Abstract
Trypanosoma cruzi, the causative agent of Chagas disease, exhibits a high genetic variability and has been classified into six discrete typing units (DTUs) named TcI through TcVI. This genetic diversity is believed to be associated with clinical characteristics and outcomes, but evidence supporting such associations has been limited. Herein, we performed a phylogenetic analysis of T. cruzi sequences of the mini-exon intergenic region obtained from a large cohort of pregnant women and newborns from Argentina, Honduras, and Mexico, to assess parasite genetic diversity and possible associations with congenital transmission. Analysis of 105 samples (including five paired samples) from maternal and umbilical cord blood indicated that T. cruzi DTU distribution was similar among pregnant women and newborns from these three countries, with a high frequency of TcII-TcV-TcVI DTUs, including mixed infections with TcI. However, phylogenetic analysis revealed that although the same parasite haplotypes circulated in these three countries, they were present at different frequencies, leading to significant geographic differences. Of importance, a strong association was observed between parasite haplotypes and congenital infection of newborns. Thus, the identification of parasite haplotypes in pregnant women, but not of parasite DTUs, may help predict congenital transmission of T. cruzi.
Chagas disease, caused by the parasitic protozoa, Trypanosoma cruzi, is widely distributed throughout the Americas, from the United States to Argentina. The parasite infects at least six million individuals in endemic areas,1 with a burden of disease 7.5 times higher than malaria, as measured by disability-adjusted life years.2 It is responsible for global health care costs of $24 billion US dollars.3 In Latin America, congenital transmission occurs in an average of 5% of approximately one million infected women,1, 4 and it is also a concern worldwide as immigrants from Latin American countries remain at risk of transmitting the parasite to their newborns.5
Trypanosoma cruzi exhibits a high genetic variability and has been classified into six discrete typing units (DTUs) named TcI through TcVI. A potential seventh DTU initially associated with bats (Tcbat) is also considered.6, 7, 8 Furthermore, analysis of TcI revealed a substructuration within this DTU, with five genotypes identified as TcIa to TcIe, possibly associated with different epidemiologic cycles.9, 10, 11, 12 Information about the relation between T. cruzi genetic diversity and geographic distribution, specific transmission cycles and hosts, clinical features of the disease and patient prognosis, drug susceptibility/resistance, and diagnosis performances remains limited. Indeed, although associations have been proposed,9, 10, 13, 14, 15 other studies have failed to detect clear relationships of T. cruzi DTUs with parasite transmission and clinical forms.16, 17, 18, 19 Most human infections in Latin America are caused by the DTUs TcI, TcII, TcV, and TcVI, which are most frequently found within domestic transmission cycles, whereas human infections with TcIII and TcIV (referred to as non-TcI DTUs) are less frequent.20 It has been suggested that TcII-TcV-TcVI, also referred to as non-TcI DTUs,19 might lead to more severe disease compared with TcI because these DTUs were detected more frequently in patients with chronic Chagas heart disease compared with asymptomatic patients from Argentina.21 In the same country, the TcIa and TcId subgenotypes have nonetheless been associated with severe heart disease, as well as reactivation after heart transplantation.22 TcId has been identified in cardiac explant samples, and TcIa has been identified in blood and skin chagoma specimens, suggesting histiotropism of this genotype.23 TcI DTU has also been reported to be predominant in the United States, Mexico, and Central America over 95% of the strains,24 but recent studies, including some from our group, are challenging this distribution, documenting the presence of non-TcI parasite strains at high frequencies in triatomines, rodents, nonhuman primates, and humans from these regions.19, 20, 25, 26, 27, 28, 29
Thus, the identification of T. cruzi DTUs from additional biological samples and broader geographic areas has become a critical point to better understand the epidemiology of Chagas disease, evaluate potential associations between strains and clinical manifestations or transmission cycles, and improve the subsequent disease control programs.29
The present study derives from a previous large observational population study about congenital transmission of T. cruzi in Argentina, Honduras, and Mexico. Initial genotyping also indicated the predominance of non-TcI parasites in some pregnant women of the three countries.19 In addition to the previous sequences analyzed by Buekens et al,19 a phylogenetic analysis of other sequences (not analyzed previously) of the mini-exon intergenic region of T. cruzi DNA, obtained from pregnant women and newborns, was performed to further assess parasite sequence diversity and explore possible associations among parasite genotypes and congenital transmission. We hypothesized that specific sequences, rather than DTUs, may be associated with congenital transmission across Latin America.
Materials and Methods
Ethics Statement
This study was approved by the Institutional Review Boards at Tulane University (New Orleans, LA) and the Ethics Committees of the Centro de Educación Médica e Investigaciones Clínicas Norberto Quirno Centro de Educacion e Investigaciones Clinicas (CEMIC, Buenos Aires, Argentina), the Facultad de Ciencias Médicas, Universidad Nacional Autónoma de Honduras (Tegucigalpa, Honduras), and the Universidad Autónoma de Yucatán (Merida, Mexico). All women provided written informed consent for participation in the study.
Study Population
A total of 105 samples [71 maternal blood (MB) and 34 cord blood (CB)] obtained from 100 women at delivery (in five cases, MB and CB from the same mothers were paired) were included in this study. Sixteen MB and 20 CB samples came from Tucuman, Argentina; 21 MB and 12 CB samples came from Santa Barbara and Intibuca, Honduras; and 34 MB and two CB samples came from Merida and Valladolid, Mexico. Samples were derived from a cohort of 495 pregnant women who were followed up to assess T. cruzi congenital transmission, and this included nine cases of confirmed congenital transmission, eight from Argentina and one from Mexico.19, 30 These 105 samples were those that were successfully genotyped. Most of the samples had a confirmed serologic diagnostic of at least one positive rapid test result, and one positive enzyme-linked immunosorbent assay (ELISA) result, and a positive T. cruzi diagnostic PCR result,19 which included 31 MB samples previously sequenced19 (Table 1). However, samples with a confirmed positive serologic diagnostic result but negative T. cruzi diagnostic PCR result, as well as samples with a discordant/negative serology (rapid test positive result with negative ELISA result and a negative PCR result and samples with negative serology but positive T. cruzi PCR result) were also included (Table 1). Although the information on the country of origin of the mothers is missing, census data show that <0.5% of women residing in this study area were born abroad.20
Table 1.
