Abstract
Background:
A benchmark of near-perfect adherence (≥95%) to antiretroviral therapy (ART) is often cited as necessary for HIV viral suppression. However, given newer, more effective ART medications, the threshold for viral suppression may be lower. We estimated the minimum ART adherence level necessary to achieve viral suppression.
Settings:
The Patient-centered HIV Care Model demonstration project.
Methods:
Adherence to ART was calculated using the proportion of days covered measure for the 365-day period before each viral load test result, and grouped into 5 categories (<50%, 50% to <80%, 80% to <85%, 85% to <90%, and ≥90%). Binomial regression analyses were conducted to determine factors associated with viral suppression (HIV RNA <200 copies/mL); demographics, proportion of days covered category, and ART regimen type were explanatory variables. Generalized estimating equations with an exchangeable working correlation matrix accounted for correlation within subjects. In addition, probit regression models were used to estimate adherence levels required to achieve viral suppression in 90% of HIV viral load tests.
Results:
The adjusted odds of viral suppression did not differ between persons with an adherence level of 80% to <85% or 85% to <90% and those with an adherence level of ≥90%. In addition, the overall estimated adherence level necessary to achieve viral suppression in 90% of viral load tests was 82% and varied by regimen type; integrase inhibitor- and nonnucleoside reverse transcriptase inhibitor-based regimens achieved 90% viral suppression with adherence levels of 75% and 78%, respectively.
Conclusions:
The ART adherence level necessary to reach HIV viral suppression may be lower than previously thought and may be regimen-dependent.
Keywords: HIV, antiretroviral therapy, viral suppression, sustained virologic response, medication adherence
INTRODUCTION
The ultimate goal of HIV care and treatment is to improve the duration and quality of life of persons with HIV. These goals are met through suppression of HIV RNA and restoration of immune function, which in turn decrease morbidity and mortality.1–4 Viral suppression has population level benefits as well, in that persons who are virally suppressed have effectively no risk of sexually transmitting HIV to uninfected partners.5 To become and remain virologically suppressed, persons with HIV must be adherent to appropriate antiretroviral therapy (ART) throughout their lifetime. Although adherence is important to the outcomes of therapy, patients face multiple barriers to consistent adherence including lack of access, treatment fatigue, stigma, and comorbid conditions.6,7
Given patients’ adherence barriers, potential history of nonadherence to therapy, and lifelong need for treatment, having a better understanding of what ART adherence level is necessary for viral suppression is valuable for clinicians when determining optimal antiretroviral (ARV) regimens for patients. Researchers have frequently set a benchmark for adherence at ≥95%. This threshold is mostly derived from a study conducted between 1997 and 1999, of persons on unboosted protease inhibitor therapy, which found that persons with adherence levels ≥95% (measured using a microelectronic monitoring system) had less virologic failure and increased CD4 lymphocyte count when compared with persons with adherence <95%.8 Other early studies showed similar results.9,10 Although 95% has long been considered the gold standard of adherence to ART, a lower adherence level of ≥90% has been set by the Pharmacy Quality Alliance.11 Given the enhanced pharmacokinetic profiles of newer ARV medications, even the lower adherence level of ≥90% may not be necessary to achieve HIV viral suppression.12 As such, we used data from a national demonstration project to estimate the minimum adherence level required for HIV viral suppression.
METHODS
Project Design and Participants
Data used for this analysis are from the Patient-centered HIV Care Model (PCHCM) demonstration project. This was a secondary analysis; the results are unrelated to the goals of the demonstration project. The PCHCM is described in detail elsewhere.13 In short, the PCHCM project partnered community-based HIV-specialty pharmacists with HIV medical providers and required the partnered pharmacists and medical providers to share patient clinical information, identify therapy-related problems, and develop therapy-related action plans. The project provided services to 765 adults with HIV at 10 project sites (made up of ≥1 medical clinic and ≥1 HIV-specialty retail pharmacy) throughout the United States from August 2014 to September 2016. Data collected for the PCHCM included, but were not limited to, prescription fulfillment data, HIV viral load test results, and participant demographics. Between 12 and 48 months of data were collected on each participant.
