Interferons can shape antiviral immune responses, but it is not well understood how they influence vaccine efficacy. We find that type I IFN preferentially promotes the production of antigen-specific IgG2c and IgA antibodies after infection with a live attenuated influenza virus or after immunization with influenza subunit vaccines. In contrast, type III IFN specifically enhances influenza virus-specific IgG1 and IgA production. The adjuvant effect of type I IFN was not dependent on TSLP, which is essential for the adjuvant effect of type III IFN. Type I IFN boosted vaccine-induced antibody production after immunization by the intranasal or the intraperitoneal route, whereas type III IFN exhibited its adjuvant activity only when the vaccine was delivered by the mucosal route. Our findings demonstrate that type I and type III IFNs trigger distinct pathways to enhance the efficacy of vaccines. This knowledge might be used to design more efficient vaccines against infectious diseases.
KEYWORDS: immunization, interferons, mucosal adjuvants
ABSTRACT
Type I and type III interferons (IFNs) can promote adaptive immune responses in mice and improve vaccine-induced resistance to viral infections. The adjuvant effect of type III IFN (IFN-λ) specifically boosts mucosal immunity by an indirect mechanism, involving IFN-λ-induced production of thymic stromal lymphopoietin (TSLP), a cytokine that activates immune cells. To date, it remained unclear whether the previously described adjuvant effect of type I IFN (IFN-α/β) would also depend on TSLP and whether type I IFN stimulates different antibody subtypes. Here, we show that after infection with a live attenuated influenza virus, mice lacking functional type I IFN receptors failed to produce normal amounts of virus-specific IgG2c and IgA antibodies. In contrast, mice lacking functional IFN-λ receptors contained normal levels of virus-specific IgG2c but had reduced IgG1 and IgA antibody levels. When applied together with protein antigen, IFN-α stimulated the production of antigen-specific IgA and IgG2c to a greater extent than IgG1, irrespective of whether the mice expressed functional TSLP receptors and irrespective of whether the vaccine was applied by the intranasal or the intraperitoneal route. Taken together, these results demonstrate that the adjuvant activities of type I and type III IFNs are mechanistically distinct.
IMPORTANCE Interferons can shape antiviral immune responses, but it is not well understood how they influence vaccine efficacy. We find that type I IFN preferentially promotes the production of antigen-specific IgG2c and IgA antibodies after infection with a live attenuated influenza virus or after immunization with influenza subunit vaccines. In contrast, type III IFN specifically enhances influenza virus-specific IgG1 and IgA production. The adjuvant effect of type I IFN was not dependent on TSLP, which is essential for the adjuvant effect of type III IFN. Type I IFN boosted vaccine-induced antibody production after immunization by the intranasal or the intraperitoneal route, whereas type III IFN exhibited its adjuvant activity only when the vaccine was delivered by the mucosal route. Our findings demonstrate that type I and type III IFNs trigger distinct pathways to enhance the efficacy of vaccines. This knowledge might be used to design more efficient vaccines against infectious diseases.
INTRODUCTION
Type I interferon (IFN) possesses adjuvant activity and can enhance the efficacy of experimental vaccines in mice (reviewed in reference 1). When used in combination with soluble protein antigens, type I IFN can stimulate the production of all IgG subclasses and can enhance long-lived immunological memory (2, 3). Furthermore, type I IFN can improve the performance of well-known vaccine adjuvants, such as CpG, CFA, alum, and MF59 (4). Type I IFN increases vaccine-induced antibody responses by directly stimulating dendritic cells (DCs), as well as B and T cells (2, 5–7). Type I IFN can stimulate DCs to promote the development of follicular helper T (Tfh) cells in lymph nodes (8). Type I IFN can further enhance adaptive immunity by protecting antiviral CD8 T cells from excessive killing by NK cells (9, 10). In contrast to the immune-enhancing effect during acute infections, type I IFN can also negatively affect production of antiviral antibodies during persisting viral infections by enhancing immune cell-mediated killing of virus-infected B cells (11–13). A clinical study in which type I IFN was used as mucosal adjuvant for an influenza vaccine yielded disappointing results, questioning the relevance of previous findings for the development of improved human vaccines (14).
