Abstract
Deriving human health risk estimates for environmental chemicals has traditionally relied on in vivo toxicity databases to characterize potential adverse health effects and associated dose-response relationships. In the absence of in vivo toxicity information, new approach methods (NAMs) such as read-across have the potential to fill the required data gaps. This case study applied an expert-driven read-across approach to identify and evaluate analogues to fill non-cancer oral toxicity data gaps for p,p’-dichlorodiphenyldichloroethane (p,p’-DDD), an organochlorine contaminant known to occur at contaminated sites in the U.S. The source analogue p,p’-dichlorodiphenyltrichloroethane (DDT) and its no-observed-adverse-effect level of 0.05 mg/kg-day were proposed for the derivation of screening-level health reference values for the target chemical, p,p’-DDD. Among the primary similarity contexts (structure, toxicokinetics, and toxicodynamics), toxicokinetic considerations were instrumental in separating p,p’-DDT as the best source analogue from other potential candidates (p,p’-DDE and methoxychlor). In vitro high-throughput screening (HTS) assays from ToxCast were used to evaluate similarity in bioactivity profiles and make inferences toward plausible mechanisms of toxicity to build confidence in the read-across approach. This work demonstrated the value of NAMs such as read-across and in vitro HTS in human health risk assessment of environmental contaminants with the potential to inform regulatory decision-making.
Keywords: Read-across, Quantitative risk assessment, Toxicokinetics, In vitro high-throughput screening
1. Introduction
With an ever-expanding list of chemicals currently in commerce that require hazard assessment, the use of alternative approaches in toxicology has gained interest from both regulatory and industry sectors. Traditionally, human health risk assessment has relied upon animal toxicity data for hazard identification and dose-response analysis of chemicals and environmental contaminants. According to the Toxic Substances Control Act (TSCA) Chemical Substance Inventory (a record of chemicals manufactured or imported into the U.S.) more than 85,000 substances are currently in commerce (U.S. EPA, 2017d). However, as of 2008 only approximately 9,912 environmentally relevant chemicals have available in vivo data and an even smaller fraction (2,767 chemicals) have been comprehensively evaluated for toxicity (Judson et al., 2009). The large universe of existing chemicals combined with the economic, technical and ethical limitations associated with the continued use of laboratory animals for toxicity testing and human health risk assessment present obvious challenges in chemical-specific data generation. As such, new approach methods (NAMs) such as read-across and high-throughput screening (HTS) technologies have emerged as promising alternatives to animal testing that can help reduce the burden or backlog of chemicals not yet evaluated for safety concerns.
In response to the U.S. National Research Council’s 2007 report entitled “Toxicity Testing in the 21st Century: A Vision and A Strategy” (NRC, 2007), substantial efforts have been made to integrate scientific and technological advances within existing risk assessment frameworks to characterize human health hazards posed by exposure to chemicals in the environment. Structure-activity relationship approaches such as chemical grouping and read-across are among the most widely used NAMs in both human health and ecotoxicity risk assessment. According to the Organisation for Economic Co-operation and Development (OECD), chemicals can be grouped based on an analogue or category approach to allow for specific properties or toxicity endpoints to be interpolated and/or extrapolated from one chemical to another (OECD, 2014). Within the International Council of Chemical Associations’ (ICCA) Cooperative Chemicals Assessment Programme and the U.S. Environmental Protection Agency’s High Production Volume (HPV) Challenge Program, significant reductions in financial and animal testing burden have been accomplished from the use of read-across and other in silico techniques to satisfy requirements for health and environmental hazard data on registered substances (Bishop et al., 2012; Stanton and Kruszewski, 2016). Likewise, 75% of the chemical assessments submitted from 2010 to 2013 under the European Union’s Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) legislation applied read-across to address data gaps for untested endpoints (Ball et al., 2016). Other novel techniques involve HTS assays that profile transcriptional or biological activity, providing mechanistic information that can support the development of adverse outcome pathways relevant to chemical exposures and aid in toxicity assessment. The U.S. EPA’s Toxicity ForeCaster (ToxCast) in its phase I and II tests has compiled a diverse database (exceeding 1,800 chemicals screened across over 700 in vitro assay endpoints) intended to inform potential human health hazards and prioritize suspect chemicals for more rigorous vetting (Richard et al., 2016).
Despite the general acceptance of read-across and HTS data as viable tools for decreasing animal use, questions remain regarding their successful implementation in quantitative risk assessment in lieu of traditional animal bioassays in the characterization of repeated dose toxicity. Specifically for read-across, the primary challenges lie in the evaluation of chemical similarity and uncertainty in the hazard prediction (Schultz et al., 2015). Concerted efforts have been made to conceptualize different ways for grouping chemicals on the basis of chemical (structure) and biological (metabolism, toxicity and mode-of-action [MOA]) properties in order to facilitate read-across (ECETOC, 2012; ECHA, 2017; OECD, 2014; Patlewicz et al., 2015; Wang et al., 2012; Wu et al., 2010). One such approach has been described by Wang et al. (2012), which considers the identification of suitable analogues for quantitative read-across based on three primary similarity contexts (structure, toxicokinetics and toxicodynamics). The present case study expands on the expert-driven read-across framework developed by Wang et al. (2012) to identify and select a single best source analogue and its corresponding point of departure (POD)1, assisting in screening-level risk assessment of the target organochlorine contaminant, p,p’-dichlorodiphenyldichloroethane (p,p’-DDD).
Organochlorine contaminants are of concern due to their toxicity and persistence in humans and wildlife. A number of adverse health outcomes have been associated with exposure to organochlorines in humans, including reproductive effects, neurobehavioral deficits, obesity, diabetes, and cancer (Androutsopoulos et al., 2013; Mrema et al., 2013). They constitute a heterogeneous class of chemicals with a chlorinated hydrocarbon structure. p,p’-DDD is both a breakdown product and a constituent often found in technical grade formulations of the insecticide, p,p’-dichlorodiphenyltrichloroethane (p,p’-DDT). Technical DDT was widely used in agricultural and other commercial applications until 1972, when it was banned in the U.S. and most parts of the world, although its application in controlling malaria outbreaks continues in some countries (UNEP, 2017). Despite restrictions imposed to regulate use and manufacturing, p,p’-DDT and two of its primary breakdown components,p,p’-DDD and dichlorodiphenyldichloroethylene (p,p’-DDE), can be readily found in the environment, animals, and human populations, mostly from past insecticide applications (ATSDR, 2002a). Bioconcentration in aquatic species and biomagnification through the food chain has been documented for p,p’-DDT-type contaminants (Borga et al., 2001; Muir et al., 2003; Skarphedinsdottir et al., 2010) and volatilization is expected from both water and soil surfaces, contributing substantially to the regional and worldwide dispersion of these chemicals (Bidleman and Leone, 2004; Kurt-Karakus et al., 2006; Shunthirasingham et al., 2016).
As part of this analysis and in the absence of adequate chemical-specific information in either humans or animals for non-cancer oral toxicity, analogues were identified and evaluated for their suitability for screening-level assessment of p,p’-DDD. Structural analogues with existing health reference values were identified for p,p’-DDD using publicly available similarity search databases (ChemIDplus, 2017; DSSTox, 2016). The availability of health reference values provides a pool of analogues with well-vetted toxicity databases that increases confidence in the prediction and facilitates the selection of a source analogue and its associated POD for quantitative read-across. The set of identified analogues was evaluated for similarity with respect to the target chemical on the basis of structural and physicochemical properties, toxicokinetics, and toxicodynamics, integrating information across the different similarity contexts to build a justification for read-across. In this particular example, toxicokinetics (with an emphasis on metabolism pathway similarities) proved most valuable in differentiating the single best source analogue compared to other similarity contexts (i.e. structure and toxicodynamics). Additionally, the utility of HTS assays from the EPA’s ToxCast database were explored to further inform the similarity justification and read-across prediction.
2. Materials and Methods
2.1. Read-across Approach
The read-across methodology applied to identify a source analogue for p,p’- DDD was adapted from Wang et al. (2012). First, structural analyses were conducted to search for potential analogues using two web-based tools that provide similarity comparisons between chemical structures on the basis of fingerprints using the Tanimoto coefficient (generally defined as the size of the intersection divided by the size of the union of two sample sets). The two web-based tools used include the National Library of Medicine’s ChemIDplus database (ChemIDplus, 2017) and U.S. EPA’s Distributed Structure-Searchable Toxicity database (DSSTox, 2016)2 A predefined similarity threshold of ≥ 50% was selected for both databases with the ability to make adjustments based on the number of analogues obtained or in accordance with any expert knowledge on the chemical class. The list of structurally similar chemicals generated from ChemIDplus was manually cross-referenced to risk assessment data repositories from the U.S. EPA’s Integrated Risk Information System (IRIS) (https://www.epa.gov/iris) and Provisional Peer-Reviewed Toxicity Value (PPRTV) electronic library (https://hhpprtv.ornl.gov/quickview/pprtvpapers.php), and the U.S. Department of Health and Human Services’ Agency for Toxic Substances and Disease Registry (ATSDR) Toxic Substance Portal (https://www.atsdr.cdc.gov/substances/index.asp). In the case of DSSTox, the search option for “IRISTR_v1b” was selected to find analogues with dose-response information from the EPA’s IRIS database. Search results from the two databases were combined, retaining only those analogues with existing non-cancer oral health reference values. The initial search for analogues via ChemIDplus and DSSTox was followed by a more comprehensive, expert-driven evaluation of chemical and biological properties as detailed below.
Evaluations of structure, physicochemical properties, toxicokinetics and toxicodynamics (including, in vitro bioactivity) were conducted to assess the suitability of the analogues identified. Commonalities in basic structural features, key functional groups, and physiochemical properties were taken into consideration for structural similarity assessment. Relevant toxicokinetic (Absorption, Distribution, Metabolism, Excretion; ADME) and toxicodynamic data were collected for the target chemical and corresponding analogues from PubMed literature searches (https://www.ncbi.nlm.nih.gov/pubmed) and available health assessment documents from the aforementioned risk assessment databases. The information was used to draw comparisons between the target and analogues regarding their toxicokinetic profiles, focusing on metabolic similarities involving common metabolic precursors, intermediates and end-products, as well as, toxic metabolites. Furthermore, the analogues were evaluated for similarities in toxicodynamics such as target organs, toxic effects, MOA and chemical class/mixture. A comparison of in vitro bioactivity for the target and analogues was performed using HTS data from ToxCast (see section 2.2).
Information from structural, toxicokinetic, and toxicodynamic evaluations for the target and analogues was pooled together and analyzed for consistency and concordance to select a single best source analogue for screening-level toxicity assessment of the target. During this approach, greater emphasis was given to toxicodynamic and/or toxicokinetic similarity over structural similarity particularly when associated with the expected toxicity, and recognizing any interdependence between chemical and biological similarities. The following overarching similarities were considered when evaluating the suitability of analogues in accordance with Wang et al. (2012): 1) a similar biological response, toxic effect, or MOA; 2) a shared metabolite/precursor or metabolism pathway; 3) a relative potency factor (RPF) or toxicity equivalent factor (TEF) approach applied in the context of a chemical class/mixture. Analogues were excluded if they did not demonstrate commonality from one or more of the similarities above or had significantly different physicochemical properties or toxicokinetic profiles that set them apart from other analogues and/or the target chemical. From the remaining analogues ranked on the basis of toxicodynamic/toxicokinetic similarities, considerations for structural similarity and/or health protectiveness of the toxicity value (i.e. POD) were taken into account to further refine the selection of the single best source analogue for read-across. The POD from the selected source analogue was subsequently proposed in the derivation of non-cancer oral health reference values for the target.
