Abstract

In vitro experiments previously published demonstrated the ability of fullerenes to decrease the capability of organophosphorus (OP) compounds to inhibit acetylcholinesterase. Experiments described herein demonstrate molecular level affinity interactions between fullerenes and the OP test compound paraoxon with NMR spectroscopy. The calculated binding constant of 19 M–1 indicates that this binding was not covalent.
Introduction
Acetylcholinesterase (AChE)-inhibiting organophosphorus (OP) compounds are toxic substances used as insecticides and nerve gases. Cholinergic poisoning associated with AChE inhibition can be rapid and severe.1 Although protection from OP compounds can be provided by protective clothing, poisonings still occur and rapid treatment with atropine and pralidoxime may be needed for survival. However, situations in which large numbers of people are exposed, such as occurred when an OP compound was released in the subway system of Tokyo in 1995, have prompted the search for additional protectants.2,3
Fullerenes are spherical structures containing 60–80 carbons in their respective molecular structures. They have been proposed for a variety of medical uses, including those based on their antioxidant property and their potential capability to deliver drugs and genes.4−6 The C80 metallofullerenes used in this study have also been proposed for use as MRI contrast agents.7,8
Previous results demonstrated protection from OP-induced AChE inhibition in incubates of SH-SY5Y human neuroblastoma cells or hen brain homogenates containing a series of OP compounds plus fullerenes.9 This protective effect was demonstrated using a wide range of OP concentrations with a wide variety of derivatized fullerenes. Delays in OP-induced toxic effects were also demonstrated in vivo.10−12 The experiments outlined in this article were designed to investigate the mode of action of the OP–fullerene interaction at the molecular level to help explain how derivatized fullerenes decrease OP toxicity. The data generated using nuclear magnetic resonance (NMR) suggest that molecular binding of an OP compound to a fullerene provides a possible mechanism for fullerene reduction of OP-induced AChE inhibition seen in previous studies.9−12
Materials and Methods
Test Materials
For these studies, paraoxon-ethyl (97.5% pure, Sigma-Aldrich, St. Louis, MO) and hydroxylated fullerene nanomaterials (Luna NanoWorks, Danville, VA) were used. The fullerenes listed in Table 1 include a C70-OH fullerene and a C80 gadolinium trimetasphere metallofullerene (Gd3N@C80(OH)n).
Table 1. HPLC Analysis of the Incubation Mixture between the Fullerenes and the Paraoxona.
| sample | paraoxon recovery (%) | p-nitrophenol |
|---|---|---|
| paraoxon control | 100 | not detected |
| Gd3N@C80(OH)n | 99 | not detected |
| C60(OH)n | 96 | not detected |
| C60(COOH) | 102 | not detected |
| C70-bis malonate | 98 | not detected |
| C60-pyrrolidine | 97 | not detected |
| Sc3N@C60(OH)n | 100 | not detected |
Detection limit for p-nitrophenol: <10–7 M.
The fullerene compounds were prepared by functionalizing the surface of the cage molecule with water solubilizing small molecule groups. C60(OH)n has approximately 14 hydroxyl groups on the surface of fullerene C60. C60(COOH) and C60-pyrrolidine have the same number of six carboxylic acid and pyrrolidine groups, respectively. C70(OH)n has about 20 hydroxyl groups. M3N@C80(OH)n has 24 hydroxyl groups for both M as Sc and Gd. C70-Bis-malonate has two malonic acid groups attached to the fullerene surface (Figure 1).
Figure 1.
Structures of test products.
Chromatographic Methods for the Examination of OP–Fullerene Interactions
Thin-Layer Chromatography (TLC)
Initial experiments were done with a mixture of C70-bis-malonate fullerene (C70-OH) and paraoxon using silica as the stationary phase and chloroform/methanol/trifluoroacetic acid as the mobile phase (50:45:5) in molar ratios of 1:1, 4:1, and 10:1.
High-Performance Liquid Chromatography (HPLC)
HPLC analysis was also used to examine possible covalent OP–fullerene interactions. The hypothesis was that the fullerene could form a covalent bond with the phosphorus of paraoxon, releasing the leaving group p-nitrophenol. For these analyses, each of the six fullerenes listed in Table 1 was tested. The respective fullerene solutions were made up in a 0.1 M phosphate buffer at pH 8.0. Each of the individual solutions was spiked with 100 μL of 1 μM paraoxon made up in the same buffer before incubation for 25 min at 37 °C. A portion of the final sample volumes was aliquotted into 2 mL amber autosampler vials and loaded into the autosampler of an Agilent 1100 Series HPLC. The respective solutions (20 μL) were injected for analysis. The analytes of interest were separated on an Agilent Zorbax XDB C18 column (150 mm × 4.6 mm × 5 μm) using a gradient mobile phase composition that started in the aforementioned phosphate buffer before increasing the concentration of methanol with a constant flow rate of 0.8 mL/min. UV detection and quantification of paraoxon and p-nitrophenol were performed at 270 and 312 nm, respectively.