We recently showed that type III IFN (IFN-λ) exhibits strong adjuvant activity on protein vaccines when applied to the airway mucosa of mice (15). Furthermore, wild-type (WT) mice produced higher levels of virus-specific IgG1 and IgA than IFN-λ receptor-deficient (Ifnlr1−/−) mice when infected with a live attenuated influenza virus strain (15), and infected WT mice showed stronger CD8 T cells responses than those of Ifnlr1−/− mice (15, 16). We found that IFN-λ boosts adaptive immune responses after intranasal immunization of mice by an indirect mechanism which involves production of thymic stromal lymphopoietin (TSLP) in M cells of the upper airways. TSLP, in turn, stimulates the migration of CD103+ DCs from the airways to the mediastinal lymph nodes. This results in improved germinal center (GC) reactions and enhanced production of antigen-specific IgG1 and IgA antibodies (15). It remains unclear, however, whether distinct IgG subtypes play particular roles in antibody-mediated protection of mice or humans against influenza virus-induced disease. Interestingly, IFN-λ did not exhibit adjuvant activity on protein vaccines applied by either the intraperitoneal or the subcutaneous routes (15), indicating that the adjuvant effect of IFN-λ strictly depends on its ability to trigger the synthesis of TSLP in epithelial cells. Other studies showed that IFN-λ can also enhance antibody titers against antigens encoded by a DNA vaccine (17, 18), but the mechanism of this enhancing effect has not yet been resolved (reviewed in reference 19).
The available data suggested that type I and type III IFN differ with regard to their abilities to regulate adaptive immune responses. However, no side-by-side comparisons had to date been performed. Here, we performed such immunizations and measured the levels of antigen-specific total IgG as well as the dominant IgG1 and IgG2c subtypes in the serum of mice. Furthermore, we also measured antigen-specific IgA in bronchoalveolar lavage (BAL) fluids. We demonstrate that the two IFN systems indeed exhibit distinct adjuvant activities on influenza vaccines. Whereas the adjuvant effect of type III IFN depended on TSLP signaling, the adjuvant activity of type I IFN was not mediated by TSLP. The adjuvant effect of type I IFN was not restricted to the respiratory tract, and type I IFN did not specifically boost IgG1 production.
RESULTS
Low levels of virus-specific IgG2c and IgA antibodies in Ifnar1−/− mice after infection with live attenuated influenza virus.
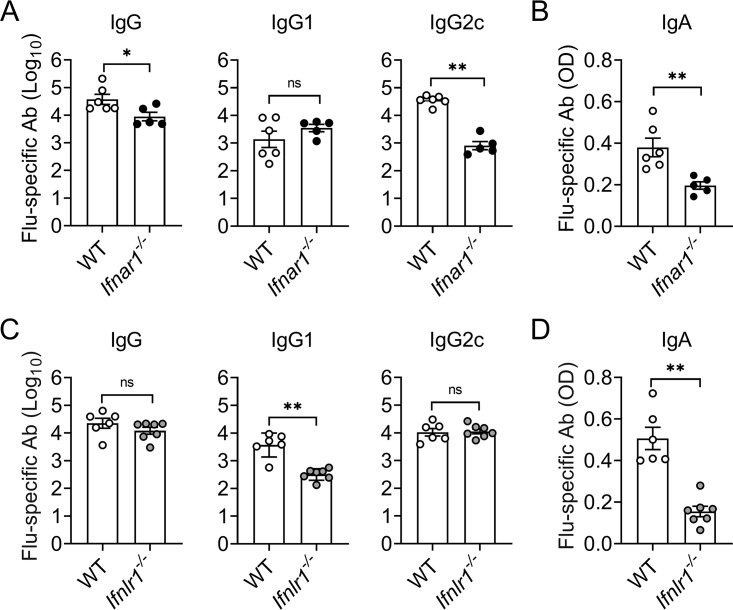
To detect possible impairments of the humoral immune response resulting from type I IFN deficiency, we infected Mx1-WT and type I IFN receptor-deficient (Mx1-Ifnar1–/–) mice with a live attenuated influenza A virus strain, designated hvPR8-ΔNS1. The hvPR8-ΔNS1 mutant virus cannot produce the NS1 virulence factor and, therefore, fails to suppress the IFN response of the host (20). Consequently, NS1-deficient influenza viruses are largely nonpathogenic in immunocompetent mice, including Mx1-Ifnar1–/–, but are pathogenic in mice lacking both functional type I and type III IFN receptors (21). Mice with functional Mx1 alleles were used for this experiment because available Ifnar1- and Ifnlr1-knockout animals have this particular genetic background (22). Mx1-Ifnar1–/– mice had about 20-fold reduced levels of influenza virus (Flu)-specific serum IgG2c compared with Mx1-WT mice at 21 days postinfection with hvPR8-ΔNS1. Total virus-specific serum IgG was also slightly reduced, whereas serum IgG1 levels were as in Mx1-WT mice (Fig. 1A). Flu-specific IgA levels in BAL fluids of Mx1-Ifnar1–/– mice at day 21 postinfection were also about 2-fold reduced compared with those of Mx1-WT mice (Fig. 1B). This picture contrasted the situation in mice lacking a functional IFN-λ system. Mx1-Ifnlr1–/– mice infected with hvPR8-ΔNS1 showed a strongly reduced ability to produce Flu-specific IgG1 but not IgG2c (Fig. 1C). In agreement with our earlier findings (15), virus-specific IgA levels in BAL fluids were about 3-fold reduced compared with Mx1-WT mice (Fig. 1D). Taken together, these results revealed strikingly different consequences of type I and type III IFN receptor deficiencies on the composition of virus-induced serum IgG antibodies.
