Abstract
Numerous studies have reported that angiotensin (Ang) II, nephrin, and podocin serve pivotal roles in podocyte injury, and thus can lead to the occurrence of proteinuria and the progression of kidney diseases. This study aimed to investigate the effects of Ang II on the production of nephrin and podocin, and their relationship with podocyte injury. We also aimed to determine whether nephrin, podocin and caspase-9 production depends on the PI3K/Akt/nuclear factor (NF)-κB signaling pathway in cultured mouse podocytes. We treated mouse podocytes with different doses of Ang II (10−9, 10−8, 10−7 and 10−6 mol/l) for 12, 24, and 48 h to analyse cell viability, and at 10−6 mol/l Ang II for 12, 24, and 48 h to evaluate cell apoptosis. Cells were treated with 10−6 mol/l of Ang II and/or LY294002 (inhibitor of Akt) or 740Y-P (activator of PI3K) for 48 h to detect Akt, phosphorylated (phospho)-Akt, p65 NF-κB, and phospho-p65 NF-κB, nephrin, podocin and caspase-9 expression, and podocyte apoptosis. Treatment with Ang II suppressed the viability and promoted the apoptosis of podocytes in a dose- and time-dependent manner. Ang II decreased phospho-Akt, phospho-p65 NF-κB, nephrin, and podocin and increased caspase-9 expression, while podocyte apoptosis was promoted. LY294002 further enhanced Ang II-induced downregulation of Akt and p65 NF-κB activation, as well as upregulation of caspase-9 mRNA and protein, and promoted the apoptosis of podocytes. Of note, 740Y-P restored Ang II-induced downregulation of Akt and p65 NF-κB activation, and upregulation of caspase-9, and decreased podocyte apoptosis. Interestingly, LY294002 and 740Y-P were determined to have no notable effects on the expression of nephrin and podocin. The data suggested that Ang II could regulate the expression of nephrin, podocin and caspase-9. Collectively, our findings suggested that the PI3K/Akt/NF-κB survival axis may serve a pivotal role in podocyte injury.
Keywords: Ang II, PI3K/Akt/NF-κB, podocyte, apoptosis, caspase-9
Introduction
Chronic kidney disease (CKD) and end-stage renal disease (ESRD) are global health problems worldwide (1). Pre-renal (decreased renal perfusion pressure), intrinsic renal (pathology of the vessels, glomeruli or tubules-interstitium) or post-renal (obstructive) are the three disease processes, which contribute to CKD (2). It is difficult to determine the true incidence and prevalence of CKD due to the asymptomatic nature of early to moderate CKD. The prevalence of CKD in the general population is ~10–14% (3). Worldwide, CKD accounted for 2,968,600 (1%) of disability-adjusted life-years and 2,546,700 (1–3%) of life-years lost in 2012 (4). CKD is a growing global public health problem with notable socioeconomic burden (1). Thus, an in-depth study of CKD pathogenesis is particularly important for its prevention and treatment.
Podocytes are cells that encapsulate glomerular capillaries in the Bowman's capsule of the kidney. Podocytes are named as of their long protrusions projecting from the cells, which are called foot processes or pedicels. Neighbouring podocytes interdigitate to overlap the basal lamina, which is closely associated with the glomerular capillaries. The pedicels of the podocytes intersect, in which spaces between the cells exist, known as slit diaphragms. These regions contain several cell-surface proteins, including nephrin, podocin and CD2-associated protein that cover the slits (5). Destruction of the filtration slits or the podocytes can result in kidney malfunction (6). Numerous studies in the past two decades have suggested that the damage and loss of podocytes are common clinical observations presented in the early stages of glomerular diseases, as well as in CKDs including minimal change disease, focal segmental glomerulosclerosis, diabetic nephropathy, membranous nephropathy and lupus nephritis (7,8).
Numerous lines of evidence have revealed that nephrin and podocin play important roles in the pathogenesis of podocyte injury. Several reports have revealed a decrease in the expression of nephrin in various proteinuria nephropathy and animal models of glomerular diseases (9,10). Hence, the deficiency of nephrin is considered as a pathologic feature of glomerular injury (11). In vivo, mild proteinuria, foot process regression, filtration slit narrowing, mesangial proliferation and sclerosis, glomerular basement membrane thickening, sub-endothelial zone widening and podocyte apoptosis were observed in mice with long-term nephrin downregulation (12). Gene mutations coding for nephrin can lead to congenital steroid-resistant nephrotic syndrome of the Finnish type, which is characterised by foot process dysmorphogenesis and the lack of inter-cellular junction formation, and is associated with considerably severe proteinuria at birth (13,14). In addition, podocin inactivation may contribute to the onset of focal segmental glomerulosclerosis and nephrotic syndrome (15); mice with podocin deficiency develop proteinuria before delivery and usually succumb from renal failure caused by massive mesangial sclerosis with a median survival time of 12 weeks (16). Electron microscopy analysis of kidney samples from these animals revealed extensive fusion of podocyte foot processes and the lack of a slit diaphragm in the remaining foot process junctions (17). Collectively, these studies suggest that nephrin and podocin serve pivotal roles in the pathogenesis of podocyte injury.
As one of the main effector hormones of the renin- angiotensin system (RAS), angiotensin (Ang II) plays an important role in the normal physiological maintenance of renal homeostasis (18). Abnormal secretion of Ang II is involved in the development of kidney diseases (19). Several studies have reported that Ang II promotes podocyte injury (20–22). Additionally, studies in vitro and in vivo have revealed that podocytes present decreased nephrin expression and increased apoptotic rates at high Ang II concentrations (23,24); however, the mechanism for Ang II-induced podocyte injury remains unclear. Few studies have investigated whether Ang II induces podocyte injury via the PI3K/Akt/nuclear factor (NF)-κB pathway. Several reports have demonstrated that the PI3K/Akt/NF-κB signaling pathway is implicated in kidney diseases (25–29). Hu et al (30) found that the PI3K/Akt signaling pathway serves a pivotal role in epithelial-mesenchymal transition of renal tubular epithelial cells, which is induced by Ang II.
In this study, we performed in vitro experiments to determine the effects of the PI3K/Akt/NF-κB signaling pathway on Ang II-induced podocyte injury. In addition, we examined the relationship between the PI3K/Akt/NF-κB signaling pathway, and nephrin, podocin and caspase-9 synthesis in Ang II-treated cultured mouse podocytes.
Materials and methods
Cell lines and cell culture
Mouse podocytes, which were purchased from the Cell Center of Fudan University (FDCC-MSN059), were cultured and differentiated as described previously (31). Briefly, the cells were cultivated at 33°C (permissive conditions) for proliferation in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) with 10 U/ml mouse recombinant γ-interferon (Invitrogen; Thermo Fisher Scientific, Inc.). Podocytes were maintained at 37°C without γ-interferon (non-permissive conditions) to induce differentiation for at least 2 weeks.
Treatment of cultured podocytes at 37°C with Ang II
We treated the mouse podocytes with different concentrations of Ang II (10−9, 10−8, 10−7 and 10−6 mol/l) for 12, 24 and 48 h for cell viability assays, and with 10−6 mol/l of Ang II for 12, 24 and 48 h for cell apoptosis assays. Cells were also treated with 10−6 mol/l of Ang II and or/LY294002 (inhibitor of Akt) or 740Y-P (activator of PI3K) for 48 h (untreated cells served as control) prior to detecting Akt, phosphorylated (phospho)-Akt, p65 NF-κB, phospho-p65 NF-κB, nephrin, podocin and caspase-9 expression, and podocyte apoptosis.
