Abstract
Devimistat (CPI-613®) is a novel lipoate analog that inhibits the tricarboxcylic acid cycle at two key carbon entry points. Through its inhibition of pyruvate dehydrogenase and a-ketoglutarate dehydrogenase complexes, devimistat inhibits the entry of glucose and glutamine derived carbons, respectively. Pancreatic cancer is dependent on mitochondrial function for enhanced survival and aggressiveness. In a Phase I study of modified FOLFIRINOX, in combination with devimistat for metastatic pancreatic cancer patients, there was a 61% objective response rate including a 17% complete response rate. This report outlines the rationale and design of the AVENGER 500 study, a Phase III clinical trial of devimistat in combination with modified FOLFIRINOX compared with FOLFIRINOX alone for patients with previously untreated metastatic adenocarcinoma of the pancreas. Clinical trial registration: NCT03504423
Keywords: : CPI-613, FOLFIRINOX, metastatic, pancreatic cancer, Phase III
Pancreatic cancer is an extremely deadly disease, with a 5-year survival rate of only 8.5%. In 2018, there were an estimated 55,440 new cases of pancreatic cancer (SEER Database). Despite this relative low incidence, pancreatic cancer became the third leading cause of cancer-related deaths in 2016, and is expected to be the second cause of death by 2030 [1].
Patients with pancreatic cancer most commonly present with abdominal pain, weight loss, asthenia and anorexia. Obstructive jaundice is a common manifestation of tumors in the head of the pancreas [2]. Because the disease typically does not present with recognizable/distinctive symptoms, it is commonly diagnosed at an advanced stage with limited treatment options [2]. Approximately half of pancreatic cancer patients present with metastatic disease for whom there are no curative therapies. Current standard therapy for these patients consists of either gemcitabine combined with nab-paclitaxel or a regimen of 5-fluorouracil (5-FU), irinotecan and oxaliplatin (FOLFIRINOX [FFX]). These regimens produce a median survival of 8.5 and 11.1 months, respectively [3,4].
Pancreatic cancer is driven by KRAS mutations in up to 90% of cases [2]. Mutated KRAS drives multiple metabolic alterations within the tumor cell including increased glycolysis, increased autophagy and a process of macropinocytosis, whereby the tumor actively engulfs and digests material in the extracellular space (reviewed by Ryan et al. [2]). The highly desmoplastic and hypovascularized tumor environment of pancreatic cancer induces metabolic dependencies on many of these mutant KRAS driven alterations (reviewed by Vaziri-Gohar et al. [5]). In this low nutrient environment, pancreatic cancer cells are particularly dependent on mitochondrial function including the tricarboxcylic acid (TCA) cycle [6,7].
Devimistat is a novel nonredox active lipoate analog that mimics the catalytic intermediates of PDH and ketoglutarate dehydrogenase (KGDH) resulting in inhibition of these complexes by regulatory networks [8,9]. In a Phase I trial for patients with previously untreated metastatic pancreatic cancer, devimistat was given in combination with modified FOLFIRINOX (mFFX). In the 18 patients treated at the maximally tolerated dose, the overall objective response rate (ORR) (complete response [CR] + partial response [PR]) was 61%. This was associated with a progression-free survival (PFS) of 9 months and a median overall survival (OS) of 19 months [10]. These data compare favorably with observed response rates of FFX of 31% and a median OS of 11.1 months [3]. Overall, the regimen was well tolerated with the most common grade 3 and 4 toxicities consistent with those seen in previous studies with FFX [10]. These compelling data were the basis for the AVENGER 500 study.
The AVENGER 500 study
This report outlines the design and the rational for the AVENGER 500 study; a multicenter open label, international, Phase III randomized trial (NCT03504423) evaluating the efficacy and safety of devimistat in combination with mFFX compared with FFX in patients with metastatic adenocarcinoma of the pancreas. The study is being conducted consistent with good clinical practice standards and as described in the US Code of Federal Regulation 21 Parts 11, 50, 54, 56 and 312 and the appropriate International Conference on Harmonization guidance documents. All participating sites will have the protocol reviewed and approved by an independent review board (IRB). All subjects will be required to read, understand and sign an IRB-approved informed consent form. An independent data monitoring committee will periodically review the safety and efficacy data.
