Abstract
Background
Numerous studies have investigated the association between hormone receptor expression and clinical outcome in ovarian carcinoma (OC); however, these have largely focussed on serous OCs, with few studies reporting specifically on endometrioid OCs (EnOC). Where analyses have been stratified by histotype, expression has been assessed using the percentage of positive tumor cells, without accounting for nuclear expression intensity.
Methods
Here we assess the expression levels of progesterone receptor (PR), estrogen receptor alpha (ER) and androgen receptor (AR) using histoscore – a nuclear scoring method incorporating both proportion of positive cells and the intensity of nuclear staining – across a cohort of 107 WT1 negative EnOCs.
Results
Hierarchical clustering by PR, ER and AR histoscores identified four EnOC subgroups (PR+/ER+, PR+/ER−, PR−/ER+ and PR−/ER−). EnOC patients in the PR+/ER+ and PR+/ER− groups displayed favorable outcome (multivariable HR for disease-specific survival 0.05 [0.01–0.35] and 0.05 [0.00–0.51]) compared to the PR−/ER+ group. Ten-year survival for stage II PRhigh and PRlow cases was 94.1% and 42.4%. ERhigh EnOC patients (PR+/ER+, PR−/ER+) had higher body mass index compared to ERlow cases (P = 0.015) and high grade serous OC patients (P < 0.001).
Conclusion
These data demonstrate that endometrioid OC cases with high PR expression display markedly favorable outcome. Stage II EnOCs with high PR expression represent potential candidates for de-escalation of first-line therapy. Future work should seek to characterise the sensitivity of PR and ER positive EnOCs to endocrine therapy.
Keywords: Endometrioid ovarian carcinoma, Progesterone receptor, Estrogen receptor, Androgen receptor, Ovarian cancer, Body mass index
Highlights
-
•
We identify four subgroups of endometrioid ovarian carcinoma (EnOC) defined by hormone receptor expression patterns.
-
•
EnOC patients in the PRhigh (PR+/ER+, PR+/ER−) groups demonstrate markedly favorable outcome.
-
•
Stage II EnOC patients in the PRhigh groups display a ten-year survival of approximately 95%.
-
•
ERhigh (PR+/ER+, PR−/ER+) EnOC patients had a higher body mass index vs ERlow cases and high grade serous carcinoma patients.
1. Introduction
Ovarian cancer is the most lethal cancer of the female genital tract, and accounts for over 180,000 deaths per year worldwide [1]. The vast majority of cases are ovarian carcinomas (OCs), comprising five core histological subtypes: high grade serous (HGS), endometrioid, clear cell, low grade serous (LGS) and mucinous OC [2].
Endometrioid OCs (EnOCs) account for approximately 10% of cases, are associated with favorable prognosis compared to other histotypes, and are often diagnosed at FIGO stage I or II [[2], [3], [4]]. EnOCs are frequently estrogen receptor alpha (ER) and/or progesterone receptor (PR)-positive, and display WT1 negativity [5]. The routine use of WT1 immunohistochemistry (IHC) is known to improve the reproducibility of EnOC diagnosis, aiding the distinction of these cases from HGS or LGS OCs which can demonstrate morphological resemblance to EnOC [6,7].
Reports of the prognostic impact of hormone receptor expression in OC have largely focussed on serous cases, with studies almost ubiquitously focusing on either these tumors alone [[8], [9], [10], [11]], or mixed patient populations dominated by OCs of serous histology [[12], [13], [14], [15], [16], [17]]. Mixed histology studies interrogating survival without stratifying by histological subtype have the potential to be confounded by the known differential expression of hormone receptors across these subtypes [6,18], which are now recognised to demonstrate markedly distinct clinical outcome [3,4].
A number of studies have included EnOC cases when investigating the prognostic impact of hormone receptor positivity on OC outcome [[13], [14], [15], [16],[19], [20], [21], [22]]; however, the majority of these studies have included fewer than 30 EnOC cases in the context of mixed histology cohorts [[14], [15], [16],19,20]. One study collected a relatively large series of EnOCs (84 cases), but did not perform histotype-specific analysis of outcome [13]. The Ovarian Tumor Tissue Analysis Consortium investigated the prognostic impact of ER and PR expression specifically in EnOC, where investigators used a three-tier taxonomy for quantifying expression magnitude, reporting a survival benefit in ER-positive and PR-positive cases [21], which was mirrored in an independent study of from Rambau et al. [22]. However, as with many other investigations of hormone receptor expression in OC, these studies defined positivity by the proportion of marker-positive tumor cells (as <1%, 1–50% and ≥50% positive tumor nuclei) without accounting for intensity of nuclear staining. Quantification of both the proportion of positive cells and the intensity of nuclear expression may allow for a more granular assessment of expression patterns. Notably, the most widely used scoring systems for hormone receptor positivity incorporate both of these measures [23].
Androgen receptor (AR) expression has also been associated with favorable outcome in some OC cohorts [11,14,17]; however, the clinical impact of AR expression specifically in EnOC is poorly defined.