Cell viability assay
Cell viability was examined using a Cell Counting Kit-8 (CCK-8, Beijing Solarbio Life Sciences) assay. The podocytes were seeded in 96-well plates at a density of 5×103 cells per well and cultured at 37°C in 5% CO2 for 12 h, and then treated with Ang II. After incubation for 0, 12, 24 and 48 h, 10 µl of CCK-8 solution was added to each well and incubated for another 1–4 h at 37°C. The absorbance was measured with a multi-mode microplate reader, TriStar LB 941 (Berthold Technologies) at 450 nm.
Cell apoptosis assay
Apoptosis was measured using a flow cytometer (FACSCanto II, BD Biosciences), and an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) double-stain assay was performed in accordance with the manufacturer's protocols (FITC-AnnexinV/PI, BD Biosciences). After incubation of the podocytes as described above, each supernatant was collected in the centrifuge tubes and cells from each group were washed thrice with PBS, trypsinized, centrifuged (400 × g at room temperature) for 5 min, adjusted to 1×106/ml and suspended in binding buffer containing Annexin V-FITC and PI. After incubation for 15 min at room temperature in the dark, the fluorescent intensity was measured using a flow cytometer (FACSCanto II; BD Biosciences). The apoptotic rate was calculated by summing the rate of annexin V+/PI− and annexin V+/PI+ cells, and was calculated using FlowJo 7.6.1 (Tree Star, Inc.).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
RT-qPCR analysis was performed to determine the mRNA expression of nephrin, podocin, and caspase-9. Briefly, 2 µg of total RNA from each sample of 5×106 cells was used for the synthesis of first strand cDNA using a FastKing RT kit (Tiangen Biotech Co., Ltd.), according to the manufacturer's protocols. Following the first strand cDNA synthesis, qPCR was carried out in a 20 µl reaction volume containing 1X SuperReal PreMix Plus (Tiangen Biotech Co., Ltd.), 0.6 µl of each of the specific forward and reverse primers, and 2 µl of cDNA template. qPCR was performed with an initial denaturation step at 95°C for 3 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec in a LightCycler® 96 system (Roche Diagnostics). GAPDH was used as an endogenous housekeeping control. The mRNA expression levels were quantified by using the 2−∆∆Cq method (32). The data shown are representative of the mean of three experiments. The primers for each gene are listed in Table I.
Table I.
Primers of reverse transcription-quantitative polymerase chain reaction.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| GAPDH | AACTTTGGCATTGTGGAAGG | GGATGCAGGGATGATGTTCT |
| Nephrin | TCCTGCTGCGATGGTGGTTG | GTCTGGGTTGCCTCCGATGG |
| Podocin | TGAGGATGGCGGCTGAGAT | GGTTTGGAGGAACTTGGGT |
| Caspase-9 | ACCTGGTGCCTGTGGTCCTG | GCTCCGCCAGAACCAATGTCC |
Western blot analysis
The podocytes were washed twice with PBS after harvesting, and lysed in ice-cold radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology) with freshly added 0.01% protease inhibitor cocktail (CW Biotech), and were incubated on ice for 30 min. Protein concentration was measured via a bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology). A total of 50 µg of protein was subjected to electrophoresis using SDS-PAGE (8%) and was electrotransferred to a nitrocellulose membrane (GE Healthcare). Thereafter, the membranes were incubated for 1 h in 5% skimmed milk prepared with Tris-buffered saline containing 0.05% Tween-20 at room temperature. Subsequently, the membranes were incubated with primary antibodies at 4°C overnight, including rabbit anti-nephrin (1:500; cat. no. ab227806, Abcam), anti-podocin (1:500; cat. no. 20384-1-AP; ProteinTech Group, Inc.), anti-Akt (1:500; cat. no. ab179463, Abcam), anti- phospho-Akt (1:500; cat. no. ab192623), anti-p65 NF-κB (1:500; cat. no. 8242S, Cell Signalling Technology, Inc.), anti-phospho-p65 NF-κB (1:500; cat. no. 3033S, Cell Signalling Technology, Inc.), and anti-caspase-9 (1:500; cat. no. TA346902, ZSBIO) which presented two bands, one indicates pro-caspase-9, with a size of 46 KDa, whereas the other one indicates cleaved-caspase-9 with a size of 35 KDa. After washing with TBST, the membrane was incubated with horseradish peroxidase-linked anti-rabbit secondary antibody (cat. no. SA00001-2; ProteinTech Group, Inc.; 1:2,500) at room temperature for 2 h and visualised for immunoreactivity. Additionally, the membranes were processed to detect GAPDH (1:1,000; cat. no. 6-004-1-1g, ProteinTech Group, Inc.), which served as a loading control. Western blots were visualized using enhanced chemiluminescence (cat. no. CW0048; CWBIO) and the images were scanned by Quantity One software (Bio-Rad Laboratories, Inc.) and the original intensity of each specific band was quantified with a freeware image analysis software (ImageJ 6.0; National Institutes of Health).
Statistical analysis
SPSS 23.0 statistical software (IBM Corp.) was used for data analysis. Normal distribution and homogeneity of variance were tested for all data. The data were expressed as mean ± standard deviation. For cell viability analysis, statistical significance was determined by multivariate analysis. One-way ANOVA was used for analysis of variance among multiple groups; the Least Significant Difference test was used with homogeneity of variance, whereas a Games Howell test was used when no homogeneity in variance was observed. Each group was repeated three times. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of Ang II on cell viability
The viability of the podocytes was assessed after treatment with Ang II, which was presumed to decrease with increasing Ang II concentrations for 12, 24 and 48 h, as observed by the CCK-8 assay. After 12, 24 and 48 h of incubation with Ang II at concentrations of 10−9, 10−8, 10−7 and 10−6 mol/l, we observed a dose- and time-dependent decrease in the cell viability compared with the controls (Fig. 1). Therefore, this indicated that Ang II could inhibit cell viability, and effectively induce dose- and time-dependent cytotoxicity. Based on these results, we decided to treat cells at a dose of 10−6 mol/l Ang II for 48 h for the following studies.
Figure 1.
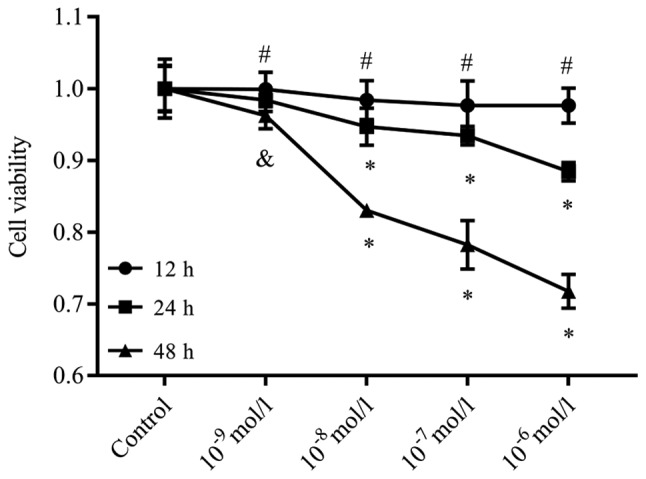
Ang II suppresses the viability of podocytes. The viability of the podocytes had a tendency to decrease as the concentrations of Ang II increased for 12, 24 and 48 h, as determined by a Cell Counting Kit-8 assay. After 12, 24 and 48 h of incubation with Ang II at concentrations of 10−9, 10−8, 10−7 and 10−6 mol/l, we observed a dose- and time-dependent decrease in cell viability compared with the controls. #P>0.05, &P<0.05, *P<0.01 vs. Control. The data are presented as the mean ± standard deviation from three independent experiments. For cell viability analysis, statistical significance was determined by multivariate analysis. Ang II, angiotensin II.