FIG 1.
Different impacts of type I and type III IFN receptor deficiencies on antibody production after infection with live attenuated influenza virus. Mx1-WT (open symbols), Mx1-Ifnar1–/– (closed symbols), and Mx1-Ifnlr1–/– (gray symbols) mice were infected with 105 PFU of NS1-deficient influenza virus (hvPR8-△NS1) by the intranasal route. Levels of influenza virus (Flu)-specific IgG in serum (A, C) and Flu-specific IgA in BAL fluid (B, D) were determined by ELISA on day 21 postinfection. Each symbol represents an individual animal. Data are expressed as mean ± SEM. *, P < 0.05; **, P < 0.01 by Mann-Whitney test; ns = no significant difference.
IFN-α broadly boosts TSLP-independent antibody production.
To determine whether IFN-α can improve antibody responses against influenza virus subunit vaccines, we used the universal influenza vaccine candidate CTA1-3M2e-DD, designated M2e, in which the evolutionarily conserved extracellular domain of the influenza A virus M2 protein is fused to the cholera toxin A1 ADP-ribosylation enzyme (23). When applied intranasally to mice, 1 μg M2e vaccine stimulated low levels of M2e-specific serum antibodies after a single booster immunization (Fig. 2A). When the M2e vaccine was enriched with 1 μg hybrid human IFN-αB/D, serum levels of M2e-specific IgG1 and IgG2c increased about 10-fold compared with those of the plain M2e vaccine (Fig. 2A). Furthermore, M2e-specific IgA levels in the BAL fluids were significantly higher in mice receiving the IFN-α-enriched M2e vaccine compared with those of mice immunized with the plain M2e vaccine (Fig. 2B). In contrast, when mice were immunized with M2e vaccine enriched with 1 μg mouse IFN-λ2, IgG1 levels in serum and IgA levels in BAL fluids were strongly increased but IgG2c remained unaffected (Fig. 2A and B).
FIG 2.
Exogenous IFN-α stimulates IgG and IgA production after intranasal immunization with M2e fusion construct irrespective of TSLP receptor expression. (A, B) WT mice were immunized by intranasal application of 1 μg CTA1-3M2e-DD (M2e) in the presence or absence of 1 μg IFN-αB/D or 1 μg IFN-λ2. Ten days after a single booster immunization, blood (A) and BAL fluid (B) was analyzed for M2e-specific IgG and IgA by ELISA. Each symbol represents an individual animal. Data are expressed as mean ± SEM. **, P < 0.01 by Mann-Whitney test. (C, D) WT and Tslpr−/− mice were immunized by intranasal application of 1 μg CTA1-3M2e-DD (M2e) in the presence or absence of 1 μg IFN-αB/D. Ten days after booster immunization, blood (C) and BAL fluids (D) were analyzed for M2e-specific IgG and IgA by ELISA. Each symbol represents an individual animal. Data are expressed as mean ± SEM. **, P < 0.01 by Mann-Whitney test; ns = no significant difference.
Previous work demonstrated that the adjuvant effect of IFN-λ is strictly dependent on TSLP (15). To determine whether the immune-stimulating effect of IFN-αB/D similarly depends on TSLP, we immunized groups of WT and Tslpr−/− mice by the intranasal route with M2e vaccine containing or lacking human IFN-αB/D and measured antibody levels in the serum and in BAL fluids after a single booster immunization. IFN-αB/D exhibited strong adjuvant activity and boosted serum IgG1 and IgG2c levels (Fig. 2C) as well as IgA levels in BAL (Fig. 2D) irrespective of whether the immunized mice possessed functional TSLP receptors. Taken together, these results revealed a striking mechanistic difference between the adjuvant activities of type I and type III IFNs.