Histoscore is a nuclear staining intensity scoring method which incorporates both the proportion of positive tumor nuclei and the intensity of nuclear staining [24]. We and others have demonstrated the utility of ER histoscore for identifying OCs that derive the greatest benefit from endocrine therapy, and this method is routinely used to identify good candidates for endocrine treatment regimens locally within our centre [[25], [26], [27]].
Here we report on hormone receptor expression across a cohort of EnOCs identified following contemporary pathology review utilizing immunohistochemistry (IHC) for WT1. We quantify expression of ER, PR and AR using histoscore in order to determine the prognostic impact of hormone receptor expression patterns specifically in EnOC.
2. Materials and methods
2.1. Cohort collection
We recently identified a cohort of 107 WT1 negative EnOCs following contemporary pathology review of OC cases (n = 289) with documented endometrioid histology from the Edinburgh Ovarian Cancer Database (full data to be reported elsewhere). Tumor material was reviewed using H&E stained slides and IHC with antibodies for WT1 (DAKO, clone 6F-H2; 1:1000 dilution) and p53 (DAKO, clone DO-7; 1:50 dilution); metastases from primary uterine carcinomas, as defined by clinicopathological criteria [28], were excluded. Use of human tumor tissue for research was approved by South East Scotland Human Annotated BioResource (Lothian NRS Bioresource ethics reference 15/ES/0094-SR494). Demographics of the 107 WT1 negative EnOCs are summarised in Table 1.
Table 1.
Demographics of 107 WT1 negative EnOCs.
| N | %/range | ||
|---|---|---|---|
| Age at diagnosis | Median years | 57 | 28–88 |
| Body mass index | Median | 25.5 | 18.0–44.0 |
| Disease grade | G1 EnOC | 80 | 74.7 |
| G2 EnOC | 19 | 17.8 | |
| G3 EnOC | 8 | 7.5 | |
| FIGO stage at diagnosis | I | 47 | 44.8 |
| II | 42 | 40.0 | |
| III | 10 | 9.5 | |
| IV | 6 | 5.7 | |
| NA | 2 | ||
| RD following debulking | <2 cma | 93 | 90.3 |
| ≥2 cm | 10 | 9.7 | |
| NA | 4 | ||
| p53 IHC pattern | AP | 11 | 10.5 |
| AN | 0 | 0.0 | |
| WT | 94 | 89.5 | |
| NE | 2 | ||
EnOC, endometrioid ovarian carcinoma; NA, not available; RD, residual disease; IHC, immunohistochemistry; AP, aberrant diffuse nuclear positive; AN, aberrant null; WT, wild-type; NE, not evaluable.
Due to the retrospective nature of this cohort and the historic classification of <2 cm residual disease as optimal debulking, debulking status is not resolved beyond <2 cm in this cohort.
2.2. Clinical annotation
Correlation of molecular data to clinicopathological variables and clinical outcome in patients with ovarian carcinoma was approved by NHS Lothian Research and Development (reference 2007/W/ON/29). Clinical data were retrieved from the Edinburgh Ovarian Cancer Database, wherein data regarding the diagnosis and management of ovarian cancer patients treated at the Edinburgh Cancer Centre are entered prospectively as part of routine care, alongside archived patient records. Body mass index (BMI) was calculated using patient height and weight as recorded at time of first chemotherapy administration. Adjuvant treatment regimens and staging information is summarised in Table S1. Patients underwent 3-monthly clinical follow-up for 2 years and 6-monthly follow-up for a further 3 years, after which patients were tracked via yearly contact with their general practitioner to determine patient status. Radiological imaging was used to determine relapse upon a significant rise in CA125 or if patients presented with symptomatic deterioration.
2.3. Immunohistochemical staining for PR, ER and AR
A human tissue microarray (TMA) was constructed to assess tumor cell positivity for markers of interest. TMAs were constructed using H&E stained slides, marked to identify tumor area by an expert pathologist (CSH), to guide tissue coring. Three 0.8 mm tissue cores were taken per EnOC case.
IHC for PR was performed using 1:50 mouse anti-human PR antibody M3569 (DAKO, clone PgR-636). ER staining was performed using 1:50 dilution of rabbit anti-human ER antibody M3643 (DAKO, clone EP1). AR staining was performed using 1:50 dilution of mouse anti-human AR antibody M3562 (DAKO, clone AR441). IHC was performed using the Leica BOND III Autostainer with epitope retrieval solution 2 for 20 min. Normal human breast tissue was used as a positive control for ER and PR, while normal human prostate tissue was used as a positive control for AR. Negative controls were performed using further sections without the addition of primary antibody.
2.4. Histoscoring of PR, ER and AR immunohistochemical staining
Marker positivity was evaluated by histoscore, a nuclear staining scoring method incorporating both proportion of positive cells and the intensity of nuclear positivity [24]. The proportion of tumor cells in each samples scored as 0 (negative), 1+ (weak positive), 2+ (moderately positive), or 3+ (strong positive) was recorded, using validated breast carcinoma control samples for comparison. Per-core histoscore was calculated as 1×(%cells 1+) + 2×(%cells 2+) + 3×(%cells 3+) (Fig. S1).