2.2. High-throughput Screening Data Analysis
In vitro bioactivity data for p,p’-DDD and the identified analogues were downloaded from the EPA’s CompTox Chemistry Dashboard (https://comptox.epa.gov/dashboard) (U.S. EPA, 2017a), which contains information on HTS assays from the ToxCast and Toxicity Testing in the 21st century (Tox21) collaboration projects (U.S. EPA, 2015). The ToxCast database includes over 700 assay endpoints that cover a variety of biological responses across ~300 signaling pathways (U.S. EPA, 2015). We narrowed the scope of the HTS screening analysis by evaluating similarities in bioactivity of most relevance to the primary health effects for the target and analogues (i.e. liver and reproductive toxicity). To examine potential mechanisms of liver toxicity, results (active/inactive, AC50, and scaled activity) from ToxCast assays tested at multiple concentrations (up to 200μM) in immortalized and primary liver cells from human and rat tissues were extracted, filtering out background control assays. Bioactivity data from in vitro assays in human liver cells were grouped based on the type of biological response or biological target using information within the ToxCast annotation library (U.S. EPA, 2015). Data in rat liver cells were only available for one of the p,p’-DDD analogues (i.e. methoxychlor) and therefore were excluded from further consideration. Subsequently, nuclear receptor assays conducted in human hepatoma HepG2 cells were investigated to identify putative target signaling pathways. The nuclear receptor assays analyzed in the positive fitting direction relative to the negative control (‘up’) were mapped to the target gene, filtering out non-specific responses from assays analyzed in the opposite direction (‘dn’). Lastly, ToxCast bioactivity data and model predictions for the estrogen receptor alpha/beta (ERα/β) and androgen receptor (AR) were obtained from Judson et al. (2015) and Kleinstreuer et al. (2016), respectively, to make comparisons of endocrine disrupting activity related to potential mechanisms of reproductive toxicity for p,p’-DDD and the analogues. Judson et al. (2015) and Kleinstreuer et al. (2016) analyzed a suite of HTS ToxCast assays encompassing several key signaling events in the pathways of these steroid hormone receptors (e.g. receptor binding, dimerization, co-factor recruitment, transcription factor-DNA binding, RNA transcription and agonist/antagonist transactivation). Computational models predicting ER/AR agonist and antagonist activities were developed for a library of 1812 chemicals, taking into account non-specific responses such as cytotoxicity (Judson et al., 2015; Kleinstreuer et al., 2016). We used Microsoft Excel and Power View 2016 for the data analysis and visualization presented herein.
3. Results
Case Study: p,p’-DDD (CASRN 72-54-8)
3.1. Analogue Identification and Structural Similarity Evaluation
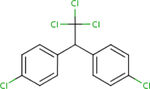
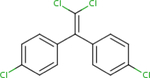
Analogues for p,p’-DDD were identified based on the search strategy described in the Materials and Methods (section 2.1). The ChemIDplus similarity search yielded a total of 119 identifications with the predefined threshold of ≥50% similarity; however, only three had published non-cancer oral health reference values from searched toxicity databases: p,p’-DDT (ATSDR, 2002a; U.S. EPA, 1987), p,p’-DDE (U.S. EPA, 2017c), and p,p’- dimethoxydiphenyltrichloroethane (methoxychlor) (ATSDR, 2002b; U.S. EPA, 1990). No additional candidates were found by searching DSSTox but findings from the two structural databases provided similar rankings (i.e. similarity scores) for the analogues identified (Table 1). Based on similarity estimates from ChemIDplus and DSSTox (77% and 96%, respectively), p,p’- DDT is the closest structural analogue to p,p’-DDD, followed by p,p’-DDE (67 and 61%, respectively), and methoxychlor (65 and 52%, respectively). All three analogues met the initial search criteria (≥50% structural similarity and availability of non-cancer oral health reference values) and were carried forward for further structural similarity evaluation.
Table 1.
Analogues Identified for p,p’-DDD
| Target Chemical | Analoguesa | |||
|---|---|---|---|---|
| Name | p,p’-Dichlorodiphenyl dichloroethane (p,p’-DDD) | p,p’-Dichlorodiphenyl trichloroethane (p,p’-DDT) | p,p’-Dichlorodiphenyl dichloroethylene (p,p’-DDE) | p,p’-Dimethoxydiphenyl trichloroethane (Methoxychlor) |
| CASRN | 72-54-8 | 50-29-3 | 72-55-9 | 72-43-5 |
| Structure | 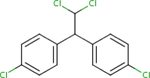 |
 |
 |
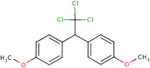 |
| ChemlDplus similarity score (%) | 100 | 77 | 67 | 65 |
| DSSTox similarity score (%) | 100 | 96 | 61 | 52 |
Identified analogues represent a set of structurally similar chemicals prefiltered on the basis of availability of non-cancer health reference values for oral exposure from regulatory databases as described in section 2.1.
p,p’-DDD and analogues (Table 1) are part of the DDT-type insecticide class that shares a common substituted diphenylalkane structure containing three or more chlorine atoms (Coats, 1990). Quantitative Structure-Activity Relationship (QSAR) analyses have suggested the importance of the para (p-) functional groups with regards to the insecticidal activity and physicochemical properties of DDT analogues (Coats, 1990). Although all four DDT-related organochlorines exist in other less common isomeric forms (namely o p’- isomers vs. p,p’- isomers), the focus of this study will be on the p,p’- isomers based on the availability of health reference values for these analogues. In the case of p,p’-DDT, p,p’-DDD, and p,p’-DDE, the p- positions are occupied by chlorine atoms. Conversely, these aromatic positions are replaced by methoxy groups (O-CH3) in the methoxychlor molecule, contributing to its greater metabolic activity and biodegradation in contrast to the p,p’-chlorinated organochlorines (i.e. p,p’-DDT,p,p’-DDE and p,p’-DDD) (Kapoor et al., 1970). In general, physicochemical properties relevant to bioaccumulation and environmental fate (e.g. molecular weight, water solubility, and logKow) for the target and analogues are comparable (Table 2). Of particular importance is the highly lipophilic nature of DDT-type insecticides evident by their poor water solubility and high log octanol-water partition coefficients (log Kow). The lipophilicity of these chemicals combined with slow rates of abiotic and biotic degradation allows for their accumulation in aquatic animals (measured as bioconcentration factors [BCF]; Table 2) particularly as it pertains to p,p’-DDT,p,p’-DDE, and p,p’-DDD. Notably, methoxychlor is a less bioaccumulative environmental contaminant than the p,p’-chlorinated organochlorines, as reflected by its relatively lower BCF values (Table 2).
Table 2.
Relevant Physicochemical and Environmental Properties for p,p’-DDD and Analoguesa
| Chemical | p,p’-DDD | p,p’-DDT | p,p’-DDE | Methoxychlor |
|---|---|---|---|---|
| Molecular weightb | 320.045 | 354.4901 | 318.0292 | 345.651 |
| Melting point (°C)b | 109.5 | 108.5 | 89 | 87 |
| Boiling point (°C)b | 350 | Not available | 336 | 346 |
| Vapor Pressure (mm Hg at 25 °C)b | 1.35 × 10−6 | 1.60 × 10−7(20 °C) | 6.0 × 10−6 c | 2.58 × 10−6 |
| Henry’s Law Constant (atm-m3/mole at 25 °C)b | 6.60 × 10−6 | 8.32 × 10−6 | 4.16 × 10−5 | 2.03 × 10−7 |
| Water solubility (mg/L) at 25 °Cb | 0.09 | 0.0055 | 0.04 | 0.1 |
| Log Kowb | 6.02 | 6.91 | 6.51 | 5.08 |
| Bioconcentration factor (BCF)d | 2,710–51,000 | 600 – 84,500 | 27,500–81,000 | 138–8,300 |
Values represent experimental measures
Data were sourced from ChemIDplus (2017) unless otherwise specified
In summary, p,p’-DDT and p,p’-DDE demonstrate similarities in basic structural features (chlorinated diphenylalkane structure), key functional groups (p,p’-chlorine substituents), and physicochemical properties that support their suitability as structural analogues of p,p’-DDD. In contrast, the presence of p,p’-methoxy groups in methoxychlor are expected to enhance its biodegradation in animals and in the environment, which could result in differences in the toxicokinetic and toxicodynamic profile of this chemical in relation to p,p’-DDD and the other analogues; thus, methoxychlor can be considered a less suitable structural analogue.
3.2. Toxicokinetic Similarity Evaluation
Absorption and Distribution: Absorption rates were on the order of 70–100% in rats for p,p’-DDT and >90% in mice for methoxychlor, demonstrating that these chemicals are almost completely absorbed via the intestinal tract following oral administration (Kapoor et al., 1970; Keller and Yeary, 1980; Rothe et al., 1957). No data on the rate or extent of oral absorption for p,p’-DDD and p,p’-DDE could be located. However, oral bioavailability is anticipated to be similar for the target and the analogues based on similarities in physicochemical properties discussed previously (e.g. water solubility, log Kow). Once absorbed into the body, p,p’-DDT, p,p’-DDD, and p,p’-DDE are stored and retained in human fat tissue after long-term exposure (5–20 mg administered daily for up to 183 days) (Morgan and Roan, 1971), consistent with the lipophilic nature of these chemicals. p,p’-DDT, p,p’-DDD, and p,p’-DDE have also been detected in human breast milk (Gebremichael et al., 2013; Hassine et al., 2012; Kunisue et al., 2006; Malarvannan et al., 2009) and in maternal and cord blood of delivering mothers (Channa et al., 2012; Sala et al., 2001; Waliszewski et al., 2000). Studies in pregnant rats provide support for placental and lactational transfer of p,p’-DDT and p,p’-DDE and the preferential partitioning of these chemicals into animal fat (Woolley and Talens, 1971; You et al., 1999). Methoxychlor can also be found in fat after repeated dietary administration to rats but it is rapidly removed from fat stores after cessation of exposure (Harris et al., 1974; Kunze et al., 1950), suggesting that unlike the p,p’-chlorinated organochlorines, methoxychlor does not appear to persist in the body.
