current study, we included term neonates; thus, gestational age may be a confounding factor in the consistency in AL parameters of oxidative stress between maternal and cord and/or neonatal blood.
More questions than answers arise from this study. Why are there differences in means and correlations for the AL parameters of oxidative stress between the MFM and GOP pregnancies? Did the MFM group, as one might assume, have more medical risk factors (e.g., previous PTB, PE) that changed their oxidative stress levels? Do the differences in oxidative stress levels between the groups suggest different degrees of allostasis response patterns (McEwen, 1998)? For example, MFM levels of SOD did not change over time. Would this be considered an inactive response pattern if the variability we observed in the GOP group’s levels would be considered appropriate for the changing redox environment? Since the overall trend of for both groups increased over time, why the variability in the antioxidant levels? Although it is difficult to speculate based on the results of this study, alone, we hypothesize that the differences in oxidative stress levels between the two groups may reflect different degrees of AL and multiple maladaptive response patterns. These patterns should be examined using a more individualized and personalized medicine approach.
Summary
This study is the first to report circulating perinatal levels using EPR spectroscopy and to describe AL parameters of oxidative stress throughout pregnancy, at birth, and in neonates in high-risk versus low-risk pregnancies based on obstetrical practice group. Our limited analyses remain inconclusive but may provide support for the revised model of AL and complications of prematurity, which suggests that maternal physiologic dysregulation during pregnancy is associated with physiologic dysregulation in neonates and, consequently, with an increased risk of AL and complications of prematurity in the neonatal period.
Acknowledgments
The authors wish to thank the staff and patients at Nebraska Medicine and the Olson Center, Jocelyn Jones, Colton T. Roessner, and Ellen Steffensmeier.
Authors’ Note: All authors have participated in the design, execution, and analysis of the study and in the reviewing of the final manuscript.
Author Contributions: T. A. Moore contributed to conception, design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. I. M. Ahmad contributed to acquisition and analysis, critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. K. K. Schmid contributed to conception, design, and analysis; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. A. M. Berger contributed to conception, design, analysis, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. R. J. Ruiz contributed to conception, design, analysis, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. R. H. Pickler contributed to conception, design, analysis, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. M. C. Zimmerman contributed to conception, design, acquisition, analysis, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by NIH 1K01NR014474-01 and a University of Nebraska Medical Center Edna Ittner Pediatric Support grant.
References
- Ahmad I. M., Temme J. B., Abdalla M. Y., Zimmerman M. C. (2016). Redox status in workers occupationally exposed to long term low levels of ionizing radiation: A pilot study. Redox Report: Communications in Free Radical Research, 21, 139–145. doi:10.1080/13510002.2015.1101891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G. J., Jauniaux E. (2011). Oxidative stress. Best Practice & Research Clinical Obstetrics & Gynaecology, 25, 287–299. doi:10.1016/j.bpobgyn.2010.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2016). Retrieved from https://www.cdc.gov/socialdeterminants/data/index.htm
- Dietze T. R., Rose F. F., Moore T. A. (2016). Maternal variables associated with physiologic stress and perinatal complications in preterm infants. Journal of Neonatal-Perinatal Medicine, 9, 271–277. doi:10.3233/NPM-16915134 [DOI] [PubMed] [Google Scholar]
- Ferguson K. K., McElrath T. F., Chen Y., Loch-Caruso R., Mukherjee B., Meeker J. D. (2015). Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. American Journal of Obstetrics and Gynecology, 212, 208.e1–208.e8. doi:10.1016/j.ajog.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giurgescu C. (2009). Are maternal cortisol levels related to preterm birth? Journal of Obstetric, Gynecologic & Neonatal Nursing, 38, 377–390. [DOI] [PubMed] [Google Scholar]