Diagnostic of Trypanosoma cruzi Infection in Pregnant Women and Newborns Included in the Study, Compared by Country
| Type of test | Argentina |
Honduras |
Mexico |
Total (N = 105) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MB (n = 16) | CB (n = 20) | Total (N = 36) | MB (n = 21) | CB (n = 12) | Total (N = 33) | MB (n = 34) | CB (n = 2) | Total (N = 36) | ||
| Rapid tests | ||||||||||
| Both rapid test results positive | 16 (100.0) | 17 (85.0) | 33 (91.7) | 18 (85.7) | 10 (83.3) | 28 (84.8) | 13 (38.2) | 0 | 13 (36.1) | 74 (70.5) |
| Only Stat-Pak result positive | 0 | 2 (10.0) | 2 (5.6) | 2 (9.5) | 1 (8.3) | 3 (9.1) | 8 (23.5) | 2 (100.0) | 10 (27.8) | 15 (14.3) |
| Only T-Detect result positive | 0 | 1 (5.0) | 1 (2.70) | 1 (4.8) | 1 (8.3) | 2 (6.1) | 11 (32.4) | 0 | 11 (30.6) | 14 (13.3) |
| Both test results negative | 0 | 0 | 0 | 0 | 0 | 0 | 2 (5.9) | 0 | 2 (5.6) | 2 (1.9) |
| ELISA Wiener | ||||||||||
| Positive result | 15 (93.8) | 18 (90.0) | 33 (91.7) | 19 (90.5) | 11 (91.7) | 30 (90.9) | 9 (26.5) | 0 | 9 (25.0) | 72 (68.6) |
| Negative result | 1 (6.3) | 2 (10.0) | 3 (9.1) | 2 (9.5) | 1 (8.3) | 3 (9.1) | 24 (70.6) | 2 (100.0) | 26 (72.2) | 32 (30.5) |
| Inconclusive result | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.9) | 0 | 1 (2.8) | 1 (0.9) |
| Diagnostic PCR | ||||||||||
| Positive result | 14 (87.5) | 18 (90.0) | 32 (88.9) | 18 (87.5) | 12 (100.0) | 30 (90.9) | 34 (100.0) | 2 (100.0) | 36 (100.0) | 98 (93.3) |
| Negative result | 2 (12.5) | 2 (10.0) | 4 (11.1) | 3 (14.3) | 0 | 3 (9.1) | 0 | 0 | 0 | 7 (6.7) |
Data are expressed as n (%).
CB, cord blood; ELISA, enzyme-linked immunosorbent assay; MB, maternal blood.
Blood Samples and DNA Extraction
Maternal MB and cord CB blood samples were obtained at the time of delivery, as described by Buekens et al.19, 30 These samples were mixed with an equal volume of 6 mol/L guanidine HCl–0.2 mol/L EDTA solution immediately after blood collection (blood sample diluted in guanidine-EDTA). DNA extraction, PCR amplification of the intergenic region of the mini-exon gene of T. cruzi,31 and the sequencing and editing of PCR amplicons were performed as previously described.19
Phylogenetic Analysis
All T. cruzi mini-exon sequences of sufficient quality were included in the phylogenetic analysis. In addition to the previous 31 sequences analyzed by Buekens et al,19 a phylogenetic analysis was performed, including 74 new sequences. Eight reference sequences covering DTUs TcII to TcVI and 12 references for the TcI genotypes TcIa, TcIb, and TcId were also included for comparison (https://www.ncbi.nlm.nih.gov/genbank). The reference sequences for non-TcI sequences were from strains MN TcV (GenBank accession number AY367128.1), SC43 TcV (AY367127.1), Tu18 TcII (AY367125.1), CL TcVI (U57984.1), CANIII TcIV (AY367123.1), S52RH1NOLA TcIV (KM376441), M5631 TcIII (AY367126), and M6241 TcIII (AF050522). The references for TcI sequences including the three subgenotypes TcIa, TcIb, and TcId were from strains CGC TcIa (AM259467), 92090802Pcl1 USA TcIa (JQ581481.1), TVC TcIa (AM259476), Sebas1 TcIb (FJ713388), Td11 TcIb (AM259475.1), FcHcl1 TcIb (KF220693), RN01 TcId (GQ398815), SilvioX10 TcId (FJ713407), VTH TcId (FJ713405), PALV2-2Cl5 TcIe (GQ398812), LGNcl8 TcIe (GU903131), and AS TcIe (FJ713356.1).
To avoid artifactual gaps due to important sequence differences, non-TcI and TcI sequences were analyzed separately. The analysis was performed on the Phylogeny.fr platform (http://www.phylogeny.fr, last accessed December 20, 2018). The manually edited sequences were aligned with MUSCLE version 3.8.31 (European Bioinformatics Institute, Cambridge, UK), configured for highest accuracy MUSCLE with default settings. After alignment, ambiguous regions (ie, containing gaps and/or poorly aligned) were removed with Gblocks version 0.91b (Institut de Biologia Evolutiva, Barcelona, Spain) using the following parameters: i) minimum length of a block after gap cleaning: 10, ii) no gap positions were allowed in the final alignment, iii) all segments with contiguous nonconserved positions >8 were rejected, and iv) minimum number of sequences for a flank position: 85%. The phylogenetic tree was inferred using the Maximum Likelihood ML method implemented in the PhyML program version 3.1/3.0 (Information Génomique et Structurale, Marseille, France) aLRT. The HKY85 substitution model was selected, assuming an estimated proportion of invariant sites and four γ-distributed rate categories to account for rate heterogeneity across sites. The tree and the robustness of the nodes were evaluated by bootstrap on 1000 replications by heuristic search. Graphs and phylogenetic tree were generated with TreeDyn version 198.3 (Génétique et Evolution des Maladies Infectieuses Centre de Recherche, Montpellier, France). TcI and non-TcI haplotypes were identified on the basis of their respective sequence alignments, and their proportions were determined according to the number of sequences of each haplotype.
Statistical Analysis
The χ2 test was used to compare the distribution of the maternal PCR genotyping, the distribution of cord blood PCR genotyping, and the distribution of the total PCR genotyping among the countries. When ≥25% of the cells of the table had expected counts <5, the Fisher exact test was used. Odds ratios were calculated with their 95% CIs for haplotype analysis.
Results
PCR Genotyping of T. cruzi
On the basis of amplicon size, PCR genotyping was successful in all 105 samples, 71 MB and 34 CB, including in samples with negative T. cruzi diagnostic PCR result and samples with negative/inconclusive serology. Infection was found with only TcI in 4 of 105 samples (3.8%), with only non-TcI in 72 of 105 samples (68.6%), and coinfection with TcI and non-TcI in 29 of 105 samples (27.6%) (Table 2). In case of the paired MB and CB samples from the same mother, non-TcI DTUs were identified in both MB and CB samples in four cases; and in one case, non-TcI was detected in the MB samples and a mixed infection TcI and non-TcI was detected in the CB sample. Overall, the distribution of DTUs did not show any significant difference among the three countries (Table 2), with a predominance of non-TcI in the three countries, as noted before.19
Table 2.