Independent Variable—Adherence to ART
Adherence to ART was calculated as the proportion of days covered (PDC). The PDC is a pharmacy claims-based metric that reflects the proportion of days for which a person has medication available during a measurement period. The ART PDC is calculated by dividing the number of days of ART coverage during the measurement period by the length of the measurement period; adjustments are made for fill days’ supply and for days with overlapping medication supply.14 ART coverage was defined as at least 3 ARV medication components (excluding cobicistat). Data were extracted from PCHCM pharmacy fulfillment records.
Adherence to ART was calculated for the 365-day period before each viral load test result, and was grouped into one of 5 PDC categories (<50%, 50% to <80%, 80% to <85%, 85% to <90%, and ≥90%). Adherence was calculated overall and by ARV regiment type. ARV regimens were grouped into 4 categories: (1) integrase inhibitor (INSTI)-based, (2) nonnucleoside reverse transcriptase inhibitor (NNRTI)-based, (3) protease inhibitor (PI)-based (included both boosted and nonboosted regimens), and (4) “all other types” (all regimens not categorized as an INSTI-, NNRTI- or PI-based (eg, INSTI-two-NRTI-PI). The INSTI-, NNRTI-, and PI-based regimens each included one medication of the main component (eg, one INSTI medication) and 2 nucleoside reverse transcriptase inhibitors (NRTI) as the “backbone” of the therapy. Because a person could be on more than one regimen during the 365-day adherence measurement period, a primary regimen type was identified as the regimen that was used for the plurality of the measurement period.
Outcome Variable—HIV Viral Suppression
The dependent variable for the analysis was a dichotomous indicator of HIV viral suppression, defined as an HIV viral load of <200 HIV RNA copies/mL.15 The suppression value of <200 copies/mL was based on the U.S. Department of Health and Human Services recommended definition of virologic failure.1 Each viral load test result was included in the analysis if the test had a corresponding 365 days of ART fulfillment data before the test result (so that each viral load would have a corresponding PDC value). Each viral load test result with a corresponding PDC value was included in the analysis even when multiple viral load test results for the same participant had overlapping adherence windows (ie, a person had more than one viral load test within a 365-day adherence measurement period). Laboratory test result data were abstracted from project clinic records.
Statistical Analysis
Bivariate and multivariable binomial regression analyses were used to determine factors associated with viral suppression. Generalized estimating equations with an exchangeable working correlation matrix were used to account for correlation within subjects. Odds ratios with 95% confidence intervals (CI) were calculated, separately, for each demographic factor (age, sex, race/ethnicity, and insurance type), categorical PDC level, and ARV regimen type. All demographic variables were considered for inclusion in the final multivariable model. The final model was determined using forward stepwise variable selection, and included all factors that were significant at the 0.05 level. In addition, probit regression models were used to create dose-response curves and to estimate the adherence level required to achieve viral suppression in 90% of HIV viral load tests, overall and by regimen category. All data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Of the 765 participants, 570 had ≥1 HIV viral load test result with a corresponding PDC value and were, therefore, eligible for the analysis. More than half of these individuals were aged ≥50 years (53%), non-black or of unknown race/ ethnicity (59%), men (78%), and nonprivately insured (85%) (Table 1). Each person’s record contributed a median of 4 viral load test results (interquartile range [IQR]: 2–6) to the analysis. More than half (67%) of the 2427 viral load test results, included in the analysis, coincided with a PDC ≥90% in the previous 365 days and most (90%) were <200 copies/mL. The primary regimen types were: INSTI-based (31%), “all other” (30%), NNRTI-based (21%), and PI-based (18%) (Table 1).
TABLE 1.