Objectives
The coprimary objectives are to evaluate the PFS and ORR (CR + PR) of devimistat plus mFFX versus FFX (control arm). These end points were chosen to facilitate an interim futility analysis that would allow for limiting the number of patient recruitment to go on further if the activity of devimistat is less than anticipated. Should the activity of the experimental arm be sufficient to justify completion of the trial, it will be sufficiently powered to test for a difference in PFS. ORR will be evaluated by an independent, blinded, central review according to RECIST guideline version 1.1 [11]. The secondary objectives are to evaluate OS, duration of response (DOR), safety, pharmacokinetics (PK) and patient-reported outcomes (PROs) by FACT hepatobiliary symptom index (FHSI-18). Exploratory objectives include assessment of PK/PD parameters of dose/exposure responses for devimistat on efficacy (e.g., ORR), safety (e.g., corrected Q-T interval) and exploratory biomarkers using diagnostic biopsies and blood/plasma samples.
Study design
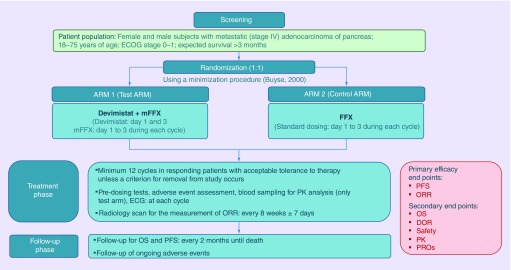
This is a Phase III, global, prospective, open label, multicenter, randomized, two-arm trial comparing CPI-613 in combination with mFFX (test arm or arm 1) with FFX (control arm or arm 2) in patients with metastatic adenocarcinoma of the pancreas. The comparator arms were chosen in consultation with the US FDA. Full dose FFX was felt to be the current standard of care for good performance status patients with metastatic pancreatic cancer. An open label design was chosen as the chemotherapy treatment on each arm differs in dose (irinotecan and oxaliplatin doses are lower on the experimental arm) double blinding the study would have represented significant logistical challenges and clinicians would not know what doses of chemotherapy patients were being treated with. The study consists of a screening/randomization period, a treatment period and a follow-up period (Figure 1). During the screening period, determination of patient eligibility, baseline characteristics, disease evaluation and clinical assessments are performed. Patients of both the sexes with metastatic (stage IV) adenocarcinoma of pancreas with 18–75 years of age, Eastern Cooperative Oncology Group (ECOG) performance status 0–1 and expected survival more than 3 months will be included in this study. A total of 500 patients will be randomized in 1:1 ratio to arm 1 or arm 2 using a minimization procedure [12]. The minimization algorithm will use the variance method to minimize overall imbalances between the treatment arms with respect to center, performance status (0 vs 1) and primary tumor location (head vs body or tail of the pancreas). Patients will be either treated with CPI-613 (days 1 and 3) in combination with mFFX (day 1–3) during each cycle (14 days cycle) or treated with standard dose of FFX (day 1–3) during each cycle (14 days cycle). Treatment will be given for minimum of 12 cycles until confirmed progression, unacceptable toxicity, withdrawal of consent or any other protocol-specified criterion for withdrawal occurs. At each cycle, predosing tests, adverse event assessment, blood sampling for PK analysis (only test arm) and ECG measurement will be conducted. Radiology scan for the measurement of ORR will be done in every 8 weeks ±7 days. When discontinuing study treatment during this trial, the investigator should make every effort to contact the patient and to perform a final evaluation. Also, the reason(s) for withdrawal from the study must be recorded. Survival and poststudy treatment will be documented bimonthly after patient completes treatment on this trial. All patients will be followed until death. Follow-up will be also done for ongoing adverse events. The study opened to accrual in November 2018 and is recruiting patients from approximately 96–105 sites in North America, Europe and Asia-Pacific.