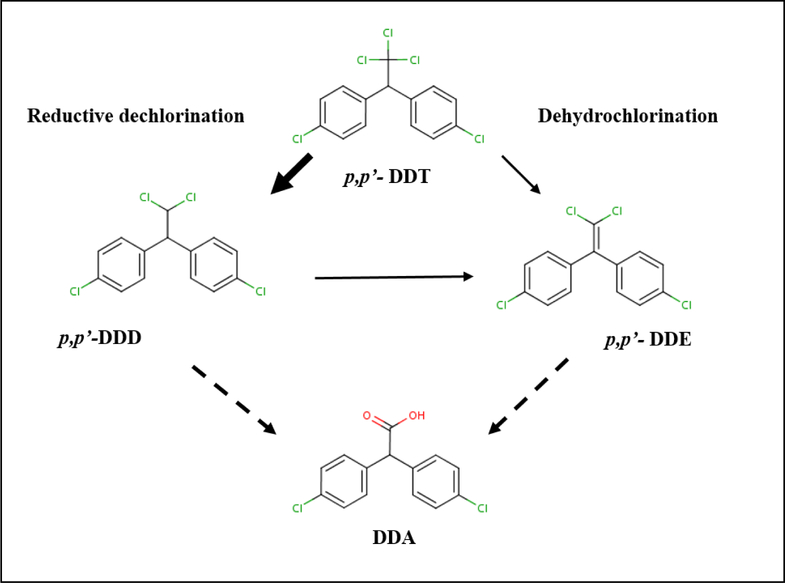
Metabolism: p,p’-DDD and p,p’-DDE are primary intermediary metabolites of p,p’-DDT, resulting from initial dechlorination in the aliphatic portion of the parent molecule (Figure 1). Dechlorination of p,p’-DDT to p,p’-DDD is a reductive process, while conversion of p,p’-DDT to p,p’-DDE occurs by dehydrochlorination. Evidence in humans and animals suggests that metabolic breakdown of p,p’-DDT via the p,p’-DDD pathway is favored comparatively to the p,p’-DDE pathway (Morgan and Roan, 1971; Peterson and Robison, 1964). Metabolism of p,p’- DDD to p,p’-DDE can be inferred from the isolation of DDE from excreta samples of DDD-exposed animals (Table 3). p,p’-DDT and p,p’-DDD are eventually oxidized and excreted from the body in the form of 2,2-bis(p-chlorophenyl) acetic acid (DDA), the primary urinary metabolite detected in humans, rats, mice, and hamsters for these chemicals (Gold and Brunk, 1982; 1983; Peterson and Robison, 1964; Roan et al., 1971). Comparative oral studies in mice revealed remarkable similarities in the metabolism of p,p’-DDT and p,p’-DDD based on patterns of excreted metabolites (Table 3). Metabolism of p,p’-DDE is largely inefficient compared to p,p’-DDD and p,p’-DDT. As such, p,p’-DDE is the major component recovered from urine or feces from DDE-exposed mice (Table 3). In humans, the apparent metabolic inactivity of p,p’-DDE has been attributed to its greater affinity for storage in fat tissue (Morgan and Roan, 1971). Metabolism of p,p’-DDE to DDA and ring hydroxylated DDE products has been demonstrated in rats via intraperitoneal injection, which suggests some commonalities in metabolic pathways between p,p’-DDD and p,p’-DDE in this species and administration route (Fawcett et al., 1987). Additionally, methylsulfonyl metabolites of p,p’-DDE (formed by interaction of arene oxide DDE intermediates with GSH followed by the mercapturic acid pathway) have been detected in humans and marine animals (Chu et al., 2003a; Chu et al., 2003b; Larsson et al., 2004; Linderholm et al., 2007) and could represent an important metabolic pathway for this chemical.
Figure 1. Basic Metabolic Scheme for p,p’-DDT.
Conversation of p,p’-DDT to p,p’-DDD via reductive dechlorination occurs more readily than dehydrochlorination of p,p’-DDT to p,p’-DDE. Both, p,p’-DDD and p,p’-DDE, can be oxidized to the primary urinary metabolite, of 2,2-bis(p-chlorophenyl) acetic acid (DDA). Figure has been adapted from ATSDR (2002a).
Table 3.
Comparative Disposition Studies for p,p’-DDD and Analogues in Mice Exposed via the Oral Route
| Chemical | Dose | Metabolites and other excretion products (%) | Source |
|---|---|---|---|
| p,p’-DDD | 500 mg/kg | 2,2-bis(p-chlorophenyl)acetic acid, DDA (95); parent chemical (4); 2-hydroxy-DDA (1); 2,2-bis(p-chlorophenyl)ethanol, DDOH (<1); DDE (<1); l-chloro-2,2-bis(/p-chlorophenyl)ethene, DDMU (<1) in urine within 72 ha | (Gold and Brunk, 1982) |
| p,p’-DDT | 100 mg/kg | DDA (86); DDE (9); DDD (3); 2-hydroxy-DDA (1); parent chemical (<1); DDMU (<1); DDOH (<1) in urine within 72 ha | (Gold and Brunk, 1982) |
| p,p’-DDE | 20 mg/kg | Mostly excreted as parent chemical in urine and feces (~7 and 4, respectively) within 72 h. 3’-hydroxy-DDE was the only metabolite identified (<1 in urine and 2 in feces)b | (Gold and Brunk, 1986) |
| Methoxychlor | 50 mg/kg | Mono-hydroxy methoxychlor (30); bis-hydroxy-methoxychlor (23); bis-hydroxy-diphenylacetic acid and bis-hydroxy-benzophenone (11); parent compound (8); bis-hydroxy-dichloroethylene (1) in urine and feces over an 11-day periodb | (Kapoor et al., 1970); (ATSDR, 2002b) |
Values expressed as % of excreted dose
Values expressed as % of administered dose
The major metabolic pathway for methoxychlor in mice involves sequential demethylation at its p-positions (O-demethylation) yielding mono- and bis-hydroxy methoxychlor derivatives that are preferentially excreted via the feces (Table 3). These demethylated metabolites possess greater endocrine disrupting activity compared to methoxychlor (Bulger et al., 1978; Charles et al., 2000; Maness et al., 1998; Sumida et al., 2001) and are likely to be involved in the reproductive effects of the parent chemical; thus, metabolic activation of methoxychlor is expected through this pathway. Aliphatic dechlorination represents a minor oxidation pathway for methoxychlor that produces metabolites such as bis-hydroxydiphenyl acetic acid, bis-hydroxybenzophenone, and bis-hydroxy-dichloroethylene (Table 3). Differences in metabolite disposition patterns between methoxychlor and p,p’-DDD are consistent with overall differences in the metabolic profile of these organochlorines (Table 3).
Excretion: Evidence collected in human volunteers demonstrates that elimination of p,p’- DDT, p,p’-DDD, or p,p’-DDE from fat stores or via the urine occurs slowly over months and even years after termination of exposure (5–20 mg administered daily for up 183 days) (Morgan and Roan, 1971; Roan et al., 1971). The suggested ranking for elimination rates based on these findings decreases in the following order p,p’-DDD > p,p’-DDT > p,p’-DDE (Morgan and Roan, 1974). No excretion data are available for methoxychlor in humans for comparative purposes. Nevertheless, studies in laboratory animals indicate that methoxychlor is less bioaccumulative than the p,p’-chlorinated organochlorines. The elimination half-life of methoxychlor from body fat in sheep was 10 days compared to 26 days for p,p’-DDD, 90 days for p,p’-DDT, and 223 days for p,p’-DDE (Reynolds et al., 1976). Similarly, the rate of excretion for methoxychlor in mice was rapid, achieving 98.3% elimination within 24 hours versus 1.02% reported for DDT and its metabolites (Kapoor et al., 1970).
Altogether, p,p’-DDT is a metabolic precursor to p,p’-DDD and a suitable analogue of p,p’-DDD on the basis of toxicokinetic attributes, including preferential partitioning into fat, similar metabolism and excretion pathways (both chemicals are predominantly converted to the urinary metabolite, DDA), and prolonged elimination rates particularly in humans. The other analogues are less suitable. Although p,p’-DDE is stored and highly retained in fat, it is less metabolically active than p,p’-DDD and p,p’-DDT. Biotransformation pathways and metabolite patterns for methoxychlor differ from p,p’-DDD and p,p’-DDT. Methoxychlor is also more rapidly metabolized and cleared via the feces and does not appear to persist in fat.
3.3. Toxicodynamic Similarity Evaluations
Experimental data in laboratory animals are available for p,p’-DDD and its analogues for comparison of toxicity endpoints and associated dose-response relationships. p,p’-DDT, p,p’- DDD, and p,p’-DDE demonstrate similarly low acute toxicity in hamsters (LDs > 5,000 mg/kg), although p,p’-DDT is consistently more acutely toxic than p,p’-DDD, p,p’-DDE, and methoxychlor in rats, mice or rabbits (Table 4). The central nervous system is a target organ of toxicity for these organochlorines in animal lethality studies (Table 4). Likewise, neurological effects have been observed in humans with acute, high-dose DDT exposures (Garrett, 1947; Hsieh, 1954).
Table 4.
Acute Lethality Data for p,p’-DDD and Analoguesa
| Chemical | p,p’-DDD | p,p’-DDT | p,p’-DDE | Methoxychlor |
|---|---|---|---|---|
| Rat Oral LD50 (mg/kg) | 113 | 87 | 880 | 1,855 |
| Mouse Oral LD50 (mg/kg) | 600 (LDLo)b | 135 | 700 | 510 |
| Hamster Oral LD50 (mg/kg) | >5,000 | >5,000 | >5,000 | Not available |
| Rabbit Skin LD50 (mg/kg) | 1,200 | 300 | Not available | >6,000 |
| Effects | Excitement, convulsions or effect on seizure threshold, tremor, skin irritation (with topical exposure) | Ataxia, muscle weakness, tremor | Not specified | Convulsions or effect on seizure threshold, ataxia, excitement |
Data were sourced from ChemIDplus (2017)
Value represents the lowest dose associated with lethality (lowest lethal dose; LDLO).
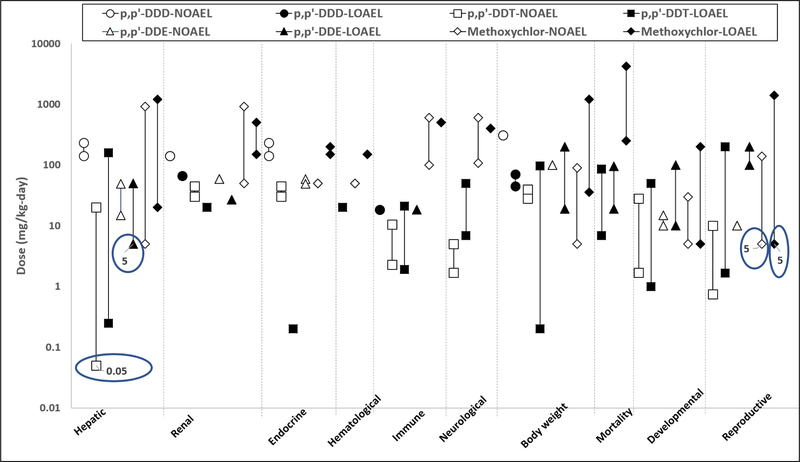
Comparison of effects in animals for p,p’-DDD and its analogues after repeated oral administration inform of potential adverse health outcomes for this group of organochlorines that include hepatic, renal, endocrine, hematological, immune, neurological, general (i.e. body weight and mortality), developmental, and reproductive toxicities (Figure 2). The liver is a primary target organ for p,p’-DDT and p,p’-DDE, and PODs used in the derivation of non-cancer oral health reference values for these chemicals are based on hepatic effects in rats (Table 5). Non-neoplastic effects in the liver for p,p’-DDT and p,p’-DDE include increased liver weight and hepatocellular changes such as hypertrophy, fatty degeneration and necrosis (Table A-1). Methoxychlor appears to be less potent than p,p’-DDT and p,p’-DDE with respect to liver effects. Indeed, liver toxicity induced by methoxychlor occurs at higher doses compared to p,p’-DDT and p,p’-DDE (LOAELs range from 0.25–160, 5–50, and 20–1,200 mg/kg-day for p,p’-DDT, p,p’-DDE, and methoxychlor, respectively) and is characterized by less severe responses (i.e. changes in liver weight and serum biomarkers and gross liver lesions) (Table A-1). Available animal studies on the effects of p,p’-DDD in the liver or other organs have generally focused on assessment of carcinogenicity (NCI, 1978; Tomatis et al., 1974), providing limited information on non-cancer endpoints (Figure 2). Nevertheless, p,p’-DDT, p,p’-DDD, and p,p’-DDE are well-known liver carcinogens in rodents and demonstrate similar carcinogenic potencies based on published cancer oral slope factors (Table 5). In contrast, evidence of liver carcinogenicity with methoxychlor exposure is inconclusive (Table 5). The data suggest that the liver is a relevant target organ of toxicity for these chemicals, particularly the p,p’-chlorinated organochlorines.