Characteristics of Patients and Viral Load Tests Included in the Analysis, Patient-Centered HIV Care Model, 2014–2016, United States
| Characteristic | Total |
|---|---|
| Patient characteristics, n = 570 | |
| Median age (years [IQR]) | 49 (40–57) |
| Median no. of viral load tests (IQR) | 4 (2–6) |
| Characteristic, n (%) | |
| Age (yrs) | |
| <50 | 268 (47) |
| ≥50 | 302 (53) |
| Race/Ethnicity | |
| Black, non-Hispanic | 231 (41) |
| All other/unknown | 339 (59) |
| Sex | |
| Male | 444 (78) |
| Female | 126 (22) |
| Medical insurance | |
| Private insurance | 83 (15) |
| Nonprivate insurance | 487 (85) |
| Viral load test characteristics, n = 2427* | |
| PDC category, n (%) | |
| <50% | 202 (8) |
| 50% to <80% | 277 (11) |
| 80% to <85% | 162 (7) |
| 85% to <90% | 173 (7) |
| ≥90% | 1613 (67) |
| Viral load test results, n (%) | |
| <200 copies/mL | 2180 (90) |
| ≥200 copies/mL | 247 (10) |
| ARV regimen category, n (%)† | |
| INSTI-based | 756 (31) |
| NNRTI-based | 513 (21) |
| PI-Based | 427 (18) |
| All other‡ | 731 (30) |
| INSTI-NRTI(2)-PI§ | 122 (17) |
| INSTI -NNRTI-PI§ | 79 (11) |
| INSTI -PI§ | 65 (9) |
| INSTI -NNRTI-NRTI(2)§ | 53 (7) |
| NRTI(2)-PI(2)§ | 43 (6) |
| NNRTI-NRTI(2)-PI§ | 41 (6) |
| INSTI -NNRTI-NRTI§ | 40 (6) |
Viral load tests with PDC values and included in the analysis, including the coinciding PDC category, viral load test value, and the coinciding primary ARV regimen.
INSTI-, NNRTI-, and PI-based regimens each included one medication of the main component and 2 nucleoside reverse transcriptase inhibitors (NRTI) as the “backbone” of the therapy.
“All other” category includes all regimens not categorized as an INSTI-, NNRTI-, or PI-based regimen.
The most common regimens within the “all other” category. The parentheses indicate the number of medication types within the regimen.
Bivariate and Multivariable Binomial Regression
Results from the bivariate and multivariable analyses are shown in Table 2. After adjusting for all covariates and within person correlations, persons aged ≥50 years (adjusted odds ratio [aOR] 2.33; 95% CI: 1.70 to 3.21), men (aOR 1.49; 1.07, 2.08), and privately insured persons (aOR 1.77; 1.04, 3.00) had greater odds of being virally suppressed compared with persons aged <50 years, women, and nonprivately insured persons, respectively. Non-Hispanic black persons had lower odds of suppression (aOR 0.46; 0.33, 0.64) compared with all other races and ethnicities combined (including persons with unknown race/ethnicity).
TABLE 2.