Trypanosoma cruzi PCR Genotyping in MB and CB Samples by Country
| Genotyping∗ | Argentina |
Honduras |
Mexico |
Total (N = 105) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MB (n = 16) | CB (n = 20) | Total (N = 36) | MB (n = 21) | CB (n = 12) | Total (N = 33) | MB (n = 34) | CB (n = 2) | Total (N = 36) | ||
| Non-TcI | 8 (43.7) | 17 (85.0) | 24 (66.7) | 12 (57.1) | 8 (66.7) | 20 (60.6) | 27 (79.4) | 1 (50.0) | 28 (77.8) | 72 (68.6) |
| TcI | 2 (12.5) | 1 (5.0) | 3 (8.3) | 1 (4.8) | 0 | 1 (3.0) | 0 | 0 | 0 | 4 (3.8) |
| TcI–non-TcI | 6 (43.7) | 2 (10.0) | 9 (25.0) | 8 (38.1) | 4 (33.3) | 12 (39.4) | 7 (20.6) | 1 (50.0) | 8 (22.2) | 29 (27.6) |
Data are given as n (%).
CB, cord blood; MB, maternal blood.
Statistical comparison of genotype proportions among countries: MB: exact test, P = 0.0403; CB: exact test, P = 0.1924. All samples totals by country, P = 0.2470.
Mini-Exon Intergenic Region Sequencing and Phylogenetic Analysis
DTU identification was confirmed and refined by sequencing 133 of the PCR products of the mini-exon intergenic region that were obtained from the 105 blood samples to account for mixed TcI and non-TcI infections in 28 samples. For 17 PCR products, no DNA sequence was obtained, was of unreliable quality, did not correspond to T. cruzi; and genotypes could not be confirmed. The remaining 117 sequences yielded T. cruzi sequences of sufficient quality for analysis, which confirmed the DTU identified by PCR for 115 sequences; and two sequences resulted as TcI instead of non-TcI. Thus, overall concordance between DTUs obtained by PCR genotyping and the sequencing was excellent (115/117, 98.2%).
Phylogenetic analyses of non-TcI and TcI sequences were then analyzed separately, to avoid artifactual gap caused by highly divergent sequences. For TcI sequences, the analysis involved 19 sequences of approximately 176 bp (12 sequences from MB and 7 sequences from CB). The analysis for non-TcI sequences involved 98 sequences of approximately 120 bp (66 sequences from MB and 32 sequences from CB samples).
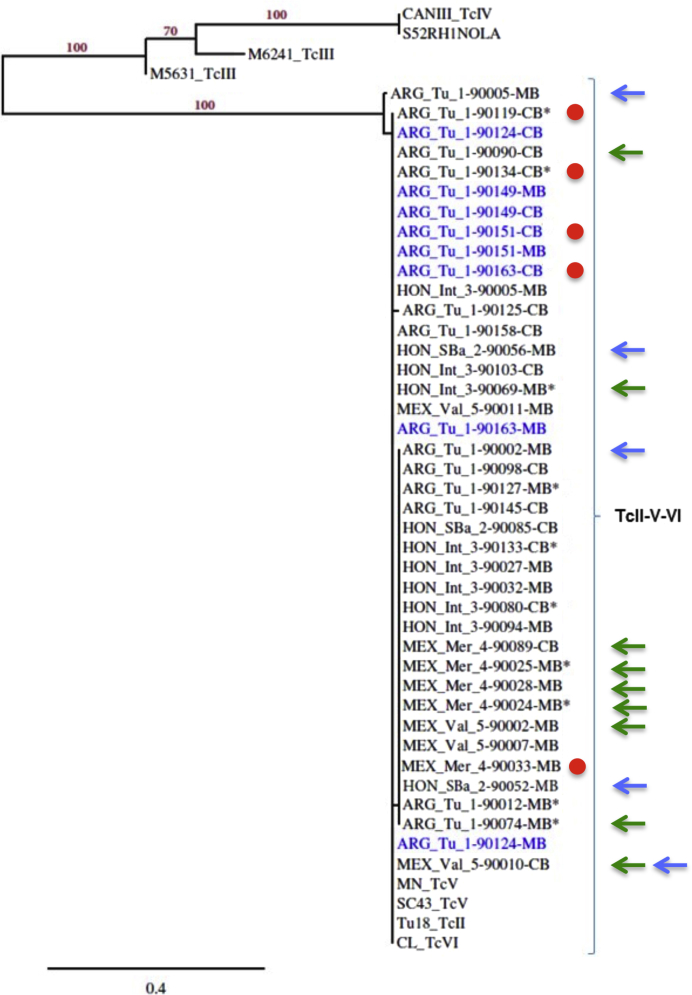
The phylogenetic analysis of sequences corresponding to TcI genotypes resulted in clear phylogenetic structure, supported by significant bootstrap values of the ML analysis (>70%) (Figure 1). The unrooted tree allowed the identification of five well-defined clusters corresponding to the TcI genotypes, TcIa boostrap value 88%, TcIb, TcIe, and TcId, with this last one being divided into two clusters with >90% bootstrap. One sequence from Honduras clustered with TcIa reference sequences, and one sequence from Argentina was more closely related to a cluster of TcIb sequences, whereas all other 17 TcI sequences clustered with TcId references, although in two different clusters. These included sequences from Argentina, Honduras, and Mexico, which suggested a lack of geographic structuring among parasites from these countries on the basis of these sequences. Eleven of the TcId sequences from one cluster were derived from cases of mixed infections with non-TcI parasites (four from Argentina, five from Honduras, and two from Mexico). Four of these TcId sequences were derived from cases that were seronegative for T. cruzi antibodies, and two were derived from cases of congenital transmission (Figure 1).
Figure 1.
Phylogenetic analysis of Trypanosoma cruzi TcI sequences. The phylogram depicting the phylogenetic relationships among 19 T. cruzi DNA sequences recovered from maternal and umbilical cord blood (MB and CB, respectively), from Argentina (ARG), Honduras (HON), and Mexico (MEX), compared with reference T. cruzi sequences from GenBank (https://www.ncbi.nlm.nih.gov/genbank; Materials and Methods), based on mini-exon intergenic gene sequencing. Bootstrap values appear on each clustering branch. Asterisks indicates cases of mixed infections with non-TcI; red circles, cases of congenital transmission; blue arrows, cases with negative PCR results; green arrows, cases with negative/inconclusive serology. n = 8 (ARG); n = 9 (HON); n = 2 (MEX). Int, Intibucá; Mer, Merida; SBa, Santa Barbara; Tuc, Tucuman.
