Abstract
Long non-coding RNAs (lncRNAs) have been implicated in the development and progression of cancer. However, the mechanisms of lncRNAs in hepatitis B virus (HBV) infection-induced hepatocellular carcinoma (HCC) remain unclear. The study aimed to reveal the roles of lncRNAs for HBV-HCC based on the hypothesis of competing endogenous RNA (ceRNA). The lncRNA (GSE27462), miRNA (GSE76903) and mRNA (GSE121248) expression profiles were collected from the Gene Expression Omnibus database. Differentially expressed lncRNAs (DELs), genes (DEGs) and miRNAs (DEMs) were identified using the LIMMA or EdgeR package, respectively. The ceRNA network was constructed based on interaction pairs between miRNAs and mRNAs/lncRNAs. The functions of DEGs in the ceRNA network were predicted using the DAVID database, which was overlapped with the known HCC pathways of Comparative Toxicogenomics Database (CTD) to construct the HCC-related ceRNA network. The prognosis values [overall survival, (OS); recurrence-free survival (RFS)] of genes were validated using the Cancer Genome Atlas (TCGA) data with Cox regression analysis. The present study screened 38 DELs, 127 DEMs and 721 DEGs. A ceRNA network was constructed among 17 DELs, 12 DEMs and 173 DEGs, including the FAM138B-hsa-miR-30c-CCNE2/RRM2 and SSTR5-AS1-hsa-miR-15b-5p-CA2 ceRNA axes. Function enrichment analysis revealed the genes in the ceRNA network that participated in the p53 signaling pathway [cyclin E2 (CCNE2), ribonucleotide reductase M2 subunit (RRM2)] and nitrogen metabolism [carbonic anhydrase 2 (CA2)], which were also included in the pathways of the CTD. Univariate Cox regression analysis revealed that six RNAs (2 DELs: FAM138B, SSTR5-AS1; 2 DEMs: hsa-miR-149, hsa-miR-7; 2 DEGs: CCNE2, RRM2) were significantly associated with OS; while seven RNAs (1 DEL: LINC00284; 3 DEMs: hsa-miR-7, hsa-miR-15b, hsa-miR-30c-2; and 3 DEGs: RRM2, CCNE2, CA2) were significantly associated with RFS. In conclusion, FAM138B-hsa-miR-30c-CCNE2/RRM2 and the SSTR5-AS1-hsa-miR-15b-5p-CA2 ceRNA axes may be important mechanisms for HBV-related HCC.
Keywords: hepatocellular carcinoma, hepatitis B virus, competing endogenous RNA, long non-coding RNA, microRNA, prognosis
Introduction
Despite the advent of effective vaccines and antiviral therapy, hepatitis B virus (HBV) infection remains one of the most important risk factors for the development of hepatocellular carcinoma (HCC), accounting for at least 50% of cases of primary liver tumors (1,2). HCC is the most common malignant disease and is associated with a poor 5-year survival rate (<20%) in most patients (3). Therefore, there still is a great need for exploring the molecular mechanisms and developing more effective therapeutic strategies for HBV-related HCC.
It is reported that in addition to ~20,000 protein-coding genes, ~90% of the human genome is non-protein-coding RNAs, including microRNAs (miRNAs, 21 nt) and long noncoding RNAs (lncRNAs, 200 nt in length) (4). Thus, aberrant expression of miRNAs (5,6) and lncRNAs (7,8) may be potential mechanisms for the progression of HBV-related HCC. miRNAs have been extensively studied to play important roles in HBV-related HCC by negatively regulating protein-coding mRNAs by directly binding to their 3′-untranslated region (3′UTR). For example, Du et al utilized miRNA microarray to reveal that miR-382-5p was upregulated in hepatitis B virus core (HBc) protein-expressed HCC cells. Transfection of miR-382-5p inhibitors abrogated the enhanced effects of HBc on cell migration and invasion by regulating the expression of deleted in liver cancer 1 (DCL-1) (9). Li et al revealed that miR-125a-5p was evidently downregulated in HBV-related HCC. Overexpression of miR-125a-5p markedly inhibited cell proliferation and induced cell apoptosis by reducing both the mRNA and protein levels of its target gene erb-b2 receptor tyrosine kinase 3 (ErbB3) (10).
The understanding of the lncRNA functions in HBV-related HCC remains limited except for a few studies that have elucidated their influence on the expression of related mRNAs. For example, Liu et al used RNA deep sequencing to demonstrate that lnc-HUR1 was significantly upregulated in HBV transgenic HepG2-4D14 cells (11). lnc-HUR1 could enhance cell proliferation by interacting with p53 to block the transcription of the pro-apoptotic gene B cell lymphoma 2-associated X (Bax) (11). A study by Jin et al revealed that hepatitis B virus × protein (HBx) induced upregulation of ZEB2-AS1 by 3.55-fold. Inhibition of ZEB2-AS1 could reverse the expression of epithelial-mesenchymal transition (EMT) markers regulated by HBx and then prevent migration and invasion of HCC cells (12). Recently, accumulating evidence has indicated that lncRNAs may also suppress miRNA functions by acting as competing endogenous RNAs (ceRNAs) in HCC (13,14). Lv et al revealed that lnRNA Unigene56159 promoted the migration and invasion of HCC cells by acting as a ceRNA for miR-140-5p to de-repress the expression of Slug and induce EMT (15). Fan et al observed that lncRNA n335586 accelerated HCC cell migration and invasion by facilitating the expression of its host gene CKMT1A by competitively binding miR-924 (16). Therefore, identification of the lncRNA-associated ceRNA axes may provide further insights into the development of HBV-HCC, which to date remains rarely reported.
The aim of the present study was to specifically identify HBV-HCC related lncRNA ceRNA axes by using microarray or sequencing data collected from the public database. The study results may provide potential diagnostic, prognostic and therapeutic biomarkers for HBV-associated HCC.
Materials and methods
Data collection and preprocessing
The HBV-HCC datasets were downloaded from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) on November 2018, including: i) GSE27462 (17) which analyzed the lncRNA expression profiling in 5 HBV-HCC and 5 matched adjacent normal tissues using microarray (Platform: GPL11269, Arraystar Human lncRNA array version 1); ii) GSE76903 (18) which explored the miRNA expression profiling in 20 HBV-HCC and 20 matched adjacent normal tissues using high throughput sequencing (Platform: GPL16791, Illumina HiSeq 2500, Homo sapiens); and iii) GSE121248 which investigated the mRNA expression profiling in 70 HBV-HCC and 37 matched adjacent normal tissues using a microarray technique [Platform: GPL570, (HG-U133_Plus_2) Affymetrix Human Genome U133 Plus 2.0 Array].