IFN-α enhances vaccine-induced serum antibody levels after intraperitoneal immunization.
IFN-λ does not boost antibody production if vaccines are applied by either the intraperitoneal or subcutaneous routes (15). Therefore, to better describe the adjuvant activity of type I IFN, we first studied whether IFN-αB/D can boost antigen-specific antibody production when the vaccine is applied via the intraperitoneal route. After a single booster immunization, M2e-specific serum IgG2c levels were at least 30-fold higher when the M2e vaccine was enriched with IFN-αB/D compared with plain M2e vaccine (Fig. 3A). As expected, the levels of total M2e-specific IgG were also significantly increased in mice immunized with the IFN-αB/D-adjuvanted M2e vaccine. In contrast, levels of M2e-specific IgG1 were not enhanced significantly (Fig. 3A).
FIG 3.
IFN-α enhances antibody production after intraperitoneal immunization with influenza subunit vaccines. (A) Mx1-WT mice were immunized by the intraperitoneal route with 1 μg CTA3M2e-DD (M2e) in the presence or absence of 1 μg IFN-αB/D. Ten days later, a single booster immunization was performed. M2e-specific IgG serum levels were determined by ELISA 10 days after the booster immunization. Each symbol represents an individual animal. Data are expressed as mean ± SEM. **, P < 0.01 by Mann-Whitney test. (B) WT mice were immunized by the intraperitoneal route with Influsplit Tetra vaccine (HA) in the presence or absence of 1 μg IFN-αB/D. Booster immunizations were performed 10 and 20 days later. HA-specific IgG serum levels were measured 10 days after the first and 10 days after the second booster immunization. Each symbol represents an individual animal. Data are expressed as mean ± SEM. *, P < 0.05; ***, P < 0.001 by two-way ANOVA with Tukey’s multiple-comparisons test; ns = no significant difference.
Next, we investigated whether IFN-αB/D has a similar stimulating effect on a commercial tetravalent influenza subunit vaccine, Influsplit Tetra, which contains hemagglutinin (HA) of two different influenza A viruses and two different influenza B viruses as its principal component. When 4 μg Influsplit Tetra (1 μg of each HA subtype present in the vaccine formulation) was applied intraperitoneally to mice, Flu-specific antibody responses remained initially low and reached high levels only if a second booster immunization was performed. Under these experimental conditions, the IFN-αB/D-adjuvanted vaccine induced about 20-fold higher levels of Flu-specific IgG2c than those of the nonadjuvanted vaccine, but it did not significantly enhance the levels of Flu-specific IgG1 (Fig. 3B). These results clearly showed that the adjuvant activity of type I IFN is not restricted to the respiratory tract mucosa.
Type I IFN enhances subunit vaccine-induced resistance of mice against influenza virus.
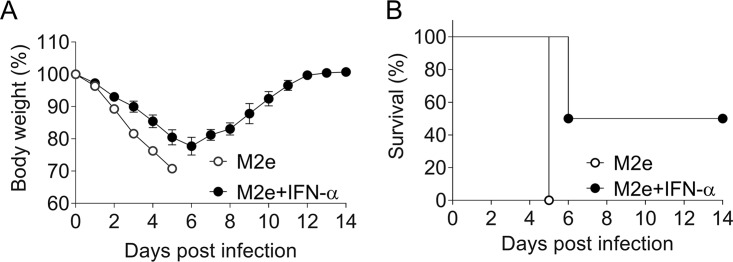
To evaluate the physiological significance of the above findings, we determined whether the 30-fold increased titers of specific serum IgG2c in Mx1-WT mice immunized with IFN-αB/D-adjuvanted M2e vaccine via the intraperitoneal route (Fig. 3A) would translate into enhanced protection against a lethal influenza virus challenge infection. Although M2e-specific antibodies are unable to neutralize the virus, they can confer some degree of influenza virus resistance by an Fcγ receptor-dependent mechanism (24). Seven weeks after the booster immunization, the immunized animals were infected with an approximately 20-fold lethal dose (103 PFU) of a highly virulent influenza A virus variant (hvPR8), and virus-induced weight loss was monitored. All mice immunized with the nonadjuvanted M2e vaccine lost weight very quickly (Fig. 4A) and they had to be euthanized on day 5 postinfection (Fig. 4B). In contrast, mice immunized with the IFN-αB/D-adjuvanted M2e vaccine lost weight less quickly (Fig. 4A), and 50% of these animals survived the challenge infection (Fig. 4B). These results demonstrated that type I IFN can improve the protective activity of an experimental influenza vaccine.