Each core was scored by two independent observers. Comparison of histoscore assessment between the two observers demonstrated excellent agreement (weighted Kappa >0.90 for all markers; Spearman's ρ ≥ 0.85, P < 0.001) (Table S2).
Per-patient histoscore was calculated as the mean of evaluable cores, weighted by percentage tumor present in each core (see Appendix A). Cores with <10% tumor cellularity were excluded from analysis. Patients with fewer than two assessable cores were considered non-evaluable for marker expression.
2.5. Statistical analysis
Statistical analyses were performed using R version 3.5.1. Relapse-free survival (RFS) was calculated as time from diagnosis to first radiological relapse. Comparisons of frequency were made using the Fisher's exact test and Chi-squared test, as appropriate. Data modality and normality was assessed by Hartigan's Dip test and the Shapiro-Wilk test. Comparisons of continuous variables were made using the unpaired t-test and Mann Whitney-U test, as appropriate.
Survival differences were assessed in the survival R package [29] using Cox proportional hazards regression models and visualised using the Kaplan-Meier method. Subgroups of EnOC were identified by hierarchical clustering of PR, ER and AR histoscores using Euclidian distance and Ward's Linkage. Correction for multiplicity of testing was performed using the Bonferroni method, where specified.
3. Results
3.1. Patterns of ER, PR and AR expression in EnOC
56.3% and 48.3% of cases demonstrated PR histoscore of ≥100 and ≥200. For ER, 60.9% and 29.9% of cases displayed a histoscore of ≥100 and ≥200. 13.2% and 3.3% of cases demonstrated an AR histoscore of ≥100 and ≥200. Hormone receptor histoscores across the EnOC cohort are summarised in Fig. S2 and Table S3. Expression distribution was non-normal for all markers (Shapiro-Wilk P < 0.001 for PR, ER and AR), and PR expression demonstrated a bimodal distribution (Fig. S2A) (Hartigan's Dip test, P < 0.0001).
Expression of PR and ER demonstrated highly significant correlation (Spearman's ρ = 0.60, P < 0.0001). Expression of PR and AR demonstrated weaker correlation that was also statistically significant (ρ = 0.34, P = 0.002), while ER and AR demonstrated significant correlation of intermediate magnitude (ρ = 0.45, P < 0.001).
3.2. PR/ER/AR-defined subgroups of EnOC
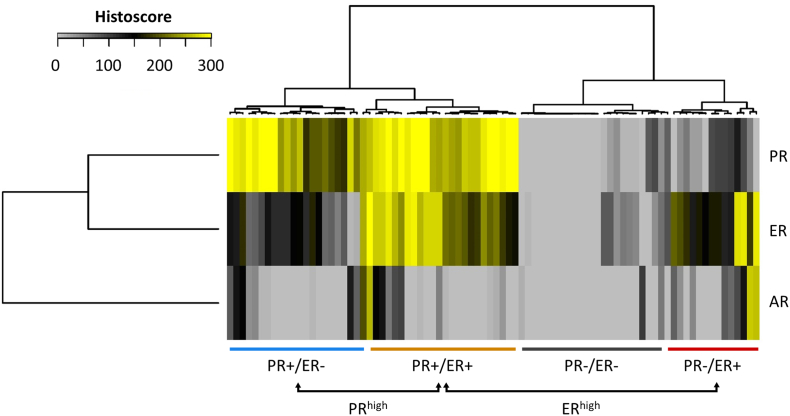
84 cases had evaluable histoscores for all three markers (n = 15 not available [NA] for PR/ER/AR; n = 2 NA for ER/PR; n = 3 NA for PR; n = 3 NA for ER). Hierarchical clustering of EnOC cases using their PR, ER and AR histoscores identified subgroups of EnOC based on hormone receptor expression patterns (Fig. 1). Four major subgroups were identified, defined largely by patterns of PR and ER expression: PR+/ER− (n = 21), PR+/ER+ (n = 25), PR−/ER+ (n = 14) and PR−/ER− (n = 24).
Fig. 1.
Hierarchical clustering by PR, ER and AR histoscores identifies subgroups of EnOC.
AR expression appeared to contribute little toward subgroup classification, likely owing to the almost ubiquitous low expression of AR across the cohort. However, AR histoscore was significantly lower in the PR−/ER− group versus the other EnOC groups (Bonferroni-adjusted P < 0.01 for all comparisons) (Fig. S3, Table S4).