The raw data were respectively preprocessed using the Linear Models for Microarray Data (LIMMA) package (version 3.34.0; http://bioconductor.org/packages/release/bioc/html/limma.html) in R (version 3.4.1; http://www.R-project.org/) (19) and oligo package (version 1.41.1; http://www.bioconductor.org/packages/release/bioc/html/oligo.html) (20) in R for the GSE27462 and GSE121248 microarray datasets. The downloaded data had been saved using the normalized_count function for GSE76903 and preprocessing was not necessary.
Differential expression analysis
The differentially expressed genes (DEGs) and lncRNAs (DELs) between HBV-HCC and adjacent normal tissues were identified using the LIMMA package (19). The differentially expressed miRNAs (DEMs) between HBV-HCC and adjacent normal tissues were screened using the EdgeR package of R software (version 3.22.3; http://www.bioconductor.org/packages/release/bioc/html/edgeR.html) (21). The |logFC(fold change)| >0.5 and false discovery rate (FDR) <0.05 were considered as the threshold value. The pheatmap package (version: 1.0.8; http://cran.r-project.org/web/packages/pheatmap) in R was used to perform hierarchical clustering based on Euclidean distance.
lncRNA-miRNA-mRNA ceRNA regulatory network construction
The DIANA-LncBase database (version 2.0; http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex-experimental) (22) was used to screen the interactions between DELs and DEMs. Only the DEL-DEM interactions with opposite expression trend were retained. The target genes of DEMs were predicted using starBase (version 2.0; http://starbase.sysu.edu.cn/index.php) which provided the prediction information from five frequently used algorithms [TargetScan (http://www.targetscan.org/vert_72/), picTar (https://pictar.mdc-berlin.de), RNA22 (https://cm.jefferson.edu/rna22/), PITA (https://genie.weizmann.ac.il/pubs/mir07/mir07_prediction.html) and miRanda (http://www.microrna.org/microrna/home.do)]. The target genes of DEMs were selected if they were predicted by at least one algorithm, and were then overlapped with the DEGs to obtain the DEM-DEG interactions. In addition, only the negative interaction pairs between DEMs and DEGs were retained. The DEL-DEM and DEM-DEG interaction pairs were integrated to obtain the DEL-DEM-DEG ceRNA axes which were used to construct the ceRNA network and visualized using the Cytoscape software (version 3.6.1; www.cytoscape.org/) (23).
The Database for Annotation, Visualization and Integrated Discovery (DAVID) online tool (version 6.8; http://david.abcc.ncifcrf.gov) (24) was used for predicting the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and Gene Ontology (GO) terms of DEGs in the ceRNA network. The pathways or terms with P-value <0.05 were considered as statistically significant. In addition, all known HCC related pathways updated to 2017 were also downloaded from the Comparative Toxicogenomics Database (CTD; http://ctd.mdibl.org/) using the key word ‘hepatocellular carcinoma’ (25), which was then overlapped with the pathways enriched by the DEGs in the ceRNA network to obtain an HCC-related ceRNA network.
Identification of prognosis-related DELs, DEMs and DEGs in the ceRNA network
The miRNA and mRNA expression profile data of the HBV-HCC samples were collected from the Cancer Genome Atlas (TCGA; http://gdc-portal.nci.nih.gov/) database. Univariate Cox regression analysis was performed to screen overall survival (OS) and recurrence-free survival (RFS)-related DELs, DEMs and DEGs using the survival package (version 2.42.6; http://cran.r-project.org/package=survival). Then, survival curves were drawn for the significant prognosis-related DELs, DEMs and DEGs by the Kaplan-Meier method with the log-rank test using the survival package. P<0.05 was considered to indicate a statistically significant difference.
Results
Differential expression analysis
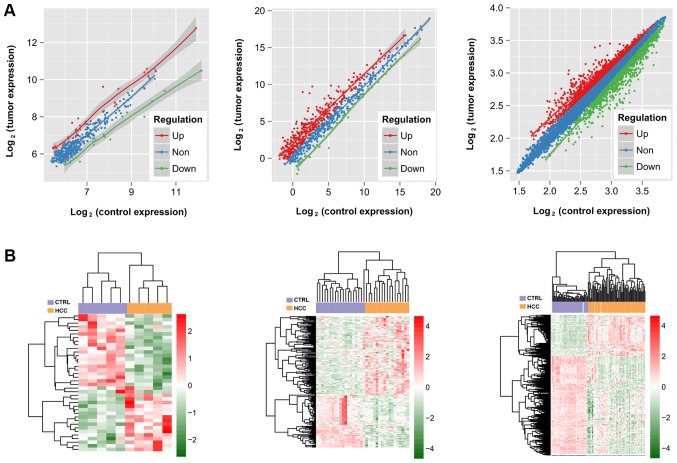
A total of 38 DELs (16 downregulated and 22 upregulated), 127 DEMs (17 downregulated and 110 upregulated) and 721 DEGs (506 downregulated and 215 upregulated) were identified between HBV-HCC and adjacent normal tissues (Fig. 1A; Table I). The heat map analysis revealed that the DELs, DEMs and DEGs could be used to distinguish the HBV-HCC samples from normal samples completely (Fig. 1B).
Figure 1.
Expression pattern of lncRNAs, miRNAs and mRNAs in HBV-HCC (HCC group) and adjacent normal control tissues (CTRL group). (A) Scatter plots of lncRNAs (left), miRNAs (middle) and mRNAs (right) between two groups. The values of the × and y axes in the scatter plot are the averaged normalized signal values of the group (log2 scaled); (B) Heat map showing differentially expressed lncRNAs (left), miRNAs (middle) and mRNAs (right) between two groups. Red and blue denote upregulated and downregulated expression, respectively. lncRNAs, long non-coding RNAs; miRNAs, microRNAs; HBV, hepatitis B virus; HCC, hepatocellular carcinoma.
Table I.