Figure 2. Range of Effect levels for Noncancer Oral Toxicity for p,p’-DDD and Analogues from Repeated-Dose Animal Studies.
Graph displays the range of effect levels (no-observed-adverse-effect levels [NOAEL] and lowest-observed-adverse-effect levels [LOAEL]) for noncancer endpoints from repeated-dose animal toxicity studies via oral administration reported by ATSDR (2002a, b) and U.S. EPA (2017 b, c). Studies for DDT, DDD, and methoxychlor include technical grade and analytical formulations of the p,p’ isomers. Circles note PODs used in the derivation of oral reference doses (RfDs) and minimal risk levels (MRLs) for these chemicals described in more detail in Table 5.
Table 5.
Comparison of Oral Health Reference Values for p,p’-DDD and Analogues
| Chemical | p,p’-DDD | p,p’-DDT | p,p’-DDE | Methoxychlor |
|---|---|---|---|---|
| POD for subchronic RfD/MRL (mg/kg-day) | NDa | 0.05 (NOAEL) | 5 (LOAEL) | 5 (LOAEL) |
| Critical effect | NDa | Liver lesions in rats exposed for 15–27 weeks | Increased relative liver weight in male offspring rats exposed during gestation and lactation | Precocious puberty in female rats (i.e., accelerated vaginal opening) exposed in utero, during lactation and after weaning |
| Source | (U.S. EPA, 2017b) | (ATSDR, 2002a) | (U.S. EPA, 2017c) | (ATSDR, 2002b) |
| POD for chronic RfD (mg/kg-day) | NDa | 0.05 (NOAEL) | 5 (LOAEL) | 5.01 (NOAEL) |
| Critical effect | NDa | Liver lesions in rats exposed for 15–27 weeks | Increased relative liver weight in male offspring rats exposed during gestation and lactation | Excessive litter loss in rabbits exposed during gestation (i.e., spontaneous abortion) |
| Source | (U.S. EPA, 2017b) | (U.S. EPA, 1987) | (U.S. EPA, 2017c) | (U.S. EPA, 1990) |
| Carcinogenicity classification | B2; probable human carcinogen | B2; probable human carcinogen | B2; probable human carcinogen | D; not classified as to human carcinogenicity |
| OSF (mg/kg-day)−1 | 2.4 × 10−1 | 3.4 × 10−1 | 3.4 × 10−1 | ND |
| Basis | Liver tumors in mice | Liver tumors in mice and rats | Liver tumors in mice and hamsters | Human data is unavailable and animal evidence is inconclusive |
| Source | (U.S. EPA, 1988c) | (U.S. EPA, 1988a) | (U.S. EPA, 1988d) | (U.S. EPA, 1988b) |
Toxicity data for p,p’-DDD is insufficient for the derivation chemical-specific oral health reference values; however, screening-level values have been developed for this chemical by U.S. EPA (2017b) based on a read-across approach.
Abbreviations: LOAEL, lowest-observed-adverse-effect level; ND, not determined; NOAEL, no-observed-adverse-effect level; MRL, minimal risk level; OSF, oral slope factor; POD, point of departure; RfD, oral reference dose.
Another important aspect of the toxicity of p,p’-DDT, p,p’-DDE and methoxychlor centers on effects within the reproductive system among developing and adult animals (Figure 2). Methoxychlor treatment can cause deleterious effects on the male and female reproductive system, namely decreased fertility, adverse pregnancy outcomes, altered sexual maturation and mating behavior, as well as gross and histopathological changes in reproductive organs (Table A-2). Furthermore, non-cancer oral health reference values for methoxychlor are derived from PODs based on reproductive effects in female rats and rabbits (Table 5). p,p’-DDE is known to disrupt sexual development and function in males, although it also exerts some effects on the female reproductive system (Table A-2). The reproductive toxicity of p,p’-DDT is primarily associated with adverse pregnancy outcomes and decreased fertility in males and females (Table A-2). Comparison of reproductive/developmental LOAELs from repeated-dose studies in animals suggests the following potency ranking: p,p’-DDT > methoxychlor > p,p’-DDE (Figure 2). No reproductive or developmental toxicity studies are available for p,p’-DDD, although some epidemiological accounts have found associations between the levels of p,p’-DDD and other organochlorine contaminants in body fluids with possible alterations in reproductive and developmental measures in humans (Al-Saleh et al., 2012; Dalvie et al., 2004; Freire et al., 2014; Guo et al., 2014; Pant et al., 2007; Pant et al., 2004; Perry et al., 2006; Pines et al., 1987; Saxena et al., 1980; Saxena et al., 1981; Tyagi et al., 2015). The reproductive effects of p,p’-DDT,p,p’-DDE and methoxychlor in animals coincide with the endocrine disrupting activity of these chemicals involving the interaction with steroid hormone receptors such as the ER and AR (Charles et al., 2000; Kelce et al., 1995; Maness et al., 1998; Soto et al., 1997; Sumida et al., 2001). Overall, similarities with respect to adverse health effects are apparent between p,p’-DDD and the identified analogues, pointing to possible shared toxicity targets (primarily the liver and reproductive system).
3.4. Bioactivity Similarity Evaluations
3.4.1. Analysis of ToxCast HTS data from in vitro assays in human liver cells.
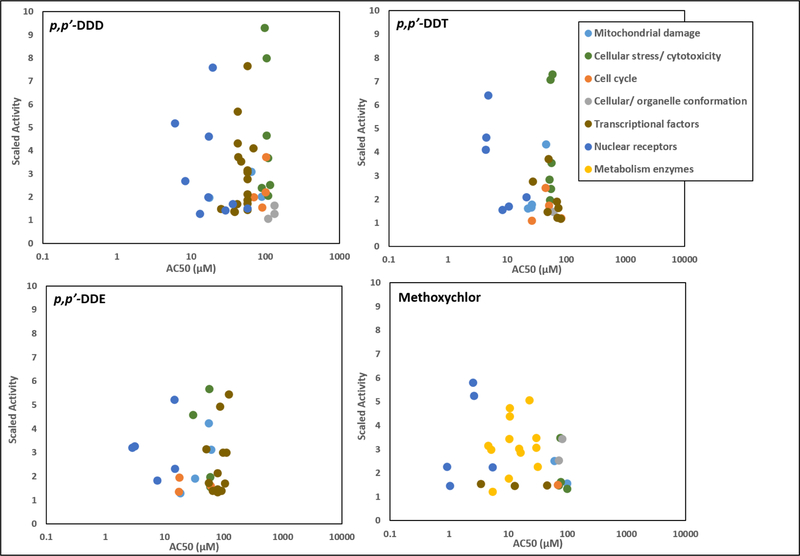
The availability of HTS data from ToxCast allowed for direct comparisons of in vitro bioactivity and potential mechanisms of toxicity of interest to this group of organochlorines. First, assays conducted in immortalized and primary liver cells derived from human tissue (Table A-3) were sourced from the U.S. EPA’s ToxCast database to provide insights into possible mechanisms of hepatotoxicity, a sensitive target organ effect in animal bioassays for p,p’-DDD and the analogues (Figures 2 and Table 5). Generally, p,p’-DDD, p,p’-DDT ,p,p’-DDE and methoxychlor displayed similar bioactivity patterns in human liver cells. p,p’-DDD had the highest total number of active assays (28%; 52 out of 185 unique assay endpoints), followed by p,p’-DDE (19%; 36 out of 185), p,p’-DDT (17%; 32 out of 185), and methoxychlor (13%; 31 out of 247).p,p’-DDD and the analogues induced both cell- and gene- specific changes in human hepatoma HepG2 cells (Figure 3 and Table A-3).p,p’-DDD, p,p’-DDT, p,p’-DDE, and methoxychlor were associated with decreased mitochondrial mass and membrane potential and markers of oxidative stress and cytotoxicity (Table A-3). Results for cell cycle-related assays were inconsistent across p,p’- DDD and the analogues, suggesting both induction and repression of cell cycle arrest responses (Table A-3). Changes in cellular/organelle conformation were reported for p,p’-DDD, p,p’-DDT, and methoxychlor (Table A-3). p,p’-DDD and p,p’-DDT caused alterations in microtubule conformation and /or nuclear size, which could be interpreted as coinciding with the observed effects on cell cycle arrest and cytotoxicity for these chemicals. Methoxychlor also showed alterations in microtube conformation, albeit opposite to p,p’-DDD. General upregulation of transcription factor activity, (including nuclear receptor activity) was reported for p,p’-DDD, p,p’-DDT, p,p’-DDE and methoxychlor (Table A-3). In addition, methoxychlor was tested in primary human hepatocytes, demonstrating transcriptional induction of cytochrome P450 (CYP450) and uridine glucuronosyltransferase (UGT) enzymes involved in metabolism (Table A-3). p,p’-DDD, p,p’-DDT, andp,p’-DDE were not tested in these assays, preventing a direct comparison of CYP450 and UGT transcriptional activity.
Figure 3. Bioactivity data for p,p’-DDD and Analogues in ToxCast Assays Conducted in Human Hepatoma HepG2 Cells and Primary Human Hepatocytes.
Scatterplots show AC50 and scaled activity values for p,p’-DDD, p,p’-DDT, p,p’-DDE and methoxychlor from in vitro assays visualized according to the type of biological response or biological target. AC50 values refer to the concentration that elicits half maximal response and the scaled activity refers to the response value divided by the activity cutoff (U.S. EPA, 2015). Metabolism enzyme-related assays were conducted in human primary hepatocytes and all other in vitro assays were measured in HepG2 cells. Assays for which chemicals were inactive are not displayed. Further information on AC50 values for individual assays can be found in Table A-3.
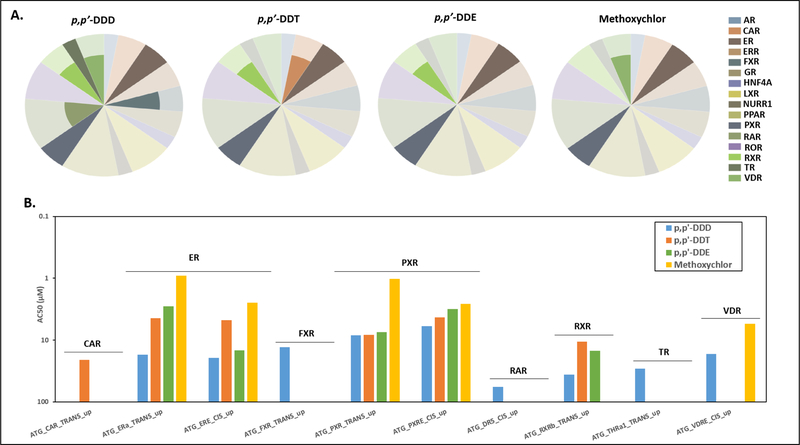
In general, organochlorine-mediated induction of nuclear receptor activities occurred at lower concentrations (i.e. AC50 values) compared to other categories of bioactivity responses in human liver cells, including those associated with cytotoxicity (Figure 3). As such, nuclear receptor assays were investigated more closely to evaluate potential signaling pathways upstream of the observed cell-specific changes. In this context, p,p’-DDD, p,p’-DDT, p,p’-DDE, and methoxychlor demonstrated consistencies in the in vitro responses for a number of human steroid/xenobiotic-sensing receptors (Figure 4A). Of particular importance, the ER and pregnane X receptor (PXR) were sensitive targets of all four chemicals (Figure 4B). p,p’-DDD and its analogues displayed inducible activity for the ER and PXR in 2 out of 2 independent assays for each receptor. The ranked order of potency for the ER and PXR based on a comparison of AC50 values is approximately: methoxychlor > p,p’- DDE > p,p’-DDT > p,p’-DDD. All chemicals with the exception of methoxychlor were also active in an assay for the retinoid X receptor (RXR), while p,p’-DDD and methoxychlor were active in a vitamin D receptor (VDR) activity assay (Figure 4). The farnesoid X receptor (FXR), retinoid acid receptor (RAR) and thyroid hormone receptor (TR) were targets for p,p’-DDD, and the constitutive androstane receptor (CAR) was a target for p,p’-DDT (Figure 4). In summary, ToxCast assays conducted mostly in HepG2 cells demonstrated similarities between p,p’-DDD and the identified analogues in responses characterizing mitochondrial damage, cellular stress and cytotoxicity. Additionally, these organochlorines elicited the upregulation of specific human steroid/xenobiotic-sensing receptors (most notably the human ER and PXR) which may be relevant to the mechanisms of hepatotoxicity of these chemicals.