The phylogenetic tree of non-TcI sequences (Figure 2) allowed the partial resolution of the non-TcI DTUs, with significant bootstrap values of the ML analysis of >80%, and three main clusters of sequences were identified. The first cluster included reference sequences corresponding to TcIV DTU with a bootstrap of 100%, and a second cluster included reference sequences for the TcIII DTU; none of the samples clustered with TcIII or TcIV. All 98 sequences from this cohort of MB and CB samples clustered with reference sequences from DTUs TcII, TcV, and TcVI, with a significant bootstrap of 100%, but these three DTUs could not be resolved any further with the available sequence data. Again, sequences from Argentina, Honduras, and Mexico could not be distinguished, suggesting a lack of geographic differentiation of T. cruzi within DTUs for these sequences. In four of five cases for which paired samples of MB and CB from the same mother were obtained, identical sequences were recovered in each paired sample (Figure 2). From the cluster of TcII, TcV, and TcVI sequences, five sequences from Argentina, four sequences from Honduras, and two sequences from Mexico were derived from cases of mixed infections with TcI. Interestingly, the nine sequences, eight from Argentina and one from Mexico, corresponding to confirmed cases of congenital transmission, seemed to cluster together within the TcII-TcV-TcVI cluster; and sequences from seronegative samples seemed more frequent in another branch of this cluster (Figure 2). This observation suggested potential association between parasite haplotype and congenital transmission.
Figure 2.
Phylogenetic analysis of Trypanosoma cruzi non-TcI sequences. The phylogram depicting the phylogenetic relationships among selected T. cruzi DNA sequences representative from a total 98 sequences (https://www.ncbi.nlm.nih.gov/genbank; accession numbers MH629842 to MH629958) recovered from maternal and umbilical cord blood (MB and CB, respectively), with T. cruzi infection from Argentina (ARG), Honduras (HON), and Mexico (MEX), compared with reference T. cruzi sequences from GenBank (see Materials and Methods), based on mini-exon intergenic gene sequencing. Bootstrap values appear on each clustering branch. Text in blue indicates MB-CB paired samples. Asterisks indicate cases of mixed infections with TcI; red circles, cases of congenital transmission; blue arrows, cases with negative PCR results; green arrows, cases with negative/inconclusive serology. n = 28 (ARG); n = 24 (HON); n = 34 (MEX). Int, Intibucá; Mer, Merida; SBa, Santa Barbara; Tu, Tucuman; Val, Valladolid.
Therefore, T. cruzi haplotype distribution and their characteristics were then examined in more detail. Despite limited sequence variations within the TcII-TcV-TcVI group, seven variable positions were identified in the sequences, corresponding to up to 10 haplotypes, although only two non-TcI-H1 and non-TcI-H2 accounted for >77% of the sequences (Table 3). The third most frequent haplotype presented a sequence ambiguity that could not be resolved, suggesting it may represent a mixture of haplotypes non-TcI-H1/H2 in unknown proportions. Similarly, seven TcI haplotypes were identified, including one TcIa and one TcIb, and the other five corresponding to TcId (Table 4). Interestingly, statistically significant differences in haplotype frequencies were identified among the three countries, with non-TcI-H1 being the most frequent haplotype in Argentina and non-TcI-H2 being the most frequent haplotype in Honduras and Mexico (Figure 3A). The unresolved non–TcI-H1/H2 haplotype was also more frequent in Mexico, but rare in Honduras and Argentina. More important, a significant association of congenital transmission cases was detected with T. cruzi haplotypes, with most cases associated with the non–TcI-H1 haplotype (Figure 3B), which was 21.12 times more likely to be present in congenital transmission cases than other parasite haplotypes (95% CI, 2.52–177.59). Comparison of haplotype proportions between MB and CB samples also revealed a significant difference (Figure 3C), clearly indicating that T. cruzi haplotypes present in CB are not a random subset of haplotypes present in MB. Haplotype non–TcI-H1 was thus 6.62 times more likely to be present in CB compared with MB (95% CI, 2.63–16.65). On the other hand, haplotype non–TcI-H2 was less likely to be found in CB samples compared with MB (odds ratio, 0.40; 95% CI, 0.16–0.99); and haplotype non–TcI-H1/H2 was only found in MB samples, but not in CB samples. Finally, T. cruzi haplotypes were also significantly associated with a negative ELISA result, with the non–TcI-H1/H2 haplotype being overrepresented among samples with a negative ELISA result (Figure 3D). On the other hand, no significant association was observed between T. cruzi haplotypes and T. cruzi PCR results (Figure 3E).
Table 3.
Non-TcI Trypanosoma cruzi Haplotypes and Their Frequency
| Haplotype | n (%) | Position in reference sequences |
||||||
|---|---|---|---|---|---|---|---|---|
| 13 | 21 | 75 | 76 | 86 | 93 | 117 | ||
| TU18, SC43, CL | G | T | C | G | G | G | G | |
| Non–TcI-H1 | 33 (35.5) | |||||||
| Non–TcI-H2 | 39 (41.9) | A | ||||||
| Non–TcI-H1/H2 | 13 (13.9) | R | ||||||
| Non–TcI-H3 | 2 (2.1) | S | ||||||
| Non–TcI-H4 | 1 (1.1) | S | R | |||||
| Non–TcI-H5 | 1 (1.1) | G | C | |||||
| Non–TcI-H6 | 1 (1.1) | A | R | |||||
| Non–TcI-H7 | 1 (1.1) | G | A | |||||
| Non–TcI-H8 | 1 (1.1) | G | ||||||
| Non–TcI-H9 | 1 (1.1) | A | ||||||
CL, reference TcVI; SC43, reference TcV; TU18, reference TcII.
Table 4.
TcI Trypanosoma cruzi Haplotypes and Their Frequency, Comparing with Reference Strains
| Haplotype | n (%) | Position in reference sequence |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 26 | 76 | 102 | 119 | 142 | 145 | 147 | 165 | ||
| Silvio X10 (TcId) | T | T | C | Y | T | C | C | T | A | |
| TcI-H1 (TcId) | 13 (68.4) | C | ||||||||
| TcI-H2 (TcId) | 1 (5.3) | C | C | |||||||
| TcI-H3 (TcId) | 1 (5.3) | T | G | |||||||
| TcI-H4 (TcId) | 1 (5.3) | T | G | C | ||||||
| TcI-H5 (TcId) | 1 (5.3) | G | ||||||||
| TcI-H6 (TcIa) | 1 (5.3) | C | G | T | C | C | ||||
| TcI-H7 (TcIb) | 1 (5.3) | C | G | G | ||||||
Silvio X10, reference sequence TcId.