Factors Associated With HIV Viral Suppression (HIV RNA Levels <200 Copies/mL), Patient-centered HIV Care Model, 2014–2016, United States
| HIV RNA <200 copies/mL (n
= 2427) |
||||
|---|---|---|---|---|
| Bivariate OR (95% CI) | P | Multivariable aOR (95% CI) | P | |
| Age ≥50 yrs* | 3.16 (2.09 to 4.78) | <0.0001 | 2.33 (1.70 to 3.21) | <0.0001 |
| Black, non-Hispanic† | 0.30 (0.20 to 0.44) | <0.0001 | 0.46 (0.33 to 0.64) | <0.0001 |
| Males | 2.51 (1.64 to 3.84) | <0.0001 | 1.49 (1.07 to 2.08) | 0.0174 |
| Private insurance‡ | 2.08 (1.05 to 4.15) | 0.0364 | 1.77 (1.04 to 3.00) | 0.0344 |
| PDC level | ||||
| <50% | 0.16 (0.10 to 0.25) | <0.0001 | 0.16 (0.11 to 0.23) | <0.0001 |
| 50% to <80% | 0.29 (0.20 to 0.43) | <0.0001 | 0.30 (0.19 to 0.47) | <0.0001 |
| 80% to <85% | 0.50 (0.32 to 0.76) | 0.0013 | 0.49 (0.23 to 1.04) | 0.0627 |
| 85% to <90% | 0.59 (0.37 to 0.93) | 0.0228 | 0.96 (0.49 to 1.90) | 0.9138 |
| ≥90% | REF | — | REF | — |
| ARV regimen category§ | ||||
| INSTI-based | 1.55 (0.94 to 2.54) | 0.0832 | 1.38 (0.90 to 2.13) | 0.1391 |
| NNRTI-based | 1.52 (0.88 to 2.65) | 0.1367 | 1.21 (0.75 to 1.94) | 0.4342 |
| PI-Based | REF | — | REF | — |
| All others | 0.79 (0.48 to 1.31) | 0.3598 | 0.51 (0.34 to 0.77) | 0.0013 |
Persons aged ≥50 years were compared with persons aged <50 years.
Black, non-Hispanic persons were compared with persons of all other race/ethnicities and persons with unknown race/ethnicity combined.
Persons with private insurance were compared with persons without private insurance.
INSTI-, NNRTI-, and PI-based regimens each included one medication of the main component and 2 nucleoside reverse transcriptase (NRTI) drugs as the “backbone” of the therapy.
There were no significant differences in the adjusted odds of viral suppression for persons with a PDC of 80% to <85% or 85% to <90% compared with that of persons with a PDC of ≥90%. Persons within the “all other” regimen category had lower adjusted odds (aOR 0.51; 0.34, 0.77) of suppression when compared with persons on PI-based regimens. Persons on INSTI- or NNRTI-based regimens did not have significantly different adjusted odds of suppression when compared with persons on PI-based regimens.
Probit Regression
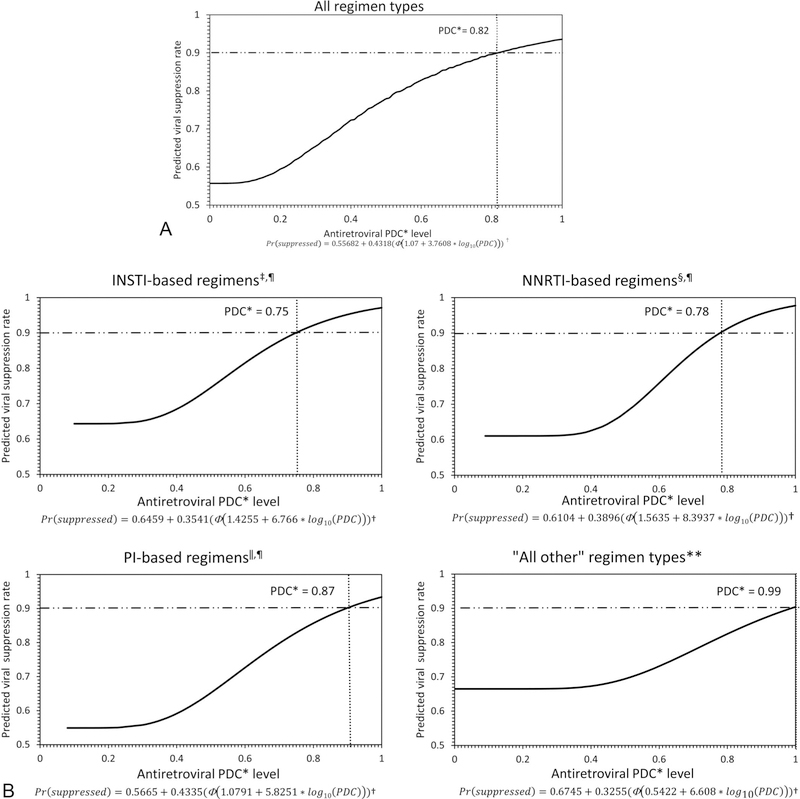
Results from the probit regression model are presented in Figure 1. Among all regimen types, the adherence level required to achieve viral suppression in 90% of HIV viral load tests was 82% (Fig. 1A). The adherence level required to achieve viral suppression in 90% of tests varied depending on ARV regimen type: 75% for INSTI-based, 78% for NNRTI-based, 87% for PI-based, and 99% for “all other” regimen types (Fig. 1B).