- Glynn L. M., Schetter C. D., Hobel C. J., Sandman C. A. (2008). Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 27, 43–51. [DOI] [PubMed] [Google Scholar]
- Haas D. M., Parker C. B., Wing D. A., Parry S., Grobman W. A., Mercer B. M.…Willinger M. (2015). A description of the methods of the nulliparous pregnancy outcomes study: Monitoring mothers-to-be (nuMoM2b). American Journal of Obstetrics & Gynecology, 212, 539.e1–539.e24. doi:10.1016/j.ajog.2015.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins C. L., Davies M. J. (2014). Detection and characterisation of radicals in biological materials using EPR methodology. Biochimica Et Biophysica Acta, 1840, 708–721. doi:10.1016/j.bbagen.2013.03.034 [DOI] [PubMed] [Google Scholar]
- Hux V. J., Catov J. M., Roberts J. M. (2014). Allostatic load in women with a history of low birth weight infants: The national health and nutrition examination survey. Journal of Women’s Health, 23, 1039–1045. doi:10.1089/jwh.2013.4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latendresse G. (2009). The interaction between chronic stress and pregnancy: Preterm birth from a biobehavioral perspective. Journal of Midwifery & Women’s Health, 54, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekharu R., Pradhan R., Sharma R., Sharma D. (2014). A study of lipid peroxidation and antioxidant enzymes in normal pregnancy. Journal of Medical Science, 3, 55–56. [Google Scholar]
- McEwen B. S. (1998). Protective and damaging effects of stress mediators. The New England Journal of Medicine, 338, 171–179. [DOI] [PubMed] [Google Scholar]
- Menon R., Fortunato S. J., Milne G. L., Brou L., Carnevale C., Sanchez S. C.…Taylor R. N. (2011). Amniotic fluid eicosanoids in preterm and term births: Effects of risk factors for spontaneous preterm labor. Obstetrics and Gynecology, 118, 121–134. doi:10.1097/AOG.0b013e3182204eaa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. A., Ahmad I. M., Zimmerman M. C. (2018). Oxidative stress and preterm birth: An integrative review. Biological Research for Nursing, 20, 497–512. doi:10.1177/1099800418791028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. A., Berger A. M., Wilson M. E. (2014). A new way of thinking about complications of prematurity. Biological Research for Nursing, 16, 72–82. doi:10.1177/1099800412461563 [DOI] [PubMed] [Google Scholar]
- Moore T. A., Wilson M. E., Schmid K. K., Anderson-Berry A., French J. A., Berger A. M. (2013). Relations between feeding intolerance and stress biomarkers in preterm infants. Journal of Pediatric Gastroenterology and Nutrition, 57, 356–362. doi:10.1097/MPG.0b013e3182953093 [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. (2017). Peristats. Retrieved from www.marchofdimes.org/peristats
- Rosanna D. P., Salvatore C. (2012). Reactive oxygen species, inflammation, and lung diseases. Current Pharmaceutical Design, 18, 3889–3900. [DOI] [PubMed] [Google Scholar]
- Schieber M., Chandel N. S. (2014). ROS function in redox signaling and oxidative stress. Current Biology, 24, R453–R462. doi:10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J. B., Sharma A., Bahadur A., Vimala N., Satyam A., Mittal S. (2006). Oxidative stress markers and antioxidant levels in normal pregnancy and pre-eclampsia. International Journal of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics, 94, 23–27. [DOI] [PubMed] [Google Scholar]
- Sikkema J. M., van Rijn B. B., Franx A., Bruinse H. W., de Roos R., Stroes E. S., van Faassen E. E. (2001). Placental superoxide is increased in pre-eclampsia. Placenta, 22, 304–308. [DOI] [PubMed] [Google Scholar]
- Wallace M. E., Harville E. W. (2012). Predictors of healthy birth outcome in adolescents: A positive deviance approach. Journal of Pediatric and Adolescent Gynecology, 25, 314–321. doi:10.1016/j.jpag.2012.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yüksel S., Yigit A. A. (2015). Malondialdehyde and nitric oxide levels and catalase, superoxide dismutase, and glutathione peroxidase levels in maternal blood during different trimesters of pregnancy and in the cord blood of newborns. Turkish Journal of Medical Sciences, 45, 454–459. [PubMed] [Google Scholar]