Figure 1. . Avenger 500 trial schema.
DOR: Duration of response; ECG: Electrocardiogram; ECOG: Eastern Cooperative Oncology Group; ORR: Objective response rate; PFS: Progression-free survival; PK: Pharmacokinetics; PROs: Patient-reported outcomes.
Key eligibility criteria
In order to be eligible to participate in this study, an individual must have previously untreated histologically or cytologically confirmed metastatic adenocarcinoma of the pancreas, ECOG performance status 0–1, be between 18 and 75 years of age, have measurable disease, expected survival >3 months, women of child-bearing potential (i.e., women who are premenopausal or not surgically sterile) must use accepted contraceptive methods and must have a negative serum or urine pregnancy test within 1 week prior to treatment initiation, adult subjects of child-bearing potential must agree to use double barrier contraceptive measure, oral contraception or avoidance of intercourse during the study and for 6 months after last study dose is received, at least 2 weeks must have elapsed from any prior surgery with resolution of any sequela. Laboratory values ≤2 weeks prior to randomization must be platelet count ≥100,000 cells/mm3 or ≥100 bil/l; absolute neutrophil count ≥1500 cells/mm3 or ≥1.5 bil/l; hemoglobin ≥9 g/dl or ≥90 g/l; adequate hepatic function (AST/Serum Glutamic-Oxaloacetic Transaminase [SGOT] ≤3 × upper normal limit [UNL]; [ALT/Serum Glutamic Pyruvic Transaminase (SGPT)] ≤3 × UNL [≤5 × UNL if liver metastases present]; bilirubin ≤1.5 × UNL; serum albumin >3.0 g/dl); serum creatinine clearance CLcr >30 ml/min; adequate coagulation function (International Normalized Ratio must be <1.5 unless on therapeutic blood thinners). No evidence of active infection and no serious infection within the past 30 days, mentally competent, ability to understand and willingness to sign the informed consent form.
Key exclusion criteria include: endocrine or acinar pancreatic carcinoma, known cerebral metastases, CNS or epidural tumor, prior treatment with any chemotherapy for metastatic adenocarcinoma of the pancreas, completion of a gemcitabine-based adjuvant chemotherapy regimen within less than 6 months at the time of screening, receipt of neoadjuvant or adjuvant FFX therapy, presence of clinically significant abdominal ascites, patients receiving any other standard or investigational treatment for their cancer or any other investigational agent for any indication within the past 2 weeks prior to initiation of study treatment, serious medical illness that would potentially increase patients’ risk for toxicity, any active-uncontrolled bleeding and any patients with a bleeding diathesis (e.g., active peptic ulcer disease), any condition or abnormality which may, in the opinion of the investigator, compromise the safety of patients, unwilling or unable to follow protocol requirements, active heart disease including but not limited to symptomatic congestive heart failure (NYHA class 3 or 4), symptomatic coronary artery disease, symptomatic angina pectoris or symptomatic myocardial infarction, evidence of active infection, or serious infection within the past 30 days, patients who have received cancer immunotherapy of any type within the past 2 weeks prior to initiation of study treatment (steroids given for supportive care or in response to allergic reactions are allowed at any time), requirement for immediate palliative treatment of any kind including surgery, prior malignancy except for the following: adequately treated basal or squamous cell skin cancer, in situ cervical cancer, adequately treated cancer from which the patient has been disease free for at least 3 years prior to screening, unwilling or unable to avoid the concomitant use of strong CYP3A4 inducers or inhibitors during treatment with irinotecan, marked baseline prolongation of QT/QTc interval (e.g., repeated demonstration of a QTc interval >480 ms (CTCAE grade 1) using Fredericia’s QT correction formula (i.e., QTcF), a history of additional risk factors for TdP (e.g., heart failure, hypokalemia and family history of long QT syndrome), or the use of concomitant medications that prolong the QT/QTc intervals.