Nuclear Magnetic Resonance Spectroscopy for the Examination of OP–Fullerene Interactions
Additional interactions of OP and fullerene were examined by the complexation of paraoxon with two different hydroxylated fullerenes. The test fullerenes were the C80 derivative containing gadolinium (Gd3N@C80(OH)n) and a nonmetallic C70 derivative (C70-OH). All proton (1H) and phosphorus (31P) NMR spectra were collected on a Bruker Advance III 600 NMR instrument (600 MHz, 242 MHz, respectively). Prior to the NMR experiments, the purity of paraoxon was checked in deuterated chloroform (CDCl3) with TMS by 1H NMR.
Each of the NMR experiments with paraoxon and the fullerenes C70-OH or Gd3N@C80(OH)n was run in deuterium oxide (D2O) with phosphoric acid (H3PO4) 0.04% as the internal reference (chemical shift δ = 0.00 ppm). Proton-decoupled 31P NMR spectra were collected for each sample. Increasing quantities of fullerenes were added to 0.5 mL of a fresh solution of paraoxon 0.5 mg/mL (1.8 mM) in D2O. The mixture was homogenized with a vortex mixer, and 10 μL of H3PO4 2% in D2O were added. The solution was then transferred to a clean, dry NMR tube for analysis. NMR spectra data were acquired 15 min after preparing the tube.
Results
TLC
No changes in migration distances among the fullerene alone and the fullerene + paraoxon were observed when analyzed at t = 0, 30 min, and 18 h. These results suggest that paraoxon was not forming a covalent bond with the fullerene molecule.
High-Performance Liquid Chromatography (HPLC)
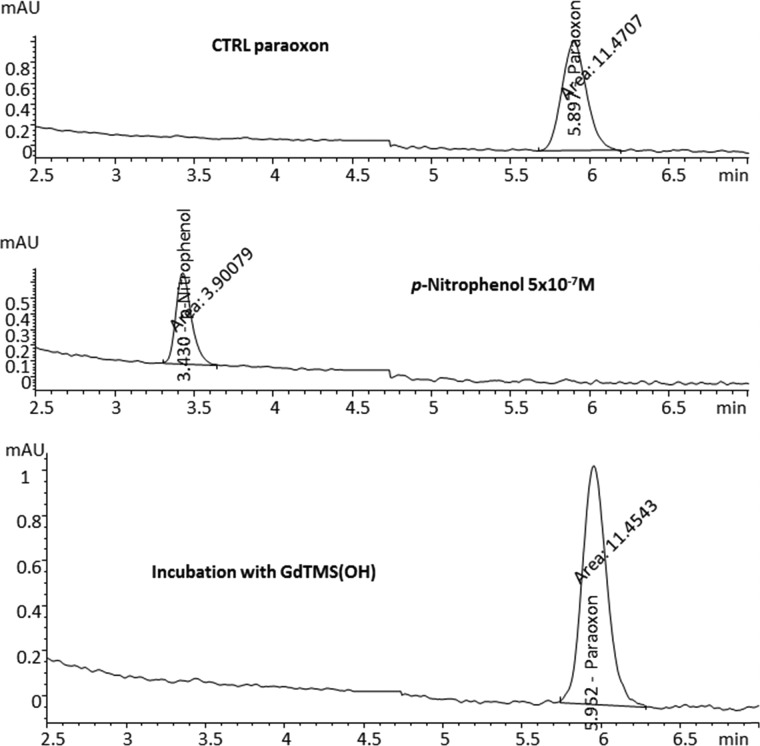
No significant changes in paraoxon concentration were observed and the metabolite of paraoxon, p-nitrophenol, was not detected in any of the incubated mixtures. The detection limit for p-nitrophenol was set at a concentration of <10–7 M (Table 1 and Figure 2). These results further substantiate TLC results, indicating that paraoxon was not forming a covalent bond with the fullerene molecule.
Figure 2.