Differentially expressed lncRNAs, miRNAs and mRNAs between HCC and adjacent normal tissues.
| mRNA | miRNA | lncRNA | ||||||
|---|---|---|---|---|---|---|---|---|
| Symbol | FDR | logFC | Symbol | FDR | logFC | Symbol | FDR | logFC |
| HAMP | 3.03E-15 | −4.16 | hsa-miR-9-5p | 2.36E-15 | 4.35 | KIAA0087 | 6.00E-04 | 0.86 |
| KCNN2 | 4.13E-24 | −3.99 | hsa-miR-431-5p | 1.71E-13 | 4.02 | SNHG9 | 1.73E-03 | −1.44 |
| CXCL14 | 7.95E-28 | −3.98 | hsa-miR-301b | 8.99E-13 | 3.87 | LINC02203 | 2.01E-03 | 1.81 |
| CNDP1 | 1.25E-19 | −3.75 | hsa-miR-216b-5p | 1.59E-12 | 3.87 | H19 | 3.55E-03 | 0.97 |
| FCN2 | 6.81E-23 | −3.64 | hsa-miR-483-3p | 1.51E-11 | 3.61 | WWC2-AS2 | 5.30E-03 | 0.82 |
| IGFBP3 | 1.61E-16 | −2.08 | hsa-miR-7974 | 1.45E-10 | 3.48 | HNF1A-AS1 | 1.00E-02 | 0.87 |
| RRM2 | 1.78E-15 | 2.25 | hsa-miR-323a-3p | 3.79E-10 | 3.37 | TTTY6B | 1.10E-02 | 0.70 |
| STEAP3 | 4.73E-13 | −1.48 | hsa-miR-483-5p | 4.78E-10 | 3.29 | FAM138B | 1.27E-02 | 1.16 |
| ALDH1B1 | 9.83E-12 | −1.16 | hsa-miR-200c-3p | 8.07E-10 | 3.25 | WEE2-AS1 | 1.30E-02 | 0.54 |
| ACSL4 | 1.83E-11 | 2.81 | hsa-miR-183-5p | 1.92E-09 | 3.15 | HLA-F-AS1 | 1.38E-02 | −0.90 |
| CCNE2 | 7.91E-10 | 1.42 | hsa-miR-410-3p | 2.90E-09 | 3.11 | LINC00284 | 1.80E-02 | −0.61 |
| GLS2 | 1.03E-09 | −2.40 | hsa-miR-182-5p | 8.38E-07 | 2.53 | SNHG15 | 1.83E-02 | −0.54 |
| CA2 | 6.67E-09 | −1.24 | hsa-miR-34c-5p | 3.03E-06 | 2.46 | DLGAP1-AS1 | 2.01E-02 | −0.71 |
| GADD45B | 7.04E-09 | −1.25 | hsa-miR-7-5p | 3.77E-05 | 2.14 | KMT2E-AS1 | 2.12E-02 | −0.68 |
| ACAA2 | 2.51E-08 | −1.01 | hsa-miR-299-3p | 4.17E-05 | 2.11 | LINC00339 | 2.14E-02 | −0.72 |
| FAS | 2.03E-07 | −1.03 | hsa-miR-15b-5p | 1.37E-04 | 1.94 | MAP3K14-AS1 | 2.19E-02 | 0.76 |
| ACSL1 | 6.24E-07 | −1.09 | hsa-miR-30c-2-3p | 1.97E-03 | −1.61 | LINC00909 | 2.72E-02 | −0.60 |
| THBS1 | 1.46E-06 | −1.56 | hsa-miR-149-5p | 9.85E-03 | 1.35 | SRRM2-AS1 | 3.78E-02 | −0.99 |
| ACADSB | 1.30E-05 | −1.03 | hsa-miR-214-5p | 1.89E-02 | −1.22 | LINC00928 | 4.00E-02 | −0.75 |
| CPS1 | 1.75E-03 | −1.24 | hsa-miR-128-3p | 2.36E-02 | 1.17 | RUSC1-AS1 | 4.06E-02 | −1.03 |
lncRNAs, long non-coding RNAs; miRNAs, microRNAs; HCC, hepatocellular carcinoma; FC, fold change; FDR, false discovery rate; CCNE2, cyclin E2; RRM2, ribonucleotide reductase M2 subunit.
Function enrichment for DEGs
GO terms and KEGG pathway enrichment analyses were performed for the DEGs to predict their functions by using DAVID software. The results revealed that 37 GO terms (including 17 biological processes, 7 cellular components and 13 molecular functions) were enriched for all the DEGs, mainly involving GO:0055114~oxidation reduction [ribonucleotide reductase M2 subunit (RRM2)], GO:0048545~response to steroid hormone stimulus [carbonic anhydrase 2 (CA2)] and GO:0007049~cell cycle [cyclin E2 (CCNE2)] (Fig. 2). Furthermore, 6 KEGG pathways were also enriched, including hsa00830:Retinol metabolism, hsa00982:Drug metabolism, hsa00980:Metabolism of xenobiotics by cytochrome P450, hsa00071:Fatty acid metabolism, hsa04610:Complement and coagulation cascades and hsa00260:Glycine, serine and threonine metabolism (Fig. 2).
Figure 2.
Function enrichment analyses for the differentially expressed genes. (A) GO enrichment. (B) KEGG pathway enrichment. GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
CeRNA network construction
By searching the DIANA-LncBasev2 database, 74 DEL-DEM interaction pairs with the opposite expression trend were predicted between 18 DELs (12 downregulated and 6 upregulated) and 20 DEMs (2 downregulated and 18 upregulated). Subsequently, the target genes of the aforementioned 20 DEMs were predicted with the starBase database. After removing the DEMs and DEGs with the consistent expression trend, 287 DEM-DEG interaction pairs between 12 DEMs (2 downregulated and 10 upregulated) and 173 DEGs (126 downregulated and 47 upregulated) were retained. By integrating the DEL-DEM and DEM-DEG interaction pairs, a DEL-DEM-DEG ceRNA network was constructed, which consisted of 202 nodes (17 DELs: 2 downregulated and 15 upregulated; 12 DEMs: 2 downregulated and 10 upregulated; 173 DEGs: 126 downregulated and 47 upregulated) (Fig. 3). In this ceRNA network, the FAM138B-hsa-miR-30c-CCNE2/RRM2 and SSTR5-AS1-has-miR-15b-5p-CA2 ceRNA axes were included.
Figure 3.
ceRNA interaction network of lncRNA-miRNA-mRNA. Square nodes represent lncRNAs; triangle nodes represent miRNAs; circular nodes represent mRNAs. Orange, upregulated; blue, downregulated. ceRNA, competing endogenous RNA; lncRNAs, long non-coding RNAs; miRNAs, microRNAs.