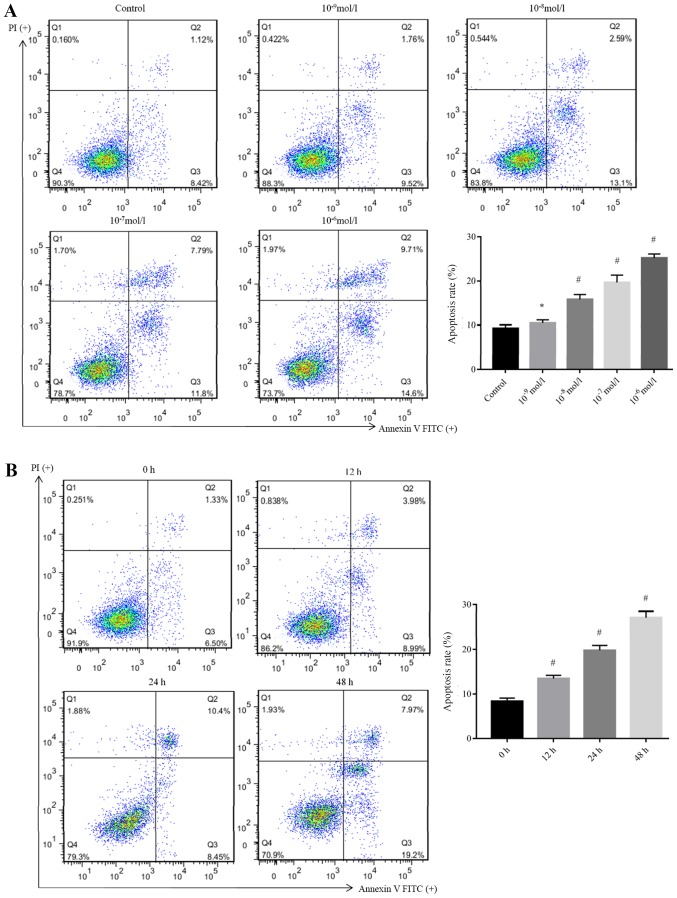
Ang II promotes podocyte apoptosis
To determine whether the cytotoxic effect of Ang II was due to the induction of apoptosis, the podocytes were treated with different doses of Ang II for 48 h; 10−6 mol/l was selected for administration at different time intervals (12, 24 and 48 h). Subsequently, cell apoptosis was determined by Annexin V-FITC/PI staining and flow cytometry analysis. As presented in Fig. 2, the apoptotic rate (sum of annexin V+/PI− and annexin V+/PI+ population/all cells events) of podocytes were significantly increased after treatment with Ang II from 15.80±1.12% (10−8 mol/l), 19.69±1.59% (10−7 mol/l) to 25.17±0.91% (10−6 mol/l), respectively, when compared with the control (9.25±0.81%). However, no significant difference was observed between the cells incubated with Ang II at concentrations of 10−9 mol/l (9.3%) and the control. Additionally, the apoptotic rates of podocytes were significantly increased after treatment with Ang II from 13.50±0.66% (12 h) and 19.80±1.08% (24 h) to 27.16±1.39% (48 h) when compared with 0 h (8.37±0.71%). This demonstrated that Ang II treatment induced apoptosis in these cells in a dose- and time-dependent manner.
Figure 2.
Ang II promotes apoptosis of podocytes. The apoptosis of podocytes was determined via flow cytometry after treatment with Ang II at concentrations of 10−9, 10−8, 10−7 and 10−6 mol/l. (A) Apoptotic rates of podocytes were significantly increased after treatment with Ang II from 10.43±0.74, 15.80±1.12, 19.69±1.59 and 25.17±0.91%, respectively, when compared with the control (9.25±0.81%). *P>0.05, #P<0.05 vs. Control. (B) Cell apoptosis of podocytes after treatment with Ang II at 10−6 mol/l for different time intervals (12, 24 and 48 h) as determined by flow cytometry. The apoptotic rates of podocytes were significantly increased after treatment with Ang II from 13.50±0.66% (12 h), 19.80±1.08% (24 h) and 27.16±1.39% (48 h) when compared with 0 h (8.37±0.71%). #P<0.05 vs. Control. The data are presented as the mean ± standard deviation from three independent experiments. For cell apoptosis analysis, statistical significance was determined one-way ANOVA followed by the Least Significant Difference test. Ang II, angiotensin II; FITC, fluorescein isothiocyanate; PI, propidium iodide.
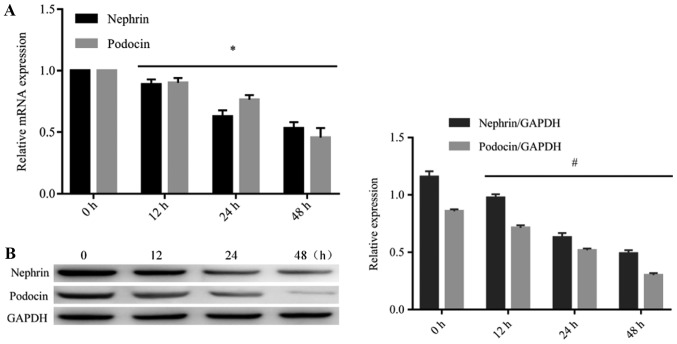
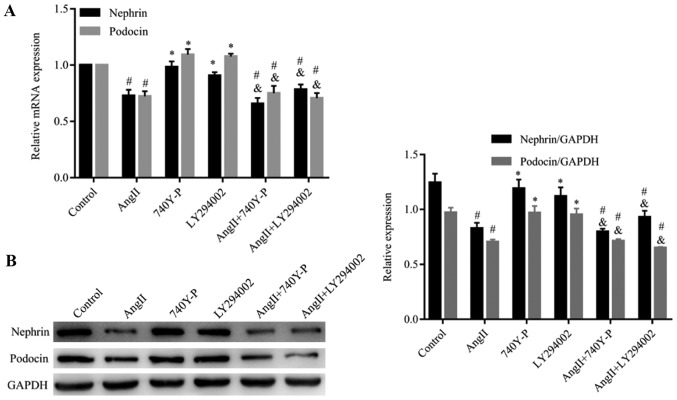
Ang II downregulates the expression of nephrin and podocin in podocytes
Western blotting and RT-qPCR analysis were performed to detect the expression of nephrin and podocin in podocytes after treatment with Ang II. As presented in Fig. 3, the mRNA and protein levels of nephrin and podocin were significantly reduced in the podocytes after treatment with Ang II (10−6 mol/l) at different time intervals (12, 24 and 48 h), when compared with 0 h. These findings were consistent with cell apoptosis.
Figure 3.
Ang II inhibits the expression of nephrin and podocin mRNA and protein. To determine the effects of Ang II on the expression of nephrin and podocin mRNA and protein, the expression of nephrin and podocin mRNA and protein after treatment with Ang II at 10−6 mol/l for different time intervals (12, 24 and 48 h) was determined by reverse transcription-quantitative polymerase chain reaction and western blotting. (A) Podocytes treated with Ang II significantly reduced the expression levels of nephrin and podocin mRNA in a time-dependent manner. *P<0.05. (B) Podocytes treated with Ang II significantly reduced the expression levels of nephrin and podocin protein in a time-dependent manner. #P<0.05. The data are presented as the mean ± standard deviation from three independent experiments. For western blot analysis, the quantification was performed by ImageJ 6.0 software; statistical significance was determined one-way ANOVA followed by the Least Significant Difference test. Ang II, angiotensin II.
