Abstract
With the introduction of new drugs with different mechanisms of action, multiple myeloma (MM) patients’ outcomes have improved. However, the efficacy seen in clinical trials is often not seen in real-world settings and data on the effectiveness of MM therapies are needed. INSIGHT MM is a prospective, global, non-interventional, observational study that is enrolling approximately 4200 patients with newly diagnosed or relapsed/refractory MM, making it the largest study of its kind to date. The study aims to describe contemporary, real-world patterns of patient characteristics, clinical disease presentation, therapies chosen, clinical outcomes (response, treatment duration, time-to-next-therapy, progression-free and overall survival), safety, healthcare resource utilization and quality of life. One interim analysis has been conducted to date; current accrual is approximately 3094 patients.
Trial registration number: NCT02761187
Keywords: : multiple myeloma, observational study, treatment
Lay abstract
Survival of patients with myeloma, a type of bone marrow cancer affecting the production of healthy blood cells, has improved due to the development and approval of new treatments. Treatments are approved based on clinical trials; however, limited data are available that confirm the effectiveness of these same treatments in real-world routine clinical practice. INSIGHT MM is a large, global study that will follow approximately 4200 myeloma patients from real-world settings for a minimum of 5 years, describing their clinical characteristics, type of therapy received and their response to treatment, including survival and toxicity. This review describes the study aims and methods.
Multiple myeloma (MM) is the second most common hematologic malignancy, with an estimated incidence of 30,770 new cases and 12,770 deaths in the USA in 2018 [1] and an estimated 48,300 new cases in Europe in 2018 [2]. Survival rates have improved dramatically over the past 20 years; the United States National Cancer Institute SEER program estimated a 5-year relative survival of 29.3% for patients with MM who were diagnosed in 1990–1992, compared with 52.4% for those diagnosed in 2008–2014 [3]. In the UK, the estimated 5-year net survival for male and female patients diagnosed with MM between 2011 and 2015, was 51.9% and 50.8%, respectively, compared with 22.4% and 21.9%, respectively, for those diagnosed between 1990 and 1991 [4,5]. In a Swedish population-based study, the 5-year relative survival rate improved from 0.28 to 0.41 for patients diagnosed with MM in 1973–1982 and 2003–2013, respectively [6]. Furthermore, MM is two- to threefold more common in African–Americans (AAs) compared with European–Americans and in AAs it is the most common hematologic malignancy, a disparity that may be due to a different underlying genetic predisposition between these groups [7].
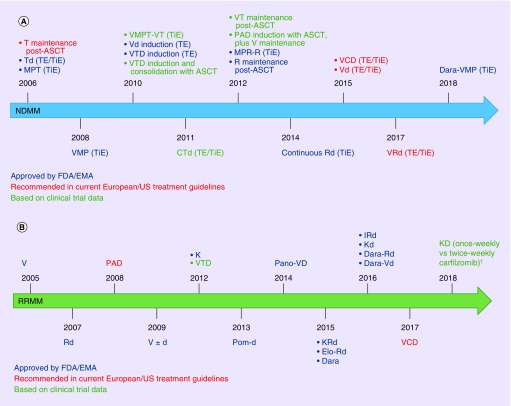
This improvement in outcomes for patients with MM has been attributed to the introduction of high-dose chemotherapy plus autologous stem cell transplant, as well as the development, approval and use of multiple new drugs and combination regimens in both the newly diagnosed MM (NDMM) and relapsed/refractory MM (RRMM) settings [8]. Over the past 20 years, 12 therapies and agents with different mechanisms of action, including immunomodulatory drugs, proteasome inhibitors (PIs), monoclonal antibodies (mAbs), as well as a histone deacetylase (HDAC) inhibitor, a Bcl-2 inhibitor and a selective inhibitor of nuclear export (SINE) agent, have been approved or have entered advanced phases of clinical testing, thus expanding the potential treatment armamentarium against MM [8,9]. A list of key agents and regimens that are approved/recommended [10–12] or have been/are being investigated in clinical trials is reported in Tables 1, 2 and Figure 1. This is not an exhaustive list but provides an overview of the many agents and regimens available for MM treatment.
Table 1. . Key agents and regimens that are approved/recommended for newly diagnosed multiple myeloma and relapsed/refractory multiple myeloma.
| Class | Agent | Initial approval | NDMM regimens | RRMM regimens |
|---|---|---|---|---|
| Proteasome inhibitors | Bortezomib | 2003 US FDA, 2004 EMA | Vd, VCd, VTd, VRd, VMP, PAD | V ± d, VCd, PAD, VTd, VRd, Benda-Vd, V-Pom-d |
| Carfilzomib | 2012 FDA, 2015 EMA | KRd, KCd | Kd, KRd, KCd, K-Pom-d | |
| Ixazomib | 2015 FDA, 2016 EMA | IRd | IRd, Id, I-Pom-d | |
| Immunomodulatory drugs | Thalidomide | 2006 FDA, 2008 EMA | Td, MPT, CTd, VTd, VTd-PACE | VTd-PACE |
| Lenalidomide | 2006 FDA, 2007 EMA | Rd, MPR-R, CRd | Rd, Benda-Rd, CRd | |
| Pomalidomide | 2013 FDA and EMA | – | Pom-d, Pom-Cd | |
| Monoclonal antibodies | Daratumumab | 2015 FDA, 2016 EMA | Dara-VMP | Dara-Rd, Dara-Vd, Dara, Dara-Pom-d |
| Elotuzumab | 2015 FDA, 2016 EMA | – | Elo-Rd, Elo-Vd, Elo-Pom-d | |
| Histone deacetylase inhibitors | Panobinostat | 2015 FDA and EMA | – | Pano-Vd, Pano-K, Pano-Rd |
Benda-Rd: Bendamustine, lenalidomide, dexamethasone; Benda-Vd: Bendamustine, bortezomib, dexamethasone; CRd: Cyclophosphamide, lenalidomide, dexamethasone; CTd: Cyclophosphamide, thalidomide, dexamethasone; d: Dexamethasone; Dara: daratumumab; Dara-pom-d: Daratumumab, pomalidomide, dexamethasone; Dara-Rd: Daratumumab, lenalidomide, dexamethasone; Dara-Vd: Daratumumab, bortezomib, dexamethasone; Dara-VMP: Daratumumab, bortezomib, melphalan, prednisone; Elo-Pom-d: Elotuzumab, pomalidomide, dexamethasone; Elo-Rd: Elotuzumab, lenalidomide, dexamethasone; Elo-Vd: Elotuzumab, bortezomib, dexamethasone; EMA: European Medicines Agency; Id: Ixazomib, dexamethasone; I-Pom-d: Ixazomib, pomalidomide, dexamethasone; IRd: Ixazomib, lenalidomide, dexamethasone; KCd: Carfilzomib, cyclophosphamide, dexamethasone; Kd: Carfilzomib, lenalidomide; K-Pom-d: Carfilzomib, pomalidomide, dexamethasone; KRd: Carfilzomib, lenalidomide, dexamethasone; MPR-R: Melphalan, prednisone, lenalidomide, plus lenalidomide maintenance; MPT: Melphalan, prednisone, thalidomide; NDMM: Newly diagnosed multiple myeloma; PAD: Bortezomib, doxorubicin, dexamethasone; Pano-K: Panobinostat, carfilzomib; Pano-Rd: Panobinostat, lenalidomide, dexamethasone; Pano-Vd: Panobinostat, bortezomib, dexamethasone; Pom-Cd: Pomalidomide, cyclophosphamide, dexamethasone; Pom-d: Pomalidomide, dexamethasone; Rd: Lenalidomide, dexamethasone; RRMM: Relapsed/refractory multiple myeloma; Td: Thalidomide, dexamethasone; V: Bortezomib; VCd: Bortezomib, cyclophosphamide, dexamethasone; Vd: Bortezomib, dexamethasone; VMP: Bortezomib, melphalan, prednisone; V-Pom-d: Bortezomib, pomalidomide, dexamethasone; VRd: Bortezomib, lenalidomide, dexamethasone; VTd: Bortezomib, thalidomide, dexamethasone; VTd-PACE: Bortezomib, thalidomide, dexamethasone, cisplatin, doxorubicin, cyclophosphamide, etoposide.