Figure 3.
Distribution of Trypanosoma cruzi haplotypes among countries and populations. The proportions of T. cruzi haplotypes of the mini-exon gene partial sequence identified in Tables 3 and 4 are shown color coded as indicated. TcI haplotypes were grouped into a single category because of the low frequency of variant haplotypes. Similarly, non–TcI-H4 to non–TcI-H9 were grouped as other non-TcI haplotype because of their low frequency. A: Comparison of haplotype distribution among countries (P = 0.009 between all groups). B: Comparison of haplotypes in congenital cases and noncongenital cases (P = 0.021). C: Comparison of haplotype distribution between maternal and cord blood (MB and CB, respectively) samples (P = 0.0003). D: Comparison of haplotype distribution between confirmed seropositive and seronegative samples (P = 0.007). E: Comparison of haplotype distribution between PCR-positive and PCR-negative samples (P = 0.258).
Discussion
Since the initial observations of T. cruzi extensive genetic diversity in the 1970s, a major challenge has been to evaluate potential associations between T. cruzi genotypes and clinical manifestations, such as severity and progression of disease, response to treatment, or congenital transmission.32, 33 Numerous experimental studies comparing infections with selected parasite strains in vitro or in animal models do support the idea that different genotypes can present different biological properties.34, 35, 36, 37 However, the number of strains that can be analyzed with such approaches is necessarily limited, which makes patterns of potential associations difficult to uncover, and generalizations may be unreliable. On the other hand, epidemiologic studies provide opportunities to assess parasite diversity in large cohorts, which can shed light on the heterogeneous distribution of T. cruzi DTUs in endemic regions.9, 10, 13, 14, 15 A recent study using next-generation sequencing compared parasite genetic diversity present in congenitally infected infants and in their mothers. Although the transmission of multiple parasite genotypes between mother and fetus was detected, this approach allowed genotyping of DTUs from only three groups: TcI, TcII, TcIII/TcVI; and no specific genotype association was detected with congenital transmission.38
This study describes the DTUs circulating among one of the largest cohorts of pregnant women from parts of Argentina, Honduras, and Mexico,19 which can be considered as highly representative of the general population in the regions where the study was conducted, as demonstrated for HIV studies.39 DNA sequences from TcI and non-TcI DTUs, alone and in combination, in both maternal blood and cord blood samples were detected. The absence of TcIII and TcIV is expected given the few human cases reported infected with these two DTUs.40 On the other hand, these data show, for the first time, the presence of a high frequency of TcII-TcV-TcVI DTUs in human samples in Mexico and Honduras, as well as the presence of mixed infections with TcI and TcII-TcV-TcVI DTUs in these two countries. Overall, TcI was present in a low proportion of our samples (18%), contrary to the proposed predominance of this DTU in Central and North America.33, 41 Thus, no significant differences were detected in parasite DTUs among populations from Argentina, Honduras, and Mexico. This adds to the growing body of literature evidencing the widespread and often predominant presence of TcII-TcV-TcVI DTUs in triatomine vectors, domestic and wild mammals in Central and North America,25, 26, 42 and human cases from the southern United States.43 Taken together, these observations highlight important gaps in our understanding of the geographic distribution of T. cruzi DTUs in Central and North America, which warrants further studies to clearly identify parasite diversity circulating in these regions.
Phylogenetic analysis of T. cruzi sequences further informed on its diversity. Of the five TcI genotypes previously described (TcIa, TcIb, TcIc, TcId, and TcIe,), a high proportion of TcId, which is believed to be associated with the sylvatic cycle,9, 10, 11, 12 was detected in samples from all three countries. This is the first report of TcId in Honduras and southern Mexico. One of the samples from Argentina was closely related with TcIb, which was previously only observed in peridomestic cycles in Colombia.9, 10, 11, 12 It is also remarkable that the DTU TcIa, associated with domestic transmission cycles,9, 10, 11, 12 was only found in one sample from Honduras. For non-TcI DTUs, limited phylogenetic structure was observed, in agreement with the close evolutionary relationship among TcII, TcV, and TcVI DTUs, which require longer sequences to be differentiated.44, 45 Nonetheless, up to 10 sequence haplotypes could be identified from this group of DTUs, with significant differences in their geographic distribution among Argentina, Honduras, and Mexico. Thus, although the overall mixture of parasite DTUs is similar among the three countries, there are key differences at the level of the T. cruzi strains circulating in each country. More important, such differences are missed by PCR genotyping and require the analysis of sequence data to be detected.
This study also provides the first epidemiologic evidence associating parasite genetic diversity with biological characteristics. Indeed, T. cruzi haplotypes present in confirmed cases of congenital transmission, as well as those detected in CB samples, were not a random subset of the haplotypes present in the maternal population. Specific haplotypes present at high frequency in mothers, such as non–TcI-H2, are less likely to be found in CB samples and even less likely to be present in congenital cases. The non–TcI-H1/H2 haplotype was also only detected in mothers, but completely absent from CB and congenital cases. On the other hand, the large majority of congenital cases were associated with the non–TcI-H1 haplotype. These results suggest that parasite haplotype selection is likely occurring during congenital transmission, possibly at the level of the placenta. Previous studies showed that the distribution of DTUs in congenital cases is highly similar to that found in corresponding local populations, suggesting that all circulating DTUs may be equally transmitted; and congenital cases with TcI, TcII, TcIII, TcV, and TcVI have been reported.46 However, these studies were limited to direct PCR genotyping of parasites to identify DTUs,31, 47, 48, 49 which, as shown herein, is insufficient to uncover genetic differences that may be associated with congenital transmission. On the other hand, these results agree with observations of differences in congenital transmission after experimental T. cruzi infection with different strains in mouse models.36, 50
These findings have major implications, as they suggest that the identification of parasite haplotypes may help predict which mother is most likely to transmit T. cruzi parasites to her newborn. Thus, further studies are warranted to expand this work and fully explore the genetic diversity of parasites associated with congenital transmission, to develop effective prognosis tools. In addition, the observation that CB haplotype distribution differed from that of MB samples strongly suggests that the detection of T. cruzi parasite sequences from these CB samples is highly unlikely to be due to contamination of samples with MB, and the high rate of PCR-positive CB samples (69.8%) observed in a previous study19 is rather due to the high sensitivity of our PCR protocol. It also supports the hypothesis that T. cruzi DNA may be present in many of these CB samples without live parasites and without leading to congenital transmission, as suggested before,19, 50 which raises questions about the usefulness of PCR for the diagnosis of congenital infections.46, 51 Finally, a significant association of parasite haplotype with serologic diagnostic was observed, as specific T. cruzi haplotypes were associated with negative/inconclusive ELISA test results despite T. cruzi infection evidenced by PCR and genotyping. This further highlights the importance of circulating parasites for the accuracy of serologic diagnostic and the limitation of current commercial tests for Central and North America.52 Thus, it is likely that some maternal and congenital cases were missed in our previous study, which relied exclusively on serology for inclusion and follow-up of cases.19 Additional studies including increased numbers of paired samples from mothers and umbilical cord blood, as well as additional samples from Mexico, should help further assess these associations among parasite genotypes and congenital transmission.