HPLC chromatograms of the paraoxon (270 nm, top) and p-nitrophenol (312 nm, middle) control standards and the incubation mixture of paraoxon with Gd3N@C80(OH)n (bottom), labeled in the figure as GdTMS(OH).
No major change in the chemical shift was observed after the addition of sodium chloride to the mixture of C70-OH, even after long incubation times. Sodium chloride may not be the best salt to deaggregate the fullerenes as it was difficult to get a strong enough signal in the presence of sodium chloride. Sodium phosphate buffer has been reported to be a stronger salt to disrupt aggregation.14
NMR Results for Gd3N@C80(OH)n
(Table 2 and Figure 3) demonstrated an upfield change in the chemical shift as the concentration of fullerene was increased. Band broadening in the NMR signal was also observed, most likely as a result of strong paramagnetic interferences associated with the increasing inorganic Gd content. Longer acquisition times were used in an attempt to compensate for this observed effect but did not produce ideal peak shapes necessary for the quantitative NMR analysis. As a result, subsequent studies described herein were performed using the C70-OH fullerene. In doing so, higher concentrations of the fullerene were able to be used while maintaining ideal peak shapes, thereby allowing observable chemical shift changes (Δδ) within a reasonable amount of spectral collection time (Table 3 and Figure 4).
Table 2. 31P NMR Data for Paraoxon-Gd3N@C80(OH)n Fullerene Interactionsa.
| [Gd3N@C80(OH)n] (M) | Gd:paraoxon ratio | δ (ppm) | Δδ (ppm) |
|---|---|---|---|
| 0.0 × 10 | 0 | 6.49 | N/A |
| 3.6 × 10–3 | 2:1 | 6.71 | 0.22 |
| 9.0 × 10–3 | 10:1 | 6.97 | 0.48 |
Δδ (ppm) represents the observed chemical shift (δ) change for that specific fullerene-containing sample vs the δ observed for the paraoxon control.
Figure 3.
31P NMR spectra of paraoxon with increasing concentrations of the Gd3N@C80(OH)n fullerene. The peak for the internal reference standard, H3PO4, is shown at 0.00 ppm.
Table 3. 31P NMR Data for Paraoxon–C70-OH Fullerene Interactionsa.
| [C70-OH] (M) | C70:paraoxon ratio | δ (ppm) | Δδ (ppm) | 1/[C70] (M)−1 | 1/Δδ (ppm–1) |
|---|---|---|---|---|---|
| 6.3 × 10–2 | 70:1 | 9.10 | 3.03 | 15.769 | 0.330 |
| 4.5 × 10–2 | 50:1 | 8.96 | 2.89 | 22.076 | 0.346 |
| 3.6 × 10–2 | 40:1 | 8.82 | 2.75 | 27.595 | 0.364 |
| 2.7 × 10–2 | 30:1 | 8.6 | 2.53 | 36.793 | 0.395 |
| 1.8 × 10–2 | 20:1 | 7.69 | 1.62 | 55.190 | 0.617 |
| 9.1 × 10–3 | 10:1 | 6.97 | 0.90 | 110.380 | 1.111 |
| 4.5 × 10–3 | 5:1 | 6.58 | 0.51 | 220.760 | 1.961 |
| 0.00 | 0 | 6.07 | N/A | N/A | N/A |
Δδ (ppm) represents the observed chemical shift (δ) change for that specific fullerene-containing sample vs the δ observed for the paraoxon control.
Figure 4.

31P NMR spectra of paraoxon with decreasing concentrations of the C70-OH fullerene. The peak for the internal reference standard, H3PO4, is shown at 0.00 ppm.
Plotting the difference in the 31P NMR chemical shift (Δδ) versus fullerene concentration resulted in a binding isotherm curve that reaches a plateau at around 0.03 M (Figure 5). The binding constant (Kb) could be calculated from the double reciprocal plot of the binding isotherm plot (Figure 6) following the Benesi–Hildebrand equation.13
where the binding constant (Kb) was calculated by dividing the intercept by the slope from the double reciprocal plot (1/Δδ) versus 1/[C70] and was determined to be 19 (M)−1.
Figure 5.
Binding isotherm curve generated by 31P NMR data. Incubation of paraoxon with increasing concentrations of C70-OH.
Figure 6.
Benesi–Hildebrand plot for the binding of paraoxon with the C70-OH fullerene.