Functional mechanism of PI3K/Akt signaling pathway in podocyte injury induced by Ang II
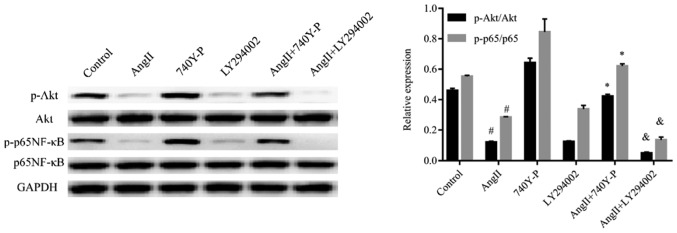
The PI3K/Akt pathway has been assumed to be involved in various types of kidney diseases. Hence, we proposed that Ang II may induce podocyte injury via the PI3K/Akt signaling pathway. To examine our hypothesis, we used western blotting to analyse the expression of proteins produced by genes located downstream of Ang II-induced cells (Figs. 4–6). The results indicate that treatment with Ang II caused a significant decrease in nephrin and podocin expression compared with the control (Fig. 5); however, an increase in caspase-9 expression was observed (Fig. 6). In addition, the phospho-Akt protein expression levels were significantly reduced in the Ang II-treated group when compared with the control levels, whereas Akt protein expression was not significantly changed (Fig. 4). Our results indicated that Ang II may exert its effect via inhibition of the PI3K/Akt signaling pathway.
Figure 4.
Ang II decreases the phosphorylation of Akt and inactivates p65 NF-κB in podocytes. Ang II reduced the expression of p-Akt and p-p65 NF-κB. Representative western blots of p-Akt and p-p65 NF-κB for 48 h expression in 10−6 M Ang II-treated podocytes are presented. Compared with the control group, Ang II decreased the phosphorylation levels of Akt and p65 NF-κB. #P<0.05 vs. Control. Compared with the Ang II incubated group, the combination of Ang II + 740Y-P restored the phosphorylation levels of Akt and NF-κB. *P<0.05 vs. Ang II. Stimulation of podocytes with Ang II + LY294002 further reduced the phosphorylation levels of Akt and p65 NF-κB vs. Ang II. &P<0.05. The data are presented as the mean ± standard deviation from three independent experiments. For western blot analysis, the quantification was performed by ImageJ 6.0 software; statistical significance was determined one-way ANOVA followed by the Least Significant Difference test. Ang II, angiotensin II; p, phosphorylated; NF-κB, nuclear factor-κB.
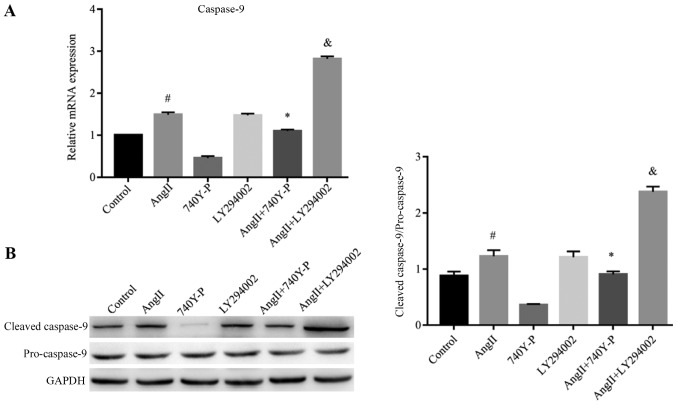
Figure 6.
Ang II induces the expression of caspase-9 via PI3K/Akt/nuclear factor-κB signaling. Representative reverse transcription-quantitative polymerase chain reaction and western blots of caspase-9 (A) mRNA and (B) protein expression in 10−6 M Ang II-treated podocytes for 48 h are presented. Compared with the control group, Ang II stimulated podocytes to increase the expression of caspase-9 mRNA and protein. #P<0.05 vs. Control. Compared with the Ang II group, the combination of Ang II + 740Y-P decreased the expression of caspase-9 mRNA and protein *P<0.05 vs. Ang II. Ang II + LY294002 increased the expression of caspase-9 mRNA and protein. &P<0.05 vs. Ang II. data are presented as the mean ± standard deviation from three independent experiments. Statistical significance was determined one-way ANOVA. Ang II, angiotensin II.
Figure 5.
Effects of PI3K/Akt/NF-κB signaling on the expression of nephrin and podocin mRNA and protein. Ang II reduced the expression of nephrin and podocin mRNA and protein; however, PI3K/Akt/NF-κB signaling was determined to have no effect on the expression of nephrin and podocin. (A) Reverse transcription-quantitative polymerase chain reaction of nephrin and podocin mRNA expression in 10−6 M Ang II-treated podocytes for 48 h. Compared with the control group, Ang II decreased the expression of nephrin and podocin mRNA. #P<0.05 vs. Control. No significant difference was observed between the control and 740Y-P and LY294002 groups. *P>0.05. Compared with the Ang II group, no significant difference was observed in the combination of Ang II + 740Y-P and Ang II + LY294002 groups. &P>0.05. The expression of nephrin and podocin mRNA in Ang II + 740Y-P and Ang II + LY294002 groups were decreased #P<0.05 vs control group (B) Western blotting of nephrin and podocin protein expression in 10−6 M Ang II-treated podocytes for 48 h. Compared with the control group, Ang II decreased the expression of nephrin and podocin mRNA. #P<0.05 vs. Control. No significant difference was observed between the control and 740Y-P and LY294002 groups *P>0.05. Compared with the Ang II group, no difference was observed in the combination of Ang II + 740Y-P and Ang II + LY294002 groups. &P>0.05. The expression of nephrin and podocin protein in Ang II + 740Y-P and Ang II + LY294002 groups were decreased #P<0.05 vs. control group. The data are presented as the mean ± standard deviation from three independent experiments. Statistical significance was determined one-way ANOVA. Ang II, angiotensin II; NF-κB, nuclear factor-κB.
In order to confirm the contribution of the PI3K/Akt signaling pathway in functioning of Ang II, an activator (740Y-P, 50 µg/ml) (33) and an inhibitor (LY294002, 5 µM) (34) of the PI3K/Akt signaling pathway were added to the cells which had been treated with and without Ang II, respectively (Figs. 4 and 5). When compared with the control group, 740Y-P increased the expression levels of phospho-Akt. In contrast, LY294002 inhibited the expression of phospho-Akt. Additionally, LY294002 further decreased the expression levels of phospho-Akt in cells, which were pre-treated with Ang II, when compared with those treated with Ang II alone. In contrast, 740Y-P restored the expression levels of phospho-Akt in the podocytes. Interestingly, the expression of nephrin and podocin did not change significantly when the cells were treated with LY294002 and 740Y-P compared with Ang II treatment alone (Figs. 5A and B). Our results suggested that the PI3K/Akt signaling pathway has no effect on the expression of nephrin and podocin.
Activity of PI3K/Akt-regulated Ang II-induced apoptosis via modulation of NF-κB activity
To investigate the mechanism of Ang II-induced inhibition of NF-κB modulated by PI3K/Akt inhibition, the levels of phospho-p65 NF-κB protein were analysed after Ang II treatment followed by 740Y-P or LY294002 treatment or both. Pre-treatment of podocytes with LY294002 significantly enhanced the Ang II-induced decrease in the levels of phospho-p65 NF-κB. In contrast, phospho-p65 NF-κB decreased significantly after treatment with Ang II and increased by pre-treatment with 740Y-P. This suggests that the PI3K/Akt signaling pathway is associated with the regulatory activity of NF-κB (Fig. 4).