Conclusions
Our study confirms that T. cruzi DTU distribution is similar among pregnant women and newborns from Argentina, Honduras, and Mexico, but highlights the limitations of direct PCR genotyping of parasites. Indeed, detailed sequence analysis revealed that although the same parasite haplotypes circulate in these three countries, they are present at different frequencies, leading to significant geographic differences. More important, sequence analysis revealed a strong association of parasite haplotypes with the presence of parasite DNA in cord blood, and with congenital infection of newborns. Thus, the identification of parasite haplotypes in pregnant women may help predict congenital transmission of T. cruzi, and this warrants further studies to fully explore the genetic diversity of parasites associated with congenital transmission in this and other large cohorts.
Acknowledgments
We thank the members of the Congenital Chagas Working Group in Argentina, Mexico, and Honduras, where the samples were collected; Pascal Deblandre [Université Libre de Bruxelles (ULB)] for technical help; and the Fonds Emile Defay and the Foundation David & Alice Van Buuren (ULB) for support.
Footnotes
Supported by National Institute of Allergy and Infectious Diseases grant R01AI083563 (P.B.) and Eunice Kennedy Shriver National Institute of Child Health and Human Development grant T32HD057780 (P.B.).
Disclosures: None declared.
References
- 1.WHO Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;90:33–43. [PubMed] [Google Scholar]
- 2.Bern C. Chagas’ disease. N Engl J Med. 2015;373:456–466. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 3.Lee B.Y., Bacon K.M., Bottazzi M.E., Hotez P.J. Global economic burden of Chagas disease: a computational simulation model. Lancet. 2013;13:342–348. doi: 10.1016/S1473-3099(13)70002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard E.J., Xiong X., Carlier Y., Sosa-Estani S., Buekens P. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121:22–33. doi: 10.1111/1471-0528.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coura J.R., Vinas P.A. Chagas disease: a new worldwide challenge. Nature. 2010;465:S6–S7. doi: 10.1038/nature09221. [DOI] [PubMed] [Google Scholar]
- 6.Marcili A., Lima L., Valente V.C., Valente S.A., Batista J.S., Junquiera A.C.V., Souza A.I., da Rosa J.A., Campaner M., Lewis M.D., Llewellyn M.S., Miles M.A., Texeira M.M. Comparative phylogeography of Trypanosoma cruzi TCIIc: new hosts, association with terrestrial ecotopes, and spatial clustering. Infect Genet Evol. 2009;9:1264–1274. doi: 10.1016/j.meegid.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Zingales B., Miles M.A., Campbell D.A., Tibayrenc M., Macedo A.M., Teixeira M.M., Schijman A.G., Llewellyn M.S., Lages-Silva E., Machado C.R., Andrade S.G., Sturm N.R. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Lima L., Espinosa-Alvarez O., Ortiz P.A., Trejo-Varon J.A., Carranza J.C., Pinto C.M., Serrano M.G., Buck G.A., Camargo E.P., Teixeira M.M. Genetic diversity of Trypanosoma cruzi in bats, and multilocus phylogenetic and phylogeographical analyses supporting Tcbat as an independent DTU (discrete typing unit) Acta Trop. 2015;151:166–177. doi: 10.1016/j.actatropica.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Herrera C., Bargues M.D., Fajardo A., Montilla M., Triana O., Vallejo G.A., Guhl F. Identifying four Trypanosoma cruzi I isolate haplotypes from different geographic regions in Colombia. Infect Genet Evol. 2007;7:535–539. doi: 10.1016/j.meegid.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Herrera C., Guhl F., Falla A., Fajardo A., Montilla M., Adolfo Vallejo G., Bargues M.D. Genetic variability and phylogenetic relationships within Trypanosoma cruzi I isolated in Colombia based on miniexon gene sequences. J Parasitol Res. 2009;2009:897364. doi: 10.1155/2009/897364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falla A., Herrera C., Fajardo A., Montilla M., Vallejo G.A., Guhl F. Haplotype identification within Trypanosoma cruzi I in Colombian isolates from several reservoirs, vectors and humans. Acta Trop. 2009;110:15–21. doi: 10.1016/j.actatropica.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Cura C.I., Mejia-Jaramillo A.M., Duffy T., Burgos J.M., Rodriguero M., Cardinal M.V., Kjos S., Gurgel-Goncalves R., Blanchet D., De Pablos L.M., Tomasini N., da Silva A., Russomando G., Cuba C.A., Aznar C., Abate T., Levin M.J., Osuna A., Gurtler R.E., Diosque P., Solari A., Triana-Chavez O., Schijman A.G. Trypanosoma cruzi I genotypes in different geographical regions and transmission cycles based on a microsatellite motif of the intergenic spacer of spliced-leader genes. Int J Parasitol. 2010;40:1599–1607. doi: 10.1016/j.ijpara.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zingales B., Andrade S.G., Brinones M.R.S., Campbell D.A., Chiari E., Fenandes O., Guhl F., Lages-Silva E., Macedo A.M., Machado C.R., Miles M.A., Romanha A.J., Sturm N.R., Tibayrenc M., Schijman A.G. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 14.Spotorno O.A.E., Cordova L., Solari I.A. Differentiation of Trypanosoma cruzi I subgroups through characterization of cytochrome b gene sequences. Infect Genet Evol. 2008;8:898–900. doi: 10.1016/j.meegid.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Llewellyn M.S., Miles M.A., Carrasco H.J., Lewis M.D., Yeo M., Vargas J., Torrico F., Diosque P., Valente V., Valente S.A., Gaunt M.W. Genome-scale multilocus microsatellite typing of Trypanosoma cruzi discrete typing unit I reveals phylogeographic structure and specific genotypes linked to human infection. PLoS Pathog. 2009;5:e1000410. doi: 10.1371/journal.ppat.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez C.I., Ortiz S., Solari A. Colombian Trypanosoma cruzi major genotypes circulating in patients: minicircle homologies by cross-hybridization analysis. Int J Parasitol. 2010;40:1685–1692. doi: 10.1016/j.ijpara.