Discussion
The results suggest that derivatized fullerenes interact with OP compounds in a manner much like cyclodextrins that have previously been shown to decrease the OP capability to inhibit AChE.15−17 The binding constant of three cyclodextrins incubated with paraoxon ranged from 110 to 182 mole/L in a publication that provided these data.13 The OP aggregate with a derivatized fullerene in experiments described here has a binding constant about 10 times lower than cyclodextrin–OP aggregates. This may be because there is no cavity in the fullerene to complex the OP molecule. Most of the complexation studies done with OP compounds have been done with proteins where the hydrophobic and π–π interactions are larger. For example, the binding constant of paraoxon to human bovine albumin was found to be 1.914 × 10–4 mole/L.18 The binding constant of paraoxon to the C70-OH is modest if we compare it to the binding constant of immobilized paraoxon to cholinesterase (3084–6327 M–1 depending on the enzyme source).19
Another study examined the interaction of cyclodextrins with two OP nerve gases and published disassociation (Kd) values of 7–9 mM.20 The Kd of the paraoxon–cyclodextrin aggregates contrasts with the Kd (0.34 mM) reported for paraoxon with human recombinant acetylcholinesterase, a difficult disassociation.21
OP compounds interact with solubilized fullerenes, but the difference in binding constants between OP compounds with cholinesterases versus OP compounds with solubilized fullerenes is vast. Also, although not specifically calculated for the compounds used here, the disassociation constant for OP–fullerene is highly likely to be greater than for OP–AChE. This suggests that solubilized fullerenes are likely to only have a minor role for prophylaxis of potentially severe OP toxicity after dermal exposure, including toxicity associated with OP nerve agents. However, further exploration may be warranted to determine if, especially in combination with other protectants, they could be used after dermal exposure to OP compounds occurs, especially if signs of toxicity had not appeared before treatment could commence. Topical exposures to toxic OP compounds are relevant, and time for absorption is longer by this route of exposure, so substances like fullerenes that interact with OP compounds but do not have intrinsic toxicities could have future value in amelioration of OP-induced toxicities especially if applied alone or in combination before potential exposure or after exposure and before onset of symptoms. Further experiments are needed to determine such potentially beneficial possibilities.
Acknowledgments
This work was supported by the CounterAct Program, NIH Office of the Director, NINDS grant U01NS063723. The authors have no conflicts of interest. The experimental work was done at Virginia Tech. Dr Geraldine Magnin and Dr Philippe Bissel have since relocated to Manhattan, KS (Kansas State University). Dr Zhiguo Zhou has relocated to Zymeron Corporation, Durham NC.
The authors declare no competing financial interest.
References
- Lotti M.Clinical toxicology of anticholinesterase agents in humans. In Hayes Handbook of Pesticide Toxicology; Elsevier Inc.: Amsterdam, 2001; pp 1542–1589. [Google Scholar]
- Costa L. G.Effects of pesticides. In Casarett & Doull’s Toxicology the Basic Science of Poisons, 8th ed.; Klaassen C. D., Ed.; McGraw Hill Medical: NY, 2013; pp 933–980. [Google Scholar]
- Keyes D. C.Chemical terrorism. In Medical Response to Terrorism, Preparedness and Clinical Practice;Keyes D. C., Ed.; Lippincott Williams & Wilkins: Philadelphia, 2005; pp 2–15. [Google Scholar]
- Bakry R.; Vallant R. M.; Najam-ul-Haz M.; Rainer M.; Szabo Z.; Huck C. W.; Bonn G. K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [PMC free article] [PubMed] [Google Scholar]
- Li T.; Xiao L.; Yang J.; Ding M.; Zhou Z.; LaConte L.; Jin L.; Dorn H. C.; Li Z. Trimetallic nitride endohedral fullerenes carboxyl-Gd3N@C80: a new theranostic agent for combating oxidative stress and resolving inflammation. ACS Appl. Mater. Interfaces 2017, 9, 17681–17687. 10.1021/acsami.7b04718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Joslin S.; Dellinger A.; Ehrich M.; Brooks B.; Ren Q.; Rodeck U.; Lenk R.; Kepley C. L. A novel class of compounds with cutaneous wound healing properties. J. Biomed. Nanotechnol. 2010, 6, 605–611. 10.1166/jbn.2010.1157. [DOI] [PubMed] [Google Scholar]