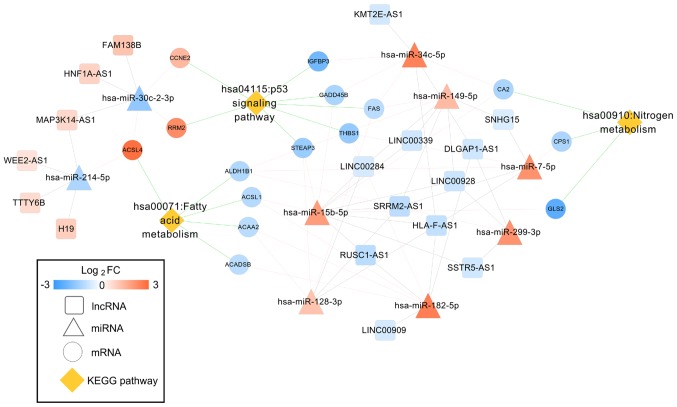
Function enrichment analysis with DAVID revealed that the genes in the ceRNA network were involved in 3 significant KEGG pathways, including the hsa04115:p53 signaling pathway (CCNE2, RRM2), hsa00071:Fatty acid metabolism and hsa00910:Nitrogen metabolism (CA2) (Table II). These three pathways were also included in the 244 KEGG pathways that had been demonstrated to be associated with HCC in the CTD database. Thus, the DEL-DEM-DEG interaction pairs that were associated with these three pathways were extracted to form the HCC-related ceRNA network (Fig. 4).
Table II.
KEGG pathways enriched for the genes in the ceRNA network.
| Term | P-value | Genes |
|---|---|---|
| hsa04115:p53 signaling pathway | 2.83E-04 | STEAP3, CCNE2, RRM2, FAS, GADD45B, THBS1, IGFBP3 |
| hsa00071:Fatty acid metabolism | 1.96 E-03 | ACAA2, ACADSB, ACSL1, ALDH1B1, ACSL4 |
| hsa00910:Nitrogen metabolism | 3.82 E-02 | GLS2, CA2, CPS1 |
KEGG, Kyoto Encyclopedia of Genes and Genomes; ceRNA, competing endogenous RNA; CCNE2, cyclin E2; RRM2, ribonucleotide reductase M2 subunit.
Figure 4.
HCC-related ceRNA interaction network of lncRNA-miRNA-mRNA. Diamond nodes represent lncRNAs; triangle nodes represent miRNAs; circular nodes represent mRNAs; rhombus nodes represent HCC pathways. Orange, upregulated; blue, downregulated. HCC, hepatocellular carcinoma; ceRNA, competing endogenous RNA; lncRNAs, long non-coding RNAs; miRNAs, microRNAs.
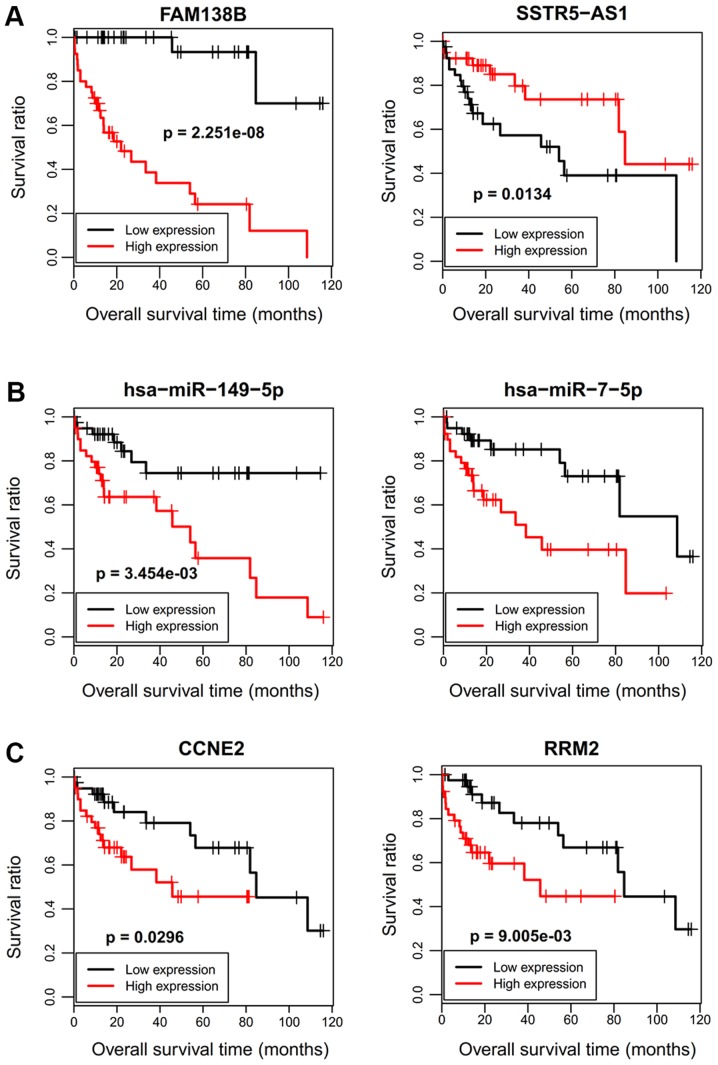
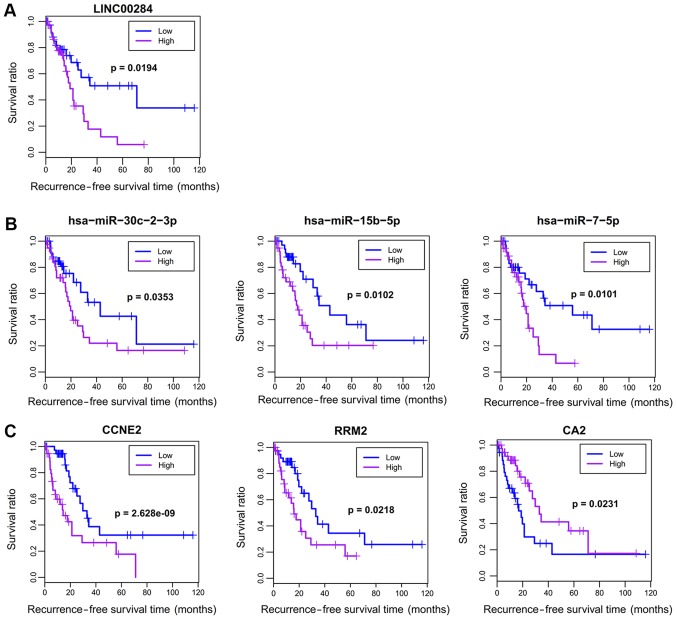
Identification of prognosis-related DELs, DEMs and DEGs in the ceRNA network
Eighty HBV-related HCC samples having OS and RFS information were collected from TCGA database. Univariate Cox regression analysis was used to screen OS- and RFS-related DELs, DEMs and DEGs from the HCC-related ceRNA network in these samples. The results revealed that six RNAs (2 DELs: FAM138B and SSTR5-AS1; 2 DEMs: hsa-miR-149 and hsa-miR-7; 2 DEGs: CCNE2 and RRM2) were significantly associated with OS, and seven RNAs (1 DEL: LINC00284; 3 DEMs: hsa-miR-7, hsa-miR-15b, hsa-miR-30c-2; and 3 DEGs: RRM2, CCNE2, CA2) were significantly associated with RFS (Table III). Survival curves were drawn for these significant prognosis-related DELs, DEMs and DEGs by Kaplan-Meier according to their expression levels in the sequencing data. As anticipated, patients with a high expression of FAM138B, hsa-miR-149-5p, hsa-miR-7-5p, hsa-miR-15b-5p, CCNE2 and RRM2 had poor survival, while patients with low expression of SSTR5-AS1 and CA2 possessed poor survival (Figs. 5 and 6). However, patients with low expression of LINC00284 and hsa-miR-30c-2 had excellent survival, which was not consistent with what we anticipated (Fig. 6). Accordingly, our results indicated the SSTR5-AS1-hsa-miR-15b-5p-CA2 ceRNA axis may be especially important for the development and prognosis of HBV-related HCC by influencing nitrogen metabolism.