Table 2. . Agents and regimens that have been/are being investigated in clinical trials and are not yet approved for multiple myeloma treatment.
| Class | Agent | NDMM regimens | RRMM regimens |
|---|---|---|---|
| Proteasome inhibitors | Carfilzomib | KMP (NCT01818752), KCRd (NCT01554852) |
KD once-weekly versus twice-weekly (NCT02412878)† |
| Ixazomib | ITd (NCT03608501) | ITd (NCT02410694) | |
| Monoclonal antibodies | Daratumumab | Dara-Rd (NCT02252172), Dara-RVd (NCT02874742), Dara-VTd (NCT02541383) |
Dara-Kd (NCT03158688) |
| Elotuzumab | Elo-Rd (NCT01335399) | Elo-Td (NCT01632150) | |
| Isatuximab | Isa-RVd (NCT03319667) | Isa-Pom-d (NCT02990338), Isa-Kd (NCT03275285) |
|
| BCL-2 inhibitor | Venetoclax | – | Venetoclax-Vd, Venetoclax-Dara-Vd (NCT03701321) |
| SINE agent | Selinexor | – | Selinexor-Vd (NCT03110562) |
| Checkpoint inhibitors | Pembrolizumab | Pembrolizumab-Rd (NCT02579863) | Pembrolizumab-Rd (NCT02036502), Pembrolizumab-Pom-d (NCT02576977) |
| Adoptive cell therapy | Anti-CD19-CAR-T | – | Anti-CD19-CAR-T (NCT02135406) |
| Anti-CD138-CAR-T | Anti-CD138-CAR-T (NCT01886976) | ||
| Anti-BCMA-CAR-T | – | Anti-BCMA-CAR-T (NCT02215967, NCT02658929, NCT03090659, NCT02546167) | |
| ADC | Anti-BCMA-ADC | – | Anti-BCMA-ADC (NCT02064387) |
| BiTE | Anti-BCMA/CD3-BiTE | – | Anti-BCMA/CD3-BiTE (NCT02514239) |
†Carfilzomib 20/70 mg/m2 administered once-weekly, carfilzomib 20/27 mg/m2 administered twice-weekly.
ADC: Antibody–drug conjugate; BCL-2: B-cell lymphoma 2; BCMA: B cell maturation antigen; BiTE: Bi-specfic T-cell engager; CAR-T: Chimeric antigen receptor T cells; CD: Cluster of differentiation; Dara-Kd: Daratumumab, carfilzomib, dexamethasone; Dara-Rd: Daratumumab, lenalidomide, dexamethasone; Dara-RVd: Daratumumab, lenalidomide, bortezomib, dexamethasone; Dara-Vd: Daratumumab, bortezomib, dexamethasone; Dara-VTd: Daratumumab, bortezomib, thalidomide, dexamethasone; Elo-Rd: Elotuzumab, lenalidomide, dexamethasone; Elo-Td: Elotuzumab, thalidomide, dexamethasone; Isa-Kd: Isatuximab, carfilzomib, dexamethasone; Isa-Pom-d: Isatuximab, pomalidomide, dexamethasone; Isa-RVd: Isatuximab, lenalidomide, bortezomib, dexamethasone; ITd: Ixazomib, thalidomide, dexamethasone; KCRd: Carfilzomib, cyclophosphamide, lenalidomide, dexamethasone; KD: Carfilzomib, dexamethasone; KMP: Carfilzomib, melphalan, prednisone; MM: Multiple myeloma; NDMM: Newly diagnosed MM; Pom-d: Pomalidomide, dexamethasone; Rd: Lenalidomide, dexamethasone; RRMM: Relapsed/refractory MM; SINE: Selective inhibitor of nuclear export; Vd: Bortezomib, dexamethasone.
Figure 1. . Timeline of key agents and treatment combinations that are approved/recommended or have been investigated in Phase III clinical trials.
In the (A) NDMM and (B) RRMM settings.
Not all regimens or agents are approved or available in all countries.
†Carfilzomib twice-weekly (20/27 mg/m2) versus once-weekly (20/70 mg/m2).
ASCT: Autologous stem cell transplant; CTd: Cyclophosphamide, thalidomide, dexamethasone; d: Dexamethasone; Dara: Daratumumab; Dara-Rd: Daratumumab, lenalidomide, dexamethasone; Dara-Vd: Daratumumab, bortezomib, dexamethasone; Dara-VMP: Daratumumab, bortezomib, melphalan, prednisone; Elo-Rd: Elotuzumab, lenalidomide, dexamethasone; EMA: European Medicines Agency; EMN: European Myeloma Network; ESMO: European Society for Medical Oncology; IRd: Ixazomib, lenalidomide, dexamethasone; K: Carfilzomib; Kd/D, carfilzomib, dexamethasone; KRd: Carfilzomib, lenalidomide, dexamethasone; MPR-R: Melphalan, prednisone, lenalidomide, plus lenalidomide maintenance; MPT: Melphalan, prednisone, thalidomide; NDMM: Newly diagnosed multiple myeloma; PAD: Bortezomib, doxorubicin, dexamethasone; Pano-VD: Panobinostat, bortezomib, dexamethasone; Pom-d: Pomalidomide, dexamethasone; R: Lenalidomide; Rd: Lenalidomide, dexamethasone; RRMM: Relapsed/refractory multiple myeloma; T: Thalidomide; Td: Thalidomide, dexamethasone; TE: Transplant-eligible; TiE: Transplant-ineligible; V: Bortezomib; VCD: Bortezomib, cyclophosphamide, dexamethasone; Vd: Bortezomib, dexamethasone; VMP: Bortezomib, melphalan, prednisone; VMPT: Bortezomib, melphalan, prednisone, thalidomide; VRd: Bortezomib, lenalidomide, dexamethasone; VT: Bortezomib, thalidomide; VTD: Bortezomib, thalidomide, dexamethasone.
Mirroring the emergence of new therapies with different mechanisms of action, MM treatment strategies have evolved, with more complex combination regimens being used, such as triplets versus doublets [13]. Additionally, there is now an increased use of continuous or long-term maintenance therapy approaches, which have been shown in clinical trials to prolong survival and delay disease progression, compared with fixed-duration or short-term treatment plans [14].
As MM treatment options become more complex, choosing an appropriate agent or combination of agents in routine clinical practice becomes increasingly challenging [15]. Although recommendations and guidelines for treatment sequencing are available, these vary between countries and global regions [10,12], and different agents and regimens are approved in different countries; as a result, there is currently no uniform, global standard-of-care treatment for patients with MM, in either the NDMM or RRMM setting.
Background & rationale
In this context, the availability of real-world data is increasingly important to our understanding of global variations in treatment practices and the impact of anti-MM therapies in the real-world setting. Despite the efficacy improvements seen with anti-MM agents in the context of clinical trials, the same results are not always achieved in the real-world setting in terms of the effectiveness of a regimen, with poorer long-term outcomes reported for real-world patients versus clinical trial-enrolled patients [16,17]. This discrepancy is likely due to multiple factors, including the rigorous study protocols followed in clinical trials [16] and the strict eligibility criteria used to select patients for enrollment [18]. For example, of the patients enrolled in the CONNECT-MM registry, 40% were found to be ineligible for inclusion in randomized controlled clinical trials (RCT) based on common exclusion criteria (M-protein ≤1.0 g/dl, creatinine >2.5 mg/dl, low absolute neutrophil count [≤1.5 × 109/l] and low hemoglobin [≤8 g/dl]) [19]. Importantly, RCT-ineligible patients had a significantly lower 3-year survival rate than RCT-eligible patients (63% vs 70%; p < 0.05) [19], suggesting that clinical trial findings may not translate to the overall MM population. In the CoMMpass registry, 22% of patients met the study definition of ineligibility for clinical trials [20]. A recent analysis estimated that only 25%, 35%, 41% and 47%, respectively, of USA real-world patients meet the eligibility criterial of the ASPIRE (NCT01080391), TOURMALINE-MM1 (NCT01564537), ELOQUENT-2 (NCT01239797) and POLLUX (NCT02076009) RCTs, investigating lenalidomide/dexamethasone-based triplets in RRMM [21]. Additionally, specific patient populations, such as elderly or frail patients, patients with comorbidities, advanced disease or aggressive and rapidly progressing disease, as well as some ethnic or racial minorities, are typically under-represented in clinical trials [19,22,23], and their omission may therefore contribute to the gap between efficacy and effectiveness. Other factors such as toxicities, the burden of long-term therapy, differences in clinical care and practice patterns between treatment centers, as well as access to and cost of therapy in real-world settings may also play a role in the observed gap between trial-based and real-world outcomes [16,24].