FIGURE 1.
*PDC, proportion of days covered; † Φ is the cumulative distribution function for the standard normal distribution; ‡INSTI, integrase inhibitor; §NNRTI, nonnucleoside reverse transcriptase inhibitor; ‖PI, protease inhibitor; ¶INSTI-, NNRTI-, and PI-based regimens each included one medication of the main component and 2 nucleoside reverse transcriptase (NRTI) drugs as the “backbone” of the therapy; **includes all regimens not categorized as an INSTI-, NNRTI-, or PI-based. The horizontal dash lines represent the 90% benchmark for viral suppression and the dotted vertical lines represent the PDC level at which 90% of HIV viral load tests were suppressed (HIV RNA <200 copies/mL).
DISCUSSION
After adjusting for demographic factors, within-person correlation, and ARV regimen type, we found that the odds of viral suppression did not differ significantly between persons with adherence levels between 80% and <85% or 85% to <90% and those with the Pharmacy Quality Alliance recommended adherence level of ≥90%. In addition, the overall estimated adherence level necessary to achieve viral suppression in 90% of HIV viral load tests was 82% and varied by regimen type; 90% of viral load tests associated with INSTI-based and NNRTI-based regimens were virally suppressed at adherence levels of 75% and 78%, respectively. These results indicate that the adherence level necessary to reach viral suppression may be lower than previously believed and may be more in line with the 80% adherence threshold often applied for other chronic diseases (eg, hypertension, hypercholesterolemia).16
Previous studies on the adherence threshold required for viral suppression are heterogeneous and often differ in the outcome of interest (eg, HIV RNA level of <50, <200, or <400 copies/mL), the adherence measure (eg, self-report, pill counts, medication possession ratios, and proportion of days covered), and the threshold for failure (eg, 10% or 20% of persons not suppressed) which makes comparisons between this and other studies somewhat difficult.17–22 However, our finding of no significant difference in the adjusted odds of viral suppression with adherence between 80% and <85% or 85% to <90% and ≥90% is consistent with like studies.17–23 For example, a meta-analysis of 43 studies which evaluated adherence thresholds and virologic outcomes, found no significant difference in the pooled odds of virologic failure at adherence levels of ≥95% or ≥98%–100% and adherence levels of ≥80%–90%.21 Similarly, using adherence self-report from a cohort study of men who have sex with men, Viswanathan et al17 found that ≥80% of persons were virally suppressed (<50 copies/mL) at adherence levels between 80% and 84%.
Although the adjusted odds of suppression were not statistically different between either INSTI-based or NNRTI-based regimens when compared with PI-based regimens, PI-based regimens required a higher adherence level to achieve viral suppression. Although this analysis was not powered to evaluate differences between regimen types, the higher adherence threshold we observed for PI-based regimens, is similar to other studies.18,20,22,24,25 However, our analysis did not distinguish between boosted and unboosted PI-based regimens, which might have accounted for some of the threshold differences. Regimens categorized as “all other” had lower adjusted odds of suppression and the highest adherence level necessary for viral suppression. The higher adherence level required for viral suppression with “all other” regimen types is not surprising given that this category includes regimens not considered adequate by treatment guidelines and because this category may represent non-standard or salvage regimens for persons who had previously failed therapy.1
When examining the study’s “All regimen types” dose-response curve, high proportions of viral load tests were suppressed even with adherence levels around 60%. This result is similar to that of Gordon et al19 who found a virologic failure rate (defined as 2 consecutive viral loads test results ≥200 copies/mL) for 3 first-line ARV regimens of approximately 20% at adherence levels of roughly 60%. The modest adherence levels necessary for viral suppression, seen in this analysis, is reflective of the improved potency and efficacy of newer ARV medications.12
Non-Hispanic black persons, younger persons and women had lower odds of viral suppression even after adjustment for adherence level and regimen category; the reasons for this are unclear. However, achieving viral suppression is dependent on factors additional to adherence such as underlying drug resistance and drug–drug or drug–food interactions, which can affect effectiveness of therapy.26–28 This analysis was unable to account for these other determinants of viral suppression in any demographic group.