Table III.
Univariate Cox regression analysis to screen OS and RFS-related genes.
| OS | RFS | ||
|---|---|---|---|
| ID | Cox-p | ID | Cox-p |
| FAM138B | 0.0012 | LINC00284 | 0.011 |
| SSTR5-AS1 | 0.011 | hsa-miR-7-1 | 0.0019 |
| hsa-miR-149 | 5.00E-04 | hsa-miR-15b | 0.021 |
| hsa-miR-7-2 | 0.037 | hsa-miR-30c-1 | 0.0335 |
| CCNE2 | 0.017 | RRM2 | 0.025 |
| RRM2 | 0.042 | CA2 | 0.028 |
| CCNE2 | 0.046 | ||
OS, overall survival; RFS, recurrence-free survival; CCNE2, cyclin E2; RRM2, ribonucleotide reductase M2 subunit.
Figure 5.
Kaplan-Meier analysis displaying the correlation of differentially expressed (A) lncRNAs, (B) miRNAs and (C) genes with overall survival outcomes for patients with HBV-related HCC. lncRNAs, long non-coding RNAs; miRNAs, microRNAs; HBV, hepatitis B virus; HCC, hepatocellular carcinoma.
Figure 6.
Kaplan-Meier analysis displaying the correlation of differentially expressed (A) lncRNAs, (B) miRNAs and (C) genes with recurrence-free survival outcomes for patients with HBV-related HCC. lncRNAs, long non-coding RNAs; miRNAs, microRNAs; HBV, hepatitis B virus; HCC, hepatocellular carcinoma.
Discussion
In the present study, the expression of lncRNAs, miRNAs and mRNAs in HBV-related HCC was comprehensively analyzed and two lncRNA-associated ceRNA axes were identified: FAM138B-hsa-miR-30c-CCNE2/RRM2 and SSTR5-AS1-has-miR-15b-5p-CA2. They were involved in HBV-related HCC by influencing the p53 signaling pathway, the cell cycle and nitrogen metabolism. In addition, all the genes in these two axes were significantly associated with the OS or RFS of patients. These findings indicated that these genes may be important prognostic biomarkers and therapeutic targets for HBV-related HCC.
Although there are studies that have demonstrated the roles of lncRNAs in cancer development (including HCC) (16,26), there are almost no studies focusing on FAM138B except for one which confirmed its prognostic value in lung adenocarcinoma (27). However, in contrast to a study by Li et al (27), FAM138B was upregulated in HCC, not downregulated. This indicates that it is necessary to verify the specific roles of FAM138B in HCC. In present study, FAM138B was predicted to possibly be involved in HBV-HCC by regulating cell cycle-related genes (CCNE2 and RRM2) by sponging hsa-miR-30c, resulting in poor prognosis. Cyclin E2 (CCNE2) is the second member of the E-type cyclin family that forms a complex with cyclin-dependent kinase 2 to promote G1/S cell cycle phase transition (28). Thus, upregulation of CCNE2 may facilitate cell cycle progression and then excessive cell proliferation, ultimately leading to the development of cancer. These hypotheses have been demonstrated by previous studies. For example, Payton et al observed that the level of CCNE2 was significantly increased in primary breast tumor samples relative to normal breast tissue controls (29). Kumari et al demonstrated that CCNE2 was frequently overexpressed in the early stages of gastric carcinoma and its expression was significantly correlated with histological type, tumor location, tumor differentiation, tumor invasion and tumor metastasis (30). Xie et al demonstrated that high CCNE2 expression was associated with worse OS (hazard ratio: 1.36, 95% confidence intervals: 1.15–1.6, P<0.01) (31). The CCNE2-CDK2 complex was also reported to mediate SAMHD1 phosphorylation and abrogate the inhibitory roles of SAMHD1 on HBV replication in hepatoma cells (32). In line with these studies, our study also revealed that CCNE2 was upregulated in tumor samples of patients with HBV-related HCC and the high expression of CCNE2 predicted poor OS and RFS. Ribonucleotide reductase M2 subunit (RRM2) is a subunit of the ribonucleotide reductase that is responsible for the reduction of ribonucleotide 5′-diphosphates into 2′-deoxyribonucleotides for DNA synthesis and replication. In addition, RRM2 also plays an active role in tumor progression, which has been confirmed in several cancers. For example, Morikawa et al revealed that RRM2 was overexpressed in 72 cases (64.3%), but rarely expressed in normal gastric mucosa. RRM2 overexpression was significantly associated with presence of muscularis propria invasion and presence of Epstein-Barr virus (33). Overexpression of RRM2 promoted their invasiveness (34), while suppression of RRM2 inhibited the growth of gastric cancer cells (33). A study by Wang et al revealed that human papillomavirus E7 induced the upregulation of RRM2 and promoted the development of cervical cancer (35). In accordance with these studies, in the present study RRM2 was predicted to be induced by HBV to promote the development of HCC and the high expression of RRM2 may serve as a poor prognostic factor. Previously, overexpression of miR-30c was reported to significantly inhibit cell proliferation and delay G1/S phase transition in hepatoma cells by blocking the HBV replication and the expression of HBV pgRNA, capsid-associated virus DNA and Hbx, indicating its tumor suppressive roles (36). As anticipated, miR-30c was also revealed to be downregulated in HBV-HCC samples. However, its association with RFS was not in line with what was anticipated, which may be attributed to the small sample size. One study indicated that transfection with mimics of the miR-30 family member, miR-30b, significantly downregulated CCNE2 expression, while the miR-30b inhibitor upregulated CCNE2 in breast cancer cell lines (37). RRM2 was also predicted to be a direct target of miR-30d in lung adenocarcinoma (38). However, there was no direct evidence to demonstrate the interaction between FAM138B and miR-30c as well as between miR-30c and CCNE2 and RRM2 in HCC.