This gap between efficacy and effectiveness highlights the importance of collecting real-world data on MM disease characteristics, treatment patterns and outcomes for anti-MM agents on a country-specific and global level from unselected patient populations, to better inform treatment decisions in everyday clinical practice. Real-world data for agents such as PIs, immunomodulatory drugs, mAbs and HDAC inhibitors are emerging in MM [17,25–29], and a variety of methods have been used in the past approximately 10 years to collect and analyze real-world datasets, including pharmaceutical company-sponsored prospective/retrospective registries and formal observational studies (Table 3), as well as national registries, administrative claims studies and retrospective chart reviews [16].
Table 3. . Selection of key non-interventional, observational, registry studies in multiple myeloma.
| Registry/observational study | N (for MM patients) | Enrollment period | Patients | Geographies | Study sites | Reported race, % | Theoretical ineligibility for interventional clinical trials, %† | HRQoL assessment | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| INSIGHT MM (NCT02761187) | Current accrual of ∼3094 (based on data cut-off of 31 Oct 2018) of planned ∼4200 | July 2016–July 2019 | NDMM RRMM |
Global: USA, Europe, South America, Central America, Middle East, East Asia | 137 | 77% white, 8% black or AA, 11% Asian‡ |
39 | EORTC QLQ-C30 (Global Health Status/QoL Subscale); EORTC QLQ-MY20 (item on PN); TSQM-9 (effectiveness, convenience and global satisfaction domains) – at baseline and quarterly for a minimum of 5 years of follow-up |
[34,44] |
| CoMMpass (NCT01454297) | 1154 (actual enrollment) | July 2011–ongoing | NDMM (PI or IMiD drug, or both for initial therapy) | USA, Canada, Spain, Italy | 73 | 76% white§ | 22¶ | EORTC QLQ-C30 and QLQ-MY20 – at baseline and during 5–8 years of follow-up |
[45,46] |
| Connect MM® (NCT01081028) | 3011 (actual enrollment) | September 2009–April 2016 | NDMM | USA | 253 | 83% white†† | 40‡‡ | HRQoL in pts with NDMM – over a period of ≥8 years | [19,47,48] |
| EMMOS (NCT01241396) | 2358 (actual enrollment) | October 2010–October 2012 | NDMM RRMM |
22 countries in Europe and Africa (19 in Europe, plus Israel, Algeria, South Africa) | 161 | NR | NR | HRQoL | [49] |
| PREAMBLE (NCT01838512) | 1075 (actual enrollment) of planned 1700 | June 2012–ongoing | RRMM (PI, IMiD drug, or both for next therapy) | USA, Canada, Western Europe | 81 | NR | NR | HRQoL and work productivity and activity – over a period of ≤6 years | [50–52] |
| CLARITY (NCT03190525) | 312 (estimated enrollment) | November 2017–ongoing | RRMM | Italy | 45 | NR | NR | EORTC QLQ-C30 and QLQ-MY20 by type of treatment, and the EORTC QLQ-INFO25 – at baseline, and thereafter at 3, 6, 12 and 24 months | [53] |
| Czech RMG | >6000 (actual enrollment) | 2007–ongoing | MM (NDMM and RRMM), MGUS, plasma cell leukemia, primary AL amyloidosis and WM | Czech Republic and Slovakia | 23 | NR | NR | NR | [27,36,54 - Personal communication, unpublished data] |
| Australian and New Zealand MRDR | ∼1800 (actual enrollment) | 2012–ongoing | NDMM, MGUS, solitary plasmacytoma or plasma cell leukemia | Australia and New Zealand | 23 | NR | NR | EQ-5D-5L | [37,55 - Personal communication, unpublished data] |
| Spanish Compassionate Use Registry | 111 (actual enrollment) | 2006–2008 | RRMM (R/Rd) | Spain | 19 | NR | NR | NR | [56] |
| Dutch PHAROS registry | 1522 (actual enrollment) | 2004–2012 | NDMM and RRMM (V-/T-/R-based regimens) |
The Netherlands | NR | NR | NR | NR | [57] |
| Dutch population-based registry | 82 (actual enrollment) | NR | RRMM (Pom-d) | The Netherlands | NR | NR | NR | NR | [58] |
†Percentage of enrolled patients in each study who would not be eligible for an RCT, based on common RCT eligibility criteria.
‡Based on cohort of 1000 patients.
§Based on cohort of 799 patients.
¶Based on cohort of 848 patients.
††Based on a cohort of 2912 patients
‡‡Based on cohort of 1406 patients.
AA: African–American; AL: amyloid light-chain; EORTC: European Organization for Research and Treatment of Cancer; EQ-5D-5L: EuroQoL 5-dimension 5-level; HRQoL: Health-related quality of life; IMiD: Immunomodulatory drug; MGUS: Monoclonal gammopathy of undetermined significance; MM: Multiple myeloma; MRDR: Myeloma and Related Diseases Registry; NDMM: Newly diagnosed MM; NR: Not reported; PI: Proteasome inhibitor; PN: Peripheral neuropathy; Pom-d: Pomalidomide, dexamethasone; pts: Patients; QLQ-C30: Quality of Life Questionnaire – Core 30 module; QLQ-INFO25: Quality of Life Questionnaire – 26-item information module; QLQ-MY-20: Quality of Life Questionnaire – 20-item Multiple Myeloma Module; R: lenalidomide; RCT: Randomized controlled trial; Rd: Lenalidomide, dexamethasone; RMG: Registry of Monoclonal Gammopathies; RRMM: Relapsed/refractory MM; T: Thalidomide; TSQM-9: Treatment Satisfaction Questionnaire for Medication 9; V: bortezomib; WM: Waldenström macroglobulinaemia.
Nevertheless, currently available data on clinical presentation, treatment patterns and outcomes for MM in the community setting at a global level, as well as on patient-reported aspects such as health-related quality of life (HRQoL), are limited. Therefore, additional real-world data are needed to bridge the gap between clinical trial efficacy and routine clinical practice effectiveness of anti-MM agents and regimens, so that treating physicians can effectively incorporate these new therapies into existing treatment algorithms and ultimately improve patient outcomes [30].
INSIGHT MM study
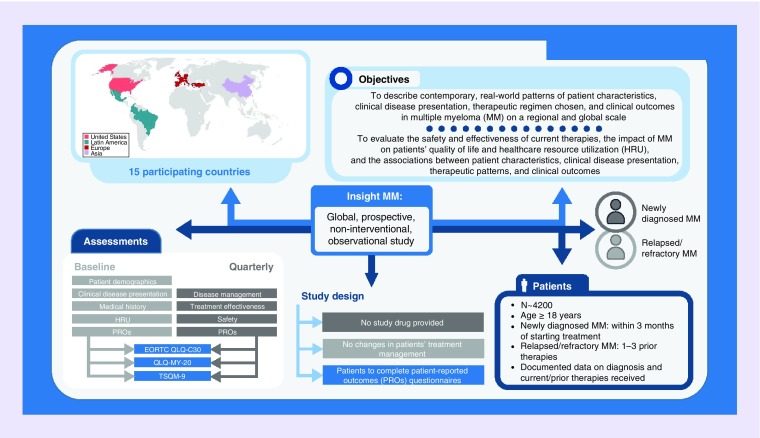
Here we describe the design and rationale for the INSIGHT MM study (NCT02761187), a large, global, prospective, non-interventional, observational study of patients with NDMM or RRMM. This is the largest and the first truly global study of its kind to date, incorporating multiyear enrollment and follow-up.
Objectives
INSIGHT MM is enrolling both NDMM and RRMM patients, and the primary objective of the study is to gain a better understanding of patient and disease characteristics both at diagnosis and at relapse, treatment regimens prescribed and associated patient outcomes in the real-world setting. Specific areas of interest include demographic data, symptoms at presentation, disease characteristics at baseline and at relapse, and assessment of the impact of MM and treatment on patients’ HRQoL and on healthcare resource utilization (HRU). Other objectives include: assessment of patient outcomes based on cohort (NDMM or RRMM), type of treatment facility (academic vs community, with academic centers being defined as those associated with a university or academic hospital), and region or country of enrollment; evaluation of the treatment regimens used; assessment of the factors associated with initiation or modification of treatment; evaluation of the associations between patient characteristics, clinical disease presentation, therapeutic regimen chosen and clinical outcomes.