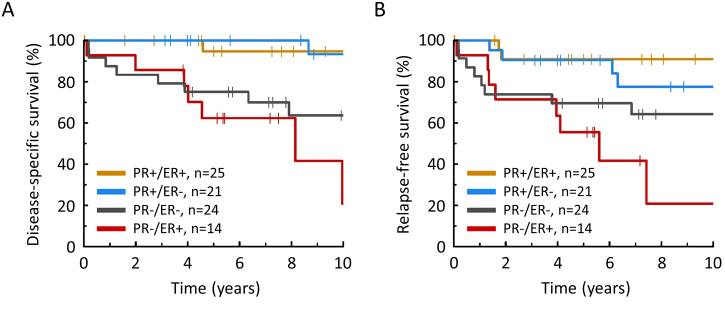
The PRhigh groups (PR+/ER+ and PR+/ER−) demonstrated favorable DSS (HR = 0.11, 95% CI 0.02–0.54 and 0.05, 95% CI 0.00–0.44, respectively) compared to the PR−/ER+ group, with the PR−/ER− group showing intermediate prognosis (HR = 0.50, 95% CI 0.18–1.40) (Fig. 2A and Table S5). These data were mirrored upon investigation of RFS (Fig. 2B, Table S5). Within stage II cases specifically, 10-year DSS in the PRhigh (n = 32) and PRlow cases (n = 15) was 94.1% and 42.4%, respectively (HR = 0.10, 95% CI 0.01–0.84) (Fig. S4).
Fig. 2.
Disease-specific (A) and relapse-free (B) survival of EnOC subgroups.
3.3. Association of EnOC subgroups with clinicopathological features in EnOC
Clinicopathological features of the four EnOC subgroups is summarised in Table 2. The PRhigh groups (PR+/ER+ and PR+/ER−) appeared to comprise a greater portion EnOCs with grade I histology (P = 0.023), early stage (FIGO I/II) at diagnosis (95.5% vs 81.6%, P = 0.074) and wild-type p53 expression pattern (P = 0.042). However, these were not significant following correction for multiple testing (Bonferroni-adjusted P = 0.070, P = 0.222 and P = 0.127). The PR+/ER− group appeared to demonstrate younger age at diagnosis, but this did not reach statistical significance (P = 0.055).
Table 2.
Demographics of EnOC subgroups.
| Group |
P-value | |||||
|---|---|---|---|---|---|---|
| PR+/ER+ | PR+/ER− | PR−/ER+ | PR−/ER− | |||
| Age at diagnosis | Median | 59.0 | 54.0 | 60.5 | 60.5 | 0.055a |
| BMI | Median | 27.2 | 23.4 | 28.9 | 24.1 | 0.015b |
| Grade | G1 EnOC | 21 (84.0%) | 18 (85.7%) | 7 (50.0%) | 16 (66.7%) | 0.023c |
| G2 EnOc | 2 (8.0%) | 2 (9.5%) | 5 (35.7%) | 5 (20.8%) | ||
| G3 EnOc | 2 (8.0%) | 1 (4.8%) | 2 (14.3%) | 3 (12.5%) | ||
| FIGO stage at diagnosis | I | 11 (45.8%) | 10 (50.0%) | 6 (42.8%) | 10 (41.7%) | 0.074d |
| II | 11 (45.8%) | 10 (50.0%) | 6 (42.8%) | 9 (37.5%) | ||
| III | 2 (8.3%) | 0 (0.0%) | 1 (7.1%) | 3 (12.5%) | ||
| IV | 0 (0.0%) | 0 (0.0%) | 1 (7.1%) | 2 (8.3%) | ||
| NA | 1 | 1 | 0 | 0 | ||
| RD following debulking | <2 cm | 22 (91.7%) | 20 (100.0%) | 12 (92.3%) | 21 (91.3%) | 0.653e |
| ≥2 cm | 2 (8.3%) | 0 (0.0%) | 1 (7.7%) | 2 (8.7%) | ||
| p53 IHC pattern | AP | 0 (0.0%) | 1 (4.8%) | 3 (21.4%) | 3 (13.0%) | 0.042f |
| AN | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| WT | 24 (100.0%) | 20 (95.2%) | 11 (78.6%) | 20 (87.0%) | ||
| NE | 1 | 0 | 0 | 1 | ||
BMI, body mass index; EnOC, endometrioid ovarian carcinoma; NA, not available; RD, residual disease; IHC, immunohistochemistry; AP, aberrant diffuse nuclear positive; AN, aberrant null; WT, wild-type; NE, not evaluable.
T test; PR+/ER− versus others.
Mann Whitney-U test: ERhigh vs ERlow.
χ2 test, PRhigh (PR+/ER+ and PR+/ER−) vs PRlow (PR−/ER+ and PR−/ER−): low grade (G1) versus high grade (G2/3).
Fisher's exact test, PRhigh vs PRlow: early (FIGO I/II) vs advanced stage (FIGO III/IV).
Fisher's exact test, PRhigh vs PRlow.
Fisher's exact test, PRhigh vs PRlow: P53 aberrant vs wild-type pattern.