A recent study by Ramnarine et al (39) revealed that SSTR5-AS1 was expressed at a low level in treatment-induced neuroendocrine prostate cancer, an aggressive variant of late-stage metastatic castrate-resistant prostate cancer compared with prostate adenocarcinoma; SSTR5-AS1 was able to identify patients more likely to develop metastatic disease from those that did not; its underlying mechanism was to interact with KDM4B, a histone demethylase that could regulate and epigenetically activate the N-Myc oncogene and lead to cancer development (40). However, the function of SSTR5-AS1 in cancer remains not fully elucidated. In the present study, it was predicted that downregulated SSTR5-AS1 may be involved in HBV-HCC by regulating CA2 via sponging of miR-15b-5p, resulting in poor prognosis. CA2 encodes one of isozymes of carbonic anhydrase which catalyzes reversible hydration of carbon dioxide and plays a pivotal role in tissue pH homeostasis. Several studies have revealed that carbonic anhydrase members (i.e., CA1, CA9 and CA12) may be upregulated to promote cancer cell growth, survival and invasiveness (41–43). The use of carbonic anhydrase inhibitors significantly inhibits the malignant characteristics of cancers and improves chemotherapy sensitivity (44,45). However, the expression of CA2 appears to be controversial in different cancers, such as upregulated in urinary bladder cancers (46), but downregulated in esophageal adenocarcinoma (47). A recent study performed by Zhang et al also implied that downregulation of CA2 promoted HCC metastasis and invasion (48). Consistent with the study by Zhang et al (48) and Nortunen et al (47), the present results also revealed that CA2 was significantly expressed at a lower level in HBV-HCC samples compared with the normal control, suggesting that CA2 may be a tumor suppressor gene for HCC. Extensive studies have revealed that miR-15b-5p was upregulated in cancer samples to promote tumorigenicity and progression (49,50), including HCC (51). Consistently, it was also revealed in the present study that miR-15b-5p was highly expressed in HBV-HCC tissues. However, there was no direct evidence to demonstrate the interaction between SSTR5-AS1 and miR-15b-5p as well as between miR-15b-5p and CA2 in HCC.
Some limitations are present in the present study. First, the sample size of our study was not large, which may be a reason leading to the expression difference in some targets (LINC00284 and hsa-miR-30c-2) with what was anticipated. Thus, more clinical samples should be further collected to confirm their expression (quantitative-polymerase chain reaction analysis) and clinical values. Second, this study was only performed to preliminarily identify the potential ceRNA mechanisms for HBV-related HCC. Subsequent cells (RNA pull-down) and animal (knockout or overexpression) experiments should be performed to validate the interaction relationships between DEMs and DELs/DEGs in HBV-related HCC.
In conclusion, the present study preliminarily indicated that FAM138B and SSTR5-AS1 may be novel prognostic biomarkers and therapeutic targets for HBV- associated HCC. They function as ceRNAs to sponge hsa-miR-30c and miR-15b-5p to regulate CCNE2/RRM2 and CA2.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All the microarray data were downloaded from the GEO database in NCBI (http://www.ncbi.nlm.nih.gov/geo/). The mRNA and miRNA Seq-data were obtained from The Cancer Genome Atlas (TCGA; http://tcga-data.nci.nih.gov/).
Authors' contributions
JX was involved in the conception and design of the study, drafted and revised the manuscript. JX, JW and YW collected and analyzed the data. JZ and FS contributed to the interpretation of the data and revised the manuscript critically for important intellectual content. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and informed consent
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, Su J, Sun CA, Liaw YF, Chen CJ, Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL-HBV) Study Group Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138:1747–1754. doi: 10.1053/j.gastro.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 2.Petruzziello A. Epidemiology of Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) related hepatocellular carcinoma. Open Virol J. 2018;12:26–32. doi: 10.2174/1874357901812010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jing L, Liang H, Yan J, Qiu M, Yan Y. Liver resection for hepatocellular carcinoma: Personal experiences in a series of 1330 consecutive cases in China. ANZ J Surg. 2018;88:E713–E717. doi: 10.1111/ans.14381. [DOI] [PubMed] [Google Scholar]
- 4.Costa FF. Non-coding RNAs: Meet thy masters. Bioessays. 2010;32:599–608. doi: 10.1002/bies.200900112. [DOI] [PubMed] [Google Scholar]
- 5.Sagnelli E, Potenza N, Onorato L, Sagnelli C, Coppola N, Russo A. Micro-RNAs in hepatitis B virus-related chronic liver diseases and hepatocellular carcinoma. World J Hepatol. 2018;27:558–570. doi: 10.4254/wjh.v10.i9.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rana MA, Ijaz B, Daud M, Tariq S, Nadeem T, Husnain T. Interplay of Wnt β-catenin pathway and miRNAs in HBV pathogenesis leading to HCC. Clin Res Hepatol Gastroenterol. 2019;43:373–386. doi: 10.1016/j.clinre.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Gong X, Wei W, Chen L, Xia Z, Yu C. Comprehensive analysis of long non-coding RNA expression profiles in hepatitis B virus-related hepatocellular carcinoma. Oncotarget. 2016;7:42422–42430. doi: 10.18632/oncotarget.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu TT, Xu XM, Hu Y, Deng JJ, Ge W, Han NN, Zhang MX. Long noncoding RNAs in hepatitis B virus-relatedhepatocellular carcinoma. World J Gastroenterol. 