Study design
INSIGHT MM is a non-interventional study and no study drug or medications are being provided. Patients are to be treated according to the current standard of care at the study site and no changes in patients’ treatment or management are required. Each patient's care is determined entirely by his/her healthcare provider. However, patients are required to complete patient self-reported outcomes (PRO) questionnaires during routine on-site clinic visits. While participating in this study, patients can also be enrolled in another observational or non-blinded, interventional study that does not prohibit participation in this trial. Eligible patients are being identified and followed prospectively. Information on patient characteristics, diagnosis and previous treatment is collected from hospital or clinic records at baseline; MM-specific disease management, safety and outcomes data are obtained during routine on-site clinic visits on a quarterly basis.
A global steering committee has been established to provide support for the study, to advise on protocol development, study conduct and activity, and to ensure that scientific validity and rigor are maintained throughout the study. The steering committee comprises 20 treating physicians from around the globe (ten from the USA, ten from elsewhere), who are considered experts in the field of MM treatment, as well as two patient representatives. Inclusion of the two patient representatives ensures that the patients’ perspective is incorporated in every aspect of the study, from study design and data collection, to the publication of the results and the communication of the findings to the wider MM community; in addition, involvement of the patient representatives ensures that patients’ concerns and needs are considered throughout the conduct of the study.
The study is being conducted in accordance with local ethical guidelines, European directives on protection of human patients in research, the Declaration of Helsinki, the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance Guidelines, Good Pharmacoepidemiology Practice Guidelines and other local or national specific, relevant guidelines, laws or regulations. All patients are required to provide written informed consent before data collection, including authorization to use their patient medical records.
Eligibility criteria
The study is enrolling NDMM and RRMM patients of ≥18 years of age. Patients with NDMM must enroll within 3 months of treatment initiation. Patients with RRMM could have received up to three prior lines of therapy before entering the study. All patients are required to have documented information available on the month and year of diagnosis, criteria met for diagnosis, stage, including International Staging System (ISS) and revised-ISS (R-ISS), prior therapies received, including duration of each prior line of treatment, whether they underwent stem cell transplant and received consolidation/maintenance therapy and whether they received any investigational therapy. In addition, all patients must be willing and able to complete the PRO instruments and sign the informed consent. Patients are excluded from the study if they report to a study site only for a second opinion, if they are participating in another trial that prohibits enrollment in this study or if they do not attend the clinic frequently enough to allow for quarterly completion of the electronic case report forms (eCRFs).
Planned sample size, study period, & selection of study sites
The target enrollment for this study is approximately 4200 patients, with approximately equal numbers of NDMM and RRMM patients to be enrolled. Patients are being enrolled over a period of approximately 3 years and will be evaluated and followed-up prospectively for a minimum of 5 years, until death, withdrawal of consent or end of study. The study is expected to end after all study patients have either completed a minimum of 5 years of follow-up, have been lost to follow-up or have died prior to study end. Patients are followed-up longitudinally and assessed on a quarterly basis to document changes in disease and/or lines of therapy.
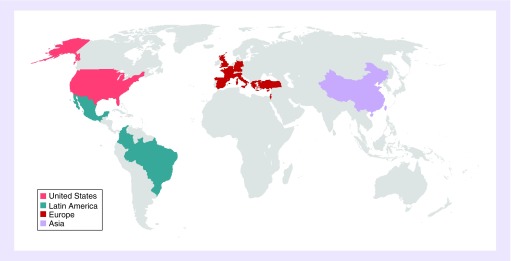
Patients are being recruited from study sites around the world, including North America, South America, Europe and Asia; 15 countries are participating (Figure 2). All participating sites are required to provide care for MM patients and to appoint a healthcare provider who agrees to oversee the study conduct and ensure that the protocol is followed correctly.
Figure 2. . INSIGHT MM: 15 participating countries.
Variables, outcome measures & end points
Measures collected during the study include assessment of patient demographics and disease characteristics, including presence of co-morbidities, disease symptoms at presentation, Eastern Cooperative Oncology Group performance status, ISS or R-ISS disease stage and cytogenetic risk. Data on patients’ frailty status, including Charlson comorbidity index, Katz index of independence in activities of daily living and Lawton instrumental activities of daily living scale, are also being collected, and may serve as an important predictor of outcomes and toxicities. Details of therapeutic regimens received, including duration of each line of therapy and whether patients have received prior stem cell transplant, are being collected and summarized. Outcome measures include overall survival (OS), progression-free survival (PFS), response per International Myeloma Working Group criteria [31] and time-to-next therapy (TTNT).
Table 4 lists variables collected during the study. These include documentation of treatment combinations prescribed, duration of treatment, sequencing of therapies, retreatment and use of continuous versus fixed-duration therapy, and if a patient has received therapy as part of a clinical trial. Patient outcomes including best response, OS, PFS and TTNT, associated with treatment regimens are being documented prospectively from study entry. Changes in treatment patterns over time are being documented by collecting information on initial and subsequent drug regimens and classes received during the study for all patients, as they move through lines of therapy. In addition, factors associated with treatment initiation and reasons for not receiving therapy are being collected. Modifications to treatment, including reasons for changes to drug, dose, schedule and duration, are also being collected. The impact of MM and treatment on patients’ HRQoL is being assessed through the use of validated PRO instruments, and data on HRU are being collected. Safety is being investigated throughout the study by collecting information on adverse events (AEs) and serious AEs leading to temporary or permanent treatment discontinuation or to drug modifications, as well as by monitoring the frequency of second primary malignancies. Information on reasons for dose modification and permanent drug discontinuation that are being collected include: AEs/toxicity, relapse due to biochemical or clinical progression, remission or death, planned therapy change or patient/family preference.
Table 4. . Patient/outcome assessments and schedule.
| Parameter | Timing | Assessment | Prospective versus retrospective |
|---|---|---|---|
| Patient demographics | Baseline | – Age – Sex – Race – Ethnicity – Height – Weight – Insurance information – Geographic region |
Prospective |
| Medical history at diagnosis | Baseline | – Date of initial diagnosis (month/year for RRMM; day/month/year for NDMM) and criteria used (ISS, R-ISS) – Stage (ISS or R-ISS) – Cytogenetics/FISH risk† – CRAB symptoms – Hematology: white blood cell count, absolute neutrophil count, hemoglobin, platelet count – Chemistry: calcium, creatinine, alkaline phosphatase, total bilirubin, aspartate aminotransferase, alanine aminotransferase, LDH, albumin, serum β2-microglobulin and BUN‡ – SPEP, serum FLC† and UPEP† – Imaging§ – History of MGUS/SMM – Frailty status: CCI, Katz Index of Independence in activities of daily living and Lawton instrumental activities of daily living scale – ECOG performance status |
NDMM patients: prospective RRMM patients: retrospective |
| Medical history prior to study inclusion | Baseline | – Bone marrow – FLC† – M-component, immunofixation – Imaging§ – GEP† – Prodromal/other plasma cell disorders (MGUS, SMM, amyloidosis) – Plasmacytoma and extramedullary disease – Central nervous system involvement – Stem cell transplant – Vaccination |
NDMM patients: prospective RRMM patients: retrospective |
| Medical evaluation | Baseline | – CCI – PN – Pneumococcal vaccine – Influenza A/B – Hypertension requiring treatment – Thromboembolism – Cardiac left ventricular function – Arrhythmias/pace-maker/AICD – Osteopenia/osteoporosis – Osteonecrosis of the jaw – Cataracts – MDS – MM-related radiation therapy – Orthopedic procedure/surgery for MM-related skeletal event – Other surgery/procedures |
Prospective |
| Quarterly | – PN – Amyloidosis – Pneumonia – Herpes zoster infection – Hypertension – Thromboembolism – Cataracts – Osteonecrosis of the jaw – Skeletal-related events – Cardiac events |
Prospective | |
| Yearly | – Height and weight – Vaccinations – MDS – Secondary primary malignancy – Frailty status: CCI, Katz Index of Independence in activities of daily living and Lawton instrumental activities of daily living scale – ECOG performance status |
Prospective | |
| Prior therapies (RRMM) | Baseline | – RRMM: number of relapses and previous lines of therapy, regimens used in each line, prior SCT or consolidation/maintenance therapy, use of investigational therapy as part of a clinical trial, response, MRD† | Retrospective |
| Disease management | Baseline and quarterly | – Current therapy: type of regimens and drugs (including duration of treatment, sequencing of therapies, retreatment and use of continuous versus fixed-duration therapy), factors associated with treatment initiation, reasons for not receiving therapy, response and MRD for each regimen†, SCT, supportive care | Prospective |
| Quarterly | – Changes or modifications to treatment: drug, schedule, dose, line of therapy and reasons for these changes – Hematology and chemistry, safety, MM-related radiation therapy and surgeries/procedures |
Prospective | |
| Effectiveness of treatment | Quarterly | – Bone marrow evaluation – FISH and/or cytogenetics† – MRD† – Imaging§ – NGS and GEP† – SPEP, serum FLC† and UPEP† – Response per IMWG criteria [31] – Progression status for each regimen – Date and cause of death |
Prospective |
| PROs | Baseline and quarterly | – EORTC QLQ-C30: two items on Global Health Status/QoL subscale – EORTC QLQ-MY-20: single item on peripheral neuropathy – TSQM-9: effectiveness, convenience and global satisfaction domains |
Prospective |
| HRU | Quarterly | – Inpatient and intensive care admissions – Reasons for admission and length of stay – Outpatient clinic visits – Emergency room visits |
Prospective |
| Safety¶ | Baseline and quarterly | – AEs and SAEs leading to treatment discontinuation (temporary and permanent) or drug modification – Frequency of secondary primary malignancy |
Prospective |
†Assessment was performed if the test was available and carried out as part of routine practice at the study sites.