Although we estimate that an overall adherence level of 82% is necessary for viral suppression in 90% of viral load tests, with lower thresholds for INSTI- and NNRTI-based regimens, these results should be used with caution. In addition to the risk of virologic rebound, poor adherence is associated with development of drug resistance (which can potentially reduce future treatment options), systemic inflammation, and higher health care use and costs.21,22,29–34 In particular, drug resistance is more common with NNRTI-based regimens in the absence of viral suppression.29 Clinicians’ message to patients should, therefore, remain “every dose every day” and be accompanied by continuous adherence counseling and support.35 These results may be useful when considering patients’ adherence barriers and optimal ARV regimens for these patients. In addition, a PDC of ≥82% may be sufficient to indicate adequate adherence when measuring PDC levels in pharmacy claims or fulfillment data not linked to viral load test results.
The study results should be viewed in light of its limitations. First, the PDC is a proxy for adherence. The PDC measures the amount of time a person has medication available, not actual pill-taking behavior; therefore, adherence was likely overestimated. In addition, the PDC does not measure 2 other important facets of adherence: whether a person takes their medication according to the prescriber’s instruction or on the prescribed schedule. However, using pharmacy fulfillment data to calculate adherence has an advantage over some other methods (eg, self-report, pill counts, and electronic monitoring) that are potentially confounded by social desirability and recall bias or expense.36 For this analysis, accurate PDC measurement required a closed pharmacy system; adherence would be underestimated if persons filled ARV prescriptions outside of the project pharmacy network. This analysis did not take into account the amount of time a person was virally suppressed; therefore, the analysis did not account for the decreased risk of virologic failure after prolonged periods of viral suppression.37 In addition, the analysis did not account for time since treatment initiation. The analysis, therefore, did not account for potentially higher adherence needed to achieve viral suppression in the first year after treatment initiation. Finally, participants in the PCHCM demonstration project represented a convenience sample which limits generalizability.
These data indicate that overall HIV viral suppression can be achieved for 90% of HIV viral load tests with an estimated ART adherence level of 82%, which is similar to that applied to other chronic diseases. The adherence threshold varied by regimen type with both INSTI-based and NNRTI-based regimens achieving viral suppression with even lower adherence levels (75% and 78%, respectively), suggesting that some regimen types may be more forgiving of missed doses than others. These results are reflective of the improved potency and efficacy of newer ARV medications. Although this analysis found an adherence level necessary for viral suppression that was lower than the conventionally reported values of ≥90% or ≥95%, clinicians should be encouraged to continue to advise patients to take all of their medications, as prescribed, to identify and address adherence barriers and to offer continuous adherence support.
Acknowledgments
Supported by the U.S. Department of Health and Human Services Secretary’s Minority AIDS Initiative fund and the Centers for Disease Control and Prevention through a co-operative agreement [grant number NU65PS004275] with the University of North Texas Health Science Center System College of Pharmacy. Walgreen Co., provided all services in-kind.