Due to the apparent differential distribution of clinicopathological features between the EnOC subgroups, a multivariable analysis for DSS was performed to account for age, stage, grade and residual disease following surgical debulking. In this model, the PRhigh groups (PR+/ER+ and PR+/ER−) demonstrated significantly favorable DSS (HR = 0.05, 95% CI 0.01–0.35 and 0.05, 95% CI 0.00–0.51, respectively) compared to the PR−/ER+ group, with the PR−/ER− group showing intermediate prognosis (HR = 0.24, 95% CI 0.06–0.99) (Tables S6 and S7).
3.4. Correlation of ER status in EnOC with body mass index at patient presentation
We have previously reported a series of 265 HGS OC cases from Edinburgh following contemporary pathology review [30]. The EnOC cohort had higher BMI at first-line chemotherapy initiation compared to the HGS OC comparator cohort (median 25.5 vs 23.9, P = 0.017) (Fig. 3). Within the EnOCs, ERhigh (PR+/ER+, PR−/ER+) cases had a higher BMI compared to ERlow cases (median 27.6 vs 23.8, P = 0.015), whose BMI was akin to that of the HGS OC cohort (median BMI 23.9, P = 0.8768). Accordingly, ERhigh EnOCs demonstrated markedly higher BMI versus the HGS OC cases (P < 0.001; Bonferroni-adjusted P = 0.002).
Fig. 3.
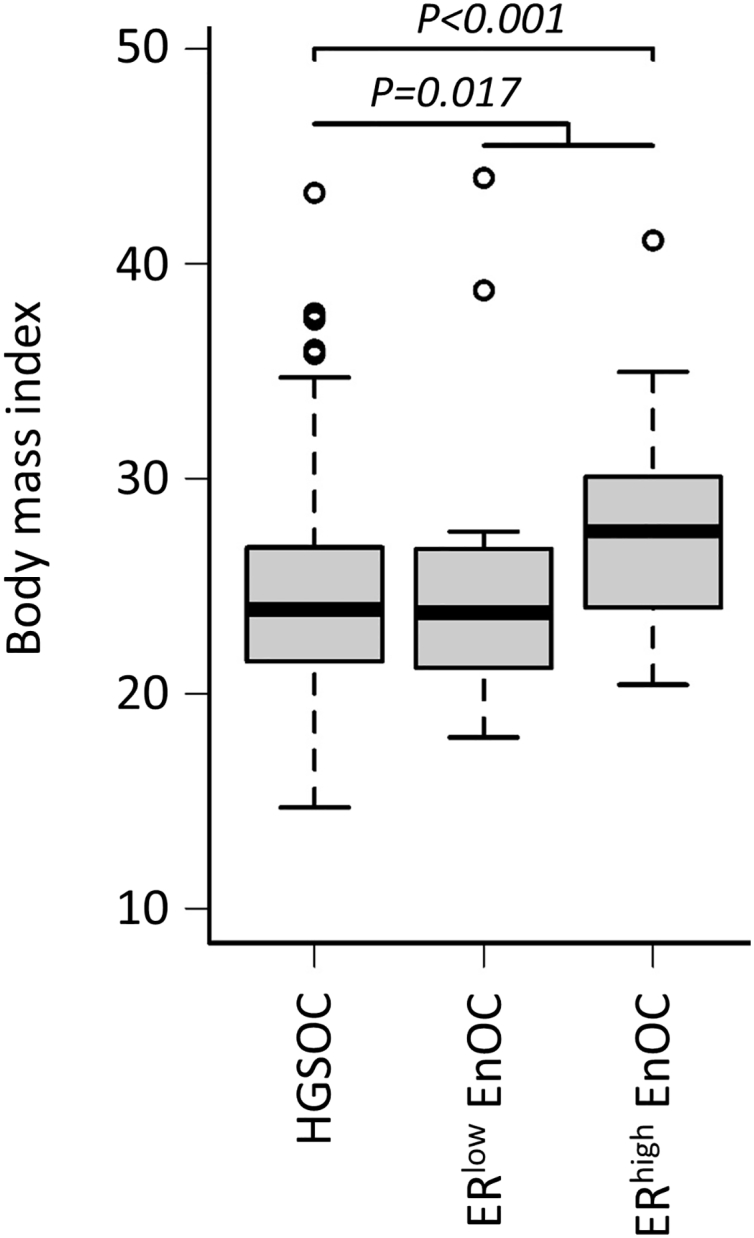
Body mass index of EnOC versus high grade serous OC patients.
4. Discussion
There has been significant research interest in the clinical impact of hormone receptor expression in OC, with regard to both patient survival [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17],22] and sensitivity to endocrine therapy [[25], [26], [27]]. Findings relating PR, ER and AR expression to clinical outcome have been mixed, with studies typically performing analysis on heterogeneous OC cohorts without stratifying by histological subtype of disease, which are now known to represent distinct clinical and molecular disease entities [3]. Moreover, methodologies for determining positivity have also been heterogeneous, with many studies classifying specimens only by the proportion of positive tumor cells, without accounting for the intensity of positive nuclear staining.