Study design & methodology
Before enrolling the patients in the study, informed consent will be obtained from the potential participants in this trial. In the screening period, medical and medication history of the patients will be reviewed, and following prestudy screening tests will be conducted to determine the eligibility of the patient to enrol in this study: physical examinations, evaluation of symptoms, vital signs, ECOG performance status, height, weight, tumor assessments (triphasic contrast computed tomography [CT] of the chest, abdomen and pelvis or MRI), CA19-9, optional blood and serum sampling, hematology, Hemoglobin A1c, coagulation function, renal function, clinical chemistry, ECG, survival assessment and serum pregnancy test for women of child-bearing potential.
After enrolling the patient in the study, before initiation of the treatment, predosing tests will be conducted. These predosing tests are: evaluation of vital signs, ECOG performance status, tumor assessments (triphasic contrast CT of the chest, abdomen and pelvis or MRI), CA19-9, hematology, coagulation function, clinical chemistry, ECG, survival assessment, FSHI-18 questionnaire and blood sampling for PK analysis. Blood sampling for PK analysis will be done only for the patients in test arm. Predosing tests will be conducted in each cycle.
Treatment on the control arm will consist of oxaliplatin, 85 mg/m2; irinotecan, 180 mg/m2; leucovorin, 400 mg/m2 and fluorouracil, 400 mg/m2 given as a bolus followed by 2400 mg/m2 given as a 46-h continuous infusion, every 2 weeks. Treatment on the experimental arm will consist of the same regimen with the following modifications; devimistat 500 mg/m2 will be given on days 1 and 3, oxaliplatin will be given at a dose of 65 mg/m2 and irinotecan will be given at a dose of 120 mg/m2. The dose and schedule of 5-FU and leucovorin is identical to the control arm.
No dose adjustment of CPI-613 is allowed in this study. But in case of toxicity related to mFFX or FFX, dose adjustment is allowed. Separate dose adjustment plan for hematological and nonhematological toxicities has been incorporated in this study. Once a dose of any of the drugs comprising FFX is decreased, re-escalation of the dose is not permitted. Patients are taken off the study if they develop the same Grade 4 toxicity despite dose reduction. Once hematologic toxicity is observed in a patient, it is not allowed to retreat the patient with mFFX or FFX until the absolute neutrophil count is ≥1.5 × 109/l and the platelet count is ≥75 × 109/l achieved. Primary prophylaxis with myeloid growth factor will be at the discretion of the treating physician and as per the ASCO guidelines.
Efficacy evaluations
The primary objectives of the study are to evaluate ORR and PFS of CPI-613 plus mFFX compared with that of FFX (control arm). Tumor response will be assessed by triphasic contrast CT of the chest, abdomen and pelvis every 8 weeks ±7 days while patients are on treatment. MRI assessments of tumor status may replace CT scans if needed for tumor response evaluation. Patients who are allergic to contrast will get an MRI and those allergic to both will have a noncontrasted study. Response category will be determined as per RECIST guideline version 1.1. The patient’s best response within the first 12 cycles of treatment as determined by the independent monitoring board will be used in the recording of the ORR for each patient. PFS is calculated from the date of randomization to the date of confirmed clinical/radiological progression or death from any cause. Patients who are lost to follow-up, withdrawn from follow-up or alive and progression free will be censored at the date of last follow-up. Secondary objectives of the study are to determine OS, DOR, safety, PRO of CPI-613 plus mFFX compared with FFX by FACT hepatobiliary symptom index (FHSI-18) and to assess PK of CPI-613. OS will be monitored every 2 months via telephone contact, review of hospital records, death records, social media, etc. as allowed by local laws after treatment termination. OS will be calculated from the day of randomization. The duration of OS will be measured until the date of death or censored at follow-up. DOR will be calculated from the date of randomization to the date of confirmed clinical/radiological progression or death from any cause. Patients who are lost to follow-up, withdraw from follow-up or alive and progression free will be censored at the date of last follow-up. PRO assessment will be done in every 4 weeks. Summary statistics of the scores will be shown by cycle (pre and post-treatment). Scores will be compared between standard and study arms.