2015;21:7208–7217. doi: 10.3748/wjg.v21.i23.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du J, Bai F, Zhao P, Li X, Li X, Gao L, Ma C, Liang X. Hepatitis B core protein promotes liver cancer metastasis through miR-382-5p/DLC-1 axis. Biochim Biophys Acta Mol Cell Res. 2018;1865:1–11. doi: 10.1016/j.bbamcr.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Li G, Zhang W, Gong L, Huang X. MicroRNA-125a-5p Inhibits Cell Proliferation and Induces Apoptosis in Hepatitis B Virus-Related Hepatocellular Carcinoma by Downregulation of ErbB3. Oncol Res. 2019;27:449–458. doi: 10.3727/096504017X15016337254623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu N, Liu Q, Yang X, Zhang F, Li X, Ma Y, Guan F, Zhao X, Li Z, Zhang L, Ye X. Hepatitis B virus-upregulated lnc-HUR1 promotes cell proliferation and tumorigenesis by blocking p53 activity. Hepatology. 2018;68:2130–2144. doi: 10.1002/hep.30098. [DOI] [PubMed] [Google Scholar]
- 12.Jin Y, Wu D, Yang W, Weng M, Li Y, Wang X, Zhang X, Jin X, Wang T. Hepatitis B virus × protein induces epithelial-mesenchymal transition of hepatocellular carcinoma cells by regulating long non-coding RNA. Virol J. 2017;14:238. doi: 10.1186/s12985-017-0903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Liu G, Li X, Dong H, Xiao W, Lu S. Long non-coding RNA SBF2-AS1 promotes hepatocellular carcinoma progression through regulation of miR-140-5p-TGFBR1 pathway. Biochem Biophys Res Commun. 2018;503:2826–2832. doi: 10.1016/j.bbrc.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 14.Sui J, Yang X, Qi W, Guo K, Gao Z, Wang L, Sun D. Long non-coding RNA linc-USP16 functions as a tumour suppressor in hepatocellular carcinoma by regulating PTEN expression. Cell Physiol Biochem. 2017;44:1188–1198. doi: 10.1159/000485449. [DOI] [PubMed] [Google Scholar]
- 15.Lv J, Fan HX, Zhao XP, Lv P, Fan JY, Zhang Y, Liu M, Tang H. Long non-coding RNA unigene56159 promotes epithelial-mesenchymal transition by acting as a ceRNA of miR-140-5p in hepatocellular carcinoma cells. Cancer Lett. 2016;382:166–175. doi: 10.1016/j.canlet.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Fan H, Lv P, Mu T, Zhao X, Liu Y, Feng Y, Lv J, Liu M, Tang H. LncRNA n335586/miR-924/CKMT1A axis contributes to cell migration and invasion in hepatocellular carcinoma cells. Cancer Lett. 2018;429:89–99. doi: 10.1016/j.canlet.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Yang F, Zhang L, Huo XS, Yuan JH, Xu D, Yuan SX, Zhu N, Zhou WP, Yang GS, Wang YZ, et al. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54:1679–1689. doi: 10.1002/hep.24563. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Chen L, Gu J, Zhang H, Yuan J, Lian Q, Lv G, Wang S, Wu Y, Yang YT, et al. Recurrently deregulated lncRNAs in hepatocellular carcinoma. Nat Commun. 2017;8:14421. doi: 10.1038/ncomms14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diboun I, Wernisch L, Orengo CA, Koltzenburg M. Microarray analysis after RNA amplification can detect pronounced differences in gene expression using limma. BMC Genomics. 2006;7:252. doi: 10.1186/1471-2164-7-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalho B, Bengtsson H, Speed TP, Irizarry RA. Exploration, normalization, and genotype calls of high-density oligonucleotide SNP array data. Biostatistics. 2007;8:485–499. doi: 10.1093/biostatistics/kxl042. [DOI] [PubMed] [Google Scholar]
- 21.Nikolayeva O, Robinson MD. edgeR for differential RNA-seq and ChIP-seq analysis: An application to stem cell biology. Methods Mol Biol. 2014;1150:45–79. doi: 10.1007/978-1-4939-0512-6_3. [DOI] [PubMed] [Google Scholar]
- 22.Paraskevopoulou MD, Georgakilas G, Kostoulas N, Reczko M, Maragkakis M, Dalamagas TM, Hatzigeorgiou AG. DIANA-LncBase: Experimentally verified and computationally predicted microRNA targets on long non-coding RNAs. Nucleic Acids Res. 2013;41:D239–D245. doi: 10.1093/nar/gks1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohl M, Wiese S, Warscheid B. Cytoscape: Software for visualization and analysis of biological networks. Methods Mol Biol. 2011;696:291–303. doi: 10.1007/978-1-60761-987-1_18. [DOI] [PubMed] [Google Scholar]
- 24.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 25.Davis AP, Grondin CJ, Johnson RJ, Sciaky D, King BL, McMorran R, Wiegers J, Wiegers TC, Mattingly CJ. The comparative toxicogenomics database: Update 2017. Nucleic Acids Res. 2017;45:D972–D978. doi: 10.1093/nar/gkw838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lan T, Ma W, Hong Z, Wu L, Chen X, Yuan Y. Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12) promotes tumorigenesis and metastasis by targeting miR-199a/b-5p in hepatocellular carcinoma. J Exp Clin Cancer Res. 2017;36:11. doi: 10.1186/s13046-016-0486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Li B, Ran P, Wang L. Identification of ceRNA network based on a RNA-seq shows prognostic lncRNA biomarkers in human lung adenocarcinoma. Oncol Lett. 2018;16:5697–5708. doi: 10.3892/ol.2018.9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauper N, Beck AR, Cariou S, Richman L, Hofmann K, Reith W, Slingerland JM, Amati B. Cyclin E2: A novel CDK2 partner in the late G1 and S phases of the mammalian cell cycle. Oncogene. 1998;17:2637–2643. doi: 10.1038/sj.onc.1202477. [DOI] [PubMed] [Google Scholar]
- 29.Payton M, Scully S, Chung G, Coats S. Deregulation of cyclin E2 expression and associated kinase activity in primary breast tumors. Oncogene. 2002;21:8529–8534. doi: 10.1038/sj.onc.1206035. [DOI] [PubMed] [Google Scholar]
- 30.Kumari S, Puneet, Prasad SB, Yadav SS, Kumar M, Khanna A, Dixit VK, Nath G, Singh S, Narayan G. Cyclin D1 and cyclin E2 are differentially expressed in gastric cancer. Med Oncol. 2016;33:40. doi: 10.1007/s12032-016-0754-8. [DOI] [PubMed] [Google Scholar]