Here, we describe a cohort of EnOCs identified through contemporary review of all available OCs diagnosed with endometrioid histology at the Edinburgh Cancer Centre, utilizing IHC for WT1 to improve the fidelity of EnOC diagnosis. We assess positivity for PR, ER and AR across 107 EnOCs – all of which were WT1 negative – using histoscore, a scoring method incorporating both the percentage of positive nuclei and the intensity of nuclear staining. Critically, we avoid sample classification by arbitrarily defined positivity thresholds, instead performing clustering of EnOCs based on their PR, ER and AR histoscores in order to identify subgroups of disease defined by patterns of hormone receptor expression. EnOC specimens displayed a low frequency of AR positivity, and AR histoscore appeared to contribute little toward the assignment of samples to the four identified subgroups.
EnOC cases demonstrating high levels of PR (PR+/ER+ and PR+/ER− groups) displayed markedly favorable clinical outcome. These data are consistent with the prolonged survival described in EnOCs with >1% PR-positive nuclei by the Ovarian Tumor Tissue Analysis consortium [21], identifying 10-year patient survival of around 80%, 60–65% and 50% in those demonstrating ≥50%, 1–50% and <1% PR positive tumor cell nuclei. The scoring method utilized in our study – incorporating both percentage positive cells and the nuclear staining intensity using histoscore – may have contributed toward the identification of a PRhigh EnOC population with exceptional 10-year survival of >90% in this EnOC cohort, providing greater granularity for distinction of tumors demonstrating moderate or strong marker positivity. While PR positivity appeared anti-correlated with features associated with poor prognosis (advanced stage at diagnosis, disease grade and patient age), multivariable analysis identified PR as an independent factor associated with prognosis.
Perhaps most notably, PRhigh stage II cases demonstrated a 10-year survival of approximately 95% compared to around 40% in the corresponding PRlow cases. These data suggest that stage II EnOC with high PR expression may be good candidates for de-escalation of adjuvant chemotherapy regimens to endocrine therapy or observation following primary surgical debulking.
For both PR and ER, the threshold for cluster separation during subgroup identification appeared to lie at a histoscore of approximately 150, and this histoscore threshold could readily be implemented to routinely stratify EnOC management and improve patient prognostication. We recently reported that HGS OCs with strong ER positivity represent those that derive the greatest benefit from endocrine therapy [26], and similar investigations are now warranted to better define the utility of endocrine therapies specifically in EnOC. Defining the efficacy of endocrine therapy in PRhigh EnOC may further highlight potential opportunities to de-escalate first-line therapy for cases diagnosed with stage II disease.
A number of studies have suggested that ER-positive OCs demonstrate favorable outcome compared to ER-negative cases [9,11,13,15,16], including a large histotype-specific study of EnOC [21]. We demonstrate highly significant correlation between PR and ER histoscore in our EnOC cohort, but did not identify a survival benefit in the PR−/ER+ group. Together, these data suggest that high ER expression may not be independently associated with better outcome in EnOC and that its association with PR expression may explain the apparent survival advantage for ER-positive EnOC patients upon univariable analysis. Indeed, in our cohort, the PR−/ER+ group demonstrated the least favorable outcome.
Upon investigation of BMI, the EnOC cohort appeared to demonstrate higher BMI versus the HGS OC comparator cohort from Edinburgh. However, upon closer investigation, ERhigh EnOCs demonstrated significantly higher BMI, while ERlow EnOCs were comparable to HGS OCs. These data suggest that increased BMI may be associated with development of ERhigh EnOC. Notably, higher BMI is known to be associated with increased risk of endometrial cancer, the majority of which display endometrioid histology and are considered hormone-driven [31].
5. Conclusions
Together, these data add to the growing evidence that hormone receptors represent clinically meaningful biomarkers of patient outcome in OC. Optimal first-line management strategies for patients with PRhigh EnOC – who appear to experience exceptional long-term survival – may now warrant re-evaluation, particularly in the context of patients diagnosed with stage II disease who would typically undergo systemic chemotherapy [32]. The relative efficacy of endocrine therapy in subgroups of EnOCs and other OC histotypes should also be investigated following the recent demonstration of greatest benefit in HGS OCs demonstrating high ER expression [26].
Future studies should seek to define how expression levels of hormone receptors – particularly PR and ER – overlap with other molecularly-defined subgroups of OC. Within HGS OCs, comparison versus BRCA1/2 mutation status may be of particular interest, given the association of these events with improved clinical outcome and sensitivity to DNA damaging agents and poly-(ADP-ribose) polymerase (PARP) inhibitors [[33], [34], [35]]. Indeed, there has already been research interest in assessing such co-occurrence [21]. Critically, these analyses will need to be performed in an OC histotype-specific manner, owing to the distinct molecular landscape demonstrate by each of these tumor types.
Acknowledgments
Acknowledgements
We extend our thanks to the patients who contributed to this study and to the Edinburgh Ovarian Cancer Database from which the clinical data reported here were retrieved.
Funding
RLH is supported by an MRC-funded Research Fellowship; BS was supported by the Oncology Endowment Fund (University of Edinburgh) and Edinburgh Lothian Health Fund. We gratefully acknowledge The Nicola Murray Foundation for their generous support of our laboratory.