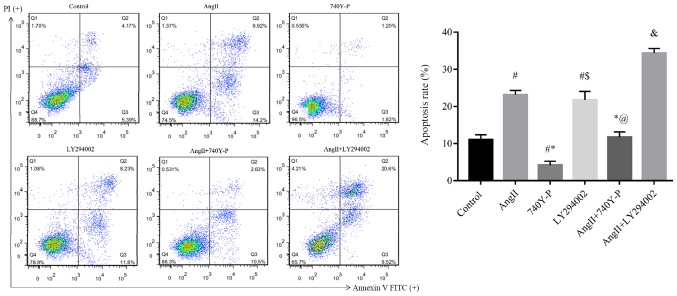
Furthermore, we focused on detecting the effect of the PI3K/Akt/NF-κB signaling pathway on the apoptosis induced by Ang II (Fig. 7). Compared with the control group (11.00±1.35%), Ang II significantly stimulated podocyte apoptosis (23.12±1.21%). Compared with the Ang II group (23.12±1.21%), the combination of Ang II + 740Y-P reduced apoptosis (11.73±1.43%), Ang II + LY294002 significantly increased apoptosis (34.36±1.24%).
Figure 7.
Ang II promotes the apoptosis of podocytes via the PI3K/Akt/NF-κB signaling pathway. Ang II induces apoptosis of podocytes via the PI3K/Akt/NF-κB signaling pathway. Representative apoptotic rates of podocytes in 10−6 M Ang II-treated podocytes for 48 h are presented. Compared with the control group, Ang II stimulated podocytes increased the rate of apoptosis. #P<0.05 vs. Control. Compared with the Ang II group, the combination of Ang II + 740Y-P decreased the rate of apoptosis. *P<0.05 vs. Ang II; stimulation of podocytes with Ang II + LY294002 increased the rate of apoptosis &P<0.05 vs. Ang II. No significant difference were observed between the Ang II and LY294002 groups. $P>0.05, and between Ang II + LY294002. And control @P>0.05. Statistical significance was determined one-way ANOVA. Ang II, angiotensin II; FITC, fluorescein isothiocyanate; NF-κB, nuclear factor-κB; PI, propidium iodide.
In order to provide further evidence of the role of this pathway in the inhibition of apoptosis after Ang II treatment, the expression levels of caspase-9 were determined by RT-qPCR and western blotting. Ang II significantly increased the expression levels of the caspase-9 at the mRNA and protein levels compared with the control. This was reversed by pre-treatment with 740Y-P, but was significantly increased by pre-treatment with LY294002 compared with Ang II treatment alone (Fig. 6), suggesting their essential role in promoting cells for apoptosis following Ang II treatment.
Thus, these preliminary results confirmed that Ang II could regulate the expression of nephrin, podocin and caspase-9 at least partly through the PI3K/Akt/NF-κB signaling pathway in podocytes, and may play a crucial role in podocyte injury.
Discussion
Podocytes, which surround the capillaries of the glomerulus constitute one of the main components of the glomerular blood filtration barrier; the unique actin-based morphological aspect of the foot processes are the most common features of kidney diseases (35). Podocyte injury and dysfunction are the major reasons for the pathogenesis of proteinuria and glomerulosclerosis (36). Thus, decoding the complex molecular and signaling mechanisms that are involved in podocyte dysfunction, is an important way to develop a treatment for proteinuria. In our present research, we investigated the involvement of PI3K/Akt/NF-κB signaling pathway in podocytes to Ang II-induced injury. Our results revealed a possible mechanism underlying podocyte injury. The expression levels of nephrin and podocin, two podocyte-specific markers, were downregulated, and the expression level of caspase-9, a pro-apoptotic factor, was upregulated in AngII-induced podocytes injury. However, the PI3K/Akt/NF-κB signaling pathway may not be involved in this process. When the physical and chemical environments are altered, cells adapt by changing several cellular processes including cell survival, proliferation, differentiation and apoptosis (37). As the central product of the RAS, Ang II promotes the progression of glomerular injury via its hemodynamic and/or non-hemodynamic effects (38). Ang II induces cell apoptosis and growth, and activates multiple signaling pathways in renal cells (39). Within the renal system, Ang II mediates various pathological processes including apoptosis via the Ang II type 1 receptor, which results in the development and progression of renal hypertrophy, extracellular matrix accumulation, and proteinuria (40). Several reports have confirmed that Ang II can promote apoptosis and suppress the proliferation of podocytes via different pathways (21,22,41,42). In accordance with other studies, in our study, we treated podocytes with different doses of Ang II for various time intervals. The results revealed that Ang II suppressed the viability and the promoted the apoptosis of podocytes in a dose- and time-dependent manner.
Protein kinase B, also referred to as Akt, is a serine/threonine-specific protein kinase that plays a crucial role in various cellular processes including apoptosis, cell proliferation, transcription, and cell migration (43). Akt activation regulates the maintenance and survival status of cellular stress fibres through the phosphorylation of various substrates (43). Lin et al (44) found that activation of the PI3K/Akt signaling pathway could inhibit kidney cell apoptosis and block the formation of interstitial fibrosis. Akt is activated by PI3K, and the PI3K/Akt signaling pathway plays a crucial role in the resistance of podocytes to apoptosis (45). In puromycin aminonucleoside (PAN)-induced podocyte injury models, Akt activity was markedly decreased, and dexamethasone inhibited podocyte apoptosis by stabilising the PI3K/Akt signaling pathway (46). Another study reported that PAN reduced Akt phosphorylation levels, while LY294002 could further promote podocyte apoptosis induced by PAN (47). The anti-apoptotic effects of PI3K/Akt on podocyte apoptosis were further supported by observations in which reduced Akt phosphorylation caused podocyte apoptosis (48).
A previous study reported that the local production of Ang II results in the progression of podocyte injury (49). It was also reported that Ang II promotes podocyte apoptosis in the cultured podocytes (21). In our study, phospho-Akt was downregulated in Ang II-treated podocytes, implying that phospho-Akt could be involved in Ang II-induced podocyte injury. Notably, LY294002 alone induced podocyte apoptosis and further promoted podocyte apoptosis induced by Ang II. In contrast, 740Y-P alleviated podocyte apoptosis induced by Ang II. Therefore, Ang II is likely to induce apoptosis by affecting the Akt activity in podocytes.
Downstream in the PI3K/Akt signaling pathway, NF-κB is a protein complex, which plays an important role in controlling the DNA transcription, cytokine production, and cell survival (50). Active NF-κB promotes cell proliferation and protects them from conditions that would lead to apoptosis. Defects in the NF-κB machinery may lead an increased susceptibility to apoptosis and cell death (51). Studies have suggested that NF-κB is involved in the intensity of proteinuria and is restricted to diseases of the renal tubules, as well as glomerular diseases (52–54).
Studies have reported that Akt can interfere with cell apoptosis via regulating apoptosis-related proteins, such as caspase-9, as well as NF-Κb (55,56). It was reported that the activation of Akt and NF-κB could promote the proliferation of human mesangial cells (57). In addition, several studies demonstrated that NF-κB is involved in podocyte injury (58,59); however, its role in the glomerulus and in the pathophysiology of podocyte diseases remains largely unexplored. To clarify the mechanism mediating the inhibitory effects of Ang II on NF-κB activation, we investigated the PI3K/Akt signaling pathway; we demonstrated that NF-κB inactivation was promoted in podocytes treated with Ang II, but was significantly enhanced by blockage of the PI3K/Akt pathway with LY249002 and restored by the activator of the PI3K/Akt pathway, 740Y-P. This suggested that inhibition of the PI3K/Akt/NF-κB pathway may represent one of the primary mechanisms for Ang II-induced apoptosis in podocytes.