The findings and conclusions of this analysis are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
APPENDIX 1. The Patient-centered HIV Care Model Team
Michael Aguirre, Osayi Akinbosoye, David M. Bamberger, Ben Bluml, Katura Bullock, Diane C. Burrell, Tim Bush, Clifton Bush, Kathy K. Byrd, Chad Cadwell, Nasima M. Camp, Roberto Cardarelli, Terri Clark, Patrick Clay, Andrew Crim, Angela Cure, Kristin Darin, Traci Dean, W. Ambrose Delpino, Michael DeMayo, Shara Elrod, Ashley L. Eschmann, David Farmer, Rose Farnan, Heather Free, Andrew Gudzelak, Andrew Halbur, Felicia Hardnett, Ronald Hazen, Heidi Hilker, John Hou, Brian Hujdich, Lisa Johnson, Heather Kirkham, James Lecounte, Sayuri Lio, Guanzhong Lo, Sondra Middleton, Brittany Mills, Stacy Muckelroy, Christopher M. Nguyen, Linda Ortiz, Glen Pietrandoni, Kim Scarsi, Jon Schommer, Michael D. Shankle, Ram Shrestha, Daron Smith, Sumihiro Suzuki, Michael S. Taitel, Gebeyehu N. Teferi, Vikas Tomer, Louis Terres, Paul J. Weidle, Carmelita Whitfield, and Jason E. Willman.
Footnotes
J.G.H., R.H., H.K., and A.D. report that they were employees of Walgreen Co., during the conduct of this study. The remaining authors have no conflicts of interest to disclose.
Members of Patient-Centered HIV Care Model Team are listed in Appendix1.
REFERENCES
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guide-lines for the Use of Antiretroviral Agents in Adults and Adolescents Living With HIV: Department of Health and Human Services. Available at: http://www.aidsinfo.nih.gov/contentfiles/adultandadolescentgl.pdf. Accessed June 5, 2018.
- 2.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28:1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Evidence of HIV treatment and viral suppression in preventing the sexual transmission of HIV. Available at: https://www.cdc.gov/hiv/pdf/risk/art/cdc-hiv-art-viral-suppression.pdf. Accessed January 10, 2019.
- 6.Shubber Z, Mills EJ, Nachega JB, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med. 2016;13:e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claborn KR, Meier E, Miller MB, et al. A systematic review of treatment fatigue among HIV-infected patients prescribed antiretroviral therapy. Psychol Health Med. 2015;20:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. [DOI] [PubMed] [Google Scholar]
- 9.Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. [DOI] [PubMed] [Google Scholar]
- 11.Pharmacy Quality Alliance. PQA’s specialty core measure set. Available at: https://www.pqaalliance.org/assets/measures/pqa%20specialty%20measures%20core%20set%20081018.pdf. Accessed January 5, 2019.
- 12.Hughes CA, Robinson L, Tseng A, et al. New antiretroviral drugs: a review of the efficacy, safety, pharmacokinetics, and resistance profile of tipranavir, darunavir, etravirine, rilpivirine, maraviroc, and raltegravir. Expert Opin Pharmacother. 2009;10:2445–2466. [DOI] [PubMed] [Google Scholar]
- 13.Byrd KK, Hardnett F, Clay PG, et al. Retention in HIV care among participants in the Patient-centered HIV Care Model: a collaboration between community-based pharmacists and primary medical providers. AIDS Patient Care STDS. 2019;33:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nau D Proportion of days covered (PDC) as a preferred method of measuring medication adherence. Available at: http://ep.yimg.com/ty/cdn/epill/pdcmpr.pdf. Accessed January 15, 2019.
- 15.HIV/AIDS Bureau performance measures. Available at: https://hab.hrsa.gov/sites/default/files/hab/clinical-quality-management/coremeasures.pdf. Accessed June 5, 2018.
- 16.Centers for Medicare and Medicaid. Medicare 2019 part C and D star ratings technical notes. Available at: https://www.cms.gov/medicare/prescription-drug-coverage/prescriptiondrugcovgenin/downloads/2019-technical-notes-preview-2.pdf. Accessed January 4, 2019.