Author contributions
Conceptualization, RLH, BS, CG, CSH; Methodology, RLH, BS, JT, MC, CG, CSH; Formal Analysis, RLH, BS; Investigation, RLH, BS, YI; Resources, TR, FN, MM, CG; Data Curation, RLH, BS, TR; Writing – Original Draft Preparation, RLH; Writing – Review & Editing, RLH, BS, YI, JT, MC, TR, FN, MM, CG, CSH; Visualization, RLH; Supervision, CG, CSH; Funding Acquisition, RLH, BS, CG.
Declaration of competing interest
RLH, none. BS, none. YI, none. JT, none. MC, none. TR, none. MM: honoraria from Tesaro, BristolMyersSquibb and Roche. FN: non-personal interests in AstraZeneca and Tesaro. CG discloses: research funding from AstraZeneca, Aprea, Nucana, Tesaro and Novartis; honoraria/consultancy fees from Roche, AstraZeneca, MSD, Tesaro, Nucana, Clovis, Foundation One, Cor2Ed and Sierra Oncology; named on issued/pending patents related to predicting treatment response in ovarian cancer outside the scope of the work described here. CSH, none.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2019.09.001.
Appendix A. Supplementary data
Supplementary material
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460:237–249. doi: 10.1007/s00428-012-1203-5. [DOI] [PubMed] [Google Scholar]
- 3.Hollis R.L., Gourley C. Genetic and molecular changes in ovarian cancer. Cancer Biol. Med. 2016;13:236–247. doi: 10.20892/j.issn.2095-3941.2016.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peres L.C., Cushing-Haugen K.L., Kobel M., Harris H.R., Berchuck A., Rossing M.A. Invasive epithelial ovarian cancer survival by histotype and disease stage. J. Natl. Cancer Inst. 2019;111:60–68. doi: 10.1093/jnci/djy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCluggage W.G. My approach to and thoughts on the typing of ovarian carcinomas. J. Clin. Pathol. 2008;61:152–163. doi: 10.1136/jcp.2007.049478. [DOI] [PubMed] [Google Scholar]
- 6.McCluggage W.G. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology. 2011;43:420–432. doi: 10.1097/PAT.0b013e328348a6e7. [DOI] [PubMed] [Google Scholar]
- 7.Kobel M., Rahimi K., Rambau P.F., Naugler C., Le Page C., Meunier L. An immunohistochemical algorithm for ovarian carcinoma typing. Int. J. Gynecol. Pathol. 2016;35:430–441. doi: 10.1097/PGP.0000000000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J.F., Hirsch M.S., Lee H., Matulonis U.A. Prognosis and hormone receptor status in older and younger patients with advanced-stage papillary serous ovarian carcinoma. Gynecol. Oncol. 2009;115:401–406. doi: 10.1016/j.ygyno.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Burges A., Bruning A., Dannenmann C., Blankenstein T., Jeschke U., Shabani N. Prognostic significance of estrogen receptor alpha and beta expression in human serous carcinomas of the ovary. Arch. Gynecol. Obstet. 2010;281:511–517. doi: 10.1007/s00404-009-1185-y. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo K., Sheridan T.B., Mabuchi S., Yoshino K., Hasegawa K., Studeman K.D. Estrogen receptor expression and increased risk of lymphovascular space invasion in high-grade serous ovarian carcinoma. Gynecol. Oncol. 2014;133:473–479. doi: 10.1016/j.ygyno.2014.03.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Z., Wen H., Bi R., Ju X., Chen X., Yang W. A clinically applicable molecular classification for high-grade serous ovarian cancer based on hormone receptor expression. Sci. Rep. 2016;6:25408. doi: 10.1038/srep25408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee P., Rosen D.G., Zhu C., Silva E.G., Liu J. Expression of progesterone receptor is a favorable prognostic marker in ovarian cancer. Gynecol. Oncol. 2005;96:671–677. doi: 10.1016/j.ygyno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Hogdall E.V., Christensen L., Hogdall C.K., Blaakaer J., Gayther S., Jacobs I.J. Prognostic value of estrogen receptor and progesterone receptor tumor expression in Danish ovarian cancer patients: from the ‘MALOVA’ ovarian cancer study. Oncol. Rep. 2007;18:1051–1059. [PubMed] [Google Scholar]
- 14.Nodin B., Zendehrokh N., Brandstedt J., Nilsson E., Manjer J., Brennan D.J. Increased androgen receptor expression in serous carcinoma of the ovary is associated with an improved survival. J. Ovarian Res. 2010;3:14. doi: 10.1186/1757-2215-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenhard M., Tereza L., Heublein S., Ditsch N., Himsl I., Mayr D. Steroid hormone receptor expression in ovarian cancer: progesterone receptor B as prognostic marker for patient survival. BMC Cancer. 2012;12:553. doi: 10.1186/1471-2407-12-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Toledo M.C., Sarian L.O., Sallum L.F., Andrade L.L., Vassallo J., de Paiva Silva G.R. Analysis of the contribution of immunologically-detectable HER2, steroid receptors and of the “triple-negative” tumor status to disease-free and overall survival of women with epithelial ovarian cancer. Acta Histochem. 2014;116:440–447. doi: 10.1016/j.acthis.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson J.M., Skovbjerg Arildsen N., Malander S., Masback A., Hartman L., Nilbert M. Sex steroid hormone receptor expression affects ovarian cancer survival. Transl. Oncol. 2015;8:424–433. doi: 10.1016/j.tranon.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prat J. New insights into ovarian cancer pathology. Ann. Oncol. 2012;23(Suppl. 10):x111–x117. doi: 10.1093/annonc/mds300. [DOI] [PubMed] [Google Scholar]