FIG 4.
Intraperitoneal immunization with M2e vaccine in the presence of IFN-α confers enhanced protection against influenza virus challenge. Mx1-WT mice (n = 6 per group) were immunized by intraperitoneal application of 1 μg CTA1-3M2e-DD (M2e) in the presence or absence of 1 μg IFN-αB/D. A single booster immunization was performed 10 days later. Seven weeks after booster immunization, all animals were infected with 103 PFU of a highly virulent influenza virus strain (hvPR8). Weight loss (A) and survival (B) were monitored. Animals were sacrificed when body weight reached the 75% limit.
DISCUSSION
Our work revealed interesting differences between type I and type III IFN with regard to their abilities to stimulate vaccine-induced production of antibodies in mice. We found that the adjuvant effect of type I IFN was not dependent on the route of vaccine administration and did not depend on the ability of the host to respond to TSLP. Furthermore, type I IFN preferentially boosted the production of antigen-specific IgG2c, whereas type III IFN mainly boosted the production of IgG1. Both IFN types boosted the production of IgA similarly well when the vaccine was applied by the intranasal route.
Ifnar1−/− mice infected with a live attenuated influenza virus produced only low levels of virus-specific IgG2c and IgA but had normal serum levels of specific IgG1 antibodies. Antibody production in WT mice was similarly skewed toward preferential production of IgG2c when a type I IFN-enriched protein vaccine was used for either intranasal or intraperitoneal immunizations. These findings are in good agreement with earlier studies that described the adjuvant effect of type I IFN for the first time (2–4). Type I IFN was reported to confer its broad vaccine adjuvant activity by acting directly on DCs as well as B and T cells (2, 5, 6). In agreement with this view, we found that the type I IFN-mediated enhancement of antibody production was independent of the route of vaccine application. Importantly, our experiments not only demonstrated that type I IFN-enriched protein vaccines induce higher serum antibody titers than nonadjuvanted vaccines but further showed that type I IFN-adjuvanted vaccines can also confer enhanced protection against influenza virus-induced disease. In stark contrast, our previous work with type III IFN demonstrated that IFN-λ exhibits adjuvant activity only if the enriched vaccine was applied via the mucosal route (15). Furthermore, Ifnlr1−/− mice failed to produce normal levels of virus-specific IgG1 and IgA in response to infection with a live attenuated influenza virus but contained normal levels of virus-specific IgG2c, in agreement with earlier findings (15). The strong IgG1 bias of the IFN-λ-mediated adjuvant effect can be explained by the fact that IFN-λ cannot act directly on the majority of immune cells in mice (25; reviewed in reference 19). IFN-λ rather triggers M cells in the upper respiratory tract to produce TSLP (15) which, in turn, primes migratory DCs to trigger the germinal center reaction, resulting in an IgG1-skewed immune response (26). Of note, TSLP is able to mimic the adjuvant effect of IFN-λ (15), and TSLP can shift antigen-specific IgG synthesis toward IgG1 by inducing Th2-cell immune responses (26). Moreover, TSLP can activate DCs to express OX40 ligand; this influences Tfh cell differentiation and programs B cells to preferentially produce IgG1 (27). Thus, the intrinsic biological properties of TSLP appear to determine the unique adjuvant effect of IFN-λ. Since the type I IFN-mediated adjuvant effect did not depend on TSLP and since type I IFN can act on all hematopoietic cells, we had expected to observe distinct IgG responses when type I IFN is used as vaccine adjuvant. We suspect that the observed difference between type I and type III IFN may be attributed to more than one mechanism. First, type I IFN could trigger interleukin-6 (IL-6) production by DCs which stimulates CXCR5+ Tfh cells to switch the immune response toward preferential production of IgG2a (8). Second, type I IFN could strengthen isotype class switching by enhancing IFN-γ release and promoting Th1 responses (7, 28). Third, type I IFN could act directly on B cells (5).
Taken together, our study highlights some unique characteristics of type I and type III IFNs as vaccine adjuvants which should be considered when using these cytokines to improve existing vaccines against diseases in livestock or humans. Unfortunately, however, it is still unclear whether IFNs exhibit similar adjuvant activities in nonrodent species.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice were purchased from Janvier Laboratories and used at 8 to 10 weeks of age. Mx1-WT, Mx1-Ifnar1–/–, Mx1-Ifnlr1–/–, and Tslpr–/– mice with C57BL/6 genetic backgrounds have been described previously (15, 22) and were bred at the local animal facility under specific-pathogen-free conditions. All experiments followed the guidelines of the Federation for Laboratory Animal Science Associations and were approved by the animal welfare committee of the Regierungspräsidium Freiburg (permits G-16/177 and G-17/74).