Figure 4. ToxCast Assays Evaluating Regulation of Nuclear Receptor Activity for p,p’-DDD and Analogues in Human Hepatoma HepG2 Cells.
Panel A shows radar plots for p,p’-DDD, p,p’-DDT, p,p’-DDE and methoxychlor, summarizing active calls from nuclear receptor assays conducted in HepG2 cells and mapped to specific target genes. The shaded area of the pie slice represents the number of active assays and the size of the pie slice refers to the total number of assays within a given nuclear receptor target gene. Further details on assay activity results can be found in Table A-4. Bar graphs compare AC50 values (concentration at half maximal response) for active assays (panel B). The scale for the AC50 values is shown in reverse order to visualize the most sensitive nuclear receptor activities (the higher bar indicates a lower AC50 value).
3.4.2. Analysis of ToxCast bioactivity data and model predictions for the ER and AR pathways
Next, a suite of ToxCast assays and model predictions profiling alterations to ER and AR activities in response to organochlorine exposure were analyzed (Table 6) to gain further understanding of the mechanisms of endocrine disruption thought to be involved in the reproductive toxicity of the p,p’-DDD analogues. Methoxychlor was highly active in ER assays (78%), while p,p’-DDD, p,p’-DDT and p,p’-DDE displayed moderate activity for this receptor (39–61%). Importantly, these chemicals targeted similar events associated with human ER signaling activity (i.e. receptor dimerization, transcriptional factor-DNA binding, RNA transcription, and agonist transactivation) (Table A-5). Likewise, p,p’-DDD and analogues were active in assays for the human AR, displaying similar activity (27–36%) and target signaling events, including co-factor recruitment and antagonist transactivation (Table A-5). Additionally, p,p’-DDE interacted with the chimpanzee AR in radioligand competitive-binding assays. ToxCast model predictions (AUC values and associated 95% confidence intervals [CI]) integrate in vitro bioactivity results and provide “concentration-specific probabilities” for ER and AR activities, ranging from 0 to 1 (Table 6). The model scores suggest that p,p’-DDT and methoxychlor are ER agonists (AUC ≥ 0.1). p,p’-DDD and p,p’-DDE also possess agonistic activity for the ER (AUC = 0.0715 and 0.0679, respectively), although estimated AUC values for these chemicals lie outside of the activity threshold (AUC < 0.1; an AUC value of 0.1 corresponds to ~100 μM, the highest concentration tested for most assays), indicating weak activity. ToxCast model predictions for the AR pathway support an antagonistic effect for p,p’- DDE (AUC = 0.251) and indicate that p,p’-DDD, p,p’-DDT and methoxychlor could also act as AR antagonists (AUC of 0.0973, 0.0642, 0.0429 for p,p’-DD, p,p’-DDT and methoxychlor, respectively). Based upon comparisons of ToxCast model scores and AC50 values, the ranked order of potency for ER activity is as follows: methoxychlor > p,p’-DDT > p,p’-DDE > p,p’- DDD; and potency for AR activity is: p,p’-DDE > p,p’-DDD > p,p’-DDT > methoxychlor (Table 6). Collectively, the bioactivity data and model predictions demonstrate similarities in the endocrine disrupting activity of the target and analogues, suggesting that to some extent all four chemicals may exhibit estrogenic and anti-androgenic properties.
Table 6.
ToxCast Bioactivity data and Model prediction scores (AUC values) for ER and AR activities for p,p’-DDD and Analoguesa
| p,p’-DDD | p,p’-DDT | p,p’-DDE | Methoxychlor | |
|---|---|---|---|---|
| ER Assays | ||||
| Active/Total Assays (%) | 7/18 (39) | 11/18 (61) | 8/18 (44) | 14/18 (78) |
| AC50 values (μM) | Range = 14.0 – 32.4 Median = 18.7 |
Range = 3.3 – 59.8 Median = 6.1 |
Range = 3.5–46.2 Median = 16.5 |
Range = 0.9 – 44.2 Median = 4.6 |
| Agonist AUC values (95% CI)b | 0.0715 (0.0342–0.0738) | 0.190 (0.181–0.231) | 0.0679 (0.0614–0.0963) | 0.254 (0.247–0.260) |
| Antagonist AUC values (95% CI) | 0 | 0 | 0 | 0 |
| AR assays | ||||
| Active/Total Assays (%) | 4/11 (36) | 3/11 (27) | 4/11 (36) | 3/11 (27) |
| AC50 values (μM) | Range = 31.0–62.8 Median = 44.8 |
Range = 17.8–72.0 Median = 47.0 |
Range = 7.0 – 58.7 Median = 29.6 |
Range = 29.3 – 40.8 Median = 34.2 |
| Agonist AUC values (95% CI) | 0 | 0 | 0 | 0 |
| Antagonist AUC values (95% CI) | 0.0973 (0.0649–0.124) | 0.0642 (0.0318–0.108) | 0.251 (0.234–0.291) | 0.0429 (0.0364–0.0465) |
Data for ER and AR activities were sourced from Judson et al., (2015) and Kleinstreuer et al., (2016), respectively.
95% CI for the ER activity model were sourced from a subsequent publication to the Judson et al., (2015) study (Watt and Judson, 2018).
AUC = area under the curve score ranging from 0–1. An AUC value of 0 indicates that the chemcial is inactive.
CI = confidence interval.
3.5. Analogue Selection
To facilitate the selection of the source analogue for p,p’-DDD, the three analogues identified were ranked based on the similarity contexts previously discussed (structure, toxicokinetics, and toxicodynamics). When considering toxicity similarity comparisons, there is consistency and concordance in target organs and associated adverse health effects for non-cancer oral toxicity, particularly among the three analogues. The liver and reproductive system are shared toxicity targets for the analogues, with p,p’-DDT exerting the highest potency based on the available in vivo evidence (Figure 2). In vivo toxicity data on p,p’-DDD is primarily restricted to carcinogenicity, and in this regard, p,p’-DDT and p,p’-DDE demonstrate close similarity to the target chemical. p,p’-DDT, p,p’-DDE, and p,p’-DDD induced liver tumors in rodents displaying analogous carcinogenic potencies (Table 5), whereas methoxychlor did not appear to have a strong carcinogenic response in animal bioassays (U.S. EPA, 1988b). Furthermore, in vitro HTS data from ToxCast revealed similarities in the bioactivity profile of p,p’-DDD and the analogues with respect to cell-specific responses and target gene pathways that provide mechanistic plausibility for the primary health effects: liver and reproductive toxicity.
Based on toxicokinetics, p,p’-DDT is the most suitable analogue for p,p’-DDD.p,p’-DDT is a metabolic precursor for p,p’-DDD and both chemicals are extensively metabolized to DDA via aliphatic dechlorination, facilitating their elimination from the body (Figure 1).p,p’-DDE, a minor metabolite of both p,p’-DDT and p,p’-DDD, can also be converted to DDA but the process is highly inefficient compared to the metabolism of p,p’-DDT and p,p’-DDD and alternate metabolic routes for p,p’-DDE have been reported (i.e. ring hydroxylation and formation of methylsulfonyl metabolites). The metabolism of methoxychlor favors a different pathway: O-demethylation (over aliphatic dechlorination as in the case of p,p’-DDD and p,p’-DDT), a putative bioactivation pathway for the critical effects (i.e. reproductive toxicity) that constitute the basis for the derivation of non-cancer oral health reference values for this analogue. Methoxychlor is also considered less bioaccumulative compared to the p,p’- chlorinated organochlorines partly due to its p,p’-methoxy groups, which allow for a more rapid metabolism and clearance from the body. As such, the selection of methoxychlor as a source analogue for p,p’-DDD could result in a underestimation of risk; therefore, methoxychlor is excluded from further consideration as a suitable analogue.
From the two remaining analogues (p,p’-DDT and p,p’-DDE), p,p’-DDT displays the highest structural similarity to p,p’-DDD, according to ChemIDplus and DSSTox scores (Table 1). The NOAEL of 0.05 mg/kg-day established for liver toxicity in DDT-exposed rats treated for 15–27 weeks provides the most health-protective POD from this group of organochlorines (Figure 2 and Table 5), and p,p’-DDT is also more acutely toxic than p,p’-DDD in lethality rodent studies (Table 4). Ultimately, p,p’-DDT is selected as the best analogue and source chemical for p,p’-DDD based largely on metabolism pathway similarities, with important considerations from in vivo toxicity testing and supportive evidence from structural similarity evaluations and in vitro bioactivity.
4. Discussion
An expert-driven read-across approach was undertaken to assist in the screening-level assessment of p,p’-DDD, a data-poor organochlorine environmental contaminant. p,p’-DDT was selected as the source analogue for p,p’-DDD, and the NOAEL of 0.05 mg/kg-day based on liver lesions in DDT-exposed rats (ATSDR, 2002a; U.S. EPA, 1987) was proposed as a surrogate POD for the derivation of non-cancer oral health reference values for p,p’-DDD. Our work demonstrates the applicability of a read-across approach where information across structure, physicochemical properties, toxicokinetics, and toxicodynamics are integrated to inform the identification and selection of analogues for quantitative read-across.
In this case study, the potential utility of toxicokinetic data (i.e. ADME) in establishing the underlying similarity for validating and selecting source analogues for quantitative read-across is emphasized. Metabolic-profile similarity has been previously proposed as the basis for grouping chemicals and building a scientific justification for read-across (ECHA, 2017; OECD, 2014; Schultz et al., 2015); however, few practical examples have been put forward thus far (Ball et al., 2014; OECD, 2014; Schultz and Cronin, 2017). Our analysis expands on such efforts, discussing key considerations for the use of toxicokinetic data in the context of read-across (e.g. common metabolism pathways, metabolic precursors, intermediates and end-products, tissue distribution and accumulation patterns, and excretion routes and rates) with an emphasis on understanding metabolism and its potential role in toxicity. For instance, methoxychlor was deemed an unsuitable analogue for the target, p,p’-DDD, on the basis of divergent metabolic pathways and overall differences in metabolite patterns (Table 3). Furthermore, bioactivation of methoxychlor through its primary metabolic pathway has been associated with the reproductive toxicity of this chemical. Conversely, similarities in metabolism surrounding a detoxification pathway for p,p’-DDD (and its precursor, p,p’-DDT) (Figure 1), were critical to differentiate p,p’-DDT as the single best analogue from the pool of potential analogues (Table 1). Additionally, information from toxicity (including non-cancer and cancer effects), in vitro bioactivity, and structural evaluations further assisted in substantiating the similarity justification for the source analogue and the use of the read-across approach to derive screening-level health reference values in the absence of sufficient chemical-specific toxicity data for the target organochlorine. In the present case study, the availability of in vivo ADME data was instrumental in facilitating similarity comparisons between the target chemical and potential analogues. Nevertheless, for chemicals with severe data deficiencies in terms of toxicokinetics, an assessment of the role of toxicokinetics/metabolism in the read-across prediction presents substantial challenges. For data-poor chemicals, in silico and in vitro data streams could be used to fill information gaps within ADME profiles. There is ongoing research to examine the use of in vitro HTS metabolism data and in silico metabolism predictions to substantiate toxicokinetic similarity and build confidence in the selection of analogues for quantitate read-across (unpublished data).