- 19.van Kruchten M., van der Marel P., de Munck L., Hollema H., Arts H., Timmer-Bosscha H. Hormone receptors as a marker of poor survival in epithelial ovarian cancer. Gynecol. Oncol. 2015;138:634–639. doi: 10.1016/j.ygyno.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 20.Gomora M.J., Morales-Vasquez F., Pedernera E., Perez-Montiel D., Lopez-Basave H., Villa A.R. Sexual steroid hormone receptors profiles in ovarian carcinoma in Mexican women. Endocr. Connect. 2018;7(9):1006–1012. doi: 10.1530/EC-18-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sieh W., Kobel M., Longacre T.A., Bowtell D.D., deFazio A., Goodman M.T. Hormone-receptor expression and ovarian cancer survival: an ovarian tumor tissue analysis consortium study. Lancet Oncol. 2013;14:853–862. doi: 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rambau P., Kelemen L.E., Steed H., Quan M.L., Ghatage P., Kobel M. Association of hormone receptor expression with survival in ovarian endometrioid carcinoma: biological validation and clinical implications. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedchenko N., Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue - a review. Diagn. Pathol. 2014;9:221. doi: 10.1186/s13000-014-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkegaard T., Edwards J., Tovey S., McGlynn L.M., Krishna S.N., Mukherjee R. Observer variation in immunohistochemical analysis of protein expression, time for a change? Histopathology. 2006;48:787–794. doi: 10.1111/j.1365-2559.2006.02412.x. [DOI] [PubMed] [Google Scholar]
- 25.Smyth J.F., Gourley C., Walker G., MacKean M.J., Stevenson A., Williams A.R. Antiestrogen therapy is active in selected ovarian cancer cases: the use of letrozole in estrogen receptor-positive patients. Clin. Cancer Res. 2007;13:3617–3622. doi: 10.1158/1078-0432.CCR-06-2878. [DOI] [PubMed] [Google Scholar]
- 26.Stanley B., Hollis R.L., Nunes H., Towler J.D., Yan X., Rye T. Endocrine treatment of high grade serous ovarian carcinoma; quantification of efficacy and identification of response predictors. Gynecol. Oncol. 2019;152:278–285. doi: 10.1016/j.ygyno.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Bowman A., Gabra H., Langdon S.P., Lessells A., Stewart M., Young A. CA125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: identification of an endocrine-sensitive subgroup. Clin. Cancer Res. 2002;8:2233–2239. [PubMed] [Google Scholar]
- 28.Ellenson L.H., Carinelli S.G., Cho K.R., Kim K.-R., Kupryjanczyk J., Prat J., Singer G. Endometrioid tumours of the ovary. In: Kurman R.J., Carcangiu M.L., Herrington C.S., Young R.H., editors. WHO Classification of Tumours of Female Reproductive Organs. WHO press; 2014. pp. 29–32. [Google Scholar]
- 29.Therneau T. 2015. A Package for Survival Analysis in S. [Google Scholar]
- 30.Hollis R.L., Churchman M., Michie C.M., Rye T., Knight L., McCavigan A. High EMSY expression defines a BRCA-like subgroup of high grade serous ovarian carcinoma with prolonged survival and hypersensitivity to platinum. Cancer. 2019;125(16):2772–2781. doi: 10.1002/cncr.32079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhaskaran K., Douglas I., Forbes H., dos-Santos-Silva I., Leon D.A., Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet (London, England) 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ledermann J.A., Raja F.A., Fotopoulou C., Gonzalez-Martin A., Colombo N., Sessa C. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013;24(Suppl. 6):vi24–vi32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 33.Tan D.S., Rothermundt C., Thomas K., Bancroft E., Eeles R., Shanley S. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J. Clin. Oncol. 2008;26:5530–5536. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- 34.Hollis R.L., Meynert A.M., Churchman M., Rye T., Mackean M., Nussey F. Enhanced response rate to pegylated liposomal doxorubicin in high grade serous ovarian carcinomas harbouring BRCA1 and BRCA2 aberrations. BMC Cancer. 2018;18:16. doi: 10.1186/s12885-017-3981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ledermann J., Harter P., Gourley C., Friedlander M., Vergote I., Rustin G. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material