Infection with live attenuated virus.
Mx1-WT, Mx1-Ifnar1–/–, and Mx1-Ifnlr1–/– mice were infected intranasally with 105 PFU of live attenuated influenza virus strain hvPR8-ΔNS1 (21). Twenty-one days after infection, blood and BAL fluids were collected as previously described (15). Subsequently, samples were used for measuring Flu-specific IgG subtypes in serum and Flu-specific IgA levels in BAL by enzyme-linked immunosorbent assay (ELISA).
Influenza subunit vaccine immunizations.
Mx1-WT and Tslpr–/– mice were anesthetized with a ketamine/xylazine mixture and immunized intranasally with 30 μl of 1 μg CTA1-3M2e-DD (M2e) (23) vaccine alone, 1 μg M2e vaccine plus 1 μg IFN-αB/D (29), or 1 μg M2e vaccine plus 1 μg IFN-λ2 (15, 30) on days 0 and 10. In some experiments, mice were immunized by the intraperitoneal routes with 1 μg M2e in the presence or absence of 1 μg IFN-αB/D in a total volume of 200 μl. Mice were sacrificed at day 10 after booster immunization to determine M2e-specific IgG subclass levels. Titers of M2e-specific IgA were determined by ELISA at day 10 post booster immunizations.
WT mice were immunized intraperitoneally with 4 μg Influsplit Tetra vaccine (GSK) (containing 1 μg HA of each influenza virus subtype) in the presence or absence of 1 μg IFN-αB/D in a total volume of 200 μl. Booster immunizations were performed 10 and 20 days later. Blood samples were collected at day 10 after the first and second booster immunization for analysis of Flu-specific IgG titers.
Influenza virus challenge.
Mx1-WT mice were immunized with 1 μg CTA1-3M2e-DD (M2e) vaccine in the presence or absence of 1 μg IFN-αB/D by the intraperitoneal route. Booster immunizations were performed 10 days later. Seven weeks after the booster immunization, mice were challenged intranasally with 103 PFU of the highly virulent influenza virus strain PR8 (hvPR8) (31). Weight loss was monitored during the following 14 days. Mice were sacrificed when they lost 25% of their original body weight.
ELISA.
Briefly, for measuring M2e-specific and Flu-specific IgG subclasses and IgA, serially diluted serum or BAL fluids were added in microtiter plates (MaxiSorp; Nunc) coated with M2e peptide (2 μg/ml; GenScript) or inactivated PR8 virus at 4°C overnight as previously described (15). Horseradish peroxidase-labeled antibodies directed against either total IgG (catalog number 62-6520; Invitrogen), IgG1 (catalog number A10551; Invitrogen), IgG2c (catalog number 1078-05; Southern Biotech), or IgA (catalog number 62-6720; Invitrogen) were used to measure antibody subtypes. After incubation, the reaction was developed with tetramethylbenzidine (Life Technologies) and stopped with 0.5 M H2SO4. Products were quantified by measuring the optical density (OD) at 450 nm. Antibody titers were defined as the highest serum dilutions resulting in OD values that were 2-fold (Flu-specific antibody) or 4-fold (M2e-specific antibody) above background. Since IgA titers in BAL fluids were relatively low, undiluted samples were used for ELISA, and the OD readings were plotted directly.
Statistical analysis.
Statistics were done using Mann-Whitney tests or two-way analysis of variance (ANOVA) followed by Tukey multiple-comparison tests. Experimental results are expressed as means ± standard errors of the means (SEM). Statistical analyses were performed using GraphPad Prism 8 software.
ACKNOWLEDGMENTS
We thank Otto Haller for helpful comments.
This work was supported by the Deutsche Forschungsgemeinschaft grant agreement STA 338/15-1 to P.S. and the Danish Council for Independent Research, Medical Research grant agreement 11-107588 to R.H.