Caspase-9 is an enzyme, which is encoded by the caspase 9 gene in humans. It is an initiator caspase, critical to the apoptotic pathway found in several tissues (60). To further investigate the apoptosis mechanism of podocytes induced by Ang II, the expression of caspase-9 was detected. The results indicated that treatment of podocytes with Ang II could increase the apoptotic rate of podocytes, as well as the expression of caspase-9. Furthermore, when compared with the treatment of podocytes with Ang II, the amount of caspase-9 was decreased after co-treating the cells with Ang II and 740Y-P; however, caspase-9 expression was increased after the cells were co-treated with Ang II and LY294002. This indicated that 740Y-P could restore the inhibitory effect and that LY294002 further promoted the activation effect of Ang II based on the levels of caspase-9. Collectively, it appears that Ang II could promote the expression of mRNA and protein of caspase-9 to induce apoptosis of podocytes. Our results are in accordance with some previous studies in which the activation of Akt inhibited apoptosis by regulating the apoptotic initiation protein, caspase-9 (61–63).
Therefore, our findings suggest that a PI3K/Akt/NF-κB signaling pathway in podocytes may be responsible for promoting apoptosis following treatment with Ang II, which is also linked to impaired upregulation of caspase-9. The results suggest that these molecular protein mediators are necessary for the involvement of the PI3K/Akt/NF-κB signaling pathway in relation to podocyte damage induced by Ang II.
Nephrin and podocin are the main structural components of the slit diaphragm. They work closely at the outer membrane of the podocyte foot processes and regulate the normal relationship between the podocytes of the epithelial cells and the basement membrane. As the structural components of the slit diaphragm, they play an important role in the appropriate functioning of the renal filtration barrier (5). Nephrin regulates several pathways in the podocyte including suppression of cell death and forming a complex with podocin (64). Mutational analysis reveals that abnormal or inefficient signaling via the nephrin-podocin complex results in the development of podocyte dysfunction and proteinuria (16). In addition, studies have indicated that decreased levels of nephrin and podocin are related to the degradation of the foot processes (64), and this may be involved in several kidney diseases including lupus nephritis (65,66).
Some researchers have reported the potential mechanism by which Ang II decreases the nephrin and podocin expression. In order to explore the mechanism of podocyte injury induced by Ang II, Yu et al (67) treated human podocytes with various concentrations of Ang II type I receptor agonistic autoantibody (AT1-AA); the results revealed that AT1-AA decreased the expression of nephrin in a dose-dependent manner and the underlying mechanism might involve activation of the transient receptor potential cation channer subfamily C member 6-calcium/calcineurin pathway (67). A similar conclusion was drawn in another study of Zhao et al (68). Additionally, Zhao et al (41) found that the activation of NOD-like receptor 3 inflammasome and mitochondrial dysfunction are related to the loss of nephrin and podocin, which was induced by Ang II. In the present study, we found that nephrin and podocin are impaired in Ang II-treated cultured podocytes; however, we were unable to reveal whether Akt is involved in the expression of nephrin and podocin proteins in our experimental model. Several other studies present a tentative link between nephrin, podocin and the PI3K/Akt signaling pathway. Yang et al (69) confirmed that Akt is a downstream intermediate of nephrin signaling. In addition, it has been reported that nephrin and podocin interact with a subunit of PI3K and subsequently activate the Akt kinase pathway, which is necessary for the regulation of actin dynamics and the cell survival (69–71). This suggests that downregulation of nephrin can regulate Akt inactivation and podocyte injury; however, the molecular mechanism of nephrin-Akt signal transduction requires further investigation.
However, there are some limitations to this study. We were unable to identify whether Akt signaling was involved in regulating the expression of nephrin and podocin proteins in our experimental model, and how Ang II induces the injury of podocytes in vivo was not determined. The relationship between the PI3K/Akt/NF-κB signaling pathway and nephrin and podocin proteins, and in vivo analysis will be investigated in our future studies.
In summary, the present study demonstrated that Ang II could induce podocyte damage via the PI3K/Akt/NF-κB signaling pathway, which could promote the development of podocyte injury following treatment with Ang II. In addition, activation of the PI3K/Akt/NF-κB survival axis may be a novel therapeutic strategy for treating Ang II-induced podocyte injury.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- Ang II
angiotensin II
- PI3K
phosphatidylinositol 3-kinase
- NF-κB
nuclear factor-κB
- RAS
renin-angiotensin system
- PAN
puromycin aminonucleoside
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81560271 and 81860296), Key Project of Scientific Research of the Guangxi Colleges and Universities (grant no. KY2015ZD092), and the Program of Natural Science Foundation of Guangxi (grant nos. 2017GXNSFDA198005 and 2018GXNSFAA281038).
Availability of data and materials
All data generated or analysed during the current study are included in this published article.
Authors' contributions
JW, SS and DF carried out the experimental work. YY and JW participated in the design of the study, and together with DF, who performed the statistical analysis. YY drafted the manuscript. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nath JD, Kashem A. Etiology and frequency of hospital admissions in maintenance hemodialysis patients in chronic kidney disease. Saudi J Kidney Dis Transpl. 2019;30:508–512. doi: 10.4103/1319-2442.256858. [DOI] [PubMed] [Google Scholar]
- 2.Vaidya SR, Aeddula NR. StatPearls. StatPearls Publishing LLC.; Treasure Island, FL: 2019. Chronic Renal Failure. [Google Scholar]
- 3.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 4.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 5.Reiser J, Altintas MM. Podocytes. F1000Res. 2016;5:F1000. doi: 10.12688/f1000research.7255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12:332–345. doi: 10.2215/CJN.05000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lal MA, Patrakka J. Understanding podocyte biology to develop novel kidney therapeutics. Front Endocrinol (Lausanne) 2018;9:409. doi: 10.3389/fendo.2018.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asanuma K. The role of podocyte injury in chronic kidney disease. Nihon Rinsho Meneki Gakkai Kaishi. 2015;38:26–36. doi: 10.2177/jsci.38.26. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 9.Doublier S, Salvidio G, Lupia E, Ruotsalainen V, Verzola D, Deferrari G, Camussi G. Nephrin expression is reduced in human diabetic nephropathy: Evidence for a distinct role for glycated albumin and angiotensin II. Diabetes. 2003;52:1023–1030. doi: 10.2337/diabetes.52.4.1023. [DOI] [PubMed] [Google Scholar]