PK evaluations
PK analysis of CPI-613 and its metabolites CPI-2850 and CPI-1810 will be performed in this study. PK sampling will be done only from the patients in the experimental arm. Blood sampling from 24 patients will be done for full PK analysis and from the remaining 226 patients for sparse PK analysis. The 24 patients in arm 1 assigned to the full PK analysis will also undergo a full ECG analysis. ECG time points are identical to PK time points for both full and sparse analysis. ECG intervals will be measured at a central ECG laboratory fully blinded to time points and subject identification. The remaining patients in arm 1 and all patients in arm 2 will undergo a sparse ECG analysis. This sparse ECG measurement will be measured at a local ECG laboratory and confirmed by central read. The full PK sample schedule will collect a sample at the time points outlined in Table 1. The sparse PK sample schedule is a single PK sample taken on day one of cycles 1–12.
Table 1. . Full pharmacokinetics schedule.
| Cycle # Day # | Time point | N | Comment |
|---|---|---|---|
| Cycle 1, Day 1 | 0 h ± 15 min (predose) 0.25 h ± 15 min 0.5 h ± 15 min 1 h ± 15 min 2 h ± 15 min 2.5 h ± 15 min 3 h ± 15 min 4 h ± 15 min 8 h ± 15 min 24 h ± 15 min |
24 patients in Arm 1 (CPI-613 + mFFX) |
Pre-infusion Day 2 |
| Cycle 1, Day 3 | 0 h ± 15 min (predose) 0.25 h ± 15 min 0.5 h ± 15 min 1 h ± 15 min 2 h ± 15 min 2.5 h ± 15 min 3 h ± 15 min 4 h ± 15 min 8 h ± 15 min 24 h ± 15 min 48 h ± 15 min |
24 patients above | Pre-infusion Day 4 Day 5 |
| Cycle 2, Day 1 | 0 h ± 15 min (predose) 0.25 h ± 15 min 0.5 h ± 15 min |
24 patients above | Pre-infusion |
| Cycle 2–12, Day 3 | 0 h ± 15 min (predose) 0.25 h ± 15 min 0.5 h ± 15 min |
24 patients above | Pre-infusion |
mFFX: Modified FOLFIRINOX; N: Number.
Pharmacodynamic/exploratory samples
Pharmacodynamic/exploratory samples are optional and a separate informed consent will be provided to patients. Blood samples will be obtained prior to treatment initiation (within 4 weeks prior to first dose), as well as prior to each restaging scan. All samples for biomarkers will be retained for up to 10 years after the end of the study and then destroyed or an extension will be requested from appropriate regulatory authorities and IRB/Institutional Ethics Committees (IECs). Samples will be used for RNA sequencing analysis, IHC for candidate biomarkers including mitochondrial SOD2, PDK1-4, PDH, KGDH and CD79a and whole-exome sequencing. Blood samples will be used as a source of circulating tumor cells and germline DNA for single nucleotide polymorphism (SNP) analysis for predictors of response, resistance or toxicity. Serum samples will be utilized to analyze the concentrations of systemic metabolites.
Safety evaluations
The Cancer Therapy Evaluation Program Active Version of the NCI Common Terminology Criteria for Adverse Events (CTCAE 4.0) will be utilized for adverse event (AE) reporting. Safety data will be reviewed by the data safety monitoring board (DSMB) at least every 6 months.
Planned study period
Dosing in both arms will continue for at least 6 months in responding patients with acceptable tolerance to therapy (with dose modifications per protocol as needed) unless a criterion for removal from study occurs. Responding patients upon completion of at least ten cycles and after having two sequential CT scans showing stable disease (no continued decrease in lesion size) will complete two additional cycles (to complete a minimum of 12 cycles) of their assigned treatment and then oxaliplatin can be dropped at the discretion of the treating physician if not already omitted. Cycles will continue until one of the criteria for removal from study are reached as outlined above.