REFERENCES
- 1.Arico E, Belardelli F. 2012. Interferon-alpha as antiviral and antitumor vaccine adjuvants: mechanisms of action and response signature. J Interferon Cytokine Res 32:235–247. doi: 10.1089/jir.2011.0077. [DOI] [PubMed] [Google Scholar]
- 2.Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14:461–470. doi: 10.1016/S1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 3.Bracci L, Canini I, Puzelli S, Sestili P, Venditti M, Spada M, Donatelli I, Belardelli F, Proietti E. 2005. Type I IFN is a powerful mucosal adjuvant for a selective intranasal vaccination against influenza virus in mice and affects antigen capture at mucosal level. Vaccine 23:2994–3004. doi: 10.1016/j.vaccine.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Proietti E, Bracci L, Puzelli S, Di Pucchio T, Sestili P, De Vincenzi E, Venditti M, Capone I, Seif I, De Maeyer E, Tough D, Donatelli I, Belardelli F. 2002. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J Immunol 169:375–383. doi: 10.4049/jimmunol.169.1.375. [DOI] [PubMed] [Google Scholar]
- 5.Le Bon A, Thompson C, Kamphuis E, Durand V, Rossmann C, Kalinke U, Tough DF. 2006. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol 176:2074–2078. doi: 10.4049/jimmunol.176.4.2074. [DOI] [PubMed] [Google Scholar]
- 6.Coro ES, Chang WL, Baumgarth N. 2006. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol 176:4343–4351. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- 7.Swanson CL, Wilson TJ, Strauch P, Colonna M, Pelanda R, Torres RM. 2010. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J Exp Med 207:1485–1500. doi: 10.1084/jem.20092695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cucak H, Yrlid U, Reizis B, Kalinke U, Johansson-Lindbom B. 2009. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity 31:491–501. doi: 10.1016/j.immuni.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Xu HC, Grusdat M, Pandyra AA, Polz R, Huang J, Sharma P, Deenen R, Kohrer K, Rahbar R, Diefenbach A, Gibbert K, Lohning M, Hocker L, Waibler Z, Haussinger D, Mak TW, Ohashi PS, Lang KS, Lang PA. 2014. Type I interferon protects antiviral CD8+ T cells from NK cell cytotoxicity. Immunity 40:949–960. doi: 10.1016/j.immuni.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Crouse J, Bedenikovic G, Wiesel M, Ibberson M, Xenarios I, Von Laer D, Kalinke U, Vivier E, Jonjic S, Oxenius A. 2014. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity 40:961–973. doi: 10.1016/j.immuni.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Moseman EA, Wu T, de la Torre JC, Schwartzberg PL, McGavern DB. 2016. Type I interferon suppresses virus-specific B cell responses by modulating CD8+ T cell differentiation. Sci Immunol 1:eaah3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sammicheli S, Kuka M, Di Lucia P, de Oya NJ, De Giovanni M, Fioravanti J, Cristofani C, Maganuco CG, Fallet B, Ganzer L, Sironi L, Mainetti M, Ostuni R, Larimore K, Greenberg PD, de la Torre JC, Guidotti LG, Iannacone M. 2016. Inflammatory monocytes hinder antiviral B cell responses. Sci Immunol 1:eaah6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fallet B, Narr K, Ertuna YI, Remy M, Sommerstein R, Cornille K, Kreutzfeldt M, Page N, Zimmer G, Geier F, Straub T, Pircher H, Larimore K, Greenberg PD, Merkler D, Pinschewer DD. 2016. Interferon-driven deletion of antiviral B cells at the onset of chronic infection. Sci Immunol 1:eaah6817 https://immunology.sciencemag.org/content/1/4/eaah6817.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couch RB, Atmar RL, Cate TR, Quarles JM, Keitel WA, Arden NH, Wells J, Nino D, Wyde PR. 2009. Contrasting effects of type I interferon as a mucosal adjuvant for influenza vaccine in mice and humans. Vaccine 27:5344–5348. doi: 10.1016/j.vaccine.2009.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye L, Schnepf D, Becker J, Ebert K, Tanriver Y, Bernasconi V, Gad HH, Hartmann R, Lycke N, Staeheli P. 2019. Interferon-lambda enhances adaptive mucosal immunity by boosting release of thymic stromal lymphopoietin. Nat Immunol 20:593–601. doi: 10.1038/s41590-019-0345-x. [DOI] [PubMed] [Google Scholar]