- 17.Viswanathan S, Detels R, Mehta SH, et al. Level of adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy (HAART). AIDS Behav. 2015;19:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viswanathan S, Justice AC, Alexander GC, et al. Adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;69:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon LL, Gharibian D, Chong K, et al. Comparison of HIV virologic failure rates between patients with variable adherence to three antiretroviral regimen types. AIDS Patient Care STDS. 2015;29:384–388. [DOI] [PubMed] [Google Scholar]
- 20.Martin M, Del Cacho E, Codina C, et al. Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort study. AIDS Res Hum Retroviruses. 2008; 24:1263–1268. [DOI] [PubMed] [Google Scholar]
- 21.Bezabhe WM, Chalmers L, Bereznicki LR, et al. Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine (Baltimore). 2016;95:e3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y, Sauer B, Zhang Y, et al. Adherence and virologic outcomes among treatment-naïve veteran patients with human immunodeficiency virus type 1 infection. Medicine (Baltimore). 2018;97:e9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutton SS, Magagnoli J, Hardin JW. Odds of viral suppression by single-tablet regimens, multiple-tablet regimens, and adherence level in HIV/ AIDS patients receiving antiretroviral therapy. Pharmacotherapy. 2017; 37:204–213. [DOI] [PubMed] [Google Scholar]
- 24.Maggiolo F, Airoldi M, Kleinloog HD, et al. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clin Trials. 2007;8:282–292. [DOI] [PubMed] [Google Scholar]
- 25.Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Ann Pharmacother. 2011;45:372–379. [DOI] [PubMed] [Google Scholar]
- 26.Fasinu PS, Gurley BJ, Walker LA. Clinically relevant pharmacokinetic herb-drug interactions in antiretroviral therapy. Curr Drug Metab. 2015; 17:52–64. [DOI] [PubMed] [Google Scholar]
- 27.Foy M, Sperati CJ, Lucas GM, et al. Drug interactions and antiretroviral drug monitoring. Curr HIV/AIDS Rep. 2014;11:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stella-Ascariz N, Arribas JR, Paredes R, et al. The role of HIV-1 drug-resistant minority variants in treatment failure. J Infect Dis. 2017; 216(suppl 9):S847–S850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bangsberg DR, Acosta EP, Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–231. [DOI] [PubMed] [Google Scholar]
- 30.Castillo-Mancilla JR, Brown TT, Erlandson KM, et al. Suboptimal adherence to combination antiretroviral therapy is associated with higher levels of inflammation despite HIV suppression. Clin Infect Dis. 2016; 63:1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn K, Lafeuille MH, Jiao X, et al. Risk factors, health care resource utilization, and costs associated with nonadherence to antiretrovirals in medicaid-insured patients with HIV. J Manag Care Spec Pharm. 2018; 24:1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haberer JE, Musinguzi N, Boum Y II, et al. Duration of antiretroviral therapy adherence interruption is associated with risk of virologic rebound as determined by real-time adherence monitoring in rural Uganda. J Acquir Immune Defic Syndr. 2015;70:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musinguzi N, Mocello RA, Boum Y II, et al. Duration of viral suppression and risk of rebound viremia with first-line antiretroviral therapy in rural Uganda. AIDS Behav. 2017;21:1735–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Wyl V, Klimkait T, Yerly S, et al. Adherence as a predictor of the development of class-specific resistance mutations: the Swiss HIV cohort study. PLoS One. 2013;8:e77691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Every dose every day. Effective interventions. Available at: https://effectiveinterventions.cdc.gov/en/care-medication-adherence/group-1/every-dose-every-day-toolkit. Accessed April 14, 2019.
- 36.Whalley Buono E, Vrijens B, Bosworth HB, et al. Coming full circle in the measurement of medication adherence: opportunities and implications for health care. Patient Prefer Adherence. 2017;11: 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenblum M, Deeks SG, van der Laan M, et al. The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One. 2009;4:e7196. [DOI] [PMC free article] [PubMed] [Google Scholar]