‡If available.
§Includes assessment of evidence of plasmacytoma, extramedullary disease, and diffuse involvement.
¶Based on the latest version the National Cancer Institute the Common Terminology Criteria for Adverse Events.
AE: Adverse event; AICD: Automatic implantable cardioverter-defibrillator; BUN: Blood urea nitrogen; CCI: Charlson comorbidity index; CRAB: hyperCalcemia, Renal insufficiency, Anemia, Bone lesions; ECOG: Eastern Cooperative Oncology Group; EORTC: European Organization for Research and Treatment of Cancer; FISH: Fluorescence in situ hybridization; FLC: Free light chain; GEP: Gene expression profiling; HRU: Healthcare resource utilization; IMWG: International Myeloma Working Group; ISS: International Staging System; LDH: Lactate dehydrogenase; MDS: Myelodysplastic syndrome; MGUS: Monoclonal gammopathy of undetermined significance; MM: Multiple myeloma; MRD: Minimal residual disease; NDMM: Newly diagnosed multiple myeloma; NGS: Next-generation sequencing; PN: Peripheral neuropathy; PRO: Patient self-reported outcomes; QLQ-C30: Quality of Life Questionnaire – Core 30 Module; QLQ-MY-20: Quality of Life Questionnaire – 20-item Multiple Myeloma Module; QoL: Quality of life; R-ISS: Revised International Staging System; RRMM: Relapsed/refractory multiple myeloma; SAE: Serious adverse event; SCT: Stem cell transplant; SMM: Smoldering multiple myeloma; SPEP: Serum protein electrophoresis; TSQM-9: 9-Item Treatment Satisfaction Questionnaire for Medication; UPEP: Urine protein electrophoresis.
Study assessments
Details of study assessments are reported in Table 4. Briefly, information on patient demographics, disease characteristics and medical history prior to study inclusion, including prior anti-MM therapies received, is collected at baseline. Disease management, effectiveness of treatment and safety are being assessed quarterly.
PROs are being collected at study inclusion and quarterly thereafter using paper forms during routine clinic visits. HRQoL is being assessed based on:
The Global Health Status/Quality of Life subscale from the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire – Core 30 module (QLQ–C30) [32];
A single item on peripheral neuropathy from the EORTC Quality of Life Questionnaire 20-item Multiple Myeloma Module (QLQ-MY-20) [32];
Nine items from the Treatment Satisfaction Questionnaire for Medication 9 (TSQM-9) covering the domains of effectiveness, convenience and global satisfaction [33].
HRU is also assessed quarterly, including rates of inpatient and intensive care unit admissions, reasons for admissions, length of stay, outpatient clinic visits and emergency room visits.
To ensure accuracy and completeness of the data, both an automatic query and a manual query process are utilized. Automatic queries are intended to resolve insufficient data entries or missed fields. Manual queries are conducted on flagged, new and changed data. Data reviews are conducted on a monthly basis at a minimum. In addition, the study coordinator and principal healthcare provider at each participating site are responsible for the quality and consistency of data in the study and will maintain accurate electronic case report forms and patient medical charts as part of the case histories.
Statistics
The planned sample size of approximately 4200 patients is intended to provide enough patients to characterize treatment in a broad population, and to maintain a reasonable level of statistical power to detect differences between subgroups. A sample size of 268 in each of any two comparison subgroups will have at least 80% power to detect a difference between two proportions, given the true difference is at least 12%.
However, no formal hypothesis will be tested in this study and all analyses are exploratory in nature. All enrolled patients are considered for inclusion in the analyses. Due to the observational nature of the study, and to address potential confounding factors and bias, adjusted regression models will be used to determine the associations between: (1) MM therapy regimens, disease attributes (e.g., disease stage and risk) and patient factors (e.g., age and frailty) and (2) clinical outcomes, HRU and HRQoL. The final analysis will take place approximately 5 years after enrollment of the last patient. Interim data summaries and formal interim analyses are being conducted as appropriate while the study is ongoing, to understand patients’ initial clinical presentations at diagnosis and relapse and the effectiveness of therapies in the real world [34,35].
Discussion
While clinical trial efficacy is critical for the MM treatment decision-making process, real-world data are becoming increasingly important as they can inform clinicians about treatment effectiveness and toxicity in a broader patient population treated outside of controlled clinical trials, with the ultimate goal of improving patients’ care and outcomes. Numerous MM observational and national registry studies have been conducted or are ongoing/being planned worldwide (Table 3 and Richardson et al. 2018 [16]), and evidence from these studies may help in elucidating the critical factors contributing to the discrepancies reported between clinical trial efficacy and real-world effectiveness as well as guiding treatment choices.
While data from several MM observational and national registry studies are already available (Table 3 and Richardson et al. 2018 [16]), INSIGHT MM is the first non-interventional, observational study that is truly global in nature, with patients being recruited from centers in 15 countries in North America, Central and South America, Europe and Asia (Figure 2). Another unique aspect of this study is the open, collaborative nature of the project and the willingness to share data beyond the investigators who participate in the trial, thereby providing a mechanism for the submission of research ideas from the broader MM community. With an increasing number of real-world MM registries being initiated in multiple geographical regions, the INSIGHT MM steering committee plans to explore the power of collating datasets and conducting collaborative analyses with country-specific MM registries and other observational studies, including the Czech Registry of Monoclonal Gammopathies [27,36] and the Australian and New Zealand Myeloma and Related Diseases Registry [37]. Partnerships between registries will increase the power of the analyses, provide a snapshot of MM treatment patterns and outcomes on a global scale, allow exploration of a wider range of clinically relevant questions as well as provide results with higher accuracy and generalizability to the whole MM population.
Inclusion of HRQoL end points in clinical trials can provide a more comprehensive evaluation of treatment outcomes by incorporating the patient's perspective. The collection of PRO data to evaluate patients’ HRQoL is a particular strength of INSIGHT MM, highlighting the patient-centric approach taken in the development of this study and the growing importance of PROs in real-world studies in MM (Table 3). In addition, several recent pivotal Phase III trials of anti-MM agents have incorporated HRQoL analyses and demonstrated that HRQoL benefits may be associated with improved outcomes [32,38,39]. Treatment-related factors, including toxicities, disease symptoms and the burden of treatment and symptom/side-effect management, can negatively affect patients’ HRQoL [24]; therefore, it is particularly important to maintain HRQoL during MM therapy, especially for patients receiving long-term/maintenance therapy. In the INSIGHT MM study, symptoms of peripheral neuropathy, one of the most important complications of MM which can adversely impact on patients’ HRQoL [40], are reported by patients through the EORTC QLQ-MY20 questionnaire. Additionally, patient satisfaction with their medication and patients’ perspectives on treatment convenience will be assessed via the TSQM-9 questionnaire to provide information on treatment adherence [33]; this is a critical challenge for patients receiving long-term/maintenance treatment with agents requiring repeated dosing and frequent clinic visits, which can impose a substantial burden [24].
Real-world data for anti-MM agents are already beginning to emerge [17,25–29] but, thanks to INSIGHT MM and other registries, in the future these data will be much more extensive and robust as more patients are enrolled, and it will become clearer which attributes, including which therapies, are having the greatest impact on patients’ outcomes and HRQoL.