Another significant aspect of this research is the use of in vitro bioactivity information from ToxCast to strengthen support for the hepatic and endocrine effects associated with this group of organochlorines, thereby reducing uncertainties associated with toxicity data gaps for the target chemical. The liver is an important target organ of toxicity for p,p’-DDD and the analogues in experimental animal models, which corresponds well with the vitro responses evaluated in HepG2 cells. These in vitro responses point to potential disruptions in mitochondrial membrane, oxidative stress, cell cycle, cellular/organelle conformation, and overall cytotoxicity (Figure 3). Moreover, activation of critical transcription factors, most remarkably of steroid/xenobiotic-sensing nuclear receptors, was observed. In particular, the human ER and PXR were sensitive molecular targets of p,p’-DDD, p,p’-DDT, p,p’-DDE and methoxychlor (Figure 4). PXR is a central transcriptional regulator of detoxification pathways, involving phase I and II enzymes (e.g. CYP450s and UGTs) and phase III transporters, and its importance for the metabolism and clearance of xenobiotics, steroid hormones, and bile acids is well-recognized (Kretschmer and Baldwin, 2005). Activation of PXR activity demonstrated correlation with the transcriptional induction of CYP450s and UGT enzymes by methoxychlor (Table A-3). p,p’- DDD and the analogues are known to be phenobarbital (PB)-type inducers of hepatic CYP3A and CYP2B (Li and Kupfer, 1998; Lubet et al., 1992; Nims et al., 1998), and such effects are in part attributable to the action of PXR (Kanno and Inouye, 2010; Lemaire et al., 2004; Mikamo et al., 2003). Despite the predominant role of PXR in the detoxification of xenobiotics, PXR activation can have undesirable effects. For example, PXR could enhance the metabolic activation of endocrine disrupting chemicals such as methoxychlor by inducing CYP450 enzymes that convert methoxychlor to demethylated metabolites with more potent estrogenic properties (Kretschmer and Baldwin, 2005). PXR has been implicated in the liver toxicity (hepatocyte hypertrophy) of pregnenolone 16α-carbonitrile (a well-established CYP3A inducer) and the DDT isomer, o,p’-DDT (Kiyosawa et al., 2008; Staudinger et al., 2001).p,p’-DDD and the analogues were also found to be active in assays for the human ER. Although activation of the human ER is mostly associated with the endocrine disrupting activity of these organochlorines, a possible involvement of ER in the hepatocarcinogenicity of technical-DDT (85% p,p’-DDT and 15% o,p’-DDT) has been described through the regulation of target genes important for cell proliferation (e.g. Ccnd1, Cyp17a1, cFos and E2f1) (Kazantseva et al., 2013). Overall, ToxCast results corroborate the shared activity of p,p’-DDD and analogues on the steroid/xenobiotic-sensing receptors, PXR and ER, hypothesized to be involved in hepatotoxic and/or liver-specific detoxification/bioactivation pathways.
The reproductive toxicity of the analogues in experimental animal models is well documented (Figure 2). In contrast, limited in vivo information exists for p,p’-DDD to characterize its potential to induce adverse effects on the reproductive system. To address this data gap and compare mechanisms of ER- and AR-mediated signaling for p,p’-DDD and its analogues that may contribute to reproductive effects, ToxCast data and model predictions for the ER and AR were evaluated. All chemicals were capable of modifying activity of the human ER, including the target chemical (Table 6). The estrogenic activity of p,p’-DDD has been previously investigated and the data reveal species-specific differences with p,p’-DDD showing no appreciable activity on rat and mouse ERs (Kelce et al., 1995; Robison et al., 1985; Tully et al., 2000). However, there is some evidence of activity for the human ER, including partial agonism for this receptor (Arnold et al., 1996; Klotz et al., 1996; Soto et al., 1997), which is in agreement with the ToxCast data and model predictions (Table 6). The in vitro estrogenic activity of p,p’-DDD and its analogues is consistent with the estrogen-like effects in female animals induced by p,p’-DDT (e.g. prolongation of estrus cycle and decreased frequency of implanted ova) and methoxyhchlor (e.g. precocious puberty, abnormal estrus cycle, decreased fertility, and pre- and post- implantation loss) (Table A-2). Furthermore, p,p’-DDD, p,p’-DDT, p,p’-DDE and methoxychlor displayed antagonism for the human AR in ToxCast assays, supporting previous findings observed among in vitro human and rat systems (Kelce et al., 1995; Maness et al., 1998) and concordant with the anti-androgenic effects of p,p’-DDE in developing and adult male animals (e.g. nipple retention, reduced anogenital distance, delayed puberty, and alterations in primary and accessory sex organs) (Table A-2). Overall, the in vitro HTS results corroborate the estrogenic and anti-androgenic mechanisms suspected to play a role in the reproductive effects of the analogues and suggest that the target chemical could potentially act via deregulation of similar endocrine pathways involving the ER and AR.
Taken together, the ToxCast HTS analysis proved to be valuable in asserting mechanistic plausibility for the two most sensitive toxicities associated with the target and identified analogues. The primary objective of the HTS analysis focused on making qualitative comparisons of in vitro bioactivity to substantiate the similarity justification for the source analogue, increasing confidence in the read-across prediction and overall approach. A notable challenge in the interpretation of the ToxCast data is the issue of metabolic competence lacking from most in vitro systems. The possible influence of metabolism in the detoxification or bioactivation of the test chemicals is often unaccounted for and could lead to either an overestimation or an underestimation of hazard. This is particularly relevant to the p,p’-DDD analogue, methoxychlor, a xenoestrogen known to be activated via hepatic metabolism. As such, caution should be exercised when extrapolating in-vitro-to-in-vivo findings for this chemical to avoid underestimation of health hazards relating to its reproductive toxicity. Research efforts are currently underway to incorporate metabolic competence into ToxCast assays and facilitate qualitative and quantitate characterization of chemcial hazards (DeGroot et al., 2018).
In conclusion, our analysis illustrates the utility of read-across in quantitative human health risk assessment of data-poor chemicals. We emphasize the application of expert-driven knowledge and integration of data from NAMs for identifying and validating analogues to inform dose-response assessment of environmental contaminants with insufficient toxicity information for evaluation via conventional assessment practices. The analysis presented here provides an illustrative case study for the practical use of read-across in formulating scientifically-based predictions of toxicity to fill regulatory data gaps. Important considerations for addressing similarity and inference towards mechanistic plausibility from in vitro HTS data were examined in order to advance efforts that strengthen the scientific evidence and acceptance of read-across techniques in human health risk assessment of environmental chemicals. Typically, chemicals detected at contaminated sites with little or no toxicity data are not considered in hazard characterizations. Thus, the incorporation of alternative methodologies and data streams into risk assessment has the potential to assist in chemical-specific cleanups and other remediation activities across the U.S.
Supplementary Material
Highlights.
A case study is presented to illustrate the identification and evaluation of analogues for quantitative read-across
Toxicokinetic similarity forms the basis for the read-across justification and source analogue selection
In vitro ToxCast assays were used to evaluate bioactivity similarity and build confidence in the read-across approach
6. Acknowledgements
The authors would like to thank Drs. Marian Olsen (U.S. EPA Region 2), Puttappa Dodmane, (Cal EPA, Sacramento, CA), Dan Petersen (U.S. EPA NCEA, Cincinnati, OH) and Grace Patlewicz and Richard Judson (U.S. EPA National Center for Computational Toxicology, Research Triangle Park, NC) for collaborative and helpful input. The views expressed in this article are those of the authors and do not reflect the views or policies of the U.S. EPA.
7. Funding
This work was supported in part by the Research Participation Program at the National Center for Environmental Assessment, Office of Research and Development, U.S. EPA, administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy and U.S. EPA.
Abbreviations
- ADME
absorption, distribution, metabolism, and excretion
- AR
androgen receptor
- DDA
bis(p-chlorophenyl) acetic acid
- p,p’-DDD
p,p’-dichlorodiphenyldichloroethane
- p,p’-DDE
p,p’-dichlorodiphenyldichloroethylene
- p,p’-DDT
p,p’-dichlorodiphenyltrichloroethane
- methoxychlor
p,p’-dimethoxydiphenyltrichloroethane
- ER
estrogen receptor
- HTS
high-throughput screening
- LOAEL
lowest-observed-adverse-effect level
- MOA
mode-of-action
- NAM
new approach methods
- NOAEL
no-observed-adverse-effect level
- OSF
oral slope factor
- POD
point of departure
- PXR
pregnane X receptor
- QSAR
quantitative structural-activity relationship
- RfD
reference dose
Footnotes
POD refers to the dose-response point that marks the starting point for low-dose extrapolation (e.g. no-observed-adverse effect level [NOAEL], lowest-observed-adverse effect level [LOAEL] or a benchmark dose) (U.S. EPA, 2012).
The EPA’s DSSTox database used in this analysis is no longer available as a stand-alone searchable platform but it has been superseded by the EPA’s CompTox Chemistry Dashboard (https://comptox.epa.gov/dashboard).