- 31.Xie L, Li T, Yang LH. E2F2 induces MCM4, CCNE2 and WHSC1 upregulation in ovarian cancer and predicts poor overall survival. Eur Rev Med Pharmacol Sci. 2017;21:2150–2156. [PubMed] [Google Scholar]
- 32.Hu J, Qiao M, Chen Y, Tang H, Zhang W, Tang D, Pi S, Dai J, Tang N, Huang A, Hu Y. Cyclin E2-CDK2 mediate SAMHD1 phosphorylation to abrogate its restriction of HBV replication in hepatoma cells. FEBS Lett. 2018;592:1893–1904. doi: 10.1002/1873-3468.13105. [DOI] [PubMed] [Google Scholar]
- 33.Morikawa T, Hino R, Uozaki H, Maeda D, Ushiku T, Shinozaki A, Sakatani T, Fukayama M. Expression of ribonucleotide reductase M2 subunit in gastric cancer and effects of RRM2 inhibition in vitro. Hum Pathol. 2010;41:1742–1748. doi: 10.1016/j.humpath.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Zhong Z, Cao Y, Yang S, Zhang S. Overexpression of RRM2 in gastric cancer cell promotes their invasiveness via AKT/NF-κB signaling pathway. Pharmazie. 2016;71:280–284. [PubMed] [Google Scholar]
- 35.Wang N, Zhan T, Ke T, Huang X, Ke D, Wang Q, Li H. Increased expression of RRM2 by human papillomavirus E7 oncoprotein promotes angiogenesis in cervical cancer. Br J Cancer. 2014;110:1034–1044. doi: 10.1038/bjc.2013.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Ma J, Wang H, Guo L, Li J. Serum microRNA-30c levels are correlated with disease progression in Xinjiang Uygur patients with chronic hepatitis B. Braz J Med Biol Res. 2017;50:e6050. doi: 10.1590/1414-431x20176050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tormo E, Adam-Artigues A, Ballester S, Pineda B, Zazo S, González-Alonso P, Albanell J, Rovira A, Rojo F, Lluch A, Eroles P. The role of miR-26a and miR-30b in HER2 + breast cancer trastuzumab resistance and regulation of the CCNE2 gene. Sci Rep. 2017;7:41309. doi: 10.1038/srep41309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arima C, Kajino T, Tamada Y, Imoto S, Shimada Y, Nakatochi M, Suzuki M, Isomura H, Yatabe Y, Yamaguchi T, et al. Lung adenocarcinoma subtypes definable by lung development-related miRNA expression profiles in association with clinicopathologic features. Carcinogenesis. 2014;35:2224–2231. doi: 10.1093/carcin/bgu127. [DOI] [PubMed] [Google Scholar]
- 39.Ramnarine VR, Alshalalfa M, Mo F, Nabavi N, Erho N, Takhar M, Shukin R, Brahmbhatt S, Gawronski A, Kobelev M, et al. The long noncoding RNA landscape of neuroendocrine prostate cancer and its clinical implications. Gigascience. 2018;7:giy050. doi: 10.1093/gigascience/giy050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J, AlTahan AM, Hu D, Wang Y, Cheng PH, Morton CL, Qu C, Nathwani AC, Shohet JM, Fotsis T, et al. The role of histone demethylase KDM4B in Myc signaling in neuroblastoma. J Natl Cancer Inst. 2015;107:djv080. doi: 10.1093/jnci/djv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hussain SA, Ganesan R, Reynolds G, Gross L, Stevens A, Pastorek J, Murray PG, Perunovic B, Anwar MS, Billingham L, et al. Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. Br J Cancer. 2007;96:104–109. doi: 10.1038/sj.bjc.6603530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang DB, Lu XK, Zhang X, Li ZG, Li CX. Carbonic anhydrase 1 is a promising biomarker for early detection of non-small cell lung cancer. Tumour Biol. 2016;37:553–559. doi: 10.1007/s13277-015-3834-z. [DOI] [PubMed] [Google Scholar]
- 44.Kazokaitė J, Ames S, Becker HM, Deitmer JW, Matulis D. Selective inhibition of human carbonic anhydrase IX in Xenopus oocytes and MDA-MB-231 breast cancer cells. J Enzyme Inhib Med Chem. 2016;31:38–44. doi: 10.1080/14756366.2016.1217854. [DOI] [PubMed] [Google Scholar]
- 45.Parkkila S, Rajaniemi H, Parkkila AK, Kivela J, Waheed A, Pastorekova S, Pastorek J, Sly WS. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci USA. 2000;97:2220–2224. doi: 10.1073/pnas.040554897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tachibana H, Gi M, Kato M, Yamano S, Fujioka M, Kakehashi A, Hirayama Y, Koyama Y, Tamada S, Nakatani T, Wanibuchi H. Carbonic anhydrase 2 is a novel invasion-associated factor in urinary bladder cancers. Cancer Sci. 2017;108:331–337. doi: 10.1111/cas.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nortunen M, Huhta H, Helminen O, Parkkila S, Kauppila JH, Karttunen TJ, Saarnio J. Carbonic anhydrases II, IX, and XII in Barrett's esophagus and adenocarcinoma. Virchows Arch. 2018;473:567–575. doi: 10.1007/s00428-018-2424-z. [DOI] [PubMed] [Google Scholar]
- 48.Zhang C, Wang H, Chen Z, Zhuang L, Xu L, Ning Z, Zhu Z, Wang P, Meng Z. Carbonic anhydrase 2 inhibits epithelial-mesenchymal transition and metastasis in hepatocellular carcinoma. Carcinogenesis. 2018;39:562–570. doi: 10.1093/carcin/bgx148. [DOI] [PubMed] [Google Scholar]
- 49.Zhao C, Li Y, Chen G, Wang F, Shen Z, Zhou R. Overexpression of miR-15b-5p promotes gastric cancer metastasis by regulating PAQR3. Oncol Rep. 2017;38:352–358. doi: 10.3892/or.2017.5673. [DOI] [PubMed] [Google Scholar]
- 50.Chen R, Sheng L, Zhang HJ, Ji M, Qian WQ. miR-15b-5p facilitates the tumorigenicity by targeting RECK and predicts tumour recurrence in prostate cancer. J Cell Mol Med. 2018;22:1855–1863. doi: 10.1111/jcmm.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y, Chen J, Liu Y, Li S, Huang P. Plasma miR-15b-5p, miR-338-5p, and miR-764 as biomarkers for hepatocellular carcinoma. Med Sci Monit. 2015;21:1864–1871. doi: 10.12659/MSM.893082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the microarray data were downloaded from the GEO database in NCBI (http://www.ncbi.nlm.nih.gov/geo/). The mRNA and miRNA Seq-data were obtained from The Cancer Genome Atlas (TCGA; http://tcga-data.nci.nih.gov/).