Statistical plan
The study will recruit 500 patients over a period of 36 month, and up to three interim analyses. An interim analysis will be done when 167 patients are evaluable for response, about 20 months after starting accrual, when approximately half of the required sample size is randomized (250 patients). The difference in ORR will be tested at the interim analysis using a Lan–DeMets Pocock type boundary for futility and efficacy. Futility will be declared (and the trial potentially stopped on the recommendation of the Independent Data Monitoring Committee) if the difference in ORR between the randomized groups is smaller than 5%, while efficacy will be declared if the difference in ORR between the randomized groups is larger than 20%, but even in this case the trial will continue until the full sample size of 500 patients are accrued. At the time interim analysis, about 125 PFS events will be available (33% information fraction). The PFS hazard ratio will be tested using a Lan–DeMets O’Brien–Fleming type boundary for efficacy. Efficacy will be declared if the PFS hazard ratio is less than 0.48, but even in this case the trial will continue until the full sample size of 500 patients are accrued. The final analysis will be done with 500 patients randomized about 40 months after starting accrual, when approximately 375 PFS events are available. Statistical significance will be reached if the PFS hazard ratio is less than 0.80, or the difference in ORR at least 11%. If the trial reaches statistical significance for either of the primary end points, OS will also be tested as a key secondary end point. At the time of the final analysis, about 290 deaths will be available (77% information fraction). The OS hazard ratio will be tested using a Lan–DeMets O’Brien–Fleming type boundary for efficacy. Conditional on PFS reaching statistical significance, efficacy will be declared if the OS hazard ratio is less than 0.74. Should OS fail to reach statistical significance, patients will be followed for death an additional period of 15 months, at which time about 381 deaths will be available, and efficacy declared for OS if the OS hazard ratio exceeds 0.80.
Discussion
Devimistat is a novel lipoate analog. It simultaneously inhibits PDH and KGDH, thereby blocking the entry of either glucose or glutamine derived carbons entry into the TCA cycle. The AVENGER 500 trial seeks to leverage this unique mechanism of action to improve the outcomes of patients suffering from metastatic pancreatic cancer. Multiple clinical trials utilizing a variety of chemotherapy combinations have shown that the response of pancreatic cancer is limited at best with limited data to support a survival benefit [13]. This is likely from a combination of tumor intrinsic and extrinsic resistance factors. The high rate of p53 mutations in pancreatic cancer contributes to resistance to chemotherapy with studies showing cells with mutated p53 have lower response to chemotherapy [14]. This is exacerbated by the highly desmoplastic microenvironment where supporting stromal cells can contribute nutrients and survival signaling to help promote tumor cell survival [15]. This data in combination with a large number of clinical studies have suggested pancreatic cancer is not ideally treated with chemotherapy alone. While the fibrotic microenvironment may be an advantage in resistance to chemotherapy it also engenders metabolic constraints on the tumor. Several studies have shown that autophagy is essential in both the tumor cell as well as in the supporting stroma consistent with metabolic stress [15,16]. Devimistat inhibits the TCA cycle and may be particularly effective against tumors that have baseline metabolic stress like pancreatic cancer. The Phase I study of devimistat plus mFFX enrolled 18 patients and showed a median OS of 19.9 months and median PFS of 9.9 months. Response rates were likewise encouraging with the CR, PR, stable disease rates of 17, 44 and 17%, respectively [10]. These data support mitochondrial metabolism as an important target for pancreatic cancer patients. The AVENGER 500 trial will provide important data on the validity of this approach and further our understanding of the role of mitochondrial metabolism in therapy response in first-line metastatic pancreatic cancer.
Executive summary.
Background
Mitochondrial metabolism is altered in tumor cells including pancreatic cells.
The tricarboxcylic acid cycle is a source of resistance to DNA damaging agents in pancreatic cancer.
Devimistat
Is a novel lipoate analog that inhibits two key tricarboxcylic acid cycle enzymes, pyruvate dehydrogenase and ketoglutarate dehydrogenase.
Devimistat impairs pancreatic cell mitochondrial metabolism and sensitizes cells to DNA damaging agents.