Conclusion
Two data analyses of the INSIGHT MM study have been reported to date. The first included 1000 patients enrolled from 13 countries (535 NDMM, 465 RRMM), and the second analysis included the first 1056 NDMM patients enrolled from 14 countries [34,35]. Both analyses highlighted marked regional differences in real-world treatment patterns in NDMM/RRMM, potentially due to differences in drug availability and treatment guidelines at academic versus community centers [34,35]. Results from a second interim analysis, which will include approximately 3000 patients, are awaited with interest.
In conclusion, these interim data analyses and, ultimately, the final study findings will provide comprehensive insights into patient and disease characteristics, treatment patterns, outcomes, patients’ HRQoL and safety of contemporary treatment approaches for NDMM and RRMM patients in community and academic settings worldwide. These data will be of substantial importance to the MM community, as they have the potential to improve our understanding of the factors contributing to the discrepancies between clinical trial efficacy and real-world effectiveness of anti-MM agents and regimens, and thereby improve patients’ outcomes.
Executive summary.
Multiple myeloma
Multiple myeloma (MM) patients’ survival has improved in the past 20 years, due to therapeutic advances, including the introduction of new drugs and combination therapies and an increased use of continuous/long-term therapy.
The anti-MM treatment armamentarium is expanding, with new agents and more complex combinations being added; in the absence of a global standard-of-care treatment for newly diagnosed MM (NDMM) or relapsed/refractory MM (RRMM) patients, and given the incurable nature of MM, treating physicians are faced with the challenge of choosing appropriate types and sequences of therapies for their patients in clinical practice.
Efficacy versus effectiveness
While improved outcomes have been observed in clinical trials, emerging outcome data from observational studies of real-world patients suggest a gap between clinical trial efficacy and real-world effectiveness.
Factors contributing to this gap include: the stringent patient eligibility criteria used for clinical trial enrollment, toxicity and treatment burden, treatment availability and access issues, as well as cost.
Real-world MM registries
Real-world data on the use of anti-MM agents and regimens in routine clinical practice are emerging.
Multiple MM observational and registry studies have been or are being conducted across the world; however, current data on disease presentation, treatment patterns and outcomes at a global level are limited.
INSIGHT MM
INSIGHT MM is the largest global, prospective, non-interventional, observational study conducted to date, enrolling approximately 4200 patients with NDMM/RRMM from 15 countries worldwide.
Objectives
To describe contemporary, real-world patterns of patient characteristics, clinical disease presentation, therapeutic regimen chosen and clinical outcomes in MM on a regional and global scale.
To evaluate the safety and effectiveness of current therapies, the impact of MM on patients’ health-related quality of life (HRQoL) and healthcare resource utilization (HRU), and the associations between patient characteristics, clinical disease presentation, therapeutic patterns and clinical outcomes.
Study design & eligibility criteria
No study drugs are being provided and no changes in patients’ treatment management are required; patients are being asked to complete patient self-reported outcomes (PRO) questionnaires.
Eligible patients are adults with NDMM (within 3 months of starting treatment) or RRMM (1–3 prior therapies), for whom information on time of diagnosis and current/prior therapies received is available to document each patient's longitudinal treatment course and associated outcomes.
Assessments
Patient demographic, clinical disease presentation and medical history are collected at baseline; disease management, treatment effectiveness, safety and HRU data are assessed quarterly.
PROs are being used to assess HRQoL at baseline and quarterly based on the European Organization for Research and Treatment of Cancer - Quality of Life Questionnaire – Core 30 module (EORTC QLQ-C30) and Quality of Life Questionnaire 20-item Multiple Myeloma Module (QLQ-MY-20; peripheral neuropathy) instruments and the Treatment Satisfaction Questionnaire for Medication-9 (TSQM-9; effectiveness, convenience and global satisfaction) questionnaire.
Supplementary Material
Acknowledgements
The authors thank all patients and their families, as well as all physicians, nurses, study coordinators and study centre staff, for participating in this study.
Footnotes
Supplementary data
An infographic accompanies this paper at the end of the references section. To download the infographic that accompanies this paper, please visit the journal website at: https://www.futuremedicine.com/doi/full/10.2217/fon-2019-0013
Author contributions
C Costello, FE Davies, G Cook, J Omel, RM Rifkin, N Puig, JA Zonder, A Spencer, X Leleu, MA Thompson and DM Stull participated in the study conception and design. C Costello, FE Davies, J Vela-Ojeda, SZ Usmani, K Weisel, JA Zonder, E Terpos, X Leleu, MA Thompson, DM Stull and V Hungria participated in the collection and assembly of data. C Costello, FE Davies, G Cook, J Vela-Ojeda, J Omel, RM Rifkin, J Berdeja, SZ Usmani, K Weisel, E Terpos, A Spencer, X Leleu, M Boccadoro, MA Thompson, D Romanus and DM Stull contributed to data analysis and interpretation. All authors participated in drafting or revising the manuscript and in the review and approval of the final version of the manuscript.
Financial & competing interests disclosure
This study was funded by Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. C Costello has acted on an advisory council or committee for Celgene, Janssen and Takeda. FE Davies has acted on an advisory council or committee for Amgen and Takeda, has received honoraria from ASH, MMRF and TRM Oncology, consulting fees from Amgen, AbbVie, Celgene, Janssen and Takeda, and reports grants/funds from Celgene and Janssen. G Cook has acted on an advisory council or committee for Celgene, Janssen and Takeda, has received honoraria from Celgene, Janssen, Sanofi and Takeda, reports consulting fees from Celgene, Janssen, Sanofi, Seattle Genetics and Takeda, and reports grants/funds from Takeda and Celgene. RM Rifkin has acted on an advisory council or committee for AbbVie, Amgen, Celgene and Takeda. J Berdeja reports research funding to his institution from AbbVie, Amgen, Bluebird, BMS, Celgene, Genentech, Glenmark, Jannssen, Novartis, Poseida, Sanofi, Takeda and Teva. N Puig has received honoraria from Janssen, Takeda, Celgene, Amgen, and The Binding Site, reports consulting fees from Janssen, Takeda, Celgene, and Amgen, and reports grants/funds from Janssen and Celgene. SZ Usmani reports consulting fees from Amgen, BMS, Celgene, Janssen, Merck, SkylineDX and Takeda, and reports holding a patent or receiving royalties from Amgen, Array Biopharma, Celgene, Janssen, Pharmacyclis, Sanofi and Takeda. K Weisel has acted on an advisory council or committee for Adaptive Biotec, Amgen, BMS, Celgene, Janssen, Juno, Sanofi and Takeda, has received honoraria from Amgen, BMS, Celgene, Janssen, Novartis and Takeda, reports consulting fees from Adaptive Biotec, Amgen, BMS, Celgene, Janssen, Juno, Sanofi and Takeda, and reports grants/funds from Amgen, Celgene, Sanofi and Janssen. JA Zonder has acted on an advisory council or committee for Alnylam, Amgen, BMS, Caelum, Celgene, Janssen, Oncopeptides and Takeda, reports consulting fees from Alnylam, Amgen, BMS, Caelum, Celgene, Janssen, Oncopeptides and Takeda, and reports grants/funds from BMS and Celgene. E Terpos has received honoraria and consulting fees from Amgen, BMS, Celgene/Genesis, Janssen, Novartis and Takeda, and reports grants/funds from Amgen, Genesis, Janssen and Takeda. A Spencer has acted on an advisory council or committee for AbbVie, Amgen, Celgene, Janssen, Servier and Takeda, has received honoraria from AbbVie, Amgen, Celgene, Janssen, Servier and Takeda, and reports grants/funds from AbbVie, Amgen, Celgene, Janssen and Takeda. X Leleu has received honoraria from Takeda. M Boccadoro has received honoraria from Amgen, Celgene, BMS, Janssen and Sanofi. MA Thompson reports ownership of stock shares from Doximity, has acted on an advisory council or committee for AIM Specialty Health, Celgene, GSK, Strata Oncology, Syapse, Takeda and VIA Oncology, and reports receiving royalties from UpToDate – Myeloma. D Romanus and DM Stull report employment with Takeda. V Hungria has received honoraria from Amgen, BMS, Celgene, Janssen and Takeda. J Vela-Ojeda and J Omel report no competing interests. Global Outcomes Research is a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. Global Medical Affairs is a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance was provided by H Johns and L Cancian of FireKite, an Ashfield company, part of UDG Healthcare plc, who provided medical writing assistance, which was funded by Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, and complied with Good Publication Practice 3 ethical guidelines (Battisti WP et al. Ann Intern Med. 163, 461–464 [2015]), and R Ferrari (Millennium Pharmaceuticals, Inc.), for editorial support. The corresponding author had full access to all the study protocol and had final responsibility for the decision to submit for publication.