8. References
- Al-Saleh I, et al. , 2012. Levels of DDT and its metabolites in placenta, maternal and cord blood and their potential influence on neonatal anthropometric measures. Sci Total Environ. 416, 62–74. [DOI] [PubMed] [Google Scholar]
- Androutsopoulos VP, et al. , 2013. A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides. Toxicology. 307, 89–94. [DOI] [PubMed] [Google Scholar]
- Arnold SF, et al. , 1996. Differential interaction of natural and synthetic estrogens with extracellular binding proteins in a yeast estrogen screen. Steroids. 61, 642–6. [DOI] [PubMed] [Google Scholar]
- ATSDR, 2002a. Toxicological Profile for DDT, DDE, and DDD. U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia. [PubMed] [Google Scholar]
- ATSDR, 2002b. Toxicological Profile for Methoxychlor. U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia. [PubMed] [Google Scholar]
- Ball N, et al. , 2014. The challenge of using read-across within the EU REACH regulatory framework; how much uncertainty is too much? Dipropylene glycol methyl ether acetate, an exemplary case study. Regul Toxicol Pharmacol. 68, 212–21. [DOI] [PubMed] [Google Scholar]
- Ball N, et al. , 2016. Toward Good Read-Across Practice (GRAP) guidance. Altex. 33, 149–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidleman TF, Leone AD, 2004. Soil-air exchange of organochlorine pesticides in the Southern United States. Environ Pollut. 128, 49–57. [DOI] [PubMed] [Google Scholar]
- Bishop PL, et al. , 2012. Animal use and lessons learned in the U.S. High Production Volume Chemicals Challenge Program. Environ Health Perspect. 120, 1631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borga K, et al. , 2001. Biomagnification of organochlorines along a Barents Sea food chain. Environ Pollut. 113, 187–98. [DOI] [PubMed] [Google Scholar]
- Browne P, et al. , 2015. Screening Chemicals for Estrogen Receptor Bioactivity Using a Computational Model. Environ Sci Technol. 49, 8804–14. [DOI] [PubMed] [Google Scholar]
- Bulger WH, et al. , 1978. Studies on the in vivo and in vitro estrogenic activities of methoxychlor and its metabolites. Role of hepatic mono-oxygenase in methoxychlor activation. Biochem Pharmacol. 27, 2417–23. [DOI] [PubMed] [Google Scholar]
- Channa K, et al. , 2012. Prenatal exposure to DDT in malaria endemic region following indoor residual spraying and in non-malaria coastal regions of South Africa. Sci Total Environ. 429, 183–90. [DOI] [PubMed] [Google Scholar]
- Charles GD, et al. , 2000. Incorporation of S-9 activation into an ER-alpha transactivation assay. Reprod Toxicol. 14, 207–16. [DOI] [PubMed] [Google Scholar]
- ChemIDplus, 2017. Chemidplus database. National Institutes of Health, U.S. Library of Medicine, Bethesda, MD: <https://chem.nlm.nih.gov/chemidplus/>. [Google Scholar]
- Chu S, et al. , 2003a. Levels and enantiomeric signatures of methyl sulfonyl PCB and DDE metabolites in livers of harbor porpoises (Phocoena phocoena) from the Southern North Sea. Environ Sci Technol. 37, 4573–8. [DOI] [PubMed] [Google Scholar]
- Chu S, et al. , 2003b. Distribution of methyl sulfone metabolites of polychlorinated biphenyls and p,p’-DDE in human tissues. Environ Health Perspect. 111, 1222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats JR, 1990. Mechanisms of toxic action and structure-activity relationships for organochlorine and synthetic pyrethroid insecticides. Environ Health Perspect. 87, 255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings AM, Gray LE Jr., 1987. Methoxychlor affects the decidual cell response of the uterus but not other progestational parameters in female rats. Toxicol Appl Pharmacol. 90, 330–6. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Gray LE Jr., 1989. Antifertility effect of methoxychlor in female rats: dose- and time-dependent blockade of pregnancy. Toxicol Appl Pharmacol. 97, 454–62. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Perreault SD, 1990. Methoxychlor accelerates embryo transport through the rat reproductive tract. Toxicol Appl Pharmacol. 102, 110–6. [DOI] [PubMed] [Google Scholar]
- Dalvie MA, et al. , 2004. The hormonal effects of long-term DDT exposure on malaria vector-control workers in Limpopo Province, South Africa. Environ Res. 96, 9–19. [DOI] [PubMed] [Google Scholar]
- DeGroot DE, et al. , 2018. mRNA transfection retrofits cell-based assays with xenobiotic metabolism. J Pharmacol Toxicol Methods. 92, 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSSTox, 2016. Distributed Structure-Searchable Toxicity Database. U.S. Environmental Protection Agency, National Center for Computational Toxicology, Research Triangle Park, NC: <https://www.epa.gov/chemical-research/distributed-structure-searchable-toxicity-dsstox-database> [Google Scholar]
- ECETOC, 2012. Category approaches, read-across, (Q)SAR Technical Report No. 116. European Centre for Ecotoxicology and Toxicology of Chemicals, Brussels, Belgium. [Google Scholar]
- ECHA, 2017. Read-accross Assessment Framework (RAAF). European Chemical Agency, Helsinki, Finland, ECHA-17-R-01-EN. [Google Scholar]
- Fawcett SC, et al. , 1987. The metabolism of 14C-DDT, 14C-DDD, 14C-DDE and 14C DDMU in rats and Japanese quail. Xenobiotica. 17, 525–38. [DOI] [PubMed] [Google Scholar]
- Freire C, et al. , 2014. Association between serum levels of organochlorine pesticides and sex hormones in adults living in a heavily contaminated area in Brazil. Int J Hyg Environ Health. 217, 370–8. [DOI] [PubMed] [Google Scholar]
- Garrett RM, 1947. Toxicity of DDT for man. J Med Assoc State Ala. 17, 74–6. [PubMed] [Google Scholar]
- Gebremichael S, et al. , 2013. Analysis of organochlorine pesticide residues in human and cow’s milk in the towns of Asendabo, Serbo and Jimma in South-Western Ethiopia. Chemosphere. 90, 1652–7. [DOI] [PubMed] [Google Scholar]
- Gold B, Brunk G, 1982. Metabolism of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)-ethane and 1,1-dichloro-2,2-bis(p-chlorophenyl)ethane in the mouse. Chem Biol Interact. 41, 327–39. [DOI] [PubMed] [Google Scholar]
- Gold B, Brunk G, 1983. Metabolism of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (DDT), 1,1-dichloro-2,2-bis(p-chlorophenyl)ethane, and 1-chloro-2,2-bis(p-chlorophenyl)ethene in the hamster. Cancer Res. 43, 2644–7. [PubMed] [Google Scholar]
- Gold B, Brunk G, 1986. The effect of subchronic feeding of 1,1-dichloro-2,2-bis(4’-chlorophenyl)ethene (DDE) on its metabolism in mice. Carcinogenesis. 7, 1149–53. [DOI] [PubMed] [Google Scholar]
- Guo H, et al. , 2014. Prenatal exposure to organochlorine pesticides and infant birth weight in China. Chemosphere. 110, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SJ, et al. , 1974. Effect of several dietary levels of technical methoxychlor on reproduction in rats. J Agric Food Chem. 22, 969–73. [DOI] [PubMed] [Google Scholar]
- Hassine SB, et al. , 2012. Determination of chlorinated pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in human milk from Bizerte (Tunisia) in 2010. Chemosphere. 89, 369–377. [DOI] [PubMed] [Google Scholar]
- HSDB, 2017. Hazardous Substances Data Bank (HSBD), A TOXNET Database. U.S. National Library of Medicine, Bethesda, MD: <https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm>. [Google Scholar]
- Hsieh HC, 1954. D.D.T. intoxication a family of Southern Taiwan. AMA Arch Ind Health. 10, 344–6. [PubMed] [Google Scholar]
- Judson R, et al. , 2009. The toxicity data landscape for environmental chemicals. Environ Health Perspect. 117, 685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson RS, et al. , 2015. Integrated Model of Chemical Perturbations of a Biological Pathway Using 18 In Vitro High-Throughput Screening Assays for the Estrogen Receptor. Toxicol Sci. 148, 137–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno Y, Inouye Y, 2010. A consecutive three alanine residue insertion mutant of human CAR: a novel CAR ligand screening system in HepG2 cells. J Toxicol Sci. 35, 515–25. [DOI] [PubMed] [Google Scholar]
- Kapoor IP, et al. , 1970. Comparative metabolism of methoxychlor, methiochlor, and DDT in mouse, insects, and in a model ecosystem. J Agric Food Chem. 18, 1145–52. [DOI] [PubMed] [Google Scholar]
- Kazantseva YA, et al. , 2013. Dichlorodiphenyltrichloroethane technical mixture regulates cell cycle and apoptosis genes through the activation of CAR and ERalpha in mouse livers. Toxicol Appl Pharmacol. 271, 137–43. [DOI] [PubMed] [Google Scholar]
- Kelce WR, et al. , 1995. Persistent DDT metabolite p,p’-DDE is a potent androgen receptor antagonist. Nature. 375, 581–5. [DOI] [PubMed] [Google Scholar]
- Keller WC, Yeary RA, 1980. A comparison of the effects of mineral oil, vegetable oil, and sodium sulfate on the intestinal absorption of DDT in rodents. Clin Toxicol. 16, 223–31. [DOI] [PubMed] [Google Scholar]
- Kiyosawa N, et al. , 2008. o,p’-DDT elicits PXR/CAR-, not ER-, mediated responses in the immature ovariectomized rat liver. Toxicol Sci. 101, 350–63. [DOI] [PubMed] [Google Scholar]
- Kleinstreuer NC, et al. , 2016. Development and Validation of a Computational Model for Androgen Receptor Activity. Chem Res Toxicol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz DM, et al. , 1996. Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ Health Perspect. 104, 1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer XC, Baldwin WS, 2005. CAR and PXR: xenosensors of endocrine disrupters? Chem Biol Interact. 155, 111–28. [DOI] [PubMed] [Google Scholar]
- Kunisue T, et al. , 2006. Contamination status of persistent organochlorines in human breast milk from Japan: Recent levels and temporal trend. Chemosphere. 64, 1601–1608. [DOI] [PubMed] [Google Scholar]
- Kunze FM, et al. , 1950. The storage of methoxychlor in the fat of the rat. Proc Soc Exp Biol Med. 75, 415–6. [DOI] [PubMed] [Google Scholar]
- Kurt-Karakus PB, et al. , 2006. Measurement of DDT fluxes from a historically treated agricultural soil in Canada. Environ Sci Technol. 40, 4578–85. [DOI] [PubMed] [Google Scholar]
- Larsson C, et al. , 2004. Enantiomeric specificity of methylsulfonyl-PCBs and distribution of bis(4-chlorophenyl) sulfone, PCB, and DDE methyl sulfones in grey seal tissues. Environ Sci Technol. 38, 4950–5. [DOI] [PubMed] [Google Scholar]
- Lemaire G, et al. , 2004. A PXR reporter gene assay in a stable cell culture system: CYP3A4 and CYP2B6 induction by pesticides. Biochem Pharmacol. 68, 2347–58. [DOI] [PubMed] [Google Scholar]
- Li HC, Kupfer D, 1998. Mechanism of induction of rat hepatic CYP2B and 3A by the pesticide methoxychlor. J Biochem Mol Toxicol. 12, 315–23. [DOI] [PubMed] [Google Scholar]
- Linderholm L, et al. , 2007. Maternal and cord serum exposure to PCB and DDE methyl sulfone metabolites in eastern Slovakia. Chemosphere. 69, 403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubet RA, et al. , 1992. A pleiotropic response to phenobarbital-type enzyme inducers in the F344/NCr rat. Effects of chemicals of varied structure. Biochem Pharmacol. 43, 1067–78. [DOI] [PubMed] [Google Scholar]
- Malarvannan G, et al. , 2009. Organohalogen compounds in human breast milk from mothers living in Payatas and Malate, the Philippines: Levels, accumulation kinetics and infant health risk. Environmental Pollution. 157, 1924–1932. [DOI] [PubMed] [Google Scholar]