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Perez A., Poveda C., Ramirez J.D., Norman F., Girones N., Guhl F., Monge-Maillo B., Fresno M., Lopez-Velez R. Prevalence of Trypanosoma cruzi's discrete typing units in a cohort of Latin American migrants in Spain. Acta Trop. 2016;157:145–150. doi: 10.1016/j.actatropica.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 18.del Puerto R., Nishizawa J.E., Kikuchi M., Iihoshi N., Roca Y., Avilas C., Gianella A., Lora J., Velarde F.U., Renjel L.A., Miura S., Higo H., Komiya N., Maemura K., Hirayama K. Lineage analysis of circulating Trypanosoma cruzi parasites and their association with clinical forms of Chagas disease in Bolivia. PLoS Negl Trop Dis. 2010;4:e687. doi: 10.1371/journal.pntd.0000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buekens P., Cafferata M.L., Alger J., Althabe F., Belizan J.M., Bustamante N., Carlier Y., Ciganda A., Del Cid J.H., Dumonteil E., Gamboa-Leon R., Garcia J.A., Gibbons L., Graiff O., Maldonado J.G., Herrera C., Howard E., Laea L.S., lopez B., Matute M.L., Ramirez-Sierra M.J., Robles M.C., Sosa-Estani S., Truyens C., Valladares C., Wesson D.M., Zuniga C. Congenital transmission of Trypanosoma cruzi in Argentina, Honduras, and Mexico: an observational prospective study. Am J Trop Med Hyg. 2018;98:478–485. doi: 10.4269/ajtmh.17-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ragone P.G., Perez Brandan C., Monje Rumi M., Tomasini N., Lauthier J.J., Cimino R.O., Uncos A., Ramos F., Alberti D'Amato A.M., Basombrio M.A., Diosque P. Experimental evidence of biological interactions among different isolates of Trypanosoma cruzi from the Chaco Region. PLoS One. 2015;10:e0119866. doi: 10.1371/journal.pone.0119866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cura C.I., Lucero R.H., Bisio M., Oshiro E., Formichelli L.B., Burgos J.M., Lejona S., Bruses B.L., Hernandez D.O., Severini G.V., Velazquez E., Duffy T., Anchart E., Lattes R., Altcheh J., Freilij H., Diez M., Nagel C., Vigliano C., Favaloro L., Merino D.E., Sosa-Estani S., Schijman A.G. Trypanosoma cruzi discrete typing units in Chagas disease patients from endemic and non-endemic regions of Argentina. Parasitology. 2012;139:516–521. doi: 10.1017/S0031182011002186. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez J.D., Guhl F., Rendon L.M., Rosas F., Marin-Neto J.A., Morillo C.A. Chagas cardiomyopathy manifestations and Trypanosoma cruzi genotypes circulating in chronic Chagasic patients. PLoS Negl Trop Dis. 2010;4:899. doi: 10.1371/journal.pntd.0000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgos J.M., Diez M., Vigliano C., Bisio M., Risso M., Duffy T., Cura C., Brusses B., Favaloro L., Leguizamon M.S., Lucero R.H., Laguens R., Levin M.J., Favaloro R., Schijman A.G. Molecular identification of Trypanosoma cruzi discrete typing units in end-stage chronic Chagas heart disease and reactivation after heart transplantation. Clin Infect Dis. 2010;51:485–495. doi: 10.1086/655680. [DOI] [PubMed] [Google Scholar]
- 24.Roellig D.M., Brown E.L., Barnabe C., Tibayrenc M., Streurer F.J., Yabsley M.J. Molecular typing of Trypanosoma cruzi isolates, United States. Emerg Infect Dis. 2008;14:1123–1125. doi: 10.3201/eid1407.080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos-Ligonio A., Torres-Montero J., Lopez-Monteon A., Dumonteil E. Extensive diversity of Trypanosoma cruzi discrete typing units circulating in Triatoma dimidiata from central Veracruz, Mexico. Infect Genet Evol. 2012;12:1341–1343. doi: 10.1016/j.meegid.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Ibanez-Cervantes G., Martinez-Ibarra A., Nogueda-Torres B., Lopez-Orduna E., Alonso A.L., Perea C., Maldonado T., Hernandez J.M., Leon-Avila G. Identification by Q-PCR of Trypanosoma cruzi lineage and determination of blood meal sources in triatomine gut samples in Mexico. Parasitol Int. 2013;62:36–43. doi: 10.1016/j.parint.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Herrera C.P., Licon M.H., Nation C.S., Jameson S.B., Wesson D.M. Genotype diversity of Trypanosoma cruzi in small rodents and Triatoma sanguisuga from a rural area in New Orleans, Louisiana. Parasit Vectors. 2015;8:123. doi: 10.1186/s13071-015-0730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrera C., Majeau A., Didier P., Falkenstein K.P., Dumonteil E. Trypanosoma cruzi diversity in naturally infected nonhuman primates in Louisiana assessed by deep sequencing of the mini-exon gene. Trans R Soc Trop Med Hyg. 2018;113:281–286. doi: 10.1093/trstmh/try119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villanueva-Lizama L., Teh-Poot C., Majeau A., Herrera C., Dumonteil E. Molecular genotyping of Trypanosoma cruzi by next-generation sequencing of the mini-exon gene reveals infections with multiple parasite DTUs in Chagasic patients from Yucatan, Mexico. J Infect Dis. 2019;12:1980–1988. doi: 10.1093/infdis/jiz047. [DOI] [PubMed] [Google Scholar]
- 30.Buekens P., Cafferata M.L., Alger J., Althabe F., Belizan J.M., Carlier Y., Ciganda A., Dumonteil E., Gamboa-Leon R., Howard E., Matute M.L., Sosa-Estani S., Truyens C., Wesson D., Zuniga C. Congenital transmission of Trypanosoma cruzi in Argentina, Honduras, and Mexico: study protocol. Reprod Health. 2013;10:55. doi: 10.1186/1742-4755-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virreira M., Alonso-Vega C., Solano M., Jijena J., Brutus L., Bustamante Z., Truyens C., Schneider D., Torrico F., Carlier Y., Svoboda M. Congenital Chagas disease in Bolivia is not associated with DNA polymorphism of Trypanosoma cruzi. Am J Trop Med Hyg. 2006;75:871–879. [PubMed] [Google Scholar]