- Li T.; Dorn H. C. Biomedical applications of metal-encapsulated fullerene nanoparticles. Small 2017, 13, 1603152 10.1002/smll.201603152. [DOI] [PubMed] [Google Scholar]
- Murphy S.; Hale A.; Reid T.; Olson J.; Kidiyoor A.; Tan J.; Zhou Z.; Jackson J.; Atala A. Use of trimetasphere metallofullene MRI contrast agent for the non-invasive longitudinal tracking of stem cells in the lung. Methods 2016, 99, 99–111. 10.1016/j.ymeth.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich M.; Van Tassell R.; Li Y.; Zhou Z.; Kepley C. L. Fullerene antioxidants decrease organophosphate-induced acetylcholinesterase inhibition in vitro. Toxicol. In Vitro 2011, 25, 301–307. 10.1016/j.tiv.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Ehrich M.; Fuhrman K.; Hinckley J.; Magnin-Bissel G.; Jortner B. S.; Werre S.; Van Tassell R.; Zhou Z.; Kepley C. L. A fullerene decreased organophosphate-induced toxicity in mice. Toxicol. Sci. Toxicologist 2011, 120, 551–552. [Google Scholar]
- Ehrich M.; Fuhrman K.; Hinckley J.; Van Tassell R.; Zhou Z.; Kepley C. Topical paraoxon toxicity reduced by fullerenes. Toxicol. Sci. Toxicologist 2012, 126, 201. [Google Scholar]
- Jortner B. S.; Hinckley J.; Hancock S.; Ehrich M. Study of behavioral and neuropathological effects 3 weeks after a single dose of organophosphate with a single dose of fullerene as a protectant. Toxicol. Sci. Toxicologist 2012, 126, 201. [Google Scholar]
- Churchill D.; Cheung J. C. F.; Park Y. S.; Smith V. H.; vanLoon G.; Buncel E. Complexation of diazinon, an organophosphorus pesticide, with α-, β-, and γ-cyclodextrin --NMR and computational studies. Can. J. Chem. 2006, 84, 702–708. 10.1139/v06-053. [DOI] [Google Scholar]
- Laus S.; Sitharaman B.; Tóth E.; Bolskar R. D.; Helm L.; Asokan S.; Wong M. S.; Wilson L. J.; Merbach A. E. Destroying gadofullerene aggregates by salt addition in aqueous solution of Gd@C(60)(OH)(x) and Gd@C(60)[C(COOH(2))](10). J. Am. Chem. Soc. 2005, 127, 9368–9369. 10.1021/ja052388+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank D.; Rougier N. M.; Vico R. V.; de Rossi R. H.; Buján E. I.; Bourne S. A.; Caira M. R. Solid-state structures and thermal properties of inclusion complexes of the organophosphate insecticide fenitrothion with permethylated cyclodextrins. Carbohydr. Res. 2010, 345, 141–147. 10.1016/j.carres.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Masurier N.; Estour F.; Froment M. T.; Lefèvre B.; Debouzy J. C.; Brasme B.; Masson P.; Lafont O. Synthesis of 2-substituted beta-cyclodextrin derivatives with a hydrolytic activity against the organophosphorylester paraoxon. Eur. J. Med. Chem. 2005, 40, 615–623. 10.1016/j.ejmech.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Wille T.; Tenberken O.; Reiter G.; Müller S.; Le Provost R.; Lafont O.; Estour F.; Thiermann H.; Worek F. Detoxification of nerve agents by a substituted beta-cyclodextrin: application of a modified biological assay. Toxicology 2009, 265, 96–100. 10.1016/j.tox.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Zhang M. F.; Xu Z. Q.; Ge Y. S.; Jiang F. L.; Liu Y. Binding of fullerol to human serum albumin: spectroscopic and electrochemical approach. J. Photochem. Photobiol., B 2012, 108, 34–43. 10.1016/j.jphotobiol.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Halámek J.; Teller C.; Zeravik J.; Fournier D.; Makower A.; Scheller F. W. Characterization of binding of cholinesterases to surface immobilized ligands. Anal. Lett. 2006, 39, 1491–1502. 10.1080/00032710600713107. [DOI] [Google Scholar]
- Désiré B.; Sant-Andre S. Inactivation of sarin and soman by cyclodextrins in vitro. Experientia 1987, 43, 395–397. 10.1007/BF01940424. [DOI] [PubMed] [Google Scholar]
- Rosenfeld C.; Sultatos L. G. Concentration-dependent kinetics of acetylcholinesterase inhibition by the organophosphate paraoxon. Toxicol. Sci. 2006, 90, 460–469. 10.1093/toxsci/kfj094. [DOI] [PubMed] [Google Scholar]