Ethical conduct of research
The authors state that the study is being conducted in accordance with local ethical guidelines, European directives on protection of human patients in research, the Declaration of Helsinki, the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance Guidelines, Good Pharmacoepidemiology Practice Guidelines and other local, national-specific relevant guidelines, laws or regulations. All patients will be required to provide written informed consent before data collection.
Open Access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute SEER, Cancer Statistics Review, 1975–2015. 2018. https://seer.cancer.gov/csr/1975_2015/results_merged/sect_18_myeloma.pdf
- 4.Cancer Research UK. Myeloma survival statistics. 2014. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/myeloma/survival#heading-Two
- 5.Office for National Statistics. Cancer survival in England: adult, stage at diagnosis and childhood – patients followed up to 2016. 2015. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancersurvivalinengland/adultstageatdiagnosisandchildhoodpatientsfollowedupto2016#cancer-survival-in-england-patients-diagnosed-between-2011-and-2015-and-followed-up-to-2016-national-statistics
- 6.Thorsteinsdottir S, Dickman PW, Landgren O, et al. Dramatically improved survival in multiple myeloma patients in the recent decade: Results from a Swedish population-based study. Haematologica. 2018;103(9):e412–e415. doi: 10.3324/haematol.2017.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baughn LB, Pearce K, Larson D, et al. Differences in genomic abnormalities among African individuals with monoclonal gammopathies using calculated ancestry. Blood Cancer J. 2018;8(10):96. doi: 10.1038/s41408-018-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Anderson KC, Richardson PG. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017;36(4):561–584. doi: 10.1007/s10555-017-9707-8. [DOI] [PubMed] [Google Scholar]
- 9.Larocca A, Mina R, Gay F, Bringhen S, Boccadoro M. Emerging drugs and combinations to treat multiple myeloma. Oncotarget. 2017;8(36):60656–60672. doi: 10.18632/oncotarget.19269. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Overview of the recent use of new agents and regimens which have been approved or are/have been under investigation for multiple myeloma (MM) treatment in the past 20 years approximately.
- 10.Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28(Suppl. 4):iv52–iv61. doi: 10.1093/annonc/mdx096. [DOI] [PubMed] [Google Scholar]
- 11.Gay F, Engelhardt M, Terpos E, et al. From transplant to novel cellular therapies in multiple myeloma: European Myeloma Network guidelines and future perspectives. Haematologica. 2018;103(2):197–211. doi: 10.3324/haematol.2017.174573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar SK, Callander NS, Alsina M, et al. NCCN guidelines insights: multiple myeloma, version 3.2018. J. Natl Compr. Canc. Netw. 2018;16(1):11–20. doi: 10.6004/jnccn.2018.0002. [DOI] [PubMed] [Google Scholar]
- 13.Offidani M, Corvatta L, Gentili S. Triplet vs. doublet drug regimens for managing multiple myeloma. Expert Opin. Pharmacother. 2018;19(2):137–149. doi: 10.1080/14656566.2017.1418856. [DOI] [PubMed] [Google Scholar]
- 14.Palumbo A, Gay F, Cavallo F, et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. J. Clin. Oncol. 2015;33(30):3459–3466. doi: 10.1200/JCO.2014.60.2466. [DOI] [PubMed] [Google Scholar]
- 15.Cook G, Zweegman S, Mateos MV, Suzan F, Moreau P. A question of class: treatment options for patients with relapsed and/or refractory multiple myeloma. Crit. Rev. Oncol. Hematol. 2018;121:74–89. doi: 10.1016/j.critrevonc.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Richardson PG, San Miguel JF, Moreau P, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 2018;8(11):109. doi: 10.1038/s41408-018-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Discusses the factors that can contribute to the discrepancies between clinical trial efficacy and real-world effectiveness and reviews non-clinical trial data of proteasome inhibitor/immunomodulatory drug-based regimens in relapsed/refractory MM.
- 17.Chari A, Romanus D, Luptakova K, et al. American Society of Hematology 59th Annual Meeting & Exposition. Atlanta, GA, USA: 9–12 December 2017. Duration of therapy (DOT) and time to next therapy (TTNT) of bortezomib, carfilzomib and ixazomib combinations with lenalidomide/dexamethasone (VRd, KRd, IRd) in patients (pts) with relapsed/refractory multiple myeloma (RRMM): clinical practice in the United States vs clinical trial experience. Presented at. [Google Scholar]
- 18.Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J. Clin. Oncol. 2017;35(33):3737–3744. doi: 10.1200/JCO.2017.73.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah JJ, Abonour R, Gasparetto C, et al. Analysis of common eligibility criteria of randomized controlled trials in newly diagnosed multiple myeloma patients and extrapolating outcomes. Clin. Lymphoma Myeloma Leuk. 2017;17(9):572–583 e572. doi: 10.1016/j.clml.2017.06.013. [DOI] [PubMed] [Google Scholar]; • Shows that, based on common clinical trial exclusion criteria, 40% of patients enrolled in the CONNECT-MM registry are ineligible for inclusion in randomized controlled clinical trials (RCTs), highlighting the strict eligibility criteria used to select patients for enrollment in RCTs.
- 20.Fiala M, Dukeman J, Stockert-Goldstein K, Tomasson M, Wildes T, Vij R. The real-world characteristics and outcomes of newly diagnosed myeloma patients ineligible for clinical trials. Clin. Lymphoma Myeloma Leuk. 2017;17(1):e55–e56. [Google Scholar]
- 21.Chari A, Romanus D, Farrelly E, et al. Randomized clinical trial (RCT) representativeness and outcomes among relapsed/refractory multiple myeloma (RRMM) patients treated in the real world (RW): comparison of ASPIRE, TOURMALINE-MM1, POLLUX, AND ELOQUENT-2 RCTS. Clin. Lymphoma Myeloma Leuk. 2018;18(Suppl. 1):S249. [Google Scholar]
- 22.Costa LJ, Hari PN, Kumar SK. Differences between unselected patients and participants in multiple myeloma clinical trials in US: a threat to external validity. Leuk. Lymphoma. 2016;57(12):2827–2832. doi: 10.3109/10428194.2016.1170828. [DOI] [PubMed] [Google Scholar]; • Compares the baseline characteristics of MM patients enrolled in USA clinical trials versus unselected patients from the SEER-18 database, which identifies specific patient types who are underrepresented in MM clinical trials.
- 23.Pulte ED, Nie L, Gormley N, et al. Survival of ethnic and racial minority patients with multiple myeloma treated with newer medications. Blood Adv. 2018;2(2):116–119. doi: 10.1182/bloodadvances.2017010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baz R, Lin HM, Hui AM, et al. Development of a conceptual model to illustrate the impact of multiple myeloma and its treatment on health-related quality of life. Support. Care Cancer. 2015;23(9):2789–2797. doi: 10.1007/s00520-015-2644-6. [DOI] [PubMed] [Google Scholar]
- 25.Jagannath S, Roy A, Kish J, et al. Real-world treatment patterns and associated progression-free survival in relapsed/refractory multiple myeloma among US community oncology practices. Expert Rev. Hematol. 2016;9(7):707–717. doi: 10.1080/17474086.2016.1195254. [DOI] [PubMed] [Google Scholar]
- 26.Romanus D, Raju A, Seal B, et al. 16th International Myeloma Workshop. New Delhi, India: 1–4 March 2017. Treatment (tx) patterns & outcomes by line of therapy (LOT) in a large United States (US) cohort of transplant-ineligible patients (pts) with multiple myeloma in the era of novel agents. Presented at. [Google Scholar]
- 27.Terpos E, Maouche N, Minarik J, Katodritou E, Jenner M, Plonkova H. American Society of Hematology 59th Annual Meeting & Exposition. Atlanta, GA, USA: 9–12 December 2017. “Real world” data on the efficacy and safety of ixazomib in combination with lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: a combined study from the Greek, Czech and UK databases. [Google Scholar]
- 28.Biran N, Vesole DH, Zhang S, et al. Real-world outcomes with panobinostat in patients with penta- and quad-refractory multiple myeloma. J. Clin. Oncol. 2017;35(Suppl. 15):e19522–e19522. [Google Scholar]
- 29.Branca A, Buros A, Yoon D, et al. Daratumumab single agent and daratumumab plus pomalidomide and dexametasone in relapsed/refractory multiple myeloma: a real life retrospective evaluation. Blood. 2016;128(22):4516. [Google Scholar]
- 30.Cavo M, Terpos E, Bargay J, et al. The multiple myeloma treatment landscape: international guideline recommendations and clinical practice in Europe. Expert Rev. Hematol. 2018;11(3):219–237. doi: 10.1080/17474086.2018.1437345. [DOI] [PubMed] [Google Scholar]
- 31.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 32.Sonneveld P, Verelst SG, Lewis P, et al. Review of health-related quality of life data in multiple myeloma patients treated with novel agents. Leukemia. 2013;27(10):1959–1969. doi: 10.1038/leu.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual. Life Outcomes. 2009;7:36. doi: 10.1186/1477-7525-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boccadoro M, Usmani SZ, Chari A, et al. 23rd Congress of the European Hematology Association. Stockholm, Sweden: 14–17 June 2018. A global treatment standard in multiple myeloma (MM) remains elusive despite advances in care over 15 years: first results from INSIGHT MM, the largest global prospective, observational MM study. [Google Scholar]; •• Preliminary data from the first 1000 patients enrolled in the INSIGHT MM study from 13 countries.