- 10.Bertuccio CA. Relevance of VEGF and nephrin expression in glomerular diseases. J Signal Transduct. 2011;2011:718609. doi: 10.1155/2011/718609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma R, Venkatareddy M, Kalinowski A, Li T, Kukla J, Mollin A, Cara-Fuentes G, Patel SR, Garg P. Nephrin is necessary for podocyte recovery following injury in an adult mature glomerulus. PLoS One. 2018;13:e0198013. doi: 10.1371/journal.pone.0198013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Chuang PY, D'Agati VD, Dai Y, Yacoub R, Fu J, Xu J, Taku O, Premsrirut PK, Holzman LB, He JC. Nephrin preserves podocyte viability and glomerular structure and function in adult kidneys. J Am Soc Nephrol. 2015;26:2361–2377. doi: 10.1681/ASN.2014040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aya K, Tanaka H, Seino Y. Novel mutation in the nephrin gene of a Japanese patient with congenital nephrotic syndrome of the Finnish type. Kidney Int. 2000;57:401–404. doi: 10.1046/j.1523-1755.2000.00859.x. [DOI] [PubMed] [Google Scholar]
- 14.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, et al. Positionally cloned gene for a novel glomerular protein-nephrin is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/S1097-2765(00)80057-X. [DOI] [PubMed] [Google Scholar]
- 15.Mollet G, Ratelade J, Boyer O, Muda AO, Morisset L, Lavin TA, Kitzis D, Dallman MJ, Bugeon L, Hubner N, et al. Podocin inactivation in mature kidneys causes focal segmental glomerulosclerosis and nephrotic syndrome. J Am Soc Nephrol. 2009;20:2181–2189. doi: 10.1681/ASN.2009040379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabatabaeifar M, Wlodkowski T, Simic I, Denc H, Mollet G, Weber S, Moyers JJ, Brühl B, Randles MJ, Lennon R, et al. An inducible mouse model of podocin-mutation-related nephrotic syndrome. PLoS One. 2017;12:e0186574. doi: 10.1371/journal.pone.0186574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roselli S, Heidet L, Sich M, Henger A, Kretzler M, Gubler MC, Antignac C. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol. 2004;24:550–560. doi: 10.1128/MCB.24.2.550-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewko B, Maryn A, Latawiec E, Daca A, Rybczynska A. Angiotensin II modulates podocyte glucose transport. Front Endocrinol (Lausanne) 2018;9:418. doi: 10.3389/fendo.2018.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu S. Role of nephrin in podocyte injury induced by angiotension II. J Recept Signal Transduct Res. 2016;36:1–5. doi: 10.3109/10799893.2014.963872. [DOI] [PubMed] [Google Scholar]
- 21.Cardoso VG, Gonçalves GL, Costa-Pessoa JM, Thieme K, Lins BB, Casare FAM, de Ponte MC, Camara NOS, Oliveira-Souza M. Angiotensin II-induced podocyte apoptosis is mediated by endoplasmic reticulum stress/PKC-δ/p38 MAPK pathway activation and trough increased Na+/H+ exchanger isoform 1 activity. BMC Nephrol. 2018;19:179. doi: 10.1186/s12882-018-0968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Ren Z, Yang Q, Ding G. Csk regulates angiotensin II-induced podocyte apoptosis. Apoptosis. 2016;21:846–855. doi: 10.1007/s10495-016-1256-z. [DOI] [PubMed] [Google Scholar]
- 23.Jia J, Ding G, Zhu J, Chen C, Liang W, Franki N, Singhal PC. Angiotensin II infusion induces nephrin expression changes and podocyte apoptosis. Am J Nephrol. 2008;28:500–507. doi: 10.1159/000113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding G, Reddy K, Kapasi AA, Franki N, Gibbons N, Kasinath BS, Singhal PC. Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol. 2002;283:F173–F180. doi: 10.1152/ajprenal.00240.2001. [DOI] [PubMed] [Google Scholar]
- 25.Chen F, Sun Z, Zhu X, Ma Y. Astilbin inhibits high glucose-induced autophagy and apoptosis through the PI3K/Akt pathway in human proximal tubular epithelial cells. Biomed Pharmacother. 2018;106:1175–1181. doi: 10.1016/j.biopha.2018.08.069. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Wang B, Guo F, Li Z, Qin G. Involvement of the TGFβ1-ILK-Akt signaling pathway in the effects of hesperidin in type 2 diabetic nephropathy. Biomed Pharmacother. 2018;105:766–772. doi: 10.1016/j.biopha.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 27.Hong J, Wang X, Zhang N, Fu H, Li W. D-ribose induces nephropathy through RAGE-dependent NF-κB inflammation. Arch Pharm Res. 2018;41:838–847. doi: 10.1007/s12272-018-1061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Wang M, Hong H, Luo C, Liu Z, Yang R. Sophocarpine attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-κB activation. Immunol Res. 2018;66:521–527. doi: 10.1007/s12026-018-9012-9. [DOI] [PubMed] [Google Scholar]
- 29.Lin N, Ji Z, Huang C. Smad7 alleviates glomerular mesangial cell proliferation via the ROS-NF-κB pathway. Exp Cell Res. 2017;361:210–216. doi: 10.1016/j.yexcr.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Hu H, Hu S, Xu S, Gao Y, Zeng F, Shui H. miR-29b regulates Ang II-induced EMT of rat renal tubular epithelial cells via targeting PI3K/AKT signaling pathway. Int J Mol Med. 2018;42:453–460. doi: 10.3892/ijmm.2018.3579. [DOI] [PubMed] [Google Scholar]
- 31.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Derossi D, Williams EJ, Green PJ, Dunican DJ, Doherty P. Stimulation of mitogenesis by a cell-permeable PI 3-kinase binding peptide. Biochem Biophys Res Commun. 1998;251:148–152. doi: 10.1006/bbrc.1998.9444. [DOI] [PubMed] [Google Scholar]
- 34.Ha TS, Park HY, Seong SB, Ahn HY. Angiotensin II induces endoplasmic reticulum stress in podocyte, which would be further augmented by PI3-kinase inhibition. Clin Hypertens. 2015;21:13. doi: 10.1186/s40885-015-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin CE, Jones N. Nephrin signaling in the podocyte: An updated view of signal regulation at the slit diaphragm and beyond. Front Endocrinol (Lausanne) 2018;9:302. doi: 10.3389/fendo.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiser J, Sever S. Podocyte biology and pathogenesis of kidney disease. Annu Rev Med. 2013;64:357–366. doi: 10.1146/annurev-med-050311-163340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong JH, Dong JY, Xie T, Lu SL. The influence of AGEs environment on proliferation, apoptosis, homeostasis, and endothelial cell differentiation of human adipose stem cells. Int J Low Extrem Wounds. 2017;16:94–103. doi: 10.1177/1534734617701575. [DOI] [PubMed] [Google Scholar]
- 38.Ren Z, Liang W, Chen C, Yang H, Singhal PC, Ding G. Angiotensin II induces nephrin dephosphorylation and podocyte injury: Role of caveolin-1. Cell Signal. 2012;24:443–450. doi: 10.1016/j.cellsig.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 40.Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension. 2001;38:635–638. doi: 10.1161/hy09t1.094234. [DOI] [PubMed] [Google Scholar]
- 41.Zhao M, Bai M, Ding G, Zhang Y, Huang S, Jia Z, Zhang A. Angiotensin ii stimulates the nlrp3 inflammasome to induce podocyte injury and mitochondrial dysfunction. Kidney Dis (Basel) 2018;4:83–94. doi: 10.1159/000488242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Yang Q, Yang J, Ma Y, Ding G. Angiotensin II induces cholesterol accumulation and injury in podocytes. Sci Rep. 2017;7:10672. doi: 10.1038/s41598-017-09733-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang ZZ, Tschopp O, Baudry A, Dummler B, Hynx D, Hemmings BA. Physiological functions of protein kinase B/Akt. Biochem Soc Trans. 2004;32:350–354. doi: 10.1042/bst0320350. [DOI] [PubMed] [Google Scholar]