Devimistat has shown promising results when combined with modified FOLFIRINOX (mFFX).
Phase III trial
AVENGER 500 is a randomized, international, Phase III study.
The primary objective is to evaluate progression-free survival and objective response rate (complete response + partial response) of CPI-613 plus mFFX versus FFX (control arm) by independent, blinded, central review by RECIST guideline version 1.1.
Secondary objective is to evaluate overall survival, duration of response, safety, pharmacokinetics and patient-reported outcomes.
Key eligibility criteria
First-line metastatic pancreatic cancer patients (18–75-years-old) with Eastern Cooperative Oncology Group performance status 0–1.
Study design
Eligible patients are randomized 1:1 to devimistat plus mFFX or to FOLFIRINOX.
Efficacy & PK evaluations
Measurement of tumor response as per RECIST guideline version 1.1.
PK samples will be collected on either a full (intensive) or sparse schedule at defined time points following administration of devimistat.
Discussion
Outcomes in first-line metastatic pancreatic cancer are poor and novel approaches are needed.
The AVENGER 500 trial will provide important new insights into the activity of an approach that combines DNA damaging agents with mitochondrial metabolism inhibition.
Acknowledgments
The authors would like to acknowledge insightful comments from the staff at Rafael Pharmaceuticals.
Footnotes
Financial & competing interests disclosures
AT Alistar has been a consultant for Rafael Pharmaceuticals, S Luther and TS Pardee are employees of Rafael Pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet 388(10039), 73–85 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N. Engl. J. Med. 371(11), 1039–1049 (2014). [DOI] [PubMed] [Google Scholar]; • An excellent review on pancreatic cancer outlining the clinical and biological features of the disease.
- 3.Conroy T, Desseigne F, Ychou M. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364(19), 1817–1825 (2011). [DOI] [PubMed] [Google Scholar]; • Reports the Phase III results of FOLOFIRINOX in metastatic pancreatic cancer patients.
- 4.Von Hoff DD, Ervin T, Arena FP. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 369(18), 1691–1703 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaziri-Gohar A, Zarei M, Brody JR, Winter JM. Metabolic dependencies in pancreatic cancer. Front. Oncol. 8, 617 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birsoy K, Possemato R, Lorbeer FK. et al. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 508(7494), 108–112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Son J, Lyssiotis CA, Ying H. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496(7443), 101–105 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zachar Z, Marecek J, Maturo C. et al. Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. J. Mol. Med. (Berl.) 89(11), 1137–1148 (2011). [DOI] [PubMed] [Google Scholar]; • Reports on the basic mechanism of devimistat in preclinical models.
- 9.Bingham PM, Stuart SD, Zachar Z. Lipoic acid and lipoic acid analogs in cancer metabolism and chemotherapy. Expert Rev. Clin. Pharmacol. 7(6), 837–846 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Alistar A, Morris BB, Desnoyer R. et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, Phase I trial. Lancet Oncol. 18(6), 770–778 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the activity of the combination of devimistat and modified FOLFIRINOX in the Phase I study.
- 11.Therasse P, Arbuck SG, Eisenhauer EA. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 92(3), 205–216 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials. a review. Control. Clin. Trials 23(6), 662–674 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Chin V, Nagrial A, Sjoquist K. et al. Chemotherapy and radiotherapy for advanced pancreatic cancer. Cochrane Database Syst. Rev. 3, CD011044 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorini C, Cordani M, Padroni C, Blandino G, Di Agostino S, Donadelli M. Mutant p53 stimulates chemoresistance of pancreatic adenocarcinoma cells to gemcitabine. Biochim. Biophys. Acta 1853(1), 89–100 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Yang A, Herter-Sprie G, Zhang H. et al. Autophagy sustains pancreatic cancer growth through both cell-autonomous and nonautonomous mechanisms. Cancer Discov. 8(3), 276–287 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Details the dependency of pancreatic cancer on autophagy in preclinical model systems.
- 16.Yang A, Rajeshkumar NV, Wang X. et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 4(8), 905–913 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]