- Maness SC, et al. , 1998. Inhibition of androgen receptor-dependent transcriptional activity by DDT isomers and methoxychlor in HepG2 human hepatoma cells. Toxicol Appl Pharmacol. 151, 135–42. [DOI] [PubMed] [Google Scholar]
- Mikamo E, et al. , 2003. Endocrine disruptors induce cytochrome P450 by affecting transcriptional regulation via pregnane X receptor. Toxicol Appl Pharmacol. 193, 66–72. [DOI] [PubMed] [Google Scholar]
- Morgan D, Roan C, 1974. The metabolism of ddt in man In Hayes WJ Jr. (Ed.), Essays in toxicology (pp. 39–97). New York, NY: Academic Press. [Google Scholar]
- Morgan DP, Roan CC, 1971. Absorption, storage, and metabolic conversion of ingested DDT and DDT metabolites in man. Arch Environ Health. 22, 301–8. [DOI] [PubMed] [Google Scholar]
- Mrema EJ, et al. , 2013. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology. 307, 74–88. [DOI] [PubMed] [Google Scholar]
- Muir D, et al. , 2003. Bioaccumulation of PCBs and chlorinated pesticides in seals, fishes and invertebrates from the White Sea, Russia. Sci Total Environ. 306, 111–31. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC), 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. National Academies Press, Washington, DC. [Google Scholar]
- NCI, 1978. Bioassays of DDT, TDE, and p,p’-DDE for possible carcinogenicity. (NCI-CG-TR 131). National Cancer Institute. U.S. Department of Health, Education, and Welfare, National Institutes of Health, Washington, DC. [PubMed] [Google Scholar]
- Nims RW, et al. , 1998. Comparative pharmacodynamics of CYP2B induction by DDT, DDE, and DDD in male rat liver and cultured rat hepatocytes. J Toxicol Environ Health A. 53, 455–77. [DOI] [PubMed] [Google Scholar]
- OECD, 2014. Guidance on Grouping of Chemicals, second edition. Series on Testing and Assessment No. 194. Organisation for Economic Co-operation and Development, Paris, France, ENV/JM/MONO(2014)4. [Google Scholar]
- Pant N, et al. , 2007. Chlorinated pesticide concentration in semen of fertile and infertile men and correlation with sperm quality. Environ Toxicol Pharmacol. 23, 135–9. [DOI] [PubMed] [Google Scholar]
- Pant N, et al. , 2004. Correlation of chlorinated pesticides concentration in semen with seminal vesicle and prostatic markers. Reprod Toxicol. 19, 209–14. [DOI] [PubMed] [Google Scholar]
- Patlewicz G, et al. , 2015. Building scientific confidence in the development and evaluation of read-across. Regul Toxicol Pharmacol. 72, 117–33. [DOI] [PubMed] [Google Scholar]
- Patlewicz G, et al. , 2018. Building Scientific Confidence in Metabolic Similarity in Read-Across through the Use of In Vitro, In Silico, and Analytical Data The Toxicologist. 162, 1 <https://www.toxicology.org/pubs/docs/Tox/2018Tox.pdf>. [Google Scholar]
- Perry MJ, et al. , 2006. A prospective study of serum DDT and progesterone and estrogen levels across the menstrual cycle in nulliparous women of reproductive age. Am J Epidemiol. 164, 1056–64. [DOI] [PubMed] [Google Scholar]
- Peterson JE, Robison WH, 1964. METABOLIC PRODUCTS OF P,P’-DDT IN THE RAT. Toxicol Appl Pharmacol. 6, 321–7. [DOI] [PubMed] [Google Scholar]
- Pines A, et al. , 1987. Some organochlorine insecticide and polychlorinated biphenyl blood residues in infertile males in the general Israeli population of the middle 1980’s. Arch Environ Contam Toxicol. 16, 587–97. [DOI] [PubMed] [Google Scholar]
- Reynolds PJ, et al. , 1976. A comparison of DDT and methoxychlor accumulation and depletion in sheep. Bull Environ Contam Toxicol. 16, 240–7. [DOI] [PubMed] [Google Scholar]
- Richard AM, et al. , 2016. ToxCast Chemical Landscape: Paving the Road to 21st Century Toxicology. Chem Res Toxicol. 29, 1225–51. [DOI] [PubMed] [Google Scholar]
- Roan C, et al. , 1971. Urinary excretion of DDA following ingestion of DDT and DDT metabolites in man. Arch Environ Health. 22, 309–15. [DOI] [PubMed] [Google Scholar]
- Robison AK, et al. , 1985. DDT supports the growth of an estrogen-responsive tumor. Toxicol Lett. 27, 109–13. [DOI] [PubMed] [Google Scholar]
- Rothe CF, et al. , 1957. Metabolism of cholorophenothane (DDT); intestinal lymphatic absorption. AMA Arch Ind Health. 16, 82–6. [PubMed] [Google Scholar]
- Sala M, et al. , 2001. Levels of hexachlorobenzene and other organochlorine compounds in cord blood: exposure across placenta. Chemosphere. 43, 895–901. [DOI] [PubMed] [Google Scholar]
- Saxena MC, et al. , 1980. Role of chlorinated hydrocarbon pesticides in abortions and premature labour. Toxicology. 17, 323–31. [DOI] [PubMed] [Google Scholar]
- Saxena MC, et al. , 1981. Organochlorine pesticides in specimens from women undergoing spontaneous abortion, premature of full-term delivery. J Anal Toxicol. 5, 6–9. [DOI] [PubMed] [Google Scholar]
- Schultz TW, et al. , 2015. A strategy for structuring and reporting a read-across prediction of toxicity. Regul Toxicol Pharmacol. 72, 586–601. [DOI] [PubMed] [Google Scholar]
- Schultz TW, Cronin MTD, 2017. Lessons learned from read-across case studies for repeated-dose toxicity. Regul Toxicol Pharmacol. 88, 185–191. [DOI] [PubMed] [Google Scholar]
- Shunthirasingham C, et al. , 2016. Atmospheric concentrations and loadings of organochlorine pesticides and polychlorinated biphenyls in the Canadian Great Lakes Basin (GLB): Spatial and temporal analysis (1992–2012). Environ Pollut. 217, 124–33. [DOI] [PubMed] [Google Scholar]
- Skarphedinsdottir H, et al. , 2010. Bioaccumulation and biomagnification of organochlorines in a marine food web at a pristine site in Iceland. Arch Environ Contam Toxicol. 58, 800–9. [DOI] [PubMed] [Google Scholar]
- Soto AM, et al. , 1997. Developing a marker of exposure to xenoestrogen mixtures in human serum. Environ Health Perspect. 105 Suppl 3, 647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton K, Kruszewski FH, 2016. Quantifying the benefits of using read-across and in silico techniques to fulfill hazard data requirements for chemical categories. Regul Toxicol Pharmacol. 81, 250–259. [DOI] [PubMed] [Google Scholar]
- Staudinger J, et al. , 2001. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos. 29, 1467–72. [PubMed] [Google Scholar]
- Sumida K, et al. , 2001. An in vitro reporter gene assay method incorporating metabolic activation with human and rat S9 or liver microsomes. Biochem Biophys Res Commun. 280, 85–91. [DOI] [PubMed] [Google Scholar]
- Tomatis L, et al. , 1974. Effect of long-term exposure to 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene, to 1,1-dichloro-2,2-bis(p-chlorophenyl)ethane, and to the two chemicals combined on CF-1 mice. J Natl Cancer Inst. 52, 883–91. [DOI] [PubMed] [Google Scholar]
- Tully DB, et al. , 2000. Six high-priority organochlorine pesticides, either singly or in combination, are nonestrogenic in transfected HeLa cells. Reprod Toxicol. 14, 95–102. [DOI] [PubMed] [Google Scholar]
- Tyagi V, et al. , 2015. Organochlorine pesticide levels in maternal blood and placental tissue with reference to preterm birth: a recent trend in North Indian population. Environ Monit Assess. 187, 471. [DOI] [PubMed] [Google Scholar]
- U.S. EPA, 1987. Integrated Risk Information System (IRIS) Summary for Chronic Oral Reference Dose (RfD) of p,p’-Dichlorodiphenyltrichloroethane (DDT) (CASRN 50-29-3) U.S. Environmental Protection Agency, National Center for Environmental Asessment, Washington, DC. [Google Scholar]
- U.S. EPA, 1988a. Integrated Risk Information System (IRIS) Summary for Carcinogenicity Assessment of p,p’-Dichlorodiphenyltrichloroethane (DDT) (CASRN 50-29-3). U.S. Environmental Protection Agency, National Center for Environmental Asessment, Washington, DC. [Google Scholar]
- U.S. EPA, 1988b. Integrated Risk Information System (IRIS) Summary for Carcinogenicty Assessment of Methoxychlor (CASRN 72-43-5). U.S. Environmental Protection Agency, National Center for Environmental Asessment, Washington, DC. [Google Scholar]
- U.S. EPA, 1988c. Integrated Risk Information System (IRIS) Summary for the Carcinogenicity Assessment of p,p’-Dichlorodiphenyl dichloroethane (DDD) (CASRN 72-54-8). U.S. Environmental Protection Agency, National Center for Environmental Asessment, Washington, DC. [Google Scholar]
- U.S. EPA, 1988d. Integrated Risk Information System (IRIS) Summary for the Carcinogenicity Assessment of p,p’-Dichlorodiphenyldichloroethylene (DDE) (CASRN 72-55-9). U.S. Environmental Protection Agency, National Center for Environmental Asessment, Washington, DC. [Google Scholar]
- U.S. EPA, 1990. Integrated Risk Information System (IRIS) Summary for Chronic Oral Reference Dose (RfD) of Methoxychlor (CASRN 72-43-5). U.S. Environmental Protection Agency, National Center for Environmental Asessment, Washington, DC. [Google Scholar]
- U.S. EPA, 2012. Benchmark Dose Technical Guidance Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC: 20460, EPA/100/R-12/001 [Google Scholar]
- U.S. EPA, 2015. ToxCast & Tox21 high-throughput assay information invitrodb_v2. Data released on October 2015. <http://www2.epa.gov/chemical-research/toxicity-forecastertoxcasttm-data>
- U.S. EPA, 2017a. CompTox Chemistry Dashboard [Chemical Activity Summary]. U.S. Environmental Protection Agency; <https://comptox.epa.gov/dashboard>. [Google Scholar]
- U.S. EPA, 2017b. Provisional Peer-Reviewed Toxicity Values for p,p’ Dichlorodiphenyldichloroethane (p,p’ DDD) (CASRN 72 54 8). U.S. Environmental Protection Agency, National Center for Environmental Asessment, Cincinnati, OH. [Google Scholar]
- U.S. EPA, 2017c. Provisional Peer Reviewed Toxicity Values for p,p’ Dichlorodiphenyldichloroethylene (p,p’ DDE) (CASRN 72-55-9). U.S. Environmental Protection Agency, National Center for Environmental Asessment, Cincinnati, OH. [Google Scholar]
- U.S. EPA, 2017d. TSCA Chemical Substance Inventory. U.S. Environmental Protection Agency; <https://www.epa.gov/tsca-inventory> [Google Scholar]
- UNEP, 2017. DDT expert group and its report on the assessment of scientific, technical, environmental and economic information on production and use of DDT for disease vector control. United Nations Environment Programme, Stockholm Convention on Persistent Organic Pollutants, Geneva, Switzerland, UNEP/POPS/COP.8/INF/6. [Google Scholar]
- Waliszewski SM, et al. , 2000. Carry-over of persistent organochlorine pesticides through placenta to fetus. Salud Publica Mex. 42, 384–90. [DOI] [PubMed] [Google Scholar]
- Wang NC, et al. , 2012. Application of computational toxicological approaches in human health risk assessment. I. A tiered surrogate approach. Regul Toxicol Pharmacol. 63, 10–9. [DOI] [PubMed] [Google Scholar]
- Woolley DE, Talens GM, 1971. Distribution of DDT, DDD, and DDE in tissues of neonatal rats and in milk and other tissues of mother rats chronically exposed to DDT. Toxicol Appl Pharmacol. 18, 907–16. [DOI] [PubMed] [Google Scholar]
- Wu S, et al. , 2010. A framework for using structural, reactivity, metabolic and physicochemical similarity to evaluate the suitability of analogs for SAR-based toxicological assessments. Regul Toxicol Pharmacol. 56, 67–81. [DOI] [PubMed] [Google Scholar]
- You L, et al. , 1999. Transplacental and lactational transfer of p,p’-DDE in Sprague-Dawley rats. Toxicol Appl Pharmacol. 157, 134–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.