- 32.Herrera C.P., Barnabe C., Breniere S.F. Complex evolutionary pathways of the intergenic region of the mini-exon gene in Trypanosoma cruzi TcI: a possible ancient origin in the Gran Chaco and lack of strict genetic structuration. Infect Genet Evol. 2013;16:27–37. doi: 10.1016/j.meegid.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 33.Zingales B. Trypanosoma cruzi genetic diversity: something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018;184:38–52. doi: 10.1016/j.actatropica.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Olmos V., Perez-Nasser N., Pinero D., Ortega E., Hernandez R., Espinoza B. Biological characterization and genetic diversity of Mexican isolates of Trypanosoma cruzi. Acta Trop. 1998;69:239–254. doi: 10.1016/s0001-706x(97)00131-9. [DOI] [PubMed] [Google Scholar]
- 35.Toledo M.J., de Lana M., Carneiro C.M., Bahia M.T., Machado-Coelho G.L., Veloso V.M., Barnabe C., Tibayrenc M., Tafuri W.L. Impact of Trypanosoma cruzi clonal evolution on its biological properties in mice. Exp Parasitol. 2002;100:161–172. doi: 10.1016/s0014-4894(02)00003-6. [DOI] [PubMed] [Google Scholar]
- 36.Hall C.A., Pierce E.M., Wimsatt A.N., Hobby-Dolbeer T., Meers J.B. Virulence and vertical transmission of two genotypically and geographically diverse isolates of Trypanosoma cruzi in mice. J Parasitol. 2010;96:371–376. doi: 10.1645/GE-2296.1. [DOI] [PubMed] [Google Scholar]
- 37.Lewis M.D., Francisco A.F., Taylor M.C., Jayawardhana S., Kelly J.M. Host and parasite genetics shape a link between Trypanosoma cruzi infection dynamics and chronic cardiomyopathy. Cell Microbiol. 2016;18:1429–1443. doi: 10.1111/cmi.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llewellyn M.S., Messenger L.A., Luquetti A.O., Garcia L., Torrico F., Tavares S.B., Cheaib B., Derome N., Delepine M., Baulard C., Deleuze J.F., Sauer S., Miles M.A. Deep sequencing of the Trypanosoma cruzi GP63 surface proteases reveals diversity and diversifying selection among chronic and congenital Chagas disease patients. PLoS Negl Trop Dis. 2015;9:e0003458. doi: 10.1371/journal.pntd.0003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eaton J.W., Rehle T.M., Jooste S., Nkambule R., Kim A.A., Mahy M., Hallett T.B. Recent HIV prevalence trends among pregnant women and all women in sub-Saharan Africa: implications for HIV estimates. AIDS. 2014;28 Suppl 4:S507–S514. doi: 10.1097/QAD.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos S.S., Cupolillo E., Junqueira A., Coura J.R., Jansen A., Sturm N.R., Campbell D.A., Fernandes O. The genetic diversity of Brazilian Trypanosoma cruzi isolates and the phylogenetic positioning of zymodeme 3, based on the internal transcribed spacer of the ribosomal gene. Ann Trop Med Parasitol. 2002;96:755–764. doi: 10.1179/000349802125002301. [DOI] [PubMed] [Google Scholar]
- 41.Bosseno M.F., Barnabe C., Magallon-Gastelum E., Lozano Kasten F., Ramsey J.M., Espinoza B., Breniere S.F. Predominance of Trypanosoma cruzi lineage I in Mexico. J Clin Microbiol. 2002;40:627–632. doi: 10.1128/JCM.40.2.627-632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodo C.L., Hamer S.A. Toward an ecological framework for assessing reservoirs of vector-borne pathogens: wildlife reservoirs of Trypanosoma cruzi across the southern United States. ILAR J. 2017;58:379–392. doi: 10.1093/ilar/ilx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia M.N., Burroughs H., Gorchakov R., Gunter S.M., Dumonteil E., Murray K.O., Herrera C.P. Molecular identification and genotyping of Trypanosoma cruzi DNA in autochthonous Chagas disease patients from Texas, USA. Infect Genet Evol. 2017;49:151–156. doi: 10.1016/j.meegid.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 44.Messenger L.A., Miles M.A., Bern C. Between a bug and a hard place: Trypanosoma cruzi genetic diversity and the clinical outcomes of Chagas disease. Expert Rev Anti Infect Ther. 2015;13:995–1029. doi: 10.1586/14787210.2015.1056158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flores-Ferrer A., Marcou O., Waleckx E., Dumonteil E., Gourbiere S. Evolutionary ecology of Chagas disease: what do we know and what do we need? Evol Appl. 2018;11:470–487. doi: 10.1111/eva.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlier Y., Truyens C. Congenital Chagas disease as an ecological model of interactions between Trypanosoma cruzi parasites, pregnant women, placenta and fetuses. Acta Trop. 2015;151:103–115. doi: 10.1016/j.actatropica.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Virreira M., Truyens C., Alonso-Vega C., Brutus L., Jijena J., Torrico F., Carlier Y., Svoboda M. Comparison of Trypanosoma cruzi lineages and levels of parasitic DNA in infected mothers and their newborns. Am J Trop Med Hyg. 2007;77:102–106. [PubMed] [Google Scholar]
- 48.Burgos J.M., Altcheh J., Bisio M., Duffy T., Valadares H.M., Seidenstein M.E., Piccinali R., Freitas J.M., Levin M.J., Macchi L., Macedo A.M., Freilij H., Schijman A.G. Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int J Parasitol. 2007;37:1319–1327. doi: 10.1016/j.ijpara.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 49.Ortiz S., Zulantay I., Solari A., Bisio M., Schijman A.G., Carlier Y., Apt W. Presence of Trypanosoma cruzi in pregnant women and typing of lineages in congenital cases. Acta Trop. 2012;124:243–246. doi: 10.1016/j.actatropica.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Cencig S., Coltel N., Truyens C., Carlier Y. Fertility, gestation outcome and parasite congenital transmissibility in mice infected with TcI, TcII and TcVI genotypes of Trypanosoma cruzi. PLoS Negl Trop Dis. 2013;7:e2271. doi: 10.1371/journal.pntd.0002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carlier Y., Truyens C. Maternal-fetal transmission of Trypanosoma cruzi. In: Tibayrenc M., Telleria J., editors. American Trypanosomiasis-Chagas Disease: One Hundred Years of Research. Elsevier; Amsterdam, the Netherlands: 2017. pp. 517–559. [Google Scholar]
- 52.Guzman-Gomez D., Lopez-Monteon A., de la Soledad Lagunes-Castro M., Alvarez-Martinez C., Hernandez-Lutzon M.J., Dumonteil E., Ramos-Ligonio A. Highly discordant serology against Trypanosoma cruzi in central Veracruz, Mexico: role of the antigen used for diagnostic. Parasit Vectors. 2015;8:466. doi: 10.1186/s13071-015-1072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]