- 35.Usmani SZ, Hungria VTM, Xavier Leleu X, et al. American Society of Hematology 60th Annual Meeting & Exposition. San Diego, CA, USA: 1–4 December 2018. Transplant status does not impact the selection of induction regimens for newly diagnosed multiple myeloma (NDMM) patients (pts) in the INSIGHT MM prospective, observational study. [Google Scholar]; •• Preliminary data form the first 1056 patients with newly diagnosed MM enrolled in the INSIGHT MM study from 14 countries.
- 36.Brozova L, Schwarz D, Snabl I, et al. Czech Registry of Monoclonal Gammopathies – technical solution, data collection and visualisation. Klin. Onkol. 2017;30(Suppl. 2):43–50. doi: 10.14735/amko20172S43. [DOI] [PubMed] [Google Scholar]
- 37.Bergin K, Moore E, McQuilten Z, et al. Design and development of the Australian and New Zealand (ANZ) myeloma and related diseases registry. BMC Med. Res. Methodol. 2016;16(1):151. doi: 10.1186/s12874-016-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delforge M, Minuk L, Eisenmann JC, et al. Health-related quality-of-life in patients with newly diagnosed multiple myeloma in the FIRST trial: Lenalidomide plus low-dose dexamethasone versus melphalan, prednisone, thalidomide. Haematologica. 2015;100(6):826–833. doi: 10.3324/haematol.2014.120121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weisel K, Dimopoulos M, Song KW, et al. Pomalidomide and low-dose dexamethasone improves health-related quality of life and prolongs time to worsening in relapsed/refractory patients with multiple myeloma enrolled in the MM-003 randomized Phase III trial. Clin. Lymphoma Myeloma Leuk. 2015;15(9):519–530. doi: 10.1016/j.clml.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Richardson PG, Delforge M, Beksac M, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26(4):595–608. doi: 10.1038/leu.2011.346. [DOI] [PubMed] [Google Scholar]
- 41.Kazandjian D, Landgren O. A look backward and forward in the regulatory and treatment history of multiple myeloma: approval of novel-novel agents, new drug development, and longer patient survival. Semin. Oncol. 2016;43(6):682–689. doi: 10.1053/j.seminoncol.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'agostino M, Boccadoro M, Smith EL. Novel immunotherapies for multiple myeloma. Curr. Hematol. Malig. Rep. 2017;12(4):344–357. doi: 10.1007/s11899-017-0397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho SF, Anderson KC, Tai YT. Targeting B cell maturation antigen (BCMA) in multiple myeloma: potential uses of BCMA-based immunotherapy. Front. Immunol. 2018;9:1821. doi: 10.3389/fimmu.2018.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terpos E, Chari A, Rifkin RM, et al. Uncovering the blind spot of clinical trials: first report of baseline characteristics of newly diagnosed (ND) and relapsed/refractory (RR) multiple myeloma (MM) patients (pts) in INSIGHT-MM, a global, prospective, observational study. Blood. 2017;130(Suppl. 1):5419. [Google Scholar]; •• Preliminary data from the first 650 patients enrolled in the INSIGHT MM study from 13 countries.
- 45.Fiala MA, Dukeman J, Stockerl-Goldstein K, Tomasson MH, Wildes TM, Vij R. The real-world characteristics and outcomes of newly diagnosed myeloma patients ineligible for clinical trials. Blood. 2016;128(22):2350. [Google Scholar]
- 46.Multiple Myeloma Research Foundation. MMRF CoMMpass Study. 2018. https://themmrf.org/we-are-curing-multiple-myeloma/mmrf-commpass-study/
- 47.Narang M, Abonour R, Durie BGM, et al. Baseline disease characteristics and diagnostic patterns for NDMM of the Connect® MM registry: observations over time in cohort 1 (2009–2011) and cohort 2 (2012–2016) Blood. 2017;130(Suppl. 1):3156. [Google Scholar]
- 48.Rifkin R, Abonour R, Durie B, et al. Treatment patterns from 2009–2015 in patients with newly diagnosed multiple myeloma in the United States: a report from the Connect® MM registry. Blood. 2016;128(22):4489. [Google Scholar]
- 49.Mohty M, Terpos E, Mateos MV, et al. Multiple myeloma treatment in real-world clinical practice: results of a prospective, multinational, non-interventional study. Clin. Lymphoma Myeloma Leuk. 2018;18(10):e401–e419. doi: 10.1016/j.clml.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 50.Vij R, Chen C, Popov S, et al. Treatment patterns and associated outcomes in patients with relapsed or refractory multiple myeloma in the US and non-US countries: Findings from PREAMBLE. Blood. 2017;130(Suppl. 1):3123. [Google Scholar]
- 51.Kuter DJ, Chen C, Popov S, Davis C, Ravi Vij R. Healthcare resource utilization and costs associated with different treatment modalities of relapsed/refractory multiple myeloma patients in the US: findings from PREAMBLE. Blood. 2017;130(Suppl. 1):3157. [Google Scholar]
- 52.Goldschmidt H, Cook G, Kuter D, et al. Lenalidomide and low-dose dexamethasone for treatment of relapsed/refractory multiple myeloma: real-world treatment patterns from the PREAMBLE study. Clin. Lymphoma Myeloma Leuk. 2017;17(1):e126–e127. [Google Scholar]
- 53.Efficace F, Boccadoro M, Palumbo A, et al. A prospective observational study to assess clinical decision-making, prognosis, quality of life and satisfaction with care in patients with relapsed/refractory multiple myeloma: the CLARITY study protocol. Health Qual. Life Outcomes. 2018;16(1):127. doi: 10.1186/s12955-018-0953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bařinová M. Registry of monoclonal gammopathies (RMG) 2019.
- 55.Spencer A. Australian and New Zealand myeloma and related diseases registry (MRDR) 2019.
- 56.Alegre A, Aguado B, Giraldo P, et al. Lenalidomide is effective as salvage therapy in refractory or relapsed multiple myeloma: analysis of the Spanish Compassionate Use Registry in advanced patients. Int. J. Hematol. 2011;93(3):351–360. doi: 10.1007/s12185-011-0785-z. [DOI] [PubMed] [Google Scholar]
- 57.Verelst S, Blommestein H, Gonzalez-McQuire S, et al. 21st Congress of the European Hematology Association. Copenhagen, Denmark: 9–12 June 2016. Overall survival in patients with symptomatic multiple myeloma in the real-world setting: a retrospective analysis of the PHAROS registry in The Netherlands. [Google Scholar]
- 58.Wester R, Dinmohamed A, Sonneveld P, Broijl A, Blijlevens N. 22nd Congress of the European Hematology Association. Madrid, Spain: 22–25 June 2017. Pomalidomide with low dose dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a prospective analysis in a population-based registry. [Google Scholar]
- 59.Willenbacher E, Balog A, Willenbacher W. Short overview on the current standard of treatment in newly diagnosed multiple myeloma. Memo. 2018;11(1):59–64. doi: 10.1007/s12254-018-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.