- 16.Hemann EA, Green R, Turnbull JB, Langlois RA, Savan R, Gale M Jr. 2019. Interferon-lambda modulates dendritic cells to facilitate T cell immunity during infection with influenza A virus. Nat Immunol 20:1035–1045. doi: 10.1038/s41590-019-0408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrow MP, Pankhong P, Laddy DJ, Schoenly KA, Yan J, Cisper N, Weiner DB. 2009. Comparative ability of IL-12 and IL-28B to regulate Treg populations and enhance adaptive cellular immunity. Blood 113:5868–5877. doi: 10.1182/blood-2008-11-190520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Wang Z, Xu Y, Zhang Z, Hua R, Liu W, Jiang C, Chen Y, Yang W, Kong W. 2017. Optimized DNA vaccine enhanced by adjuvant IL28B induces protective immune responses against herpes simplex virus type 2 in mice. Viral Immunol 30:601–614. doi: 10.1089/vim.2017.0033. [DOI] [PubMed] [Google Scholar]
- 19.Ye L, Schnepf D, Staeheli P. 14 June 2019. Interferon-lambda orchestrates innate and adaptive mucosal immune responses. Nat Rev Immunol doi: 10.1038/s41577-019-0182-z. [DOI] [PubMed] [Google Scholar]
- 20.Ferko B, Stasakova J, Romanova J, Kittel C, Sereinig S, Katinger H, Egorov A. 2004. Immunogenicity and protection efficacy of replication-deficient influenza A viruses with altered NS1 genes. J Virol 78:13037–13045. doi: 10.1128/JVI.78.23.13037-13045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochs G, Martinez-Sobrido L, Lienenklaus S, Weiss S, Garcia-Sastre A, Staeheli P. 2009. Strong interferon-inducing capacity of a highly virulent variant of influenza A virus strain PR8 with deletions in the NS1 gene. J Gen Virol 90:2990–2994. doi: 10.1099/vir.0.015727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klinkhammer J, Schnepf D, Ye L, Schwaderlapp M, Gad HH, Hartmann R, Garcin D, Mahlakoiv T, Staeheli P. 2018. IFN-lambda prevents influenza virus spread from the upper airways to the lungs and limits virus transmission. Elife 7:e33354. doi: 10.7554/eLife.33354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eliasson DG, El Bakkouri K, Schon K, Ramne A, Festjens E, Lowenadler B, Fiers W, Saelens X, Lycke N. 2008. CTA1-M2e-DD: a novel mucosal adjuvant targeted influenza vaccine. Vaccine 26:1243–1252. doi: 10.1016/j.vaccine.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 24.Van den Hoecke S, Ehrhardt K, Kolpe A, El Bakkouri K, Deng L, Grootaert H, Schoonooghe S, Smet A, Bentahir M, Roose K, Schotsaert M, Schepens B, Callewaert N, Nimmerjahn F, Staeheli P, Hengel H, Saelens X. 2017. Hierarchical and redundant roles of activating FcγRs in protection against influenza disease by M2e-specific IgG1 and IgG2a antibodies. J Virol 91:e02500-16. doi: 10.1128/JVI.02500-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sommereyns C, Paul S, Staeheli P, Michiels T. 2008. IFN-lambda (IFN-λ) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog 4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Roey GA, Arias MA, Tregoning JS, Rowe G, Shattock RJ. 2012. Thymic stromal lymphopoietin (TSLP) acts as a potent mucosal adjuvant for HIV-1 gp140 vaccination in mice. Eur J Immunol 42:353–363. doi: 10.1002/eji.201141787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pattarini L, Trichot C, Bogiatzi S, Grandclaudon M, Meller S, Keuylian Z, Durand M, Volpe E, Madonna S, Cavani A, Chiricozzi A, Romanelli M, Hori T, Hovnanian A, Homey B, Soumelis V. 2017. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J Exp Med 214:1529–1546. doi: 10.1084/jem.20150402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. 2005. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol 23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 29.Horisberger MA, de Staritzky K. 1987. A recombinant human interferon-alpha B/D hybrid with a broad host-range. J Gen Virol 68:945–948. doi: 10.1099/0022-1317-68-3-945. [DOI] [PubMed] [Google Scholar]
- 30.Dellgren C, Gad HH, Hamming OJ, Melchjorsen J, Hartmann R. 2009. Human interferon-lambda3 is a potent member of the type III interferon family. Genes Immun 10:125–131. doi: 10.1038/gene.2008.87. [DOI] [PubMed] [Google Scholar]
- 31.Grimm D, Staeheli P, Hufbauer M, Koerner I, Martinez-Sobrido L, Solorzano A, Garcia-Sastre A, Haller O, Kochs G. 2007. Replication fitness determines high virulence of influenza A virus in mice carrying functional Mx1 resistance gene. Proc Natl Acad Sci U S A 104:6806–6811. doi: 10.1073/pnas.0701849104. [DOI] [PMC free article] [PubMed] [Google Scholar]