- 44.Lin X, Jiang C, Luo Z, Qu S. Protective effect of erythropoietin on renal injury induced in rats by four weeks of exhaustive exercise. BMC Nephrol. 2013;14:130. doi: 10.1186/1471-2369-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Chen X, Yuan L, Zhang Y, Wu J, Guo N, Chen X, Liu J. Down-regulation of IRAK1 attenuates podocyte apoptosis in diabetic nephropathy through PI3K/Akt signaling pathway. Biochem Biophys Res Commun. 2018;506:529–535. doi: 10.1016/j.bbrc.2018.09.175. [DOI] [PubMed] [Google Scholar]
- 46.Yu-Shengyou, Li Y. Dexamethasone inhibits podocyte apoptosis by stabilizing the PI3K/Akt signal pathway. BioMed Res Int. 2013;2013:326986. doi: 10.1155/2013/326986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren Q, You Yu S. CD2-associated protein participates in podocyte apoptosis via PI3K/Akt signaling pathway. J Recept Signal Transduct Res. 2016;36:288–291. doi: 10.3109/10799893.2015.1101137. [DOI] [PubMed] [Google Scholar]
- 48.Wang XM, Yao M, Liu SX, Hao J, Liu QJ, Gao F. Interplay between the Notch and PI3K/Akt pathways in high glucose-induced podocyte apoptosis. Am J Physiol Renal Physiol. 2014;306:F205–F213. doi: 10.1152/ajprenal.90005.2013. [DOI] [PubMed] [Google Scholar]
- 49.Fukuda A, Wickman LT, Venkatareddy MP, Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE, Wiggins RC. Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int. 2012;81:40–55. doi: 10.1038/ki.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu P, Wang J, Yang ZW, Lou XL, Chen C. Regulatory roles of the PI3K/Akt signaling pathway in rats with severe acute pancreatitis. PLoS One. 2013;8:e81767. doi: 10.1371/journal.pone.0081767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seitz CS, Freiberg RA, Hinata K, Khavari PA. NF-kappaB determines localization and features of cell death in epidermis. J Clin Invest. 2000;105:253–260. doi: 10.1172/JCI7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markó L, Vigolo E, Hinze C, Park JK, Roël G, Balogh A, Choi M, Wübken A, Cording J, Blasig IE, et al. Tubular epithelial NF-κB activity regulates ischemic AKI. J Am Soc Nephrol. 2016;27:2658–2669. doi: 10.1681/ASN.2015070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva GE, Costa RS, Ravinal RC, Ramalho LZ, Dos Reis MA, Coimbra TM, Dantas M. NF-κB expression in IgA nephropathy outcome. Dis Markers. 2011;31:9–15. doi: 10.1155/2011/940827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun F, Teng J, Yu P, Li W, Chang J, Xu H. Involvement of TWEAK and the NF-κB signaling pathway in lupus nephritis. Exp Ther Med. 2018;15:2611–2619. doi: 10.3892/etm.2018.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang B, Tang F, Wang Z, Qi G, Liang X, Li B, Yuan S, Liu J, Yu S, He S. Upregulation of Akt/NF-κB-regulated inflammation and Akt/Bad-related apoptosis signaling pathway involved in hepatic carcinoma process: Suppression by carnosic acid nanoparticle. Int J Nanomedicin. 2016;11:6401–6420. doi: 10.2147/IJN.S101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu CJ, Lo JF, Kuo CH, Chu CH, Chen LM, Tsai FJ, Tsai CH, Tzang BS, Kuo WW, Huang CY. Akt mediates 17beta-estradiol and/or estrogen receptor-alpha inhibition of LPS-induced tumor necresis factor-alpha expression and myocardial cell apoptosis by suppressing the JNK1/2-NFkappaB pathway. J Cell Mol Med. 2009;13:3655–3667. doi: 10.1111/j.1582-4934.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng N, Wang D, Ming H, Zhang H, Yu X. BAFF promotes proliferation of human mesangial cells through interaction with BAFF-R. BMC Nephrol. 2015;16:72. doi: 10.1186/s12882-015-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ory V, Fan Q, Hamdaoui N, Zhang SY, Desvaux D, Audard V, Candelier M, Noel LH, Lang P, Guellaën G, et al. c-mip down-regulates NF-κB activity and promotes apoptosis in podocytes. Am J Pathol. 2012;180:2284–2292. doi: 10.1016/j.ajpath.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 59.Li S, Liu X, Lei J, Yang J, Tian P, Gao Y. Crocin protects podocytes against oxidative stress and inflammation induced by high glucose through inhibition of NF-κB. Cell Physiol Biochem. 2017;42:1481–1492. doi: 10.1159/000479212. [DOI] [PubMed] [Google Scholar]
- 60.Druskovic M, Suput D, Milisav I. Overexpression of caspase-9 triggers its activation and apoptosis in vitro. Croat Med J. 2006;47:832–840. [PMC free article] [PubMed] [Google Scholar]
- 61.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.RES.0000022200.71892.9F. [DOI] [PubMed] [Google Scholar]
- 63.Yuan J, Deng Y, Zhang Y, Gan X, Gao S, Hu H, Hu S, Hu J, Liu H, Li L, Wang J. Bmp4 inhibits goose granulosa cell apoptosis via PI3K/AKT/Caspase-9 signaling pathway. Anim Reprod Sci. 2019;200:86–95. doi: 10.1016/j.anireprosci.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 64.Huber TB, Kottgen M, Schilling B, Walz G, Benzing T. Interaction with podocin facilitates nephrin signaling. J Biol Chem. 2001;276:41543–41546. doi: 10.1074/jbc.C100452200. [DOI] [PubMed] [Google Scholar]
- 65.Perysinaki GS, Moysiadis DK, Bertsias G, Giannopoulou I, Kyriacou K, Nakopoulou L, Boumpas DT, Daphnis E. Podocyte main slit diaphragm proteins, nephrin and podocin, are affected at early stages of lupus nephritis and correlate with disease histology. Lupus. 2011;20:781–791. doi: 10.1177/0961203310397412. [DOI] [PubMed] [Google Scholar]
- 66.Eichinger A, Ponsel S, Bergmann C, Günthner R, Hoefele J, Amann K, Lange-Sperandio B. Cyclosporine A responsive congenital nephrotic syndrome with single heterozygous variants in NPHS1, NPHS2, and PLCE1. Pediatr Nephrol. 2018;33:1269–1272. doi: 10.1007/s00467-018-3961-z. [DOI] [PubMed] [Google Scholar]
- 67.Yu Y, Zhang L, Xu G, Wu Z, Li Q, Gu Y, Niu J. Angiotensin II type I receptor agonistic autoantibody induces podocyte injury via activation of the TRPC6-calcium/calcineurin pathway in pre-eclampsia. Kidney Blood Press Res. 2018;43:1666–1676. doi: 10.1159/000494744. [DOI] [PubMed] [Google Scholar]
- 68.Zhao Y, Wu J, Zhang M, Zhou M, Xu F, Zhu X, Zhou X, Lang Y, Yang F, Yun S, et al. Angiotensin II induces calcium/calcineurin signaling and podocyte injury by downregulating microRNA-30 family members. J Mol Med (Berl) 2017;95:887–898. doi: 10.1007/s00109-017-1547-z. [DOI] [PubMed] [Google Scholar]
- 69.Yang Q, Ma Y, Liu Y, Liang W, Chen X, Ren Z, Wang H, Singhal PC, Ding G. Angiotensin II down-regulates nephrin-Akt signaling and induces podocyte injury: Role of c-Abl. Mol Biol Cell. 2016;27:197–208. doi: 10.1091/mbc.E15-04-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu J, Sun N, Aoudjit L, Li H, Kawachi H, Lemay S, Takano T. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73:556–566. doi: 10.1038/sj.ki.5002691. [DOI] [PubMed] [Google Scholar]
- 71.Liu J, Zhang YD, Chen XL, Zhu XL, Chen X, Wu JH, Guo NF. The protective effect of the EP2 receptor on TGF-β1 induced podocyte injury via the PI3K/Akt signaling pathway. PLoS One. 2018;13:e0197158. doi: 10.1371/journal.pone.0197158